Abstract
Bacteria often possess multiple siderophore-based iron uptake systems for scavenging this vital resource from their environment. However, some siderophores seem redundant, because they have limited iron-binding efficiency and are seldom expressed under iron limitation. Here, we investigate the conundrum of why selection does not eliminate this apparent redundancy. We focus on Pseudomonas aeruginosa, a bacterium that can produce two siderophores—the highly efficient but metabolically expensive pyoverdine, and the inefficient but metabolically cheap pyochelin. We found that the bacteria possess molecular mechanisms to phenotypically switch from mainly producing pyoverdine under severe iron limitation to mainly producing pyochelin when iron is only moderately limited. We further show that strains exclusively producing pyochelin grew significantly better than strains exclusively producing pyoverdine under moderate iron limitation, whereas the inverse was seen under severe iron limitation. This suggests that pyochelin is not redundant, but that switching between siderophore strategies might be beneficial to trade off efficiencies versus costs of siderophores. Indeed, simulations parameterized from our data confirmed that strains retaining the capacity to switch between siderophores significantly outcompeted strains defective for one or the other siderophore under fluctuating iron availabilities. Finally, we discuss how siderophore switching can be viewed as a form of collective decision-making, whereby a coordinated shift in behaviour at the group level emerges as a result of positive and negative feedback loops operating among individuals at the local scale.
Keywords: iron uptake, siderophore, trade-off, fluctuating environment, phenotypic plasticity, decision-making
1. Introduction
Many bacterial species possess multiple molecular apparatuses that, although structurally different, fulfil similar functions. For instance, an individual bacterium often has multiple quorum sensing-based communication systems [1], multiple secretory apparatuses [2,3] or multiple siderophore-based iron acquisition systems [4,5]. This multiplicity raises a fundamental evolutionary question: why should natural selection maintain a costly and seemingly redundant array of different molecular mechanisms for a single cellular function? The problem becomes particularly intriguing in instances where a molecular system seems disused. This is apparently the case for siderophore-based iron acquisition systems, where so-called ‘secondary’ siderophores are usually expressed only weakly or not at all under iron limitation [4,6,7]. An evolutionary consequence of this disuse should be that selection eliminates superfluous systems [8].
Here, we address this conundrum in the context of siderophore production in the opportunistic human pathogen Pseudomonas aeruginosa. Bacteria rely on the secretion of siderophores to satisfy their need of iron in environments where iron is insoluble or host-bound [9,10]. Pseudomonas aeruginosa possesses molecular machineries to synthesize two siderophores, pyoverdine [11] and pyochelin [12]. While the synthesis [11], function [13,14] and virulence [15,16] of pyoverdine has been extensively studied, pyochelin has received much less attention. This is mainly because pyochelin is usually produced in low amounts [17] has a much lower affinity to iron (Kf = 105 M−1) [12] than pyoverdine (Kf = 1024 M−1) [11] and is less important for virulence [16]. Despite its apparent inferiority to pyoverdine under typical conditions, it has been suggested that under less stringent iron limitation, pyochelin might prove most useful [4]. It remains unclear, however, how bacteria regulate differential deployment of their two siderophores in response to local iron availability, and indeed, what benefit might accrue from different deployment configurations.
In this study, we used an integrative approach to investigate why P. aeruginosa maintains two siderophore-based iron acquisition systems, one of which seems superfluous. At the mechanistic level, we investigated whether there is a mechanism that might facilitate switching among different siderophore strategies in response to iron availability. Such switching could potentially emerge from interactions between the respective negative and positive feedback loops regulating each siderophore's synthesis. Positive feedback loops (enhancing siderophore synthesis) comprise signalling systems whereby the binding of iron-loaded siderophores to their respective receptors results in the intracellular activation of PvdS (sigma factor triggering pyoverdine synthesis [18]) and PchR (transcriptional regulator of pyochelin synthesis [19]). Negative feedback loops (reducing siderophore synthesis) operate through ferric uptake regulator (Fur). Fur forms complexes with excess iron in the cytoplasm, and then blocks siderophore production by binding upstream of PvdS [20] and PchR [19]. While these feedback loops have already been studied in detail for each siderophore separately, potential interactions between the two loops have not been examined.
At the ecological level, we tested whether iron availability is indeed the environmental cue that triggers switching between siderophore investment strategies as suggested by Cornelis [4]. Accordingly, we carried out a set of experiments in which we measured the relative production of pyoverdine and pyochelin across a range of environmental conditions. We kept the absolute iron concentration constant, but altered the relative iron availability by manipulating (i) how strongly iron is bound in the environment (through altering pH and chelator-binding strength), and (ii) the iron uptake rate (through altering temperature).
At the evolutionary level, we investigated whether switching between two siderophore systems could yield greater fitness benefits compared to having a single efficient siderophore system. A possible scenario is that pyoverdine is not only more efficient, but also more costly to produce, such that having the ability to produce a cheaper alternative might enable bacteria to trade off costs versus efficiencies of siderophores in a fluctuating environment. To test this possibility, we first quantified the costs involved in the synthesis of the two siderophores by gathering information on the number of genes and building blocks involved in their synthesis. Next, we compared the absolute fitness of wild-type strains (able to produce both siderophores) versus genetically engineered strains defective for the production of one or the other siderophore across different environments. Finally, we used computer simulations to investigate how a fluctuating environment could potentially influence the long-term competitive dynamics among producers of pyoverdine, pyochelin or both siderophores.
2. Material and methods
(a). Strains and culturing methods
We used P. aeruginosa PAO1 (ATCC 15692) as our wild-type strain, which can produce both pyoverdine and pyochelin. We further used two deletion mutants derived directly from our PAO1 strain background [21], namely: (i) PAO1ΔpvdD, a strain defective for pyoverdine production, in which the gene coding for the pyoverdine synthetase PvdD is deleted; and (ii) PAO1ΔpchEF, a strain defective for pyochelin production owing to a deletion in genes coding for pyochelin synthetases PchE and PchF.
The standard culturing method (where not otherwise specified) included the inoculation of approximately 105 bacterial cells (from overnight Luria-Bertani cultures) into 200 µl casamino acids (CAA) medium (5 g CAA, 1.18 g K2HPO4*3H2O, 0.25 g MgSO4*7H2O, per litre). We further added 100 µg ml−1 human apo-transferrin (a strong natural iron chelator) and 20 mM NaHCO3 (as cofactor) to prevent non-siderophore-mediated uptake of the residual (approx. 2 µM) iron [15,22]. We buffered the CAA medium at around pH 7 by the addition of 25 mM HEPES (Sigma-Aldrich). Cultures were incubated for 24 h under static conditions at 37°C. Subsequently, we measured growth as optical density (OD) (at 600 nm) and quantified pyoverdine and pyochelin production using the natural fluorescence properties of these molecules using a multimode plate reader (Synergy Mx, Biotek). We then standardized the relative fluorescence units (RFU) by OD (RFU/OD) [14,23].
