Abstract
Models generally predict a response in species richness to climate, but strong climate-diversity associations are seldom observed in long-term (more than 106 years) fossil records. Moreover, fossil studies rarely distinguish between the effects of atmospheric CO2 and temperature, which limits their ability to identify the causal controls on biodiversity. Plants are excellent organisms for testing climate-diversity hypotheses owing to their strong sensitivity to CO2, temperature and moisture. We find that pollen morphospecies richness in an angiosperm-dominated record from the Palaeogene and early Neogene (65–20 Ma) of Colombia and Venezuela correlates positively to CO2 much more strongly than to temperature (both tropical sea surface temperatures and estimates of global mean surface temperature). The weaker sensitivity to temperature may be due to reduced variance in long-term climate relative to in higher latitudes, or to the occurrence of lethal or sub-lethal temperatures during the warmest times of the Eocene. Physiological models predict that productivity should be the most sensitive to CO2 within the angiosperms, a prediction supported by our analyses if productivity is linked to species richness; however, evaluations of non-angiosperm assemblages are needed to more completely test this idea.
Keywords: plant diversity, carbon dioxide, temperature, Cenozoic, neotropics
1. Introduction
Understanding the controls on biodiversity is a central goal in biology [1,2]. Von Humboldt [3] noted the latitudinal gradient in diversity over two centuries ago and discussed its possible link with climate. Ever since, climate has remained on the ‘short list’ of candidate factors for driving the diversity in many biological groups [1,2].
Most studies examining climate–diversity hypotheses use present-day observations or conceptual models [1,2,4,5], but a growing number of studies have turned to the fossil record [6–12], trading space for time. Many fossil analyses focus on the Phanerozoic (last 542 Myr) marine invertebrate record compiled by Jack Sepkoski [13] and expanded by the Palaeobiology Database [14]. Unfortunately, these studies come to contradictory conclusions, ranging from a positive correlation between climate and diversity [6,7], a negative correlation [8,9] and no significant correlation [10]. Studies focused on the Cenozoic (last 66 Myr) find a weak positive climate–diversity link in planktonic foraminifera [11] and mixed relationships for North American mammals [12,15]. In short, these biological groups show, at best, an equivocal long-term (more than 106 years) relationship between diversity and climate. Furthermore, most of these studies do not distinguish between the effects of atmospheric CO2 and temperature for the simple reason that CO2 and temperature correlate broadly with one another on geologic timescales [16]; this makes differentiating their influence on biodiversity patterns difficult [9]. One attempt at this differentiation found that the Phanerozoic record of marine invertebrates is better explained by temperature than by CO2 [6].
Plants are excellent candidates for investigating climate–diversity hypotheses because as sessile primary producers many of their traits are highly sensitive to climate [17]. In particular, plants depend directly on atmospheric CO2 for food and so, compared with heterotrophic groups, plant diversity may be more strongly affected by CO2. Models support a positive relationship between plant species richness and both CO2 and temperature [1,2,4,5,18]. This is because in most plants CO2 and temperature affect productivity, as well as water- and nutrient-use efficiency. If increased productivity leads to more individuals, both in existing environments and in previously water- or nutrient-limited environments, then this may reduce extinction rates and therefore increase species richness; other traits sensitive to temperature such as metabolic strategy may also be important for affecting richness [1].
Here, we investigate how atmospheric CO2 and temperature relate to an angiosperm-dominated record of plant diversity from pollen in central Colombia and western Venezuela for the Palaeogene and early Neogene (65–20 Ma) [19]. Jaramillo et al. [19] found a significant positive correlation between pollen morphospecies richness and temperature. We seek here to test the effects of both CO2 and temperature. Owing to the multiple fundamental roles CO2 plays in plants, we hypothesize that CO2 will be at least as important as temperature for explaining the observed record of plant diversity.
2. Material and methods
(a). Datasets
The record of pollen morphospecies richness comes from Jaramillo et al. [19], who analysed 1060 samples from the Palaeogene and early Neogene (65–20 Ma) of central Colombia and western Venezuela (figure 1). Atmospheric CO2 comes from the compilation of Beerling & Royer [20] and subsequent updates [21,22]; together, there are 168 independent estimates for our studied interval (figure 1a). Benthic δ18O comes from the compilation of Zachos et al. [23] (n = 6649 for our interval; figure 1b). Most previous climate–diversity studies correlate directly with benthic δ18O; here, we transform δ18O to global mean surface temperature following the approach of Royer et al. [24], which includes an ice-volume correction, an important factor after 34 Ma. This particular transformation probably underestimates peak temperatures during the Eocene [24]. Tropical sea surface temperatures (SSTs) may have been partially decoupled from the benthic δ18O record during the Eocene [25]. Because our diversity record comes from the tropics, we also included in our analyses a compilation of tropical SST (n = 76) [25–27].
