Abstract
Insects have been extraordinarily successful in occupying terrestrial habitats, in contrast to their mostly aquatic sister group, the crustaceans. This success is typically attributed to adult traits such as flight, whereas little attention has been paid to adaptation of the egg. An evolutionary novelty of insect eggs is the serosa, an extraembryonic membrane that enfolds the embryo and secretes a cuticle. To experimentally test the protective function of the serosa, we exploit an exceptional possibility to eliminate this membrane by zerknüllt1 RNAi in the beetle Tribolium castaneum. We analyse hatching rates of eggs under a range of humidities and find dramatically decreasing hatching rates with decreasing humidities for serosa-less eggs, but not for control eggs. Furthermore, we show serosal expression of Tc-chitin-synthase1 and demonstrate that its knock-down leads to absence of the serosal cuticle and a reduction in hatching rates at low humidities. These developmental genetic techniques in combination with ecological testing provide experimental evidence for a crucial role of the serosa in desiccation resistance. We propose that the origin of this extraembryonic membrane facilitated the spectacular radiation of insects on land, as did the origin of the amniote egg in the terrestrial invasion of vertebrates.
Keywords: desiccation resistance, cuticle, Tribolium castaneum, chs (chitin synthase), zen (zerknüllt)
1. Introduction
Insects comprise three-quarters of all described animal species and their diversification represents an unparalleled episode in the course of evolution [1,2]. Insects are among the earliest land animals and their exoskeleton preadapted them for terrestrial life. However, without the ability to oviposit on land, insects would have never been able to attain this incredible diversity [2]. In insect eggs, an extraembryonic membrane, the serosa, envelops the embryo and yolk [3]. To investigate the possibility that the serosa is an evolutionary novelty in insects that protects the embryo against desiccation, we first explore the literature to examine the correlation between the humidity of the habitat and the presence of a serosa in the major arthropod groups: the chelicerates, the myriapods, the crustaceans and the hexapods (comprising entognaths and insects).
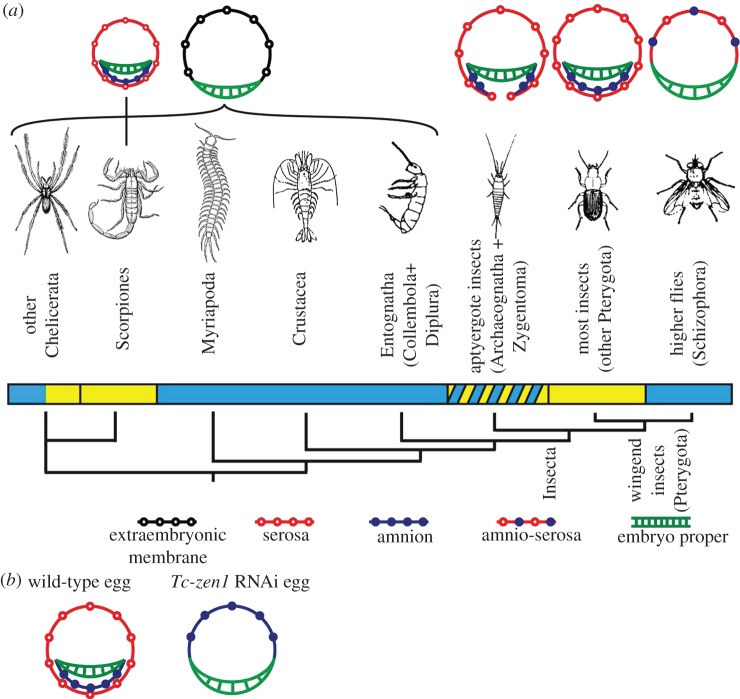
All major arthropod groups have colonized land to some extent. Chelicerates have been quite successful in terrestrial environments, because they evolved maternal adaptations to support terrestrial egg development [1,4–8]: spiders wrap their eggs in silk, scorpions are viviparous, and mites develop elaborate maternal eggshells and wax layers. Eggs of the mite Halotydeus destructor have even been reported to develop inside the maternal body, which serves as a protective envelope after death [9]. Chelicerates do not have an extraembryonic membrane that envelops the embryo (figure 1a) [15] (see e.g. [16–18] for more recent descriptions), although scorpion embryos have been reported to be covered by two extraembryonic membranes [19–21] (figure 1a).
Figure 1.
The serosa is an evolutionary novelty of insects. (a) Phylogeny of the main arthropod groups [10,11]. The bar under the groups indicates whether species in this class are generally aquatic or restricted to humid environments for reproduction (blue), or terrestrial (yellow), or if species of this class live in very different environments concerning humidity (yellow diagonal stripes). Above the groups, schematic cross-section drawings of the embryo (green) and extraembryonic membranes are shown (open black circles, extraembryonic membrane; red open circles, serosa; blue closed circles, amnion). Jura [12], Machida [13] and Machida & Ando [14] call the extraembryonic membrane in Entognatha a serosa. We, however, adopted the terminology of Anderson [15]. Although parallel evolution of two extraembryonic membranes took place in the scorpions, a serosa completely enveloping the embryo and secreting a cuticle is an evolutionary novelty of the insects. In the Schizophoran flies, a secondary reduction took place. (b) Schematic drawing of Tribolium wild-type and Tc-zen1 RNAi development. In wild-type eggs, the serosa completely envelops yolk and embryo. After Tc-zen1 RNAi, an amnion covers the yolk dorsally; the serosa is absent.
In contrast to the chelicerates, crustaceans are largely aquatic, myriapods mainly occur in tropical soil or leaf litter, and the Entognatha are restricted to humid conditions [1,6,22]. It is striking that all these arthropod groups have a single extraembryonic membrane that covers the yolk dorsally, but never envelops the embryo [15] (see e.g. [23–25] for more recent descriptions; figure 1a). In insects, however, a serosa is present that completely enfolds the embryo [3,13,14,21,26] (figure 1a). Insects occupy all terrestrial habitats and have radiated into more than a million species [1,2]. In some basal (apterygote) insects, the serosa does not close completely beneath the embryo [12]. Consistent with this, variation exists in their desiccation resistance: most apterygotes live in leaf litter, under bark and in other places with high humidity, but some are extremely resistant to desiccation (ch. 5 in [6]). In the pterygotes, the serosa completely envelops the embryo. More than two-thirds of all blastodermal cells will give rise to the serosa in most insects, suggesting an important role for this membrane [26].