(b). Measuring pyoverdine and pyochelin production
To be able to simultaneously quantify investment into the two siderophores in batch culture, we established a new method based on the fluorescent properties of the pyoverdine [23] and pyochelin [24] molecules (see the electronic supplementary material). The establishment of this method involved three steps: (i) determination of the optimal excitation (ex) and emission (em) wavelengths for pyoverdine and pyochelin fluorescence in CAA (see the electronic supplementary material, figure S1); (ii) application of a correction procedure to account for overlaps between the emission spectra of two siderophores (see the electronic supplementary material, figure S2); and (iii) demonstration of a linear relationship between the RFU and the actual siderophore concentrations in the media (see the electronic supplementary material, figure S3). For all three steps, we required purified pyoverdine and pyochelin, which we obtained by applying standard extraction protocols (see the electronic supplementary material).
(c). Mechanistic basis of siderophore switching
To examine whether interactions between positive feedback loops were involved in preferential production of one or the other siderophore, we compared the pyoverdine and pyochelin production of strains PAO1ΔpvdD and PAO1ΔpchEF (where interaction is not possible) with PAO1 (where interaction is possible) in iron-limited CAA. We inoculated 105 cells of the three strains (in 16-fold replication each) and let them grow for 24 h. Following this incubation period, we scaled all RFU/OD-values relative to the wild-type PAO1, such that pyoverdine and pyochelin production equalled one for this strain. In order to relate fluorescence measures to the actual quantities of pyoverdine and pyochelin, we extracted siderophores from culture supernatants of PAO1, PAO1ΔpvdD and PAO1ΔpchEF. We grew strains statically overnight at 37°C in 250 ml iron-limited CAA medium (in twofold replication each). The cultures, which reached a mean OD ± s.e. = 0.32 ± 0.04, were subjected to the extraction protocol described in the electronic supplementary material. Following extraction, we weighed the siderophore powder and estimated the original concentration (micromolar) in the culture (relative molecular masses, pyochelin: Mr = 324, pyoverdin: Mr = 1350; pyoverdine type I with carboyclic acid residue). To examine whether the Fur-mediated negative feedback loops were involved in switching from producing one or the other siderophore, we supplemented 105 cells of PAO1 in iron-limited CAA with 0, 1, 2, 5, 10, 20 and 40 µM of FeCl3 (in 10-fold replication for each treatment) [14,25]. After 24 h, we then compared scaled RFU/OD values across the iron-supplementation treatments.
(d). Ecological aspects of siderophore switching
We conducted a series of experiments in environments where we manipulated relative iron availability (i.e. manipulating how strongly iron is bound in the medium), while keeping the absolute iron concentration constant (approx. 2 µM). Keeping absolute iron concentration constant is essential to decouple total siderophore investment (depending on absolute iron concentration [14]) from relative siderophore production (i.e. switching from producing one or the other siderophore). For all experiments, we inoculated 105 cells of PAO1 into various media (differing in chelator strength, pH and temperature) and compared RFU/OD values for pyoverdine and pyochelin following a 24 h growth period.
With regard to chelator strength, we manipulated relative iron availability by supplementing the CAA medium with one of four organic iron chelators (in 16-fold replication for each treatment): transferrin (100 µg ml−1), citrate (100 µM), malate (100 µM) and bicarbonate (40 mM). The iron-binding affinity of transferrin is Kf = 1025.6 M−1 [9] at physiological pH. While the iron chelation behaviour of citrate is complex [26], its iron-binding affinity (approx. Kf = 1011.5 M−1 for the most simple Fe(Cit) complex) is certainly higher than that of malate (with two carboxyl groups) [27] and bicarbonate (with one carboxyl group) [28]. Note that citrate is a special case among the chelators chosen, since P. aeruginosa possesses a specific citrate receptor to take up citrate-bound iron [29].
With regard to pH, the iron-affinity of transferrin is highest at pH 7 [30]. Accordingly, to manipulate relative iron availability, we buffered the CAA medium at pH values different from the optimum (pH 5, 6, 8 and 9 in addition to pH 7; buffer strength 50 mM; 16-fold replication each) using phosphate buffer (i.e. mixing the appropriate quantities of monosodium phosphate monohydrate and disodium phosphate heptahydrate, see http://home.fuse.net/clymer/buffers/phos2.html for calculating concentrations).
With regard to temperature, we let bacteria grow in iron-limited CAA medium at 25°C, 30°C, 37°C and 42°C (in 24-fold replication each). Here, relative iron availability was manipulated indirectly by the fact that higher incubation temperatures are expected to increase the thermodynamic potential of chemical reactions and therefore speed up iron uptake rates.
(e). Fitness consequences of siderophore switching
To obtain an estimate of costs involved in the synthesis of the two siderophores, we gathered data from the ‘Pseudomonas Genome Database’ (www.pseudomonas.com) on the number of genes involved in their synthesis, the required nucleotides and amino acids to transcribe and translate those genes, and the relative molecular mass of the two siderophores. It is important to note here that both siderophores are synthetized through non-ribosomal peptide synthesis [11,12]. This means that an entire machinery of enzymes first needs to be produced, and only then are the siderophores assembled from amino acids and other chemical compounds.
To assess the fitness consequences of siderophore switching, we grew PAO1, PAO1ΔpchEF and PAO1ΔpvdD (in 4–12-fold replication) in all of the environments described above. We tracked the growth kinetics, measuring OD600 nm over a 24 h period using a multimode plate reader (Synergy Mx, Biotek). For each strain and each environment, we fitted a single non-parametric spline to the growth data, and estimated from this spline the maximum growth rate (ΔOD600 nm/h) attained during the observation period. We then generated n = 1000 bootstrap replicates from these data, and extracted the median and 95% bootstrap confidence intervals. We took these as our measures of absolute fitness.
These assays provide information on whether the ability to produce two siderophores is more beneficial than having a single siderophore system in a constant environment. However, a key prediction of our hypothesis is that switching between two siderophores pays off in a variable environment, where bacteria can possibly trade off costs versus efficiencies of their siderophores. To address this question, we used an in-silico approach, where we simulated competitive interactions among strains in a randomly fluctuating environment. Since we were interested only in the fitness consequences of siderophore switching in response to iron availability, we needed to exclude any potentially confounding influences on fitness that could derive from social exploitative interactions, such as cheating [31–33]. For instance, while PAO1ΔpvdD alone grows poorly in strongly iron-limited medium because it cannot produce pyoverdine, it typically performs well in mixed culture with PAO1 wild-type as it can exploit the pyoverdine produced by PAO1 [31–33]. Our simulation therefore purposefully excluded these types of social aspects of siderophore production. We simulated a population with a total density of OD600 nm = 1, which, at the start, comprised all three strains in equal proportions (i.e. 0.333). An environment type was selected at random from among those provided (i.e. all environments shown in figure 3), and each strain then grew at a rate sampled from a strain- and environment-specific truncated normal distribution, the parameters of which were determined from previous empirical observations (figure 3). After a number of timesteps (i.e. hours, as integers) the population was rescaled to OD600 nm = 1, and the next environment was selected at random. Intervals between switches were sampled randomly, anew each time, from a truncated normal distribution with mean = 3 timesteps, range = [1;100] and coefficient of variation = 0.5 (except for figure 4a, where a range of increasingly larger means was used, up to constancy = 1, where the interval was always equal to the full length of the simulation run). If a strain's proportion dropped below 10−10 at any point, that strain was considered extinct and could not rebound during subsequent timesteps. Simulations were run over 100 timesteps (except for figure 4b, where run length was manipulated) and with 1000-fold replication, whereafter, for each strain, means were calculated for the final proportions.