Figure 1.
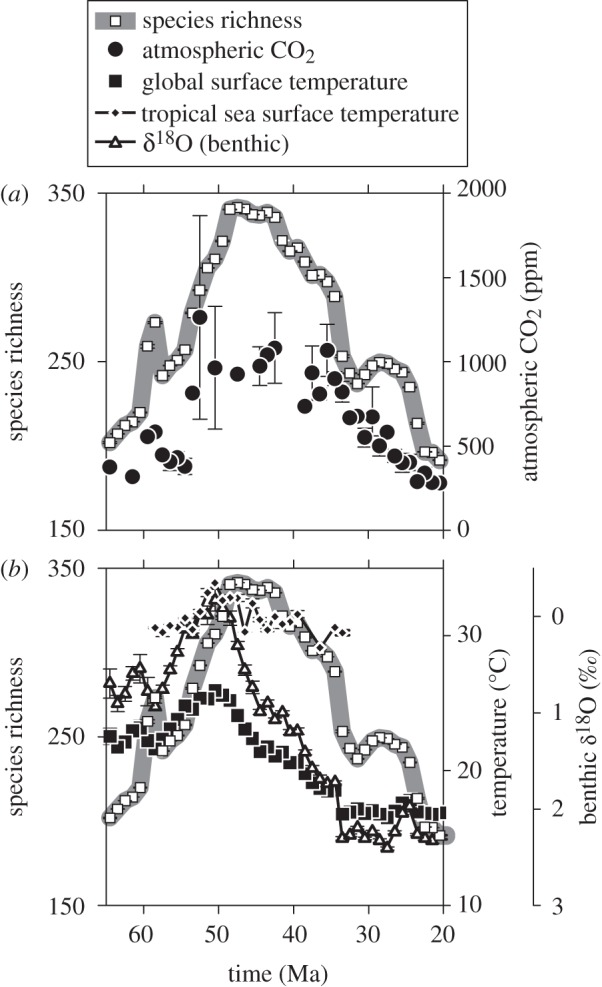
Temporal patterns in pollen morphospecies richness, atmospheric CO2 and temperature. Each data point is the mean of a 1 Myr time-step. Bars represent standard errors of the mean (many are smaller than the symbols). Species richness data are identical in (a,b).
For each dataset, we calculated arithmetic means of 1 Myr time bins. The diversity, benthic δ18O, and global mean surface temperature series have no empty bins, CO2 has 11, and tropical SST 22. We transformed each series to have a mean of zero and a standard deviation of one (z distribution). This aids in visualization, for example, the self-similarity in the patterns of benthic δ18O and estimated global mean surface temperature (figure 2b).
Figure 2.
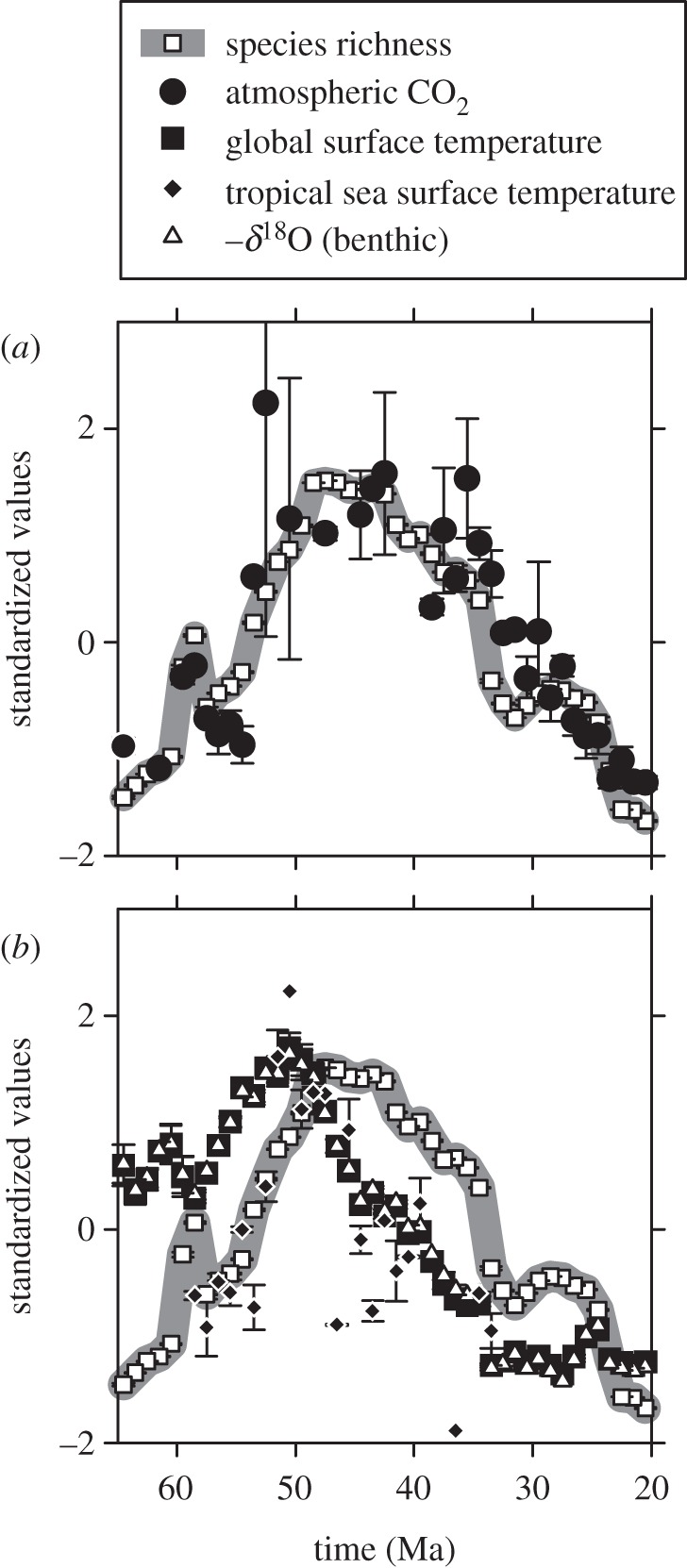
Standardized temporal patterns in pollen morphospecies richness, atmospheric CO2 and temperature. Each data point is the mean of a 1 Myr time-step, then transformed to a z distribution (series mean = 0 and s.d. = 1). Bars represent standard errors of the mean (many are smaller than the symbols). Species richness data are identical in (a,b).
One risk with inferring causation from correlational analyses is that an additional factor or factors may be important. In climate–diversity studies, particularly those encompassing the entire Phanerozoic, one such potential factor is the general rise in diversity to the present [13,14]. To minimize the influence of these types of factor, especially