Exceptionally, in a single group of higher flies (Schizophora), including Drosophila, a secondary reduction of the extraembryonic membranes to a single dorsal amnioserosa took place [27,28] (figure 1a). Eggs of these flies are generally deposited in rotting vegetable matter or moist soil, or plant or animal tissues [29–31]. There are drosophilids that occur in dry habitats such as the Sonoran desert, but their eggs develop in necrotic tissue of cacti where humidity is higher than in the surrounding air at day and reaches over 90 per cent relative humidity (RH) at night [32]. Drosophila melanogaster eggs do not survive RH below 80 per cent [33].
Overall, we find an intriguing correlation between the capacity of arthropod eggs to develop under dry conditions and the presence of a serosa that completely enfolds the embryo. Interestingly, the serosa secretes a chitinized cuticle underneath the maternal eggshell [34]. Since eggs of the mosquitoes Aedes aegypti and Anopheles gambiae gain desiccation resistance at the time of serosal cuticle secretion, the serosal cuticle has been suggested to protect the developing embryo against desiccation [35,36]. However, no experimental evidence exists because it is impossible to physically remove the zygotic serosa or serosal cuticle in insects without affecting the overlying maternal eggshell, which consists of an exochorion, endochorion and vitelline membrane [37].
To investigate the hypothesis that the serosa protects the embryo against desiccation, we use RNAi in Tribolium castaneum. In this beetle, it is possible to prevent the development of the serosa without affecting the maternal eggshell using parental Tc-zerknüllt1 (Tc-zen1) RNAi [38]. In Tc-zen1 RNAi eggs, a single amnion (the inner extraembryonic membrane that normally covers the embryo ventrally) covers the yolk dorsally and does not envelop the embryo (figure 1b). This is similar to the reduced extraembryonic membrane in Drosophila (figure 1a). Zen RNAi or mutations lead to lethal alterations of late morphogenetic movements in other insects, as does Tc-zen2 RNAi in Tribolium [38–41]. Tribolium Tc-zen1 RNAi eggs, however, can hatch under normal laboratory conditions [38]. Thus, Tribolium provides a unique system to experimentally test our hypothesis. Finally, using in situ hybridization and RNAi, we investigate whether Tc-chs1, a key enzyme in cuticle synthesis [42–44], is involved in serosal cuticle secretion and desiccation resistance of the egg.
Our results unravel a dual role for the serosa; first, a role in desiccation resistance mediated by the secreted serosal cuticle; and second, mediated by the serosal cells themselves, a role in dorsal closure, the process during which the lateral halves of the embryo meet dorsally and enclose the yolk [39].
2. Material and methods
(a). Molecular cloning and RNAi
The plasmid containing an 850 bp Tc-zen1 fragment was obtained from Falciani et al. [45]. The 333 bp Tc-chs1 fragment was cloned according to Arakane et al. [44]. Its dsRNA targets both splice variants of Tc-chs1 [44]. Non-targeting control dsRNA was synthesized from a 500 bp vector sequence (pCRII, Invitrogen) cloned with the forward primer 5′-TGCCGGATCAAGAGCTACCAA-3′ and the reverse primer 5′-TGTGAGCAAAAGGCCAGCAA-3′. dsRNA was synthesized using the MEGAscript RNAi kit (Ambion), and about 0.2 μl of a 0.5 μg μl−1 dsRNA solution was injected into pupae of the San Bernardino strain, according to Bucher et al. [46].
(b). Hatching rate assays
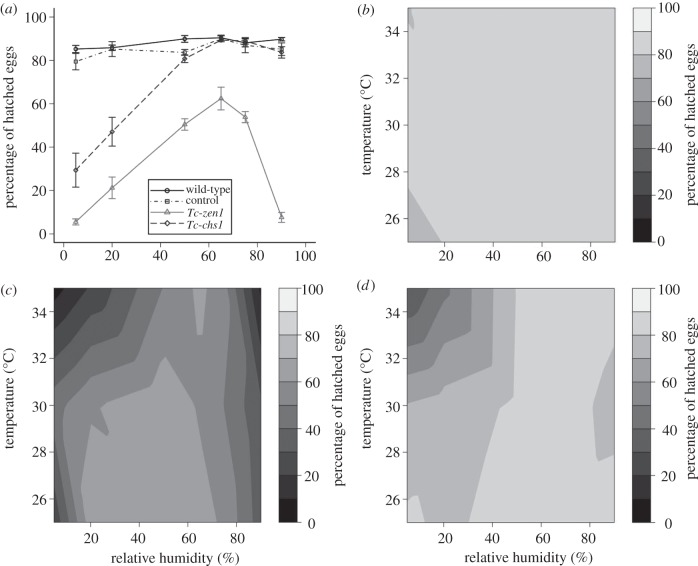
Eggs were collected at 18–24 h old and put individually in a well of a 96-well plate, and incubated at 5, 20, 50, 65, 75 or 90 per cent RH and at 25°C, 30°C or 35°C (5° below, exactly at and 5° above the temperature at which our laboratory stock is kept, respectively). Hatching rates were assayed after 4 days for 35°C, 5 days for 30°C and 8 days for 25°C. These data points were repeated three to ten times, giving rise to standard errors such as in figure 2a. Heat maps correlating hatching rates to humidity and temperature were generated using bivariate interpolation [47] in R v. 2.13.1 [48]. Five per cent RH was obtained using silica gel; 20 per cent RH was obtained adjusting a KOH solution at the bottom of a glass desiccator [49]. Relative humidities of 50, 65, 75 and 90 per cent RH were obtained in climate chambers. We excluded higher humidities to avoid condensation of liquid water. For the hatching rate assays, eggs were put into a 96-well plate with their chorion and adhering flour. To investigate the role of the exochorion, however, we removed the exochorion by washing the eggs in 50 per cent bleach for 1 min, after which they were rinsed in water, dried at room temperature and incubated at 30°C at the different humidities for 5 days. All temperatures and humidities were constantly monitored using a MicroDAQ datalogger.