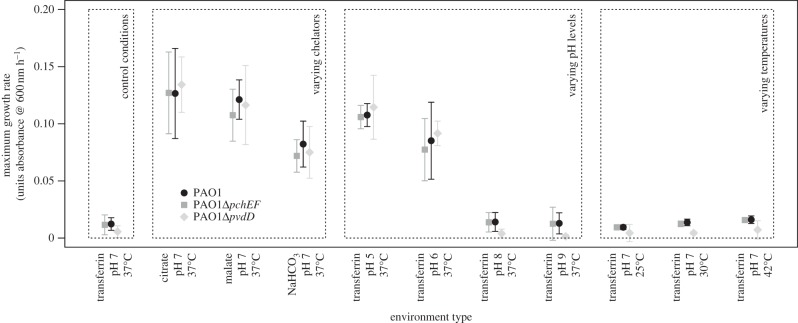
Figure 3.
Fitness consequences of siderophore switching across a range of environments. Maximal growth rate (change in OD600nm per hour) attained during monoculture growth is shown for wild-type PAO1 (black circles, capable of pyoverdine and pyochelin synthesis), PAO1ΔpchEF (dark grey squares, capable of pyoverdine production only) and PAO1ΔpvdD (light grey diamonds, capable of pyochelin production only). We manipulated iron across the different environments by using chelators with different iron-binding affinities, varying the pH of the medium and varying incubation temperature. Maximal growth rate was estimated from non-parametric splines fitted to observed growth trajectories during 24 h. Here, points represent the median parameter estimate, and error bars the 95% CIs from n = 1000 bootstrap replicate splines.
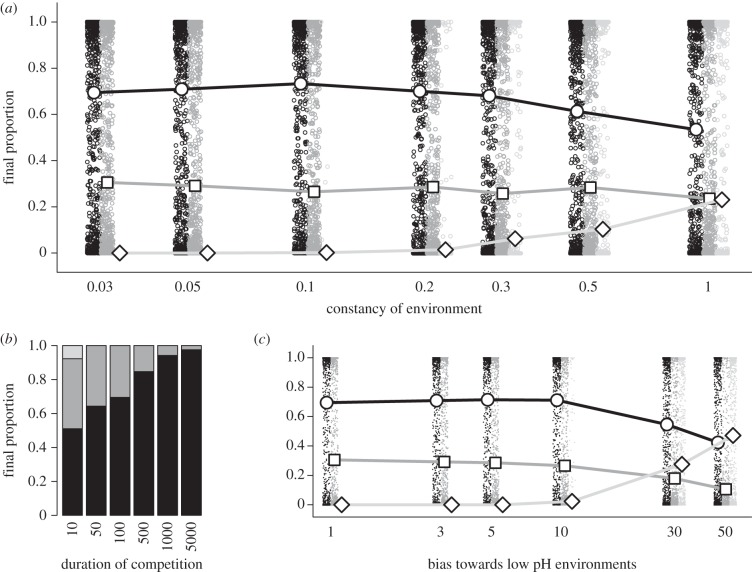
Figure 4.
Outcomes of simulated competitions among strains producing one or both siderophores. Strain PAO1ΔpchEF (dark grey, squares) produces only pyoverdine; strain PAO1ΔpvdD (light grey, diamonds) produces only pyochelin, while PAO1 WT (black, circles) produces both siderophores. Small markers in (a) and (c) indicate outcomes of individual replicates, after 100 timesteps, of a given strain in simulated competitions versus the two other strain types. In all cases, n = 1000 replicates. (a) Final proportions of the three strains in response to changing environmental constancy. All outcomes are possible in a constant environment (constancy = 1) since no one strain is a universal winner in all possible environments. Under more changeable conditions (constancy < 1), however, PAO1 generally performs best while PAO1ΔpvdD nearly always goes extinct. (b) Stacked barplot giving final strain proportions after competitions of different durations in rapidly fluctuating environments (constancy = 0.03). The success of PAO1 increased in relatively longer competitions, driving PAO1ΔpvdD to extinction and PAO1ΔpchEF to very low frequency. (c) The usually inferior PAO1ΔpvdD can prevail when environmental conditions are biased towards more acidic conditions, even given rapid environmental fluctuations (constancy = 0.03). Here, a bias of x means that environment types pH 5 and pH 6 were, respectively, x and x/2 times more likely to be sampled than other environment types.
(f). Statistical analysis
We used linear models for all analyses, unless otherwise specified. We tested whether relative siderophore production significantly differed as a function of strain type, siderophore and iron supplementation, as well as in relation to chelator strength, pH and temperature. We further tested for significant associations between pyoverdine and pyochelin production across experimental treatments. Whenever necessary, we log-transformed the dependent variables to meet the assumption of normally distributed residuals. Analyses were carried out with R. 2.15.3 (http://www.r-project.org/). In the case of growth curves analyses, we used the functions from the package ‘grofit’, v. 1.1 [34].
3. Results
(a). Interactions between feedback loops enable siderophore switching
Under stringent iron limitation, positive feedback loops should trigger high levels of siderophore production. Consistent with this view, we found that the strains PAO1ΔpchEF and PAO1ΔpvdD produced significant amounts of pyoverdine (concentration of 297 ± 11 µM; mean ± s.e.) and pyochelin (265 ± 40 µM), respectively, in media with the strong iron chelator, transferrin. The pattern differed in the wild-type strain PAO1, which showed significantly reduced pyochelin production (88 ± 23 µM, t3 = −3.80, p = 0.032), while producing similarly high amounts of pyoverdine (269 ± 19 µM, PAO1 versus PAO1ΔpchEF: t3 = −1.29, p = 0.29). We recovered the same results using fluorescence measures as proxies for pyoverdine and pyochelin production (figure 1a). Here, we observed a significant 5.7 ± 0.2-fold upregulation in pyochelin production in PAO1ΔpvdD compared with PAO1 (t93 = 35.2, p < 0.0001), whereas pyoverdine production did not differ between PAO1 and PAO1ΔpchEF (t93 = 0.2, p = 0.84). These results strongly suggest directional interference between the two positive feedback loops, whereby pyoverdine production significantly represses pyochelin-mediated signalling and, therewith, pyochelin synthesis.