those that operate on longer timescales than the temporal resolution of the analysis (1 Myr here), first differences [10,12,19] or residuals to a spline fit [6,9] are often first computed. Although a factor such as biological escalation is probably less important in a Cenozoic- versus Phanerozoic-scale study, we include a first-difference analysis. The true levels of significance from first-difference correlations are probably underestimated because all long-term trends are eliminated, while those from uncorrected correlations may be overestimated. For first differences, we do not analyse the benthic δ18O dataset owing to its near identity to global mean surface temperature (figure 2b), nor the tropical SST dataset owing to its sparseness (25 missing bins).
(b). Analyses
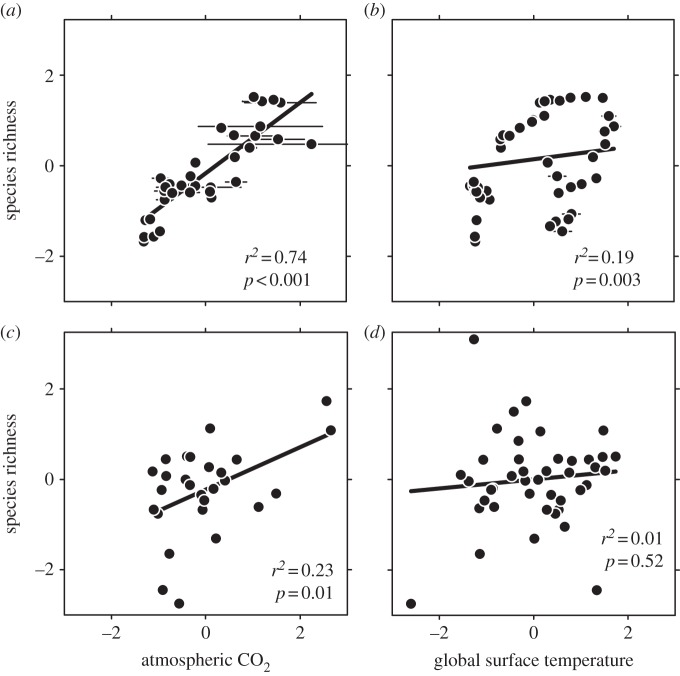
All analyses were performed in Statistica (v. 8; Stat Soft, Inc.). Each of our input series (figure 1) show temporal autocorrelation, which can inflate correlational coefficients (r of lag-1 autocorrelation = 0.91 for morphospecies richness, 0.77 for CO2, 0.95 for benthic δ18O and global surface temperature, and 0.46 for tropical SST). To minimize this effect, we computed all correlational coefficients (figure 3 and table 1) with a bootstrap routine, resampling with replacement 1000 times assuming a uniform distribution. The regressions (and associated p-values) in figure 3 are type II regressions, which account for error in both independent and dependent variables.
Figure 3.
