Abstract
Most studies in chronobiology focus on solar cycles (daily and annual). Moonlight and the lunar cycle received considerably less attention by chronobiologists. An exception are rhythms in intertidal species. Terrestrial ecologists long ago acknowledged the effects of moonlight on predation success, and consequently on predation risk, foraging behaviour and habitat use, while marine biologists have focused more on the behaviour and mainly on reproduction synchronization with relation to the Moon phase. Lately, several studies in different animal taxa addressed the role of moonlight in determining activity and studied the underlying mechanisms. In this paper, we review the ecological and behavioural evidence showing the effect of moonlight on activity, discuss the adaptive value of these changes, and describe possible mechanisms underlying this effect. We will also refer to other sources of night-time light (‘light pollution’) and highlight open questions that demand further studies.
Keywords: lunar cycle, reproduction, communication, predation, foraging, light pollution
1. Introduction
Most studies in chronobiology focus on solar cycles (daily and annual); moonlight and the lunar cycle received considerably less attention. An exception are intertidal habitats, where the effects of the lunar cycle have been studied extensively with a focus on behaviour and reproduction synchronization (reviewed by [1,2]). Terrestrial ecologists long ago acknowledged the effects of moonlight on predation success, and consequently on predation risk, foraging behaviour and habitat use [3,4].
The lunar cycle refers to the 29.5 days (lunar month) required for the Moon to orbit around the Earth, and the 24.8 h (lunar day) required for the Moon to travel by the same spot on the Earth. These two cycles give rise to several environmental cycles, such as illumination levels, tides and geomagnetic fields. As a result of the Moon's orbit around the Earth, every approximately 14.5 days the Moon, the Earth and the Sun are in approximately the same axis and this gives rise to spring tides. The 24.8-hour lunar day, on the other hand, leads to a tidal cycle of 12.4 h, with high tides when the Moon is positioned directly above the sea water or on the point diametrically opposed on the other side of the planet. Finally, the deviation of the Moon's orbit from the Earth's equatorial plane causes the amplitude of the semidiurnal tides (with a period of 12.4 h) to be asymmetrical, leading a semidiurnal inequality in the tides, in some cases so unequal that there is only one significant ebb of water every 24.8 h and not every 12.4 h [5,6]. All these environmental changes can be perceived by animals and plants, affect their behaviour, physiology, the adaptive significance of performing certain activity at a certain time and ultimately affect their fitness.
Light changes during the lunar cycle can affect rhythms in organisms in several non-mutually exclusive ways. It can represent a time cue, for example, for explosive breeding in amphibians; it can change the ability of animals to use visual cues, affecting the use of senses (e.g. for communication, navigation, prey or predator location); and it can indirectly change the biotic environment by affecting activity levels of predators, competitors and prey. In the marine realm, the Earth's biggest reproduction event known as the ‘mass spawning event’, which occurs in the Great Barrier Reef, Australia, the Moon may act as clock to choreograph sex among more than 100 species of corals at the same night, once a year.
We review the ecological and behavioural evidence for the cycling effect of moonlight on animals, discuss the adaptive value of these responses and address possible underlying mechanisms: is there evidence for a lunar day or lunar month endogenous rhythm? Does the effect of moonlight represent a ‘masking’ effect? Is moonlight intensity high enough to influence the circadian clock, and how? We also refer to artificial sources of nocturnal light (‘light pollution’) and highlight open questions that demand further studies.
2. Effects on reproduction
(a). Invertebrates
The effects of moonlight on reproduction are summarized in the electronic supplementary material, table S1. One of the most prominent examples of light-dependent behaviour in Anthozoans is the synchronized spawning at the Great Barrier Reef in Western Australia [7–10], Hawaii [11], the Caribbean [12] and Okinawa [13]. Hundreds of species of corals and other invertebrates spawn simultaneously several nights after the full Moon. The timing of annual coral spawning varies geographically around the Earth but is consistent and predictable at any location [13,14]. Environmental factors, such as sea temperature, salinity, tidal periodicity, food and day length have been suggested as inducers for gametogenesis and spawning [7,10,15], while actual spawning appears to be triggered by the level of lunar irradiance [7,13,16–18].