Figure 1.
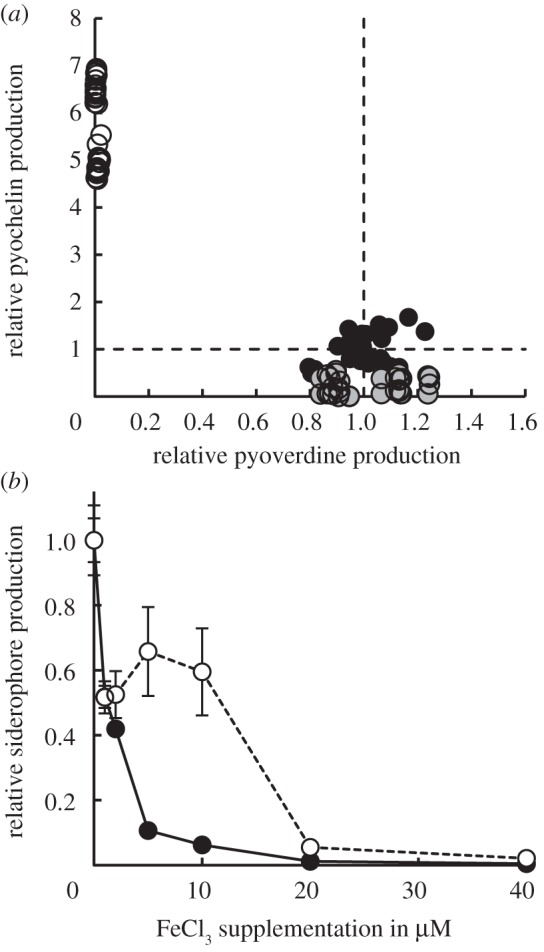
Mechanistic aspects of siderophore switching in P. aeruginosa facing iron limitation. (a) In the wild-type strain PAO1 (black circles) pyochelin synthesis was strongly reduced relative to the situation in PAO1ΔpvdD (open circles). This suggests pyoverdine-mediated suppression of pyochelin production in PAO1. By contrast, pyochelin had no effect on pyoverdine production: pyoverdine production did not differ between PAO1 and PAO1ΔpchEF (grey circles). (b) In the wild-type strain PAO1, pyoverdine production (black circles) is strongly downregulated in response to increasing iron supplementation, whereas pyochelin production (open circles) remains relatively high until approximately 10 μM FeCl3. The differences in repression sensitivities result in a range of iron availabilities where pyochelin becomes the predominant siderophore. The production of each siderophore is represented as RFUsiderophore/OD, relative to the standard medium without iron supplementation.
Under less stringent iron limitation, Fur-mediated negative feedbacks should downregulate siderophore production. Although this prediction generally held (figure 1b), we found that pyoverdine synthesis was strongly downregulated even with low amounts of supplemented iron, whereas pyochelin synthesis remained relatively high over an extended range of iron concentrations (comparison of slopes of linear regressions on log-transformed data: slopepyoverdine = −1.54 ± 0.04 versus slopepyochelin = −0.98 ± 0.08, t124 = 6.54, p < 0.0001). This difference in sensitivity resulted in pyochelin becoming the predominant siderophore at intermediate iron concentrations.
(b). Relative iron availability drives siderophore switching
We found that PAO1 always produced both pyoverdine and pyochelin, but that the relative proportions varied greatly across environments (figure 2). The addition of weaker chelators (in relation to transferrin, the strongest chelator used here) resulted in a significant decrease in pyoverdine (linear regression: F1,62 = 358, r2 = 0.85, p < 0.0001) and a significant increase in pyochelin (F1,60 = 71.9, r2 = 0.538, p < 0.0001) production (figure 2a). Across chelator treatments, there was a significant negative association between pyoverdine and pyochelin production (F1,60 = 120, r2 = 0.661, p < 0.0001).
Figure 2.
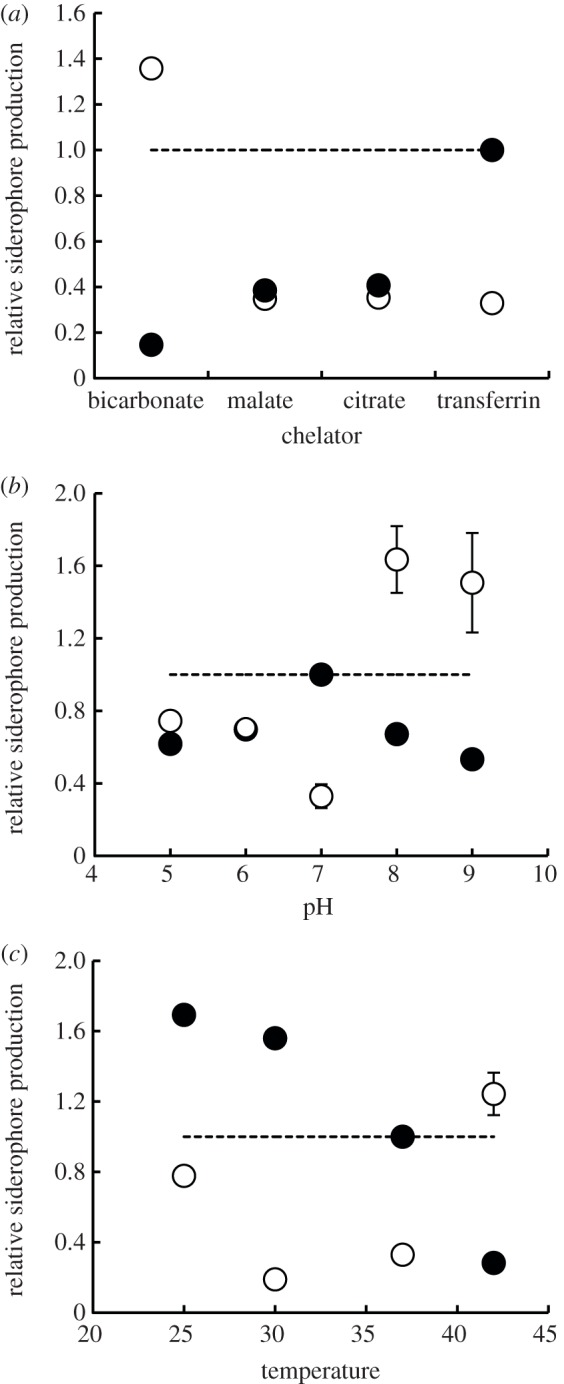
Siderophore switching as a function of relative iron availability in the environment. We manipulated relative iron availability in the medium by: (a) adding chelators with different iron-binding affinities (low to high from left to right); (b) varying the pH of the medium, which alters siderophore and/or chelator affinity to iron; and (c) varying incubation temperature, which determines the thermodynamic potential of chemical reactions and therefore the speed of iron uptake. Overall, there was a significant switch from the production of pyoverdine (filled circles) to pyochelin (open circles) when moving from environments with lower to higher relative iron availabilities. Here, the production of each siderophore is represented as a relative measure, i.e. the RFUsiderophore/OD, relative to the RFUpyoverdine/OD, measured under baseline conditions (i.e. transferrin, pH 7, 37°C). Values are given as mean±s.e. Some standard errors are smaller than symbols, and are therefore not shown.