Crossplots of atmospheric CO2 and global surface temperature against pollen morphospecies richness. (a,b) Standardized scores (identical to data in figure 2). Bars represent standard errors of the mean (both for x-axis and y-axis; many are smaller than the symbols). (c,d) Standardized first-difference scores. First differences are computed from the data in figure 1, then transformed to a z distribution (series mean = 0 and s.d. = 1). Type II regressions and their associated p-values are plotted in all panels; companion r2 values come from a bootstrap approach (see also table 1).
Table 1.
Relationships between pollen morphospecies richness, temperature and atmospheric CO2.
| r (±s.e.m.) | |
|---|---|
| z distribution | |
| richness versus CO2 | 0.86 ± 0.008 |
| richness versus benthic δ18O | −0.45 ± 0.03 |
| richness versus global surface temperature | 0.44 ± 0.03 |
| richness versus tropical sea surface temperature | 0.41 ± 0.03 |
| CO2 versus global surface temperature | 0.29 ± 0.03 |
| first difference + z distribution | |
| richness versus CO2 | 0.47 ± 0.02 |
| richness versus global surface temperature | 0.10 ± 0.03 |
| CO2 versus global surface temperature | 0.06 ± 0.03 |
To investigate the combined effects of CO2 and temperature on diversity, we ran a type II multiple linear regression with backwards stepwise reduction. This procedure sequentially removes input variables that are not contributing significantly to the explanatory power of the regression. As a cross-check, we examined these same relationships with a path analytic approach (structural equation modelling). As with the multiple regression analysis, diversity was the dependent variable and various combinations of climate were the independent variables. The models were generated under a Monte Carlo bootstrapping procedure with 1000 replicates, with the means and standard errors calculated from the bootstrapped distributions. Goodness of fit was quantified with the Akaike information criterion (AIC). Path analysis accounts for collinearity, but as a complementary test we also directly removed the covariance between standardized CO2 and global surface temperature with a partial correlation analysis. Standardized diversity was then regressed separately on the independent partitions of standardized CO2 and global surface temperature.
(c). Strengths of study design
Our study design has several advantages over many previous efforts. First and most critically, atmospheric CO2 and temperature can be more readily deconvolved for our selected time interval. Although CO2 and temperature are generally coupled during the Cenozoic [20,24], two intervals exhibit a weaker relationship. First, during the Palaeocene (66–56 Ma) CO2 was mostly under 500 ppm and similar to values during the Oligocene (33.9–23.0 Ma), while global temperatures were distinctly warmer (approx. more than 5°C; figure 1). Second, CO2 stayed fairly constant at approximately 1000 ppm from approximately 50–35 Ma, while global temperatures dropped considerably (figure 1). Importantly, these periods of partial decoupling are each recorded by multiple palaeo-CO2 methods [20]. These differences offer a rare opportunity to distinguish between the roles of CO2 and temperature on diversity.
A second advantage to our approach is that the plant record comes from a limited number of stratigraphic sections (n = 15) that were sampled for pollen in a uniform way by one laboratory group [19]. As a result, the record is largely unbiased by sampling effort. Such a bias has hindered many previous investigations, especially Phanerozoic-scale studies, because intervals with high species richness tend to be the most intensely sampled [14,28]. Tools are available to account for the bias [14], but two recent studies using them come to contradictory conclusions about Phanerozoic climate–diversity relationships in marine invertebrates [6,10].
A third advantage is that the stratigraphic sections sample a fairly uniform environment: the lowland wet neotropics. This minimizes the likelihood of major biome shifts and contrasts with most Phanerozoic global compilations which sample across many environments. The evolution of the Andes profoundly impacted the biogeography of South America, but mountain building in the north peaked largely after our studied interval (23–12 Ma) [29]. Intracanopy variations in CO2 in tropical forests (typically approx. 100 ppm [30]) presumably have not changed significantly over time and are smaller than the long-term atmospheric changes studied here (approx. 500 ppm; figure 1a).
A final advantage is that the climate records of benthic δ18O [23] and atmospheric CO2 [20] are comparatively rich for our chosen interval, allowing for a temporal resolution of 1 Myr for all analysis; this contrasts with Phanerozoic-scale studies, which are typically limited to an approximately 10 Myr resolution [6–10]. In addition, temperature estimates from the δ18O of marine carbonate become increasingly suspect in the pre-Cretaceous [31], an issue we avoid with our younger chosen interval.
3. Results
Pollen morphospecies richness [19] rises through the Palaeocene and early Eocene, with one shorter-term spike in the late Palaeocene (60–58 Ma; figure 1). Richness remains high and largely unchanging during the middle Eocene (49–42 Ma) before falling, including a sharp drop around the Eocene–Oligocene boundary (33.9 Ma; figure 1). Atmospheric CO2 shows a strikingly similar pattern, including the sharp late Palaeocene spike (figure 1a). The similarity is more apparent once the two series are transformed to the same scale (figure 2a).