Figure 2.
Eggs without a serosa or a serosal cuticle become desiccation-susceptible. (a) Hatching rates of control (grey squares), wild-type (black circles), Tc-zen1 RNAi (grey triangles) and Tc-chs1 RNAi (black diamonds) eggs at 35°C. Error bars indicate standard error among three to 10 replicates of 96 eggs. (b–d) Heat maps summarizing hatching rates of (b) control eggs, (c) Tc-zen1 RNAi eggs and (d) Tc-chs1 RNAi eggs.
(c). Embryo fixation, in situ hybridizations and immunohistochemistry
The Tc-chs1 fragment used for in situ hybridization was cloned with the forward primer 5′-AACGACTTCATCTCGCACCAACACG-3′ and the reverse primer 5′-AAATTGGCAGTTCCATGAGCCGG-3′. Embryo fixation and in situ hybridization were performed according to Shippy et al. [50]. Fluorescein isothiocyanate (FITC)-coupled wheat germ agglutinin (WGA) stainings were performed according to Rezende et al. [36], where WGA is a lectin highly specific for N-acetylglucosamine polymers, therefore specifically labelling chitinous structures. For egg size measurements, 8 per cent formaldehyde in phosphate-buffered saline (PBS) was used for fixation, and the methanol shock was omitted to prevent shape changes.
(d). Transmission electron microscopy
For electron microscopical preparations, both wild-type and Tc-chs1 RNAi eggs were collected at 37 and 63 h after egg lay (AEL) and fixed for 1 h at 30°C. Eggs were dechorionated and fixed in 5 ml heptane and 5 ml of a solution of 2.5 per cent gluteraldehyde, 2 per cent paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4). After this initial fixation, eggs were removed from the solution, washed with 70 per cent ethanol to remove the heptane and fixed for another hour in 5 ml of 2.5 per cent gluteraldehyde, 2 per cent paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4). After fixation, specimens were washed (3 × 10 min) in cacodylate buffer and placed for 1 h in 1 per cent osmium tetroxide. Then, specimens were dehydrated with increasing concentrations of ethanol and embedded in Agar100. Sections of about 70 nm thickness were contrasted with uranyl acetate and lead citrate, and studied with a JEOL 1010 transmission microscope coupled to an Olympus MegaView camera.
3. Results
(a). Serosa-less eggs are prone to desiccation
To experimentally test the hypothesis that the serosa protects against desiccation, we assayed hatching rates of serosa-less (Tc-zen1 RNAi) eggs at various humidities and three temperatures (25°C, 30°C and 35°C). Since Tc-zen1 is solely expressed in the serosa, we expect only serosa-related effects. The penetrance of the serosa-less phenotype [38] is higher than 95 per cent and this was constantly monitored in fixed material in parallel to the experiments. As a control, a non-targeting dsRNA was used (see §2a). In total, we analysed over 40 000 eggs. At 35°C, wild-type eggs and control eggs show hatching rates of about 80 per cent in all humidities (figure 2a). At this temperature, serosa-less eggs display lower hatching rates, with a peak average hatching rate of 62.4 per cent at 65 per cent RH (figure 2a). In contrast to the control and wild-type eggs, hatching rates of the serosa-less eggs decrease dramatically to 5.5 per cent at 5 per cent RH. We display the hatching rates at all three temperatures and their interpolation in heat maps (figure 2b,c). Hatching rates of control eggs and wild-type eggs are above 75 per cent for all conditions (figure 2b; electronic supplementary material, figure S1). In strong contrast to control eggs, serosa-less eggs show dramatically decreased hatching rates in low humidities at all temperatures (figure 2c). The effect is most pronounced at higher temperatures, when evaporation is maximal (figure 2c). These data provide strong evidence that the serosa is crucial for desiccation resistance.
To verify that only the zygotic serosa, but not the maternal eggshell mediates desiccation resistance, we performed a hatching rate assay on eggs from which the exochorion was removed by a bleach treatment. We found no significant differences in hatching rates between eggs with and without the exochorion (see the electronic supplementary material, figure S2). These data suggest that it is not the maternal eggshell that is required for desiccation resistance.
(b). The serosa secretes a cuticle that protects against desiccation
To differentiate the effect of the serosal cells themselves and the secreted serosal cuticle, we investigated serosal cuticle formation in Tribolium using transmission electron microscopy (TEM). In wild-type eggs, a serosal cuticle with clear chitinous layers is present, as in the lepidopteran Manduca sexta [51] (figure 3a). Next, we cloned Tc-chitin-synthase1 (Tc-chs1, also called TcCHS-A), a key enzyme involved in Tribolium cuticle production [42–44]. During early development, we detected mRNA of this gene in the serosa, but not in the embryo (figure 3c–f). After injection of 0.5 µg µl−1 Tc-chs1 dsRNA in female pupae of the San Bernardino strain, we obtained eggs. In these eggs, the serosal cuticle was severely affected (figure 3b). Amniotic and serosal cells were found, but the chitinous layers of the cuticle were absent. Thus, Tc-chs1 pRNAi is an efficient method to disrupt serosal cuticle formation. We subjected Tc-chs1 RNAi eggs to the same hatching rate assay as Tc-zen1 RNAi eggs. In eggs without a serosal cuticle, hatching rates decreased at low humidities, similar to serosa-less eggs (figure 2a,d). We conclude that it is mainly the cuticle secreted by the serosal cells that protects the egg against desiccation.
Figure 3.