With regard to pH, we detected a significant decrease in pyoverdine production (quadratic regression: F2,75 = 29.5, r2 = 0.425, p < 0.0001) and significant increase in pyochelin production (F2,73 = 13.9, r2 = 0.255, p < 0.0001) when moving away from pH 7, where transferrin's iron-binding constant is strongest (figure 2b). Across treatments, there was a significant negative association between pyoverdine and pyochelin production (linear regression: F1,74 = 39.9, r2 = 0.342, p < 0.0001).
Finally, we observed that pyoverdine production significantly decreased at higher temperatures (linear regression: F1,94 = 531, r2 = 0.848, p < 0.0001; figure 2c). The pattern for pyochelin production in function of temperature was more complex: production increased at higher temperatures (as predicted), but also increased at the lowest temperature (25°C; quadratic regression: F2,91 = 111, r2 = 0.703, p < 0.0001; figure 2c). Overall, we found a significant negative association between pyoverdine and pyochelin production across treatments (linear regression: F1,92 = 34.2, r2 = 0.263, p < 0.0001).
(c). Siderophore switching pays off in fluctuating environments
Our Pseudomonas Genome Database search revealed that pyoverdine synthesis and secretion involves 2.6 times more characterized genes (26 versus 10) and requires 3.6 times more nucleotides and amino acids to transcribe and translate those genes than pyochelin synthesis (see the electronic supplementary material, table S1). This indicates that the cost of building the siderophore synthesis machinery is substantially larger for pyoverdine than pyochelin. Moreover, the molecular mass of pyoverdine (Mr = 1350) is 4.2 times larger than that of pyochelin (Mr = 324). This suggests that not only the up-front costs associated with readying synthesis machinery, but also the ongoing production costs in terms of building blocks needed to synthesize pyoverdine, greatly exceed those for pyochelin. These findings support our hypothesis that siderophore switching could be beneficial, because it could allow bacteria to trade off costs against benefits of siderophores.
When comparing the fitnesses of the three strains (PAO1, PAO1ΔpchEF and PAO1ΔpvdD) across environments, we found significant growth differences between environments (ANOVA: F10,20 = 287.4, p < 0.0001) but not, overall, between strains (F2,20 = 1.79, p = 0.19; figure 3). However, we found that PAO1ΔpvdD grew significantly better than PAO1ΔpchEF (paired t-test: t4 = 4.72, p = 0.009) in the five environments that supported highest growth owing to increased relative iron availability (citrate, malate, bicarbonate, pH 5 and pH 6), while the reverse was true (i.e. PAO1ΔpchEF grew significantly better than PAO1ΔpvdD, t-test: t4 = 8.33, p = 0.001) in the five environments supporting the lowest growth owing to more stringent iron limitation (pH 8, pH9, temperatures 25°C–42°C). These results indicate that pyochelin and pyoverdine are indeed most beneficial when relative iron availability is increased or reduced, respectively. This observation, in turn, suggests that a strain which is able to switch between siderophores in response to changing iron availability should perform best in a fluctuating environment.
Our simulation analyses provided clear support for that hypothesis (figure 4). In simulated populations seeded with equal proportions of the three strains, PAO1 significantly outcompeted the two other single siderophore-producing strains when environmental conditions fluctuated randomly. The level of domination by PAO1 was influenced by both the frequency of environmental change (PAO1 reached higher final proportions with more rapid environmental changes, figure 4a) and the duration of the competition (PAO1 reached higher final proportions with longer competition times, figure 4b). However, we also found that a more stable coexistence of strains was possible when certain environmental conditions were encountered more frequently than others, as illustrated by a set of simulations where we gradually raised the relative probability of encountering acidic environments (figure 4c)—a scenario possibly representative of habitats such as some types of forest soils [35].
4. Discussion
Our results indicate that P. aeruginosa indeed possesses a regulatory mechanism which enables it to differentially adjust its relative investment in the production of its two siderophores, pyoverdine and pyochelin, as a function of iron availability. Moreover, our fitness comparisons across different environmental conditions, and our simulations, demonstrate when and why having two siderophores is adaptive. Switching between siderophore investment strategies seems to be beneficial, because it allows bacteria to better optimize the cost-to-benefit ratio of siderophore production across changing environmental conditions. When iron is severely limited, P. aeruginosa can focus on the production of the costly but highly efficient pyoverdine, whereas it can switch to a cheaper option, pyochelin, when iron is relatively more accessible. These results highlight that pyochelin is not redundant and that its role in iron scavenging in P. aeruginosa has been underestimated, probably owing to the fact that iron uptake has usually been studied under stringent iron limitation.
The siderophore switching mechanism we report in this study provides an example of how, via their molecular regulatory networks, bacteria can make ‘decisions’ about optimal investment strategies in response to their prevailing environmental conditions. In the present case, the decision-making process is based on a relatively sophisticated mechanism entailing interference and sensitivity differences between the two regulatory feedback loops controlling the expression of pyoverdine and pyochelin, respectively. On the one hand, preferential pyoverdine production is possible because pyoverdine production interferes with pyochelin-mediated signalling and/or pyochelin production directly, when iron is severely limited. The exact mechanism of this suppression remains yet to be determined. On the other hand, preferential pyochelin production can occur because the Fur-mediated negative feedback is more sensitive in repressing pyoverdine than pyochelin production under less stringent iron limitation.
While our results show that the decision to preferentially produce pyoverdine or pyochelin is made by individual cells in response to local iron availability (i.e. an environmental factor), the involvement of publicly shared molecules as signals in the feedback loops means that there is also a social aspect to this decision-making process. To this end, we posit that the decision to make pyoverdine or pyochelin has many of the hallmarks of the collective decision-making processes seen in animals [36–38]. Our assertion derives from the following observations. First, iron limitation per se only induces baseline expression of the genes involved in siderophore synthesis in each individual, whereas positive signalling feedbacks based on social interactions are required to fully induce the systems [18,19]. Specifically, signalling operates through iron-loaded siderophores binding to their respective receptors resulting in the upregulation of pyoverdine (and two other public goods [18]) and pyochelin [19] synthesis genes. Because siderophores are highly diffusible molecules, a producer cell typically does not receive feedback only from its own molecules, but rather receives an aggregate signal comprising molecules secreted by the entire local neighbourhood [39]. Just as the positive feedback loop that promotes siderophore production is influenced by social cues, the Fur-mediated negative feedback loop is also influenced by the actions of other individuals because the iron uptake rate, which determines the strength of Fur repression, is given by the total siderophore production of all community members [14]. Taken together, this means that the switch between producing one or the other type of siderophore is one that is largely coordinated in a group-level manner, and emerges from distributed local interactions among individuals. At this broad level, bacterial siderophore switching is qualitatively similar to the processes by which animal groups collectively determine where to travel [40], how to evade predators [41], which food sources to exploit [42] or where to site a new communal nest [43]. In each of these situations, coordination and synchrony can emerge in the absence of leadership and cognitive abilities, but rather from simple mechanisms involving positive and negative feedbacks based on repeated local (distributed) interactions among individuals [37,38].