In contrast, temperature correlates more weakly to diversity. The benthic δ18O record and its transformation to global mean surface temperature show a comparatively more gradual ramp-up to the early Eocene (the ‘early Eocene climatic optimum’), with no evidence for a late Palaeocene spike; temperatures then begin to cool immediately, with no plateau between 49 and 42 Ma (figures 1b and 2b). Tropical SST data are more sparse, but do show a rise during the Palaeocene and early Eocene similar in scale to the diversity and CO2 records; however, the steep cooling after the early Eocene climatic optimum closely mirrors the global temperature record but not the diversity and CO2 records (figure 2b).
A complementary suite of statistical analyses support a stronger association between richness and CO2 than to temperature. The correlation coefficients between CO2 and richness are much higher than between temperature and richness (table 1) whether for standardized (z distribution; figure 3a,b) or standardized first-difference (figure 3c,d) values. The regression coefficients of richness on CO2 and temperature are both highly significant (p < 0.01) but the adjusted r2s are disproportionate with CO2 and temperature explaining 73.3 per cent and 17.2 per cent of the variance in richness, respectively. Regression analysis of standardized first-difference data reveals a significant effect for CO2 (p = 0.01; adjusted r2 = 0.19) but not for global mean surface temperature (p = 0.52; adjusted r2 = −0.01). CO2 and temperature correlate only weakly (tables 1 and 2); not surprisingly, then, the partial correlation between standardized diversity and CO2 holding global surface temperature constant was significant (r = 0.77, p < 0.01), whereas the partial correlation between standardized diversity and global surface temperature holding CO2 constant was not significant (r = 0.18, p = 0.32). Interestingly, the strength of correlation between standardized diversity and both global surface temperature and CO2 improves somewhat when diversity is lagged by several million years; it is not clear if this reflects a true lag in the response of forest diversity to climate change.
Table 2.
Path analysis for pollen morphospecies richness, global surface temperature and atmospheric CO2.
| r | p-value | |
|---|---|---|
| z distribution | ||
| CO2 → richness | 0.85 | <0.001 |
| global surface temperature → richness | 0.19 | 0.02 |
| CO2 → global surface temperature | 0.29 | 0.07 |
| first difference + z distribution | ||
| CO2 → richness | 0.47 | 0.002 |
| global surface temp → richness | 0.34 | 0.06 |
| CO2 → global surface temperature | 0.06 | 0.76 |
For multiple linear regression models built to explain richness from CO2 and temperature, CO2 always has the most explanatory power. Reverse algorithms that remove independent variables of little-to-no-significance uniformly eliminate all temperature variables, leaving only a univariate model with CO2 for both standardized and first-difference data (p < 0.001 and p = 0.01, respectively). Finally, path analysis (structural equation modelling) supports the greater importance of CO2 over temperature for explaining pollen morphotype richness. AIC values are lowest for a model that includes standardized richness, CO2 and global mean surface temperature (0.099 ± 0.02 versus 0.15 ± 0.01 for substituting in tropical SST and 0.54 ± 0.03 for including both temperature variables); in this model, CO2 is the strongest correlate of richness (table 2). Path analysis of standardized first-difference data finds that only the effect of CO2 on richness is significant (table 2), in keeping with the multiple regression analysis. These same patterns are also observed from the regressions of standardized diversity on the residuals of CO2 and global surface temperature from the partial correlation analyses (F1,32 = 4.3, p < 0.01; F1,32 = 1.01, p = 0.32, respectively).
4. Discussion
Pollen morphospecies richness from the neotropics of Colombia and Venezuela [19] is more strongly correlated with atmospheric CO2 than with temperature (figure 3; tables 1 and 2). This is true even though in the CO2 dataset uncertainties are large (especially at high CO2) and some of the million year bins are empty (figure 1a). Our interpreted patterns hold whether or not the data are transformed by their first difference and whether or not the data are analysed univariately or multivariately. Tropical SST, which should be more appropriate than global mean surface temperature for correlating with tropical diversity, shows the same weaker link to richness. Atmospheric CO2 is the only dataset that mirrors the low richness values at the beginning (Palaeocene) and end (Miocene) of the time series, sustained high values during the mid-Eocene, and a short-term spike in the late Palaeocene.