Sesarma haematocheir is a terrestrial crab, common in Japan. One population of this species live as adults in the mountains above the Ogamo river, but adult females have to climb down the mountain to release their offspring into the sea. They do so only during new or full Moon, when spring tides occur [19]. This semilunar rhythm is entrained by moonlight cycles and not by tides [20,21].
The small marine chironomid Clunio marinus [22] larvae live in algae mats in the lowest intertidal zone of the European Atlantic coast. When new or full Moon is approaching, the mature larvae pupate and few days later the adults eclose at local low tide, when their habitat has fallen dry. The males eclose first, search for a female pupa, help the wingless female to eclose from the pupal case and mate with her. After mating, the males bring the females to favourable places, where the fertilized eggs are deposited; shortly afterwards the adults die. The insects use a circasemilunar clock to time these events and the moonlight cycles as zeitgeber in southern populations [23]. Northern populations use water turbulence as zeitgeber, because northern summer nights are short and the Moon is too low at the sky to be able to be a reliable zeitgeber.
Lunar rhythms in reproduction exist also in marine species that do not experience tidal fluctuations, such as the swarming of the Palolo worm Eunice viridis in the Pacific ocean [24], and in species living in tropical lakes, as plankton and mayflies [25,26]. The mayfly Povilla adusta develops in Lake Victoria, West Africa. Povilla mayflies eclose in large numbers from their pupal case 2 days after full Moon. This synchrony is necessary because adults live only 1–2 h and during this short time span they have to perform their mating flight, copulate and lay eggs. They do so during the time when the bright Moon lengthens twilight [27].
(b). Amphibians
Anurans typically use either explosive or prolonged breeding. Explosive breeders show a very high degree of synchronization. A study which analysed various parameters related to reproductive activity for a range of anuran and urodele species, sites and years found lunar periodicity in the large arrival and spawning events, the number of animals in amplexus and first sightings of individuals [28]. Female Tungara frogs (Physalaemus pustulosus) change their mate choice in response to changing light levels: in dim light, females prefer males with simple, louder calls, which indicate a closer male, than in the dark. These differences in male call preference in response to changing light levels probably reflect the increased predation risk caused by movement towards the chosen male when light levels are high [29].
(c). Birds
Several species of nightjars synchronize their nesting cycles with the lunar cycle: the whip-poor-will (Caprimulgus vociferous) hatching tends to occur during new Moon nights, so that the highest energy demand of the nestlings coincides with the period with most moonlight [30]. In the visually oriented insectivores, fierynecked nightjar (Caprimulgus pectoralis), egg laying starts with the full Moon in September and is further stimulated by the next two full Moon periods. Parents feed their brood during twilight and full Moon [31], enabling longer foraging activity which coincides with the highest energy demand of the brood.
In the moluccan megapode (Megapodius wallacei), egg laying peaks during the week after full Moon, and decreases just after new Moon. Moreover, during full Moon nights, the egg burrows are more distributed and are deeper than during new Moon [32].
(d). Mammals
In the Euroasian badger (Meles meles), squat marking and raised-leg urination, which increase in frequency during reproductively active period, as well as mattings, are highest around new Moon [33].
To sum, in many cases, Moon phase acts as a synchronizer for reproduction, allowing explosive breeders (such as amphibians), species which use external fertilization (such as anthozoans) or species with extremely short lifespan (mayflies) to synchronize reproductive activity. In these cases, Moon phase serves as a synchronizing cue between individuals, which is expected to result in increased reproductive success. In other groups, Moon phase serves as a cue for other environmental parameter (spring tides, food availability) which effect reproductive success.
3. Effects on communication
The effects of moonlight on communication are summarized in the electronic supplementary material, table S2. Moonlight presents a powerful source of light, which may greatly affect the ability to use visual communication.
(a). Birds
Eagle owls (Bubo bubo) use visual signalling for intraspecific communication. Vocal display is more frequent, and performed on higher elevation call-posts in moonlit nights, increasing the conspicuousness of the white throat feathers that appear during the vocal display [34]. Calls of the Mexican spotted owl (Strix occidentalis lucida), which does not display white plumage when calling, are more frequent during the waning and new Moon quarters [35]. Call frequency of nocturnal seabirds (petrels) are very low during moonlit nights, possibly to avoid predation [36].