In this context, siderophore-mediated signalling is comparable to quorum sensing-based signalling that is widely recognized to have evolved as a measure to coordinate group activities [1,44]. Specifically, quorum sensing is based on cells releasing small signalling molecules into the environment, which, upon uptake, induce further signal production in receiving individuals. Once signal density has reached a certain concentration (which serves as a proxy for cell density) bacteria start producing a range of extracellular public goods, such as enzymes and toxins, which are secreted in the environment in a group-coordinated manner. Thus, quorum sensing too can be understood as a collective decision-making process [45], which assures that public goods are only produced when they are beneficial, and when efficient sharing of the public good is guaranteed [46]. Evidently, collective decision-making also plays an important role in organisms as simple as bacteria.
Their capacity for switching between different siderophore investment strategies notwithstanding, we saw that bacteria still produced both pyoverdine and pyochelin in all environments (figure 2). This raises the question of why, in certain environments, P. aeruginosa does not focus exclusively on the more suitable of the two siderophores and completely repress the other? There are at least three mutually non-exclusive explanations for our observation. First, there may be mechanistic constraints [47] which prevent a complete shutdown of production of one or the other siderophore, even though it would be beneficial to do so. Our data provide some evidence that mechanistic constraints may be an issue here, in that the relative impacts of the two regulatory elements (interference and sensitivity of feedbacks) appear to vary across ecological conditions (figure 2), leading to both noisy (e.g. at high pH) and non-intuitive (e.g. at low temperature) siderophore production patterns. Moreover, it seems that under stringent iron limitation, pyoverdine represses only pyochelin-mediated signalling (i.e. the positive amplifying feedback loop) but not the baseline pyochelin production, which would explain why pyochelin is always expressed to some extent. Second, an adaptive explanation could be that the simultaneous production of both siderophores represents a form of bet-hedging behaviour, enabling bacteria to remain in a state of readiness should environmental conditions suddenly change (as seen for other bacterial traits [48,49]). Moreover, siderophores might have alternative functions not investigated in our study, such as their recently suggested roles in heavy metal tolerance [50] or decomposition of organic compounds [51].
Finally, our simulation analyses based on fitness data suggest that switching between different siderophore investment strategies confers benefits in environments with randomly fluctuating iron availabilities. The idea that natural selection should maintain different siderophore systems in fluctuating environments makes sense given that spatial and temporal ecological variability is a typical feature of most microbial habitats [52,53], and iron availability, in particular, varies widely in water [54] and soil [55] habitats. However, our simulations also show that the relative prevalence of strains producing both siderophores can decrease when the environment is less prone to change and/or is biased towards some specific conditions. Just such a scenario may apply during chronic infections in cystic fibrosis lungs, where pyoverdine-negative mutants typically accumulate over time [56–58]. One possible explanation for this observation is that siderophores are not required in the lung because iron concentration is increased (7.5–33 µM; [59,60]). Alternatively, it has been suggested that selection against pyoverdine production occurs because of cheating (i.e. pyoverdine-defective mutants experiencing fitness advantages by exploiting pyoverdine produced by others [58,61]). Our findings now suggest a third possibility, namely, that increased iron availability in cystic fibrosis lungs could favour a permanent shift in siderophore production from pyoverdine to pyochelin, such that a mutant defective for pyoverdine synthesis could spread, because it avoids costly pyoverdine production in an environment where the cheap pyochelin might suffice to do the job. These considerations highlight that care must be taken when interpreting the rising of siderophore-negative mutants, since both social interactions (i.e. cheating) and ecological conditions (i.e. iron availability) can be the underlying selective drivers.
Acknowledgements
We thank Olivia van der Reiden for help in the laboratory, Tim Cooper, Alex Hall and two anonymous referees for constructive comments.
Data accessibility
Data deposited in Dryad: http://dx.doi.org/10.5061/dryad.jp596.
Funding statement
This work was funded by several grants from the Swiss National Science Foundation and a Marie Curie Reintegration grant from the European Commission to R.K.
References
- 1.Williams P, Winzer K, Chan WC, Camara M. 2007. Look who's talking: communication and quorum sensing in the bacterial world. Phil. Trans. R. Soc. B 362, 1119–1134 10.1098/rstb.2007.2039 (doi:10.1098/rstb.2007.2039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscoso JA, Mikkelsen H, Heeb S, Williams P, Filloux A. 2011. The Pseudomonas aeruginosa sensor RetS switches Type III and Type VI secretion via c-di-GMP signalling. Environ. Microbiol. 13, 3128–3138 10.1111/j.1462-2920.2011.02595.x (doi:10.1111/j.1462-2920.2011.02595.x) [DOI] [PubMed] [Google Scholar]
- 3.Waksman G. 2012. Bacterial secretion comes of age. Phil. Trans. R. Soc. B 367, 1014–1015 10.1098/rstb.2011.0200 (doi:10.1098/rstb.2011.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis P. 2010. Iron uptake and metabolism in pseudomonads. Appl. Microbiol. Biotechnol. 86, 1637–1645 10.1007/s00253-010-2550-2 (doi:10.1007/s00253-010-2550-2) [DOI] [PubMed] [Google Scholar]
- 5.Hider RC, Kong X. 2010. Chemistry and biology of siderophores. Nat. Prod. Rep. 27, 637–657 10.1039/b906679a (doi:10.1039/b906679a) [DOI] [PubMed] [Google Scholar]