Theoretical considerations support a positive relationship between temperature and plant diversity (see §1). While some of our analyses uphold this view, others do not, most notably the first differences (figure 3c,d; tables 1 and 2). Indeed, because the diversity correlations to temperature are always weaker than those to CO2, it is possible that temperature is simply secondarily related. For a plant record from the tropics, this may make sense. First, long-term diversity records from heterotrophic groups show no consistently strong associations with benthic δ18O; coupled to this, plant productivity is sensitive to CO2, and productivity has been linked to species richness (see §1). Together, this suggests a model whereby diversity in most organisms is at best weakly linked to temperature, but diversity in primary producers (including plants) is positively linked to CO2. Second, CO2 is a globally mixed gas but climate-driven temperature changes are muted in the tropics relative to in higher latitudes [32]. Thus, the lever-arm for temperature to affect tropical diversity is comparatively shorter than that for CO2; as a result, it is plausible for temperature to be positively correlated to richness more strongly in higher latitudes than in the tropics. During the early Eocene climatic optimum (approx. 50 Ma), it is even possible that in the tropics temperature was negatively related to plant diversity because temperatures may have regularly reached lethal or near-lethal levels ([33] but see also [34]). Our analysis provides some support for such a negative coupling, because richness peaked after the climatic optimum as temperatures were sharply dropping but CO2 remained high (figure 2).
Paradoxically, major plant groups often evolve during periods of falling CO2, including the angiosperms in the Cretaceous [35,36]. These radiations were likely aided by advances in physiological performance that improved competitiveness in a falling CO2 world. For angiosperms, such adaptations may have included unprecedented high vein density, the proliferation of vessel elements and improved stomatal control [35,37–39]. We argue that these patterns are not at odds with our interpretations: within a physiological equivalent group of plants (e.g. angiosperms, the dominant group in the pollen record), diversity should be stimulated by CO2, but across groups large differences in physiological performance may cause one group to outcompete another when CO2 drops. In fact, because of their uniquely high photosynthetic rates, angiosperms may be the only plant group whose productivity is strongly stimulated by CO2 [40], and by extension whose diversity is positively linked to CO2. Additional palaeobotanical studies with non-angiosperm groups are needed to test this physiological prediction.
Although our analyses support a positive coupling between CO2 and plant species richness over long timescales, we do not consider these patterns applicable to the near future because changes in addition to CO2 are occurring (e.g. reduction in habitat area, spread of invasive species). Indeed, examples from the geological past, such as the mass extinction at the Cretaceous–Palaeogene boundary (66 Ma), provide evidence for a drop in plant diversity [41] with rising CO2 [42] when other dramatic global environmental changes are co-occurring [43].
Acknowledgements
We thank the Mellon Foundation and Menakka and Essel Bailey for generous support of the College of the Environment.
References
- 1.Erwin DH. 2009. Climate as a driver of evolutionary change. Curr. Biol. 19, R575–R583 10.1016/j.cub.2009.05.047 (doi:10.1016/j.cub.2009.05.047) [DOI] [PubMed] [Google Scholar]
- 2.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 10.1890/03-8006 (doi:10.1890/03-8006) [DOI] [Google Scholar]
- 3.Von Humboldt A. 1808. Ansichten der Natur mit wissenschaftlichen Erläuterungen. Tübingen, Germany: J.G. Cotta [Google Scholar]
- 4.Currie DJ. 2001. Projected effects of climate change on patterns of vertebrate and tree species richness in the conterminous United States. Ecosystems 4, 216–225 10.1007/s10021-001-0005-4 (doi:10.1007/s10021-001-0005-4) [DOI] [Google Scholar]
- 5.Hansen AJ, Neilson RP, Dale VH, Flather CH, Iverson LR, Currie DJ, Shafer S, Cook R, Bartlein PJ. 2001. Global change in forests: responses of species, communities, and biomes. BioScience 51, 765–779 10.1641/0006-3568(2001)051[0765:GCIFRO]2.0.CO;2 (doi:10.1641/0006-3568(2001)051[0765:GCIFRO]2.0.CO;2) [DOI] [Google Scholar]