Several species of nightjars vocalize more during moonlight nights. It is possible that the high light intensity results in increased time available for foraging and ease in moving and defending territories, as well as better ability to detect and evade predators, which result in increased call frequency [31,37,38].
(b). Mammals
Coyotes (Canis latrans) use three different types of howling: lone, group and group-yip howling. Group and group-yip howling used for territorial advertisement are more frequent during new Moon nights [39], implying increased territorial defence. Alternatively, under low visual acuity, the coyotes may prefer to hunt larger prey, which demands hunting in groups and hence communication [39].
4. Effects on foraging and predation (success and risk)
The effects of moonlight on foraging and predation are summarized in electronic supplementary material, table S3. Many studies have documented an influence of moonlight on foraging that could result from the effects of light conditions on visual detection of food items, including prey, and therefore may also influence predation risk. Many species are both predator and prey, and the demand of avoiding predation while foraging, both of which may be influenced by light, makes foraging a complex decision. In case the increased predation risk outweighs the increase in foraging success, a lower activity level during moonlight nights is expected. Conversely, if the increase in foraging success outweighs predation risk, a higher activity level during moonlight nights is expected. The effect on predation success may be caused by increased visual acuity during moonlit nights, but may also result from changes in prey activity, which may in turn respond to the lunar cycle.
(a). Invertebrates
Doodle bug larvae (Myrmeleon obscurus) dig funnel-shaped traps into the sand at the bottom of which they lurk for insect prey [40]. These funnels are rebuilt every day: around full Moon the pits are large, during new Moon small; perhaps the higher probability to catch prey during moonlit nights is worth the investment. This is an endogenous lunar rhythm, since it is observable also under continuous darkness in the laboratory [41].
The nocturnal bee, Sphecodogastra texana, shows a clear lunar rhythm in foraging. Pollen collection occurs during dusk and is extended during moonlit night; the bees do not leave their nest during new Moon nights [42]. Even for the diurnal honeybee a lunar rhythm in foraging activity is reported in North Africa (Morocco) during winter, whereas foraging shows a semilunar rhythm during the summer [43].
(b). Reptiles
Most reptiles are active during daytime; there are very few studies on the effect of Moon phase on reptiles. Most studies found that snakes are less active during full Moon nights, among them a small cryptic desert snake, the desert nightsnake (Hypsiglena chlorophaea), which feeds on other small reptiles [44], the habu (Trimeresurus flavoviridis), a venomous pit viper species endemic to the Ryukyu Islands of Japan [45], and the fish eating snake Lake Tanganyika water snake (Lycodonomorphus bicolor) [46]. Bright moonlight avoidance by snakes may be a strategy that reduces detection by visually hunting predators and may also be influenced by the activity patterns of their nocturnal rodent prey. A different response was observed in the Florida cottonmouth snakes (Agkistrodon piscivorus conanti) which are terrestrial, and feed on fish carrion dropped by nesting birds. They are nocturnal, and their only potential predators are owls. The availability of fish carrions is indifferent to light levels, eliminating the complexities of availability of the prey. Activity of these snakes is positively correlated with Moon phase, and snakes are significantly more active during full Moon nights. The higher activity during full Moon nights is the opposite of the expected response to predation risk from owls, and probably results from the better visual detection of the fish carrions [47].
Two studies looked at the effect of moonlight on habitat selection in snakes under laboratory conditions: one found that adult prairie rattlesnakes (Crotalus viridis viridis) decrease activity in general and especially in open microhabitats during moonlit nights [48]. The other tested the combined effect of moonlight and prey availability on habitat selection in the brown tree snake (Boiga irregularis) which enabled them to differentiate between the direct effect of light on perceived predation risk and prey detection. The snakes were able to detect the prey presence in the open microhabitat during both full and Moon nights, but descended from the tree canopy microhabitat only during new Moon nights, indicating that microhabitat selection in this species is a predator avoidance strategy [46].
(c). Birds
Movement of breeding eagle owls (Bubo bubo) is highest during full Moon nights. This may reflect an increase in the time needed to find prey, resulting from an effect of Moon phase on prey behaviour. Accordingly, hunting effort in this species peaked during dark nights, possibly compensating for a decreased hunting efficiency due to the decreased ability to detect prey visually [49]. However, it may also result from the time owls devote to vocal communication displays, which involve frequent and rapid movements from one call post to another, during full Moon nights (see the communication chapter in [49]).