- 6.Mossialos D, Meyer J-M, Budzikiewicz H, Wolff U, Koedam N, Baysse C, Anjaiah V, Cornelis P. 2000. Quinolobactin, a new siderophore of Pseudomonas fluorescens ATCC 17400, the production of which is repressed by the cognate pyoverdine. Appl. Environ. Microbiol. 66, 487–492 10.1128/AEM.66.2.487-492.2000 (doi:10.1128/AEM.66.2.487-492.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemos ML, Balado M, Osorio CR. 2010. Anguibactin- versus vanchrobactin-mediated iron uptake in Vibrio anguillarum: evolution and ecology of a fish pathogen. Environ. Microbiol. Rep. 2, 19–26 10.1111/j.1758-2229.2009.00103.x (doi:10.1111/j.1758-2229.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 8.Darwin CR. 1859. The origin of species. London, UK: John Murray [Google Scholar]
- 9.Ratledge C, Dover LG. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54, 881–941 10.1146/annurev.micro.54.1.881 (doi:10.1146/annurev.micro.54.1.881) [DOI] [PubMed] [Google Scholar]
- 10.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71, 413–451 10.1128/MMBR.00012-07 (doi:10.1128/MMBR.00012-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visca P, Imperi F, Lamont IL. 2007. Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 15, 22–30 10.1016/j.tim.2006.11.004 (doi:10.1016/j.tim.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 12.Youard ZA, Wenner N, Reimmann C. 2011. Iron acquisition with the natural siderophore enantiomers pyochelin and enantio-pyochelin in Pseudomonas species. Biometals 24, 513–522 10.1007/s10534-010-9399-9 (doi:10.1007/s10534-010-9399-9) [DOI] [PubMed] [Google Scholar]
- 13.Cox CD, Adams P. 1985. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect. Immun. 48, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kümmerli R, Jiricny N, Clarke LS, West SA, Griffin AS. 2009. Phenotypic plasticity of a cooperative behaviour in bacteria. J. Evol. Biol. 22, 589–598 10.1111/j.1420-9101.2008.01666.x (doi:10.1111/j.1420-9101.2008.01666.x) [DOI] [PubMed] [Google Scholar]
- 15.Meyer J-M, Neely A, Stintzi A, Georges C, Holder IA. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64, 518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takase H, Nitanai H, Hoshino K, Otani T. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68, 1834–1839 10.1128/IAI.68.4.1834-1839.2000 (doi:10.1128/IAI.68.4.1834-1839.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaille C, Reimmann C, Haas D. 2003. Isochorismate synthase (PchA), the first and rate-limiting enzyme in salicylate biosynthesis of Pseudomonas aeruginosa. J. Biol. Chem. 278, 16 893–16 898 10.1074/jbc.M212324200 (doi:10.1074/jbc.M212324200) [DOI] [PubMed] [Google Scholar]
- 18.Lamont IL, Beare P, Ochsner U, Vasil AI, Vasil ML. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 99, 7072–7077 10.1073/pnas.092016999 (doi:10.1073/pnas.092016999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel L, Bachelard A, Reimmann C. 2007. Ferripyochelin uptake genes are involved in pyochelin-mediated signalling in Pseudomonas aeruginosa. Microbiology 153, 1508–1518 10.1099/mic.0.2006/002915-0 (doi:10.1099/mic.0.2006/002915-0) [DOI] [PubMed] [Google Scholar]
- 20.Ochsner UA, Vasil ML. 1996. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc. Natl Acad. Sci. USA 93, 4409–4414 10.1073/pnas.93.9.4409 (doi:10.1073/pnas.93.9.4409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghysels B, Thi Min Dieu B, Beatson SA, Pirnay J-P, Ochsner UA, Vasil ML, Cornelis P. 2004. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology 150, 1671–1680 10.1099/mic.0.27035-0 (doi:10.1099/mic.0.27035-0) [DOI] [PubMed] [Google Scholar]
- 22.Höfte M, Buysens S, Koedam N, Cornelis P. 1993. Zinc affects siderophore-mediated high affinity iron uptake systems in the rhizosphere Pseudomonas aeruginosa 7NSK2. Biometals 6, 85–91 [DOI] [PubMed] [Google Scholar]
- 23.Ankenbauer R, Sriyosachati S, Cox CD. 1985. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect. Immun. 49, 132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox CD. 1980. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J. Bacteriol. 142, 581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiburzi F, Imperi F, Visca P. 2008. Intracellular levels and activity of PvdS, the major iron starvation sigma factor of Pseudomonas aeruginosa. Mol. Microbiol. 67, 213–227 10.1111/j.1365-2958.2007.06051.x (doi:10.1111/j.1365-2958.2007.06051.x) [DOI] [PubMed] [Google Scholar]
- 26.Silva AMN, Kong X, Parkin MC, Cammack R, Hider RC. 2009. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 40, 8616–8625 10.1039/b910970f (doi:10.1039/b910970f) [DOI] [PubMed] [Google Scholar]
- 27.Schlabach MR, Bates GW. 1975. The synergistic binding of anions and Fe3+ by transferrin. J. Biol. Chem. 250, 2182–2188 [PubMed] [Google Scholar]
- 28.Matinaho S, Karhumaki P, Parkkinen J. 2005. Bicarbonate inhibits the growth of Staphylococcus epidermidis in platelet concentrates by lowering the level of non-transferrin-bound iron. Transfusion 45, 1768–1773 10.1111/j.1537-2995.2005.00601.x (doi:10.1111/j.1537-2995.2005.00601.x) [DOI] [PubMed] [Google Scholar]
- 29.Harding RA, Royt PW. 1990. Acquisition of iron from citrate by Pseudomonas aeruginosa. J. Gen. Microbiol. 136, 1859–1867 10.1099/00221287-136-9-1859 (doi:10.1099/00221287-136-9-1859) [DOI] [PubMed] [Google Scholar]
- 30.Sipe DM, Murphy RF. 1991. Binding to cellular receptors results in increased iron release from transferrin at mildly acidic pH. J. Biol. Chem. 266, 8002–8007 [PubMed] [Google Scholar]
- 31.Griffin A, West SA, Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027 10.1038/nature02744 (doi:10.1038/nature02744) [DOI] [PubMed] [Google Scholar]
- 32.Ross-Gillespie A, Gardner A, West SA, Griffin AS. 2007. Frequency dependence and cooperation: theory and a test with bacteria. Am. Nat. 170, 331–342 10.1086/519860 (doi:10.1086/519860) [DOI] [PubMed] [Google Scholar]
- 33.Kümmerli R, van den Berg P, Griffin AS, West SA, Gardner A. 2010. Repression of competition promotes cooperation: experimental evidence from bacteria. J. Evol. Biol. 23, 699–706 10.1111/j.1420-9101.2010.01936.x (doi:10.1111/j.1420-9101.2010.01936.x) [DOI] [PubMed] [Google Scholar]
- 34.Kahm M, Hasenbrink G, Lichtenberg-Fraté H, Ludwig J, Kschischo M. 2010. Grofit: fitting biological growth curves with R. J. Stat. Softw. 33, 1–2120808728 [Google Scholar]