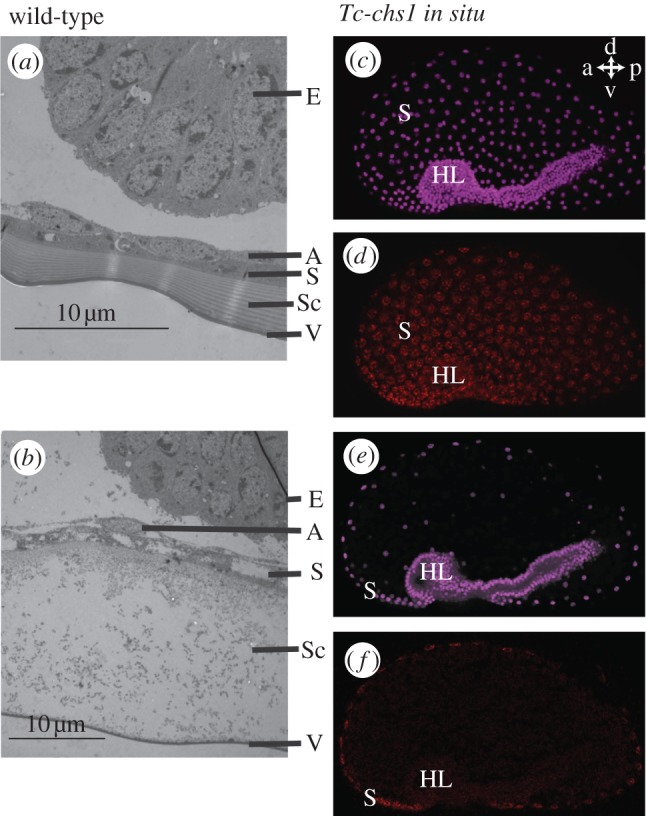
Tribolium castaneum produces a chitinized serosal cuticle. (a,b) TEM pictures of an approximately 37 h old (a) wild-type and (b) Tc-chs-1 RNAi egg. Note the absence of the chitinous layers in the Sc after Tc-chs1 RNAi. The vitelline membrane no longer sticks to the cuticle. The serosal cells might have a slightly aberrant appearance because they lost contact with the extracellular matrix, similar to the chitin-secreting epidermal cells in Drosophila chs1 (kkv) mutants [52]. (c) Nuclear DRAQ5 stain (purple) of the egg shown in (d). The serosal nuclei are widely spaced. Ventrally, the dense nuclei of the embryo proper (headlobes to the left) are prominently visible. (d) Tc-chs1 in situ hybridization during gastrulation. Tc-chs1 mRNA (red) is detected around the serosal nuclei and not in the embryo. (e,f) A single confocal plane showing an optical cross-section of the embryo shown in (c) and (d). a, anterior; p, posterior; d, dorsal; v, ventral. HL, head lobes; E, embryo; A, amnion; S, serosa; Sc, serosal cuticle; V, vitelline membrane.
Arakane et al. [44] report involvement of Tc-chs1 in epidermal cuticle formation. We cannot exclude that the epidermal cuticle contributes to the desiccation resistance found in figure 2d. However, TEM analysis shows that an epidermal cuticle is still not present when embryos are at least 65 h old (figure 4h,i). The larvae hatch when they are 82 h old. That means that the contribution of the epidermal cuticle to desiccation resistance must be relatively small. Furthermore, lactic acid digestions of Tc-chs1 RNAi eggs suggest that larval cuticle secretion is hardly affected in our RNAi treatments. Insect cuticles resist digestion by lactic acid [54], and we find larval cuticles of normal appearence after lactic acid digestions of Tc-chs1 RNAi eggs (see the electronic supplementary material, figure S4u). Together with the fact that our Tc-chs1 RNAi eggs can hatch, this could mean that injection of 0.5 µg µl−1 Tc-chs1 dsRNA in pupae of the San Bernardino strain affects the epidermal cuticle less than injection of 1 µg µl−1 in pupae of the Georgia strain [44]. Finally, chitin can be detected using WGA [36,55]. Staining of eggshells using WGA-FITC and digestions of eggs by lactic acid reveal the serosal cuticle in wild-type eggs, and confirm its absence after Tc-chs1 and Tc-zen1 RNAi (see the electronic supplementary material, figures S3 and S4).
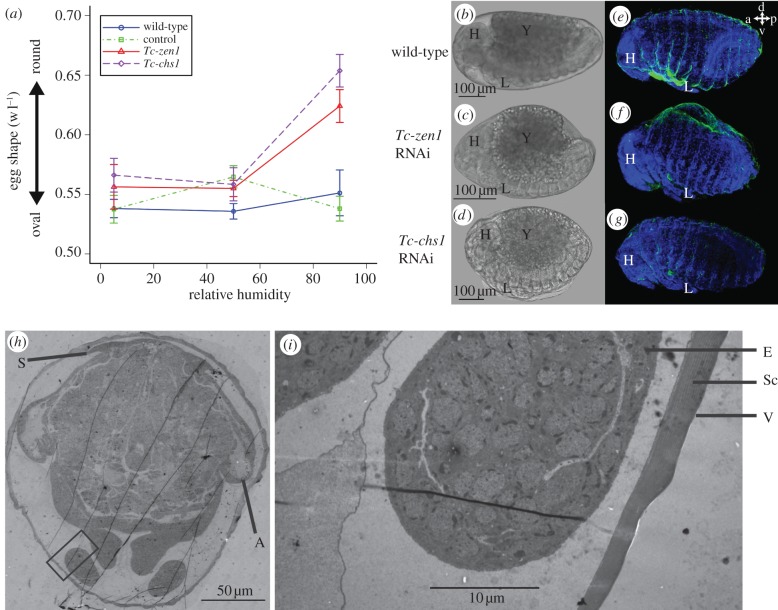
Figure 4.