Nightjars, visually oriented insectivores, exhibit increased foraging efficiency by moonlight and avoid activity at dark nights; some species enter torpor during these nights [50–53]. The whip-poor-will (Caprimulgus vociferous) increases locomotor activity, nest visits and vocalization during full Moon nights [30]. However, Moon heights and not percentages of moon-face illuminated affect foraging activity, suggesting that some lunar light is necessary to allow activity, but above a threshold level other factors are important [44]. Two species of tropical nightjars, the standard-winged nightjar (Macrodipteryx longipennis) and the long-tailed nightjar (Caprimulgus climacurus), show the greatest crepuscular activity during new Moon nights. However, foraging during crepuscular twilight impose a high risk of predation to nightjars, and when moonlight conditions allow them to forage during the night, they avoid the twilight and forage during nights. Moreover, during the wet season, Moon light promoted flight activity of insects, increasing prey availability [54]. The freckled nightjars (Caprimulgus tristigma) forage for insects in moonlight, and become torpid when light levels are low. When the full Moon was covered by clouds, the nightjars did not forage and entered torpor, indicating that effects of the lunar cycle on foraging and thermoregulation are a direct response to light conditions [53]. Australian owlet-nightjars (Aegotheles cristatus) do not increase foraging during moonlit nights, possibly because risk of predation during full Moon nights outweighs the benefits of foraging [50].
Lapwings (Vanellus vanellus) feed by day and roost by night for most of the lunar cycle, except for a few full Moon nights during summer, when the pattern is reversed [55].
Many bird species migrate at night. Decreased lunar light is correlated with increased number of departures during fall migration of land birds [56]. The arrival date of Barau's petrel (Pterodroma baraui), a tropical seabird, to their breeding colonies, occurred around the last full Moon of the austral (Southern Hemisphere) winter, and their night-time sea activity exhibited a clear cycle of about 29 days, with higher activity during full Moon nights, suggesting that nocturnal foraging is regulated by Moon phase [57].
(d). Mammals
The effect of moonlight as an indirect cue for predation risk for rodents received considerable attention during the last decades [4,58–60]. Rodents are preyed upon by owls and mammalian predators, whose predation efficiency increases during full Moon nights [4,59,61–64]; consequently, many rodent species reduce activity or shift it to more sheltered habitats [58,65–70].
Most bat species which respond to moonlight decrease their activity during moonlit nights (lunar-phobia). However, pteropodid bats feed on fruits and rely on vision for foraging, and it is expected that locating fruits will be easier during moonlit nights. Nevertheless, during full Moon nights the Mexican fruit bat (Artibeus jamaicensis [71]) decreases its activity, and the eastern tube-nosed bat (Nyctimene robinsoni) has significantly lower body temperature, reflecting reduced activity [72]. Common vampire bat Desmodus rotundus are most active during new Moon nights and darker periods of the night, and most bats do not leave their roost during full Moon nights [73]. Fruits (and prey of vampire bats) availability should be similar in full and new Moon nights, but may be easier to find during full Moon lights, so it is possible that the bats are able to forage more efficiently during moonlit nights, and therefore need to invest less time in foraging. Alternatively, the reduction in activity may reflect predation avoidance. Studies on islands, where most of the visual bat predators are absent, found no effect of lunar phase on activity [74,75], supporting the latter hypothesis.
Insectivores and fish eating bats are both predators and prey, and their own prey may respond to the lunar cycle. Thus, moonlight may increase their foraging success and predation risk. Moreover, their prey may reduce activity levels in response to high illumination and predation risk, thus reducing food availability. Many studies of insectivorous bats found no effect of Moon phase on activity levels. Others found such an effect, and attributed it to increased predation risk, decreased prey availability or both. The greater fishing bat, Noctilio leporinus, significantly increases its activity in low light conditions [76] including the dark parts of full Moon nights, suggesting that they respond directly to light conditions. Interestingly, these bats preferred to forage in well-lit areas by dock or boat lights; these areas appear to have the highest concentration of surface disturbances from fish activity, i.e. the bats appear to be responding to the effect of light on their prey behaviour [76]. Another water surface forager whose activity is negatively correlated with moonlight is Daubenton's bat (Myotis daubentonii) [77]. A study of activity levels of the white-throated round-eared bat (Lophostoma silvicolum), a gleaning insectivorous bat which relies mainly on passive acoustic cues to find prey, and its main prey item katydids, found that both were significantly more active during the dark periods associated with new Moon [78], suggesting that the reduced activity can be attributed to reduced prey availability [78].