- 6.Mayhew PJ, Bell MA, Benton TG, McGowan AJ. 2012. Biodiversity tracks temperature over time. Proc. Natl Acad. Sci. USA 109, 15 141–15 145 10.1073/pnas.1200844109 (doi:10.1073/pnas.1200844109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornette JL, Lieberman BS, Goldstein RH. 2002. Documenting a significant relationship between macroevolutionary origination rates and Phanerozoic pCO2 levels. Proc. Natl Acad. Sci. USA 99, 7832–7835 10.1073/pnas.122225499 (doi:10.1073/pnas.122225499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman DH. 2001. Global biodiversity and the ancient carbon cycle. Proc. Natl Acad. Sci. USA 98, 4305–4310 10.1073/pnas.071047798 (doi:10.1073/pnas.071047798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayhew PJ, Jenkins GB, Benton TG. 2008. A long-term association between global temperature and biodiversity, origination and extinction in the fossil record. Proc. R. Soc. B 275, 47–53 10.1098/rspb.2007.1302 (doi:10.1098/rspb.2007.1302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannisdal B, Peters SE. 2011. Phanerozoic Earth system evolution and marine biodiversity. Science 334, 1121–1124 10.1126/science.1210695 (doi:10.1126/science.1210695) [DOI] [PubMed] [Google Scholar]
- 11.Ezard THG, Aze T, Pearson PN, Purvis A. 2011. Interplay between changing climate and species’ ecology drives macroevolutionary dynamics. Science 332, 349–351 10.1126/science.1203060 (doi:10.1126/science.1203060) [DOI] [PubMed] [Google Scholar]
- 12.Alroy J, Koch PL, Zachos JC. 2000. Global climate change and North American mammalian evolution. Paleobiology 26(Suppl.), 259–288 10.1666/0094-8373(2000)26[259:GCCANA]2.0.CO;2 (doi:10.1666/0094-8373(2000)26[259:GCCANA]2.0.CO;2) [DOI] [Google Scholar]
- 13.Sepkoski JJ. 2002. A compendium of fossil marine animal genera. Bull. Am. Paleontol. 363, 1–560 [Google Scholar]
- 14.Alroy J, et al. 2008. Phanerozoic trends in the global diversity of marine invertebrates. Science 321, 97–100 10.1126/science.1156963 (doi:10.1126/science.1156963) [DOI] [PubMed] [Google Scholar]
- 15.Figueirido B, Janis CM, Pérez-Claros JA, De Renzi M, Palmqvist P. 2012. Cenozoic climate change influences mammalian evolutionary dynamics. Proc. Natl Acad. Sci. USA 109, 722–727 10.1073/pnas.1110246108 (doi:10.1073/pnas.1110246108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royer DL. 2006. CO2-forced climate thresholds during the Phanerozoic. Geochim. Cosmochim. Acta 70, 5665–5675 10.1016/j.gca.2005.11.031 (doi:10.1016/j.gca.2005.11.031) [DOI] [Google Scholar]
- 17.Beerling DJ, Woodward FI. 2001. Vegetation and the terrestrial carbon cycle. Cambridge, UK: Cambridge University Press [Google Scholar]
- 18.Barrett PM, Willis KJ. 2001. Did dinosaurs invent flowers? Dinosaur-angiosperm coevolution revisited. Biol. Rev. 76, 411–447 10.1017/S1464793101005735 (doi:10.1017/S1464793101005735) [DOI] [PubMed] [Google Scholar]
- 19.Jaramillo C, Rueda MJ, Mora G. 2006. Cenozoic plant diversity in the Neotropics. Science 311, 1893–1896 10.1126/science.1121380 (doi:10.1126/science.1121380) [DOI] [PubMed] [Google Scholar]
- 20.Beerling DJ, Royer DL. 2011. Convergent Cenozoic CO2 history. Nat. Geosci. 4, 418–420 10.1038/ngeo1186 (doi:10.1038/ngeo1186) [DOI] [Google Scholar]
- 21.Pagani M, Huber M, Liu Z, Bohaty SM, Henderiks J, Sijp W, Krishnan S, DeConto RM. 2011. The role of carbon dioxide during the onset of Antarctic glaciation. Science 334, 1261–1264 10.1126/science.1203909 (doi:10.1126/science.1203909) [DOI] [PubMed] [Google Scholar]
- 22.Grein M, Konrad W, Wilde V, Utescher T, Roth-Nebelsick A. 2011. Reconstruction of atmospheric CO2 during the early Middle Eocene by application of a gas exchange model to fossil plants from the Messel Formation, Germany. Paleogeogr. Paleoclimatol. Paleoecol. 309, 383–391 10.1016/j.palaeo.2011.07.008 (doi:10.1016/j.palaeo.2011.07.008) [DOI] [Google Scholar]
- 23.Zachos JC, Dickens GR, Zeebe RE. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283 10.1038/nature06588 (doi:10.1038/nature06588) [DOI] [PubMed] [Google Scholar]
- 24.Royer DL, Pagani M, Beerling DJ. 2012. Geobiological constraints on Earth system sensitivity to CO2 during the Cretaceous and Cenozoic. Geobiology 4, 298–310 10.1111/j.1472-4669.2012.00320.x (doi:10.1111/j.1472-4669.2012.00320.x) [DOI] [PubMed] [Google Scholar]