Tc-zen1 RNAi embryos display defects in dorsal closure at high humidity. (a) Width/length ratio (1 = perfect sphere) of control (green squares), wild-type (blue circles), Tc-zen1 RNAi (red triangles) and Tc-chs1 RNAi (purple diamonds) eggs measured in their vitelline membrane using ImageJ [53]. (b–d) Phase contrast images of a 65–75 h old (b) wild-type, (c) Tc-zen1 RNAi and (d) Tc-chs1 RNAi embryo at 90% RH. (e–g) DAPI (blue) label cell nuclei and WGA-FITC [36] (green) label chitin of the larval cuticle in (e) a dorsally closed wild-type embryo, (f) a Tc-zen1 RNAi embryo that has not completed dorsal closure, and (g) a dorsally closed Tc-chs1 RNAi embryo. a, anterior; p, posterior; d, dorsal; v, ventral. In (e), a small piece of vitelline membrane is stuck between the legs. (h) Overview TEM pictures of 65–75 h old wild-type egg during dorsal closure with dorsally condensed serosa. (i) Magnification of area boxed in (h). A layered serosal cuticle can be detected (Sc), but the serosa has condensed dorsally. No epidermal cuticle is found 65 h AEL. H, head; Y, yolk; L, legs; E, embryo; Sc, serosal cuticle; V, vitelline membrane; S, serosa; A, amnion.
(c). The serosa facilitates proper dorsal closure
Unexpectedly, hatching rates of serosa-less eggs not only decrease at low humidities, but also at relative humidities higher than 75 per cent (figure 2a,c). A process important at these high humidities might be the uptake of water by osmosis. To assess water uptake, we measured the volume of eggs lacking a serosa or serosal cuticle using microscopy, and weighed single eggs and groups of eggs on an ultrafine balance that could measure one-tenth of a microgram. However, we could not detect any consistent increase (or slower decrease) in volume or weight for Tc-zen1 or Tc-chs1 RNAi eggs, compared with control eggs. Nevertheless, by measuring width and length of eggs at 65–75 h AEL, we could detect a consistent shape change at 90 per cent RH towards a more rounded shape for both Tc-zen1 RNAi and Tc-chs1 RNAi eggs (figure 4a–d). At this time point, no epidermal cuticle is present yet (figure 4h,i). The shape change at high humidity suggests an increase in internal pressure, and thus water uptake. This would mean that our weight measurements are not sensitive enough, and that both Tc-zen1 RNAi and Tc-chs1 RNAi eggs do take up water at high humidities.
We wondered whether this shape change affects dorsal closure. During this process, which occurs at the end of embryonic development, the extraembryonic membrane actively pulls the sides of the embryo over the yolk to close dorsally [38,39,56] (electronic supplementary material, figure S5). In order to assay dorsal closure, we analysed fixed eggs a few hours before hatching. In Tc-zen1 RNAi eggs, 24 per cent and 26 per cent of the embryos did not complete dorsal closure (n = 93 and n = 65) at 5 per cent and 50 per cent RH, respectively (see the electronic supplementary material, figure S6). These proportions are not significantly different from each other (proportion test, p > 0.05 [57]). Importantly, the visible defects in dorsal closure (dorsal open phenotypes) for serosa-less eggs double to 52 per cent (n = 54) at 90 per cent RH (figure 4f; electronic supplementary material, figure S6). The occurrence of dorsal closure failure at 90 per cent RH was significantly higher than in 5 per cent RH (proportion test, p < 0.05) and than in 50 per cent RH (proportion test, p < 0.01).
We cannot exclude a severe delay in dorsal closure in Tc-zen1 RNAi eggs. However, the number of embryos that do not close dorsally matches well the number of embryos that do not hatch (figure 2a), suggesting that dorsal closure fails and is not simply delayed at these humidities. We conclude that the decreased hatching rates of serosa-less eggs at high humidity are largely caused by an increased incidence of dorsal closure defects. The amnion that is present at the dorsal side of Tc-zen1 RNAi eggs instead of the serosa (figure 1b; electronic supplementary material, figure S5) might not apply sufficient pulling force for dorsal closure, especially in rounded eggs. Despite rounding up at high humidities, Tc-chs1 RNAi eggs do not show defects in dorsal closure and hatch at the same rates as control eggs (figures 2a,d and 4e,g). This means that the serosal cuticle normally prevents shape changes at higher humidities, possibly because of its rigidity, but that this function is not required for dorsal closure. Rounded eggs can perform dorsal closure provided that serosal cells are present, which is the case in Tc-chs1 RNAi eggs.
4. Discussion
We provided experimental evidence for a role for the serosa in desiccation resistance. Tc-zen1 and Tc-chs1 pRNAi provided an excellent approach to reveal this function. It should be noted, however, that an amniotic cavity (the space between embryo and amnion) is eliminated in Tc-zen1 RNAi eggs, because of the ectopic dorsal amnion. We cannot exclude formally that the lack of an amniotic cavity causes increased desiccation sensitivity in these eggs. However, we do not think that this is the case since Tc-chs1 RNAi eggs that do possess an amniotic cavity are prone to desiccation too. It should be noted that apart from chitin, the serosal cuticle also comprises proteins, tyrosine-derived quinones responsible for sclerotization and lipids [35,58–60]. Thus, in Tc-chs1 RNAi eggs, the other components of the serosal cuticle are still produced. These other cuticular components in combination with intact serosal cells might protect slightly against desiccation and could provide an explanation for the higher viability of Tc-chs1 RNAi eggs when compared with Tc-zen1 RNAi eggs at low relative humidities (figure 2a).
It is interesting that desiccation resistance is a zygotic investment. Although protective maternal eggshells are known from Chelicerata, the present work in Tribolium shows that, in insects, it is not the maternal eggshell that protects against desiccation, but the cuticle secreted by the zygotic serosa, as suggested before for mosquitoes [36].