- 35.Baath E, Anderson T-H. 2003. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 35, 955–963 10.1016/S0038-0717(03)00154-8 (doi:10.1016/S0038-0717(03)00154-8) [DOI] [Google Scholar]
- 36.Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 10.1016/j.tree.2005.01.010 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 37.Sumpter DJT. 2006. The principles of collective animal behaviour. Phil. Trans. R. Soc. B 361, 5–22 10.1098/rstb.2005.1733 (doi:10.1098/rstb.2005.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couzin ID. 2009. Collective cognition in animal groups. Trends Cogn. Sci. 13, 36–43 10.1016/j.tics.2008.10.002 (doi:10.1016/j.tics.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 39.Kümmerli R, Brown SP. 2010. Molecular and regulatory properties of a public good shape the evolution of cooperation. Proc. Natl Acad. Sci. USA 107, 18 921–18 926 10.1073/pnas.1011154107 (doi:10.1073/pnas.1011154107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward AJW, Sumpter DJT, Couzin ID, Hart PJB, Krause J. 2008. Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953 10.1073/pnas.0710344105 (doi:10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward AJW, Herbert-Read JE, Sumpter DJT, Krause J. 2011. Fast and accurate decisions through collective vigilance in fish shoals. Proc. Natl Acad. Sci. USA 108, 2312–2315 10.1073/pnas.1007102108 (doi:10.1073/pnas.1007102108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson DE, Châline N. 2007. Modulation of pheromone trail strength with food quality in Pharaoh's ant, Monomorium pharaonis. Anim. Behav. 74, 463–470 10.1016/j.anbehav.2006.11.027 (doi:10.1016/j.anbehav.2006.11.027) [DOI] [Google Scholar]
- 43.Visscher PK. 2007. Group decision making in nest-site selection among social insects. Annu. Rev. Entomol. 52, 255–275 10.1146/annurev.ento.51.110104.151025 (doi:10.1146/annurev.ento.51.110104.151025) [DOI] [PubMed] [Google Scholar]
- 44.Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125, 237–246 10.106/j.cell.2006.04.001 (doi:10.106/j.cell.2006.04.001) [DOI] [PubMed] [Google Scholar]
- 45.Brown SP, Johnstone RA. 2001. Cooperation in the dark: signalling and collective action in quorum-sensing bacteria. Proc. R. Soc. Lond. B 268, 961–965 10.1098/rspb.2001.1609 (doi:10.1098/rspb.2001.1609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darch SE, West SA, Winzer K, Diggle SP. 2012. Density-dependent fitness benefits in quorum-sensing bacterial populations. Proc. Natl Acad. Sci. USA 109, 8259–8263 10.1073/pnas.1118131109 (doi:10.1073/pnas.1118131109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner A. 2011. Genotype networks shed light on evolutionary constraints. Trends Ecol. Evol. 26, 577–584 10.1016/j.tree.2011.07.001 (doi:10.1016/j.tree.2011.07.001) [DOI] [PubMed] [Google Scholar]
- 48.Beaumont HJE, Gallie J, Kost C, Ferguson GC, Rainey PB. 2009. Experimental evolution of bet hedging. Nature 462, 90–94 10.1038/nature08504 (doi:10.1038/nature08504) [DOI] [PubMed] [Google Scholar]
- 49.Ratcliff WC, Denison RF. 2010. Individual-level bet hedging in the bacterium Sinorhizobium meliloti. Curr. Biol. 20, 1740–1744 10.1016/j.cub.2010.08.036 (doi:10.1016/j.cub.2010.08.036) [DOI] [PubMed] [Google Scholar]
- 50.Schalk IJ, Hannauer M, Braud A. 2011. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 13, 2844–2854 10.1111/j.1462-2920.2011.02556.x (doi:10.1111/j.1462-2920.2011.02556.x) [DOI] [PubMed] [Google Scholar]
- 51.Sun G-X, Zhou W-Q, Zhong J-J. 2006. Organotin decomposition by pyochelin, secreted by Pseudomonas aeruginosa even in an iron-sufficient environment. Appl. Environ. Microbiol. 72, 6411–6413 10.1128/AEM.00957-06 (doi:10.1128/AEM.00957-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Or D, Smets BF, Wraith JM, Dechesne A, Friedman SP. 2007. Physical constraints affecting bacterial habitats and activity in unsaturated porous media: a review. Adv. Water Resour. 30, 1505–1527 10.1016/j.advwatres.2006.05.025 (doi:10.1016/j.advwatres.2006.05.025) [DOI] [Google Scholar]
- 53.Dumas Z, Kümmerli R. 2012. Cost of cooperation rules selection for cheats in bacterial metapopulations. J. Evol. Biol. 25, 473–484 10.1111/j.1420-9101.2011.02437.x (doi:10.1111/j.1420-9101.2011.02437.x) [DOI] [PubMed] [Google Scholar]
- 54.Mioni C, Howard AM, DeBruyn JM, Bright NG, Twiss MR, Applegate BM, Wilhelm SW. 2003. Characterization and field trials of a bioluminescent bacterial reporter of iron bioavailability. Mar. Chem. 83, 31–46 10.1016/S0304-4203(03)00094-X (doi:10.1016/S0304-4203(03)00094-X) [DOI] [Google Scholar]
- 55.Norrström AC. 1995. Concentration and chemical species of iron in soils from groundwater/surface water ecotones. Hydrol. Sci. J. 40, 319–329 10.1080/02626669509491418 (doi:10.1080/02626669509491418) [DOI] [Google Scholar]
- 56.De Vos D, De Chial M, Cochez C, Jansen S, Tümmler B, Meyer J-M, Cornelis P. 2001. Study of pyoverdin type and production by Pseudomonas aeruginosa isolated from cystic fibrosis patients: prevalence of type II pyoverdine isolates and accumulation of pyoverdine-negative mutations. Arch. Microbiol. 175, 384–388 10.1007/s002030100278 (doi:10.1007/s002030100278) [DOI] [PubMed] [Google Scholar]
- 57.Wiehlmann L, et al. 2007. Population structure of Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 104, 8101–8106 10.1073/pnas.0609213104 (doi:10.1073/pnas.0609213104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiricny N. 2010. The social evolution of Pseudomonas aeruginosa in the cystic fibrotic lung. PhD thesis, University of Oxford, Oxford, UK [Google Scholar]
- 59.Stites SW, Plautz MW, Bailey K, O'Brien-Ladner AR, Wesselius LJ. 1999. Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am. J. Respir. Crit. Care Med. 160, 796–801 10.1164/ajrccm.160.3.9811018 (doi:10.1164/ajrccm.160.3.9811018) [DOI] [PubMed] [Google Scholar]
- 60.Reid DW, Lam QT, Schneider H, Walters EH. 2004. Airway iron and iron-regulatory cytokines in cystic fibrosis. Eur. Respir. J. 24, 286–291 10.1183/09031936.04.00104803 (doi:10.1183/09031936.04.00104803) [DOI] [PubMed] [Google Scholar]
- 61.Kümmerli R, Griffin AS, West SA, Buckling A, Harrison F. 2009. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc. R. Soc. B 276, 3531–3538 10.1098/rspb.2009.0861 (doi:10.1098/rspb.2009.0861) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data deposited in Dryad: http://dx.doi.org/10.5061/dryad.jp596.