- 25.Pearson PN, van Dongen BE, Nicholas CJ, Pancost RD, Schouten S, Singano JM, Wade BS. 2007. Stable warm tropical climate through the Eocene Epoch. Geology 35, 211–214 10.1130/G23175A.1 (doi:10.1130/G23175A.1) [DOI] [Google Scholar]
- 26.Tripati A, Delaney ML, Zachos JC, Anderson LD, Kelly DC, Elderfield H. 2003. Tropical sea-surface temperature reconstruction for the early Paleogene using Mg/Ca ratios of planktonic foraminifera. Paleoceanography 18, 1101. 10.1029/2003PA000937 (doi:10.1029/2003PA000937) [DOI] [Google Scholar]
- 27.Sexton PF, Wilson PA, Pearson PN. 2006. Microstructural and geochemical perspectives on planktic foraminiferal preservation: ‘glassy’ versus ‘frosty’. Geochem. Geophys. Geosyst. 7, Q12P19. 10.1029/2006GC001291 (doi:10.1029/2006GC001291) [DOI] [Google Scholar]
- 28.Peters SE, Foote M. 2001. Biodiversity in the Phanerozoic: a reinterpretation. Paleobiology 27, 583–601 (doi:10.1666/0094-8373(2001)027<0583:BITPAR>2.0.CO;2) [DOI] [Google Scholar]
- 29.Hoorn C, et al. 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931 10.1126/science.1194585 (doi:10.1126/science.1194585) [DOI] [PubMed] [Google Scholar]
- 30.Quay P, King S, Wilbur D, Wofsy S, Rickey J. 1989. 13C/12C of atmospheric CO2 in the Amazon Basin: forest and river sources. J. Geophys. Res. 94, 18 327–18 336 10.1029/JD094iD15p18327 (doi:10.1029/JD094iD15p18327) [DOI] [Google Scholar]
- 31.Royer DL, Berner RA, Montañez IP, Tabor NJ, Beerling DJ. 2004. CO2 as a primary driver of Phanerozoic climate. GSA Today 14(3), 4–10 (doi:10.1130/1052-5173(2004)014<4:CAAPDO>2.0.CO;2) [DOI] [Google Scholar]
- 32.IPCC 2007. Climate Change 2007: the physical science basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Head JJ, Bloch JI, Hastings AK, Bourque JR, Cadena EA, Herrera FA, Polly PD, Jaramillo CA. 2009. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature 457, 715–717 10.1038/nature07671 (doi:10.1038/nature07671) [DOI] [PubMed] [Google Scholar]
- 34.Jaramillo C, et al. 2010. Effects of rapid global warming at the Paleocene–Eocene boundary on neotropical vegetation. Science 330, 957–961 10.1126/science.1193833 (doi:10.1126/science.1193833) [DOI] [PubMed] [Google Scholar]
- 35.McElwain JC, Willis KJ, Lupia R. 2005. Cretaceous CO2 decline and the radiation and diversification of angiosperms. In A history of atmospheric CO2 and its effects on plants, animals, and ecosystems (eds Ehleringer JR, Cerling TE, Dearing MD.), pp. 133–165 New York, NY: Springer [Google Scholar]
- 36.Beerling DJ. 2005. Evolutionary responses of land plants to atmospheric CO2. In A history of atmospheric CO2 and its effects on plants, animals, and ecosystems (eds Ehleringer JR, Cerling TE, Dearing MD.), pp. 114–132 New York, NY: Springer [Google Scholar]
- 37.Robinson JM. 1994. Speculations on carbon dioxide starvation, Late Tertiary evolution of stomatal regulation and floristic modernization. Plant, Cell Environ. 17, 345–354 10.1111/j.1365-3040.1994.tb00303.x (doi:10.1111/j.1365-3040.1994.tb00303.x) [DOI] [Google Scholar]
- 38.Boyce CK, Brodbribb TJ, Feild TS, Zwieniecki MA. 2009. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. R. Soc. B 276, 1771–1776 10.1098/rspb.2008.1919 (doi:10.1098/rspb.2008.1919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl Acad. Sci. USA 106, 10 343–10 347 10.1073/pnas.0904209106 (doi:10.1073/pnas.0904209106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyce CK, Zwieniecki MA. 2012. Leaf fossil record suggests limited influence of atmospheric CO2 on terrestrial productivity prior to angiosperm evolution. Proc. Natl Acad. Sci. USA 109, 10 403–10 408 10.1073/pnas.1203769109 (doi:10.1073/pnas.1203769109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilf P, Johnson KR. 2004. Land plant extinction at the end of the Cretaceous: a quantitative analysis of the North Dakota megafloral record. Paleobiology 30, 347–368 (doi:10.1666/0094-8373(2004)030<0347:LPEATE>2.0.CO;2) [DOI] [Google Scholar]
- 42.Beerling DJ, Lomax BH, Royer DL, Upchurch GR, Kump LR. 2002. An atmospheric pCO2 reconstruction across the Cretaceous-Tertiary boundary from leaf megafossils. Proc. Natl Acad. Sci. USA 99, 7836–7840 10.1073/pnas.122573099 (doi:10.1073/pnas.122573099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulte P, et al. 2010. The Chicxulub asteroid impact and mass extinction at the Cretaceous–Paleogene boundary. Science 327, 1214–1218 10.1126/science.1177265 (doi:10.1126/science.1177265) [DOI] [PubMed] [Google Scholar]