We also showed that dorsal closure is more robust in presence of serosal cells. In the context of dorsal closure, the serosa compacts into a condensed structure, the dorsal organ [15,39] (electronic supplementary material, figure S5e). This quick condensation is probably required for proper dorsal closure in humid conditions. The actual final dorsal closure (that is, the joining of the two lateral sides of the embryo) takes place after degeneration of the serosa and must be mediated by the amnion. It could well be that the role of an extraembryonic membrane in dorsal closure was ancestral in arthropods [13,14]. This membrane would then have differentiated into the amnion that mediates final dorsal closure, and into the serosa that was recruited to fold around the embryo and secrete a cuticle to mediate desiccation resistance.
Overall, non-insect terrestrial arthropods are generally restricted to cryptozoic environments and have undergone limited speciation [2]. Desiccation-resistant eggs must have been crucial for insect habitat expansion, as were the amniote egg and the seed for vertebrates and plants, respectively [61,62]. Using T. castaneum as a model, we have demonstrated a critical role for the insect serosa in desiccation resistance. We propose that the origin of the serosa opened up a whole new range of terrestrial oviposition sites and facilitated the spectacular radiation of insects on land.
Acknowledgements
We thank Siegfried Roth and Bas Zwaan for ideas, Rodrigo Fonseca and Jessica Cande for technical advice, Agnieszka Doroszuk, Daniel Rozen, Julia Hunn, Michael Richardson, Paul Brakefield, Hans Slabbekoorn and Siegfried Roth for critically reading the manuscript, and Kees Koops for practical assistance. G.L.R. thanks Mike Levine for the opportunity to be introduced to T. castaneum. C.G.C.J. performed RNAi, hatching experiments, egg measurements and lactic acid digests. G.L.R. performed Tc-chs1 in situ hybridization. M.v.d.Z. performed WGA-FITC stainings on egg shells and analysed dorsal closure using WGA-FITC. G.E.M.L. and C.G.C.J. performed TEM. G.L.R. and M.v.d.Z. conceived the study. C.G.C.J. and M.v.d.Z. wrote the paper. All authors analysed and discussed data, and commented on the manuscript.
Funding statement
Part of this work was financially supported by the EU Network of Excellence LifeSpan (FP6 036894). G.L.R. was supported by Faperj and CNPq. M.v.d.Z. was supported by NWO VENI grant no. 863.09.014. The authors declare no competing financial interests.
References
- 1.Grimaldi DA, Engel MS. 2005. Evolution of the insects. New York, NY: Cambridge University Press [Google Scholar]
- 2.Zeh DW, Zeh JA, Smith RL. 1989. Ovipositors, Amnions and eggshell architecture in the diversification of terrestrial arthropods. Q. Rev. Biol. 64, 147–168 10.1086/416238 (doi:10.1086/416238) [DOI] [Google Scholar]
- 3.Schwalm FE. 1988. Insect morphogenesis. Basel, Switzerland: S. Karger AG [Google Scholar]
- 4.Beament JWL. 1951. The structure and formation of the egg of the fruit tree red spider mite, Metatetranychus ulmi Koch. Ann. Appl. Biol. 38, 1–24 10.1111/j.1744-7348.1951.tb07787.x (doi:10.1111/j.1744-7348.1951.tb07787.x) [DOI] [Google Scholar]
- 5.Foelix RF. 1996. Biology of spiders, 2nd edn, p. 330 Oxford, UK: Oxford University Press [Google Scholar]
- 6.Gillott C. 2005. Entomology, 3rd edn Dordrecht, The Netherlands: Springer [Google Scholar]
- 7.Lees AD. 1948. Passive and active water exchange through the cuticle of ticks. Discuss. Faraday Soc. 3, 187–192 10.1039/df9480300187 (doi:10.1039/df9480300187) [DOI] [Google Scholar]
- 8.Witaliñski W. 1993. Egg shells in mites: vitelline envelope and chorion in Acaridida (Acari). Exp. Appl. Acarol. 17, 321–344 10.1007/bf00058596 (doi:10.1007/bf00058596) [DOI] [Google Scholar]
- 9.Norris KR. 1950. The aestivating eggs of the Red-legged earth mite, Halotydeus destructor (Tucker). Bull. Commonw. Sci. Indust. Res. Aust. 253, 1–26 [Google Scholar]
- 10.Giribet G, Edgecombe GD. 2012. Re-evaluating the arthropod tree of life. Annu. Rev. Entomol. 57, 167–186 10.1146/annurev-ento-120710-100659 (doi:10.1146/annurev-ento-120710-100659) [DOI] [PubMed] [Google Scholar]
- 11.Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW. 2010. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463, 1079–1083 See http://www.nature.com/nature/journal/v463/n7284/suppinfo/nature08742_S1.html. [DOI] [PubMed] [Google Scholar]
- 12.Jura C. 1972. Development of apterygote insects. In Developmental systems: insects (eds Counce SJ, Waddington CH.), pp. 49–94 London, UK: Academic Press Inc [Google Scholar]
- 13.Machida R. 2006. Evidence from embryology for reconstructing the relationships of hexapod basal clades. Arthropod Syst. Phylogeny 64, 95–104 [Google Scholar]
- 14.Machida R, Ando H. 1998. Evolutionary changes in developmental potentials of the embryo proper and embryonic membranes along with the derivative structures in atelocerata, with special reference to hexapoda (Arthropoda). Proc. Arthropod Embryol. Soc. Jpn 33, 1–13 [Google Scholar]
- 15.Anderson DT. 1973. Embryology and phylogeny in annelids and arthropods. Oxford, UK: Pergamon Press [Google Scholar]
- 16.Dearden PK, Donly C, Grbić M. 2002. Expression of pair-rule gene homologues in a chelicerate: early patterning of the two-spotted spider mite Tetranychus urticae. Development 129, 5461–5472 10.1242/dev.00099 (doi:10.1242/dev.00099) [DOI] [PubMed] [Google Scholar]
- 17.Mittmann B, Wolff C. 2012. Embryonic development and staging of the cobweb spider Parasteatoda tepidariorum C. L. Koch, 1841 (syn.: Achaearanea tepidariorum; Araneomorphae; Theridiidae). Dev. Genes Evol. 222, 189–216 10.1007/s00427-012-0401-0 (doi:10.1007/s00427-012-0401-0) [DOI] [PubMed] [Google Scholar]
- 18.Wolff C, Hilbrant M. 2011. The embryonic development of the central American wandering spider Cupiennius salei. Front. Zool. 8, 1–35 10.1186/1742-9994-8-15 (doi:10.1186/1742-9994-8-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korschelt E, Heider K. 1936. Vergleichende Entwicklungsgeschichte der Tiere. New York, NY: Fischer [Google Scholar]
- 20.Laurie M. 1890. The embryology of a scorpion (Euscorpius italicus). Q. J. Microsc. Sci. 31, 105–142 [Google Scholar]
- 21.Rafiqi AM. 2008. Morphological transitions and the genetic basis of the evolution of extraembryonic tissues in flies. PhD thesis, Wageningen University, Wageningen, The Netherlands [Google Scholar]
- 22.Larink O, Bilinski SM. 1989. Fine structure of the egg envelopes of one proturan and two Collembolan genera (Apterygota). Int. J. Insect Morphol. Embryol. 18, 39–45 10.1016/0020-7322(89)90034-2 (doi:10.1016/0020-7322(89)90034-2) [DOI] [Google Scholar]
- 23.Brena C, Akam M. 2012. The embryonic development of the centipede Strigamia maritima. Dev. Biol. 363, 290–307 10.1016/j.ydbio.2011.11.006 (doi:10.1016/j.ydbio.2011.11.006) [DOI] [PubMed] [Google Scholar]
- 24.Browne WE, Price AL, Gerberding M, Patel NH. 2005. Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis 42, 124–149 10.1002/gene.20145 (doi:10.1002/gene.20145) [DOI] [PubMed] [Google Scholar]
- 25.Extavour CG. 2005. The fate of isolated blastomeres with respect to germ cell formation in the amphipod crustacean Parhyale hawaiensis. Dev. Biol. 277, 387–402 (doi:10.1016/j.ydbio.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 26.Roth S. 2004. Gastrulation in other insects. In Gastrulation: from cells to embryos (ed. Stern C.), pp. 105–121 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- 27.Rafiqi AM, Lemke S, Ferguson S, Stauber M, Schmidt-Ott U. 2008. Evolutionary origin of the amnioserosa in cyclorrhaphan flies correlates with spatial and temporal expression changes of zen. Proc. Natl Acad. Sci. USA 105, 234–239 10.1073/pnas.0709145105 (doi:10.1073/pnas.0709145105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Ott U. 2000. The amnioserosa is an apomorphic character of cyclorrhaphan flies. Dev. Genes Evol. 210, 373–376 10.1007/s004270000068 (doi:10.1007/s004270000068) [DOI] [PubMed] [Google Scholar]
- 29.Ferrar P. 1987. A guide to the breeding habits and immature stages of Diptera Cyclorrhapha. Leiden, The Netherlands: Brill u.a [Google Scholar]
- 30.Colless DH, McAlpine DK. 1970. Diptera. In The insects of Australia (ed. Waterhouse DF.), pp. 656–740 Carlton, Australia: Melbourne University Press [Google Scholar]
- 31.McAlpine JF. 1989. Manual of Nearctic Diptera. Ottawa, Canada: Research Branch, Agriculture Canada [Google Scholar]
- 32.Gibbs AG, Perkins MC, Markow TA. 2003. No place to hide: microclimates of Sonoran Desert Drosophila. J. Therm. Biol. 28, 353–362 10.1016/S0306-4565(03)00011-1 (doi:10.1016/S0306-4565(03)00011-1) [DOI] [Google Scholar]
- 33.Al-Saffar ZY, Grainger JNR, Aldrich J. 1995. Influence of constant and changing temperature and humidity on the development and survival of the eggs and pupae of Drosophila melanogaster (Meigen). J. Therm. Biol. 20, 389–397 10.1016/0306-4565(94)00075-T (doi:10.1016/0306-4565(94)00075-T) [DOI] [Google Scholar]
- 34.Hinton HE. 1981. The biology of insect eggs, 1st edn Oxford, UK: Pergamon Press [Google Scholar]
- 35.Goltsev Y, Rezende GL, Vranizan K, Lanzaro G, Valle D, Levine M. 2009. Developmental and evolutionary basis for drought tolerance of the Anopheles gambiae embryo. Dev. Biol. 330, 462–470 10.1016/j.ydbio.2009.02.038 (doi:10.1016/j.ydbio.2009.02.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rezende GL, Martins AJ, Gentile C, Farnesi LC, Pelajo-Machado M, Peixoto AA, Valle D. 2008. Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Dev. Biol. 8, 82. 10.1186/1471-213x-8-82 (doi:10.1186/1471-213x-8-82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furneaux PJS, James CR, Potter SA. 1969. The egg shell of the house cricket (Acheta domesticus): an electron-microscope study. J. Cell Sci. 5, 227–249 [DOI] [PubMed] [Google Scholar]
- 38.van der Zee M, Berns N, Roth S. 2005. Distinct functions of the Tribolium zerknullt genes in serosa specification and dorsal closure. Curr. Biol. 15, 624–636 10.1016/j.cub.2005.02.057 (doi:10.1016/j.cub.2005.02.057) [DOI] [PubMed] [Google Scholar]
- 39.Panfilio KA. 2008. Extraembryonic development in insects and the acrobatics of blastokinesis. Dev. Biol. 313, 471–491 10.1016/j.ydbio.2007.11.004 (doi:10.1016/j.ydbio.2007.11.004) [DOI] [PubMed] [Google Scholar]
- 40.Panfilio KA, Liu PZ, Akam M, Kaufman TC. 2006. Oncopeltus fasciatus zen is essential for serosal tissue function in katatrepsis. Dev. Biol. 292, 226–243 10.1016/j.ydbio.2005.12.028 (doi:10.1016/j.ydbio.2005.12.028) [DOI] [PubMed] [Google Scholar]
- 41.Wakimoto BT, Turner FR, Kaufman TC. 1984. Defects in embryogenesis in mutants associated with the antennapedia gene-complex of Drosophila melanogaster. Dev. Biol. 102, 147–172 10.1016/0012-1606(84)90182-9 (doi:10.1016/0012-1606(84)90182-9) [DOI] [PubMed] [Google Scholar]
- 42.Arakane Y, Hogenkamp DG, Zhu YC, Kramer KJ, Specht CA, Beeman RW, Kanost MR, Muthukrishnan S. 2004. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem. Mol. Biol. 34, 291–304 10.1016/j.ibmb.2003.11.004 (doi:10.1016/j.ibmb.2003.11.004) [DOI] [PubMed] [Google Scholar]
- 43.Arakane Y, Muthukrishnan S, Kramer KJ, Specht CA, Tomoyasu Y, Lorenzen MD, Kanost M, Beeman RW. 2005. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol. Biol. 14, 453–463 10.1111/j.1365-2583.2005.00576.x (doi:10.1111/j.1365-2583.2005.00576.x) [DOI] [PubMed] [Google Scholar]
- 44.Arakane Y, Specht CA, Kramer KJ, Muthukrishnan S, Beeman RW. 2008. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 38, 959–962 10.1016/j.ibmb.2008.07.006 (doi:10.1016/j.ibmb.2008.07.006) [DOI] [PubMed] [Google Scholar]
- 45.Falciani F, Hausdorf B, Schroder R, Akam M, Tautz D, Denell R, Brown S. 1996. Class 3 Hox genes in insects and the origin of zen. Proc. Natl Acad. Sci. USA 93, 8479–8484 10.1073/pnas.93.16.8479 (doi:10.1073/pnas.93.16.8479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucher G, Scholten J, Klingler M. 2002. Parental RNAi in Tribolium (Coleoptera). Curr. Biol. 12, R85–R86 10.1016/S0960-9822(02)00666-8 (doi:10.1016/S0960-9822(02)00666-8) [DOI] [PubMed] [Google Scholar]
- 47.Akima H. 1978. Bivariate interpolation and smooth surface fitting for irregularly distributed data points. ACM Trans. Math. Softw. 4, 160–164 10.1145/355780.355787 (doi:10.1145/355780.355787) [DOI] [Google Scholar]
- 48.R Development Core Team 2009. R: a language and environment for statistical computing (2.9.0 ed.). Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 49.Winston PW, Bates DH. 1960. Saturated solutions for the control of humidity in biological research. Ecology 41, 232–237 10.2307/1931961 (doi:10.2307/1931961) [DOI] [Google Scholar]
- 50.Shippy TD, Ronshaugen M, Cande J, He JP, Beeman RW, Levine M, Brown SJ, Denell RE. 2008. Analysis of the Tribolium homeotic complex: insights into mechanisms constraining insect Hox clusters. Dev. Genes Evol. 218, 127–139 10.1007/s00427-008-0213-4 (doi:10.1007/s00427-008-0213-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamer A, Dorn A. 2001. The serosa of Manduca sexta (Insecta, Lepidoptera): ontogeny, secretory activity, structural changes, and functional considerations. Tissue Cell 33, 580–595 10.1054/tice.2001.0213 (doi:10.1054/tice.2001.0213) [DOI] [PubMed] [Google Scholar]
- 52.Moussian B, Schwarz H, Bartoszewski S, Nusslein-Volhard C. 2005. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J. Morphol. 264, 117–130 10.1002/jmor.10324 (doi:10.1002/jmor.10324) [DOI] [PubMed] [Google Scholar]
- 53.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 10.1038/nmeth.2089 (doi:10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wieschaus E, Nusslein-Volhard C. 1986. Looking at embryos. In Drosophila: a practical approach (ed. Roberts DB.), pp. 199–227 Oxford, UK: IRL Press [Google Scholar]
- 55.Wright CS. 1984. Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J. Mol. Biol. 178, 91–104 10.1016/0022-2836(84)90232-8 (doi:10.1016/0022-2836(84)90232-8) [DOI] [PubMed] [Google Scholar]
- 56.Solon J, Kaya-Çopur A, Colombelli J, Brunner D. 2009. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331–1342 10.1016/j.cell.2009.03.050 (doi:10.1016/j.cell.2009.03.050) [DOI] [PubMed] [Google Scholar]
- 57.Newcombe RG. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17, 857–872 10.1002/(SICI)1097-0258(19980430)17:8%3C857::AID-SIM777%3E3.0.CO;2-E (doi:10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 58.Furneaux PJS, McFarlane JE. 1965. A possible relationship between the occurrence of catecholamines and water absorption in insect eggs. J. Insect Physiol. 11, 631–635 10.1016/0022-1910(65)90145-9 (doi:10.1016/0022-1910(65)90145-9) [DOI] [PubMed] [Google Scholar]
- 59.Furneaux PJS, McFarlane JE. 1965. Identification, estimation, and localization of catecholamines in eggs of the house cricket, Acheta domesticus (L.). J. Insect Physiol. 11, 591–600 10.1016/0022-1910(65)90141-1 (doi:10.1016/0022-1910(65)90141-1) [DOI] [PubMed] [Google Scholar]
- 60.McFarlane JE. 1960. Structure and function of the egg shell as related to water absorption by the eggs of Acheta domesticus (L.). Can. J. Zool. 38, 231–241 10.1139/z60-029 (doi:10.1139/z60-029) [DOI] [Google Scholar]
- 61.Reisz RR. 1997. The origin and early evolutionary history of amniotes. Trends Ecol. Evol. 12, 218–222 10.1016/s0169-5347(97)01060-4 (doi:10.1016/s0169-5347(97)01060-4) [DOI] [PubMed] [Google Scholar]
- 62.Stewart WN. 1983. Paleobotany and the evolution of plants. New York, NY: Cambridge University Press [Google Scholar]