The fringe-lipped bat (Trachops cirrhosis) preys on frogs which it locates acoustically through the frogs calling behaviour. When the frogs detect this bat, they immediately stop calling, reducing predation risk. On dark nights, the frogs are not able to visually detect the bats, which may affect predation success of the bats, and therefore their activity levels [79].
Another possible response to moonlight is a change in microhabitat selection. A study of bat community comprising insectivorous species in Vancouver Island found no evidence for the effect of moonlight intensity. However, height of activity within the forest changed depending on Moon phase, and there was a significant interaction between moonlight and height. The authors suggest that bats adjust use of microhabitats to match prey distribution [80].
(i). Large mammalian predators
Darwin suggested that human innate fear of darkness is an adaptation for avoiding risk of predation by nocturnal predators [81]. A study of African lions found that food consumption is negatively correlated with Moon light levels. This result accords with studies showing highest hunting success during dark nights [82]. Lions were more likely to make a daytime kill after full Moon nights. Human victims were significantly more likely to be attacked during new Moon nights and during the darkest hours of the night [82]. While herbivores are most likely to be attacked during the first and third quarter, humans were most likely to be attacked during the first week after full Moon. This difference probably stems from the difference in behaviour between herbivores and humans: while herbivores remain outdoors all night, humans sleep in shelters, are not active throughout the night and are most exposed to predators during the evening. The first hours of the night are darkest during the week following full Moon, and the lions are hungriest at that time because of the low predation success during full Moon nights, producing a lunar pattern in human predation [82].
Wild maned wolves (Chysocyon brachyurys), which hunt for small rodents and ground living birds, reduce their activity during full Moon nights, possibly in response to reduced activity of their prey [83]. The reduced activity level could also result from increased predation efficiency. African wild dogs (Lycaon pictus) are considered diurnal or crepuscular. However, when more than 49 per cent of the Moon is visible, starting 7 days before full Moon, and ending 6 days after full Moon, they hunt also at night [84]. In another study, the number of pray taken by European wolves (Canis lupus lupus) was highest during dim light; dawn, dusk and moonlit nights [61,64].
Cats hunt primarily using visual and auditory cues. Moonlight increases visibility for cats, and therefore vulnerability of their prey. A study of activity levels of jaguars (Panthera onca) and their most important prey armadillos (Dasypus novemcinctus) found that armadillos were less active above ground during nights with high Moon illumination levels, while that of the jaguar shifted from armadillo habitats to other habitats [85].
(ii). Primates
Most nocturnal primates are largely dependent on moonlight for their foraging activity [86–89]. They show a remarkable lunarphilia, with nocturnal activity tracking the 24.8 h lunar periodicity. The presumed benefits include predator avoidance, competitor avoidance and optimized foraging opportunities. Nocturnal activity in primates is restricted, probably by the availability of Moon light, which may be a consequence of the importance of the visual system in primates.
To sum, moonlight effects activity pattern of a forager if it affects its ability to use its senses for locating its food, or if it affects the availability of its prey. Prey species whose predation risk is influenced by moonlight are expected to reduce their activity and/or change their microhabitat selection in response to changing moonlight levels.
5. Mechanisms underlying lunar chronobiology
(a). Clock entrainment by moonlight
In many of the above-mentioned examples, the monthly rhythms are controlled by endogenous circalunar clocks. In other cases, the endogenous circadian clocks respond to moonlight and the daily activity phases are shifted when compared with moonless nights (figure 1). In any case, the animals have to perceive the Moon phase and moonlight. The mechanisms and photoreceptors used to detect moonlight and entrain or shift the endogenous clock were revealed in only few organisms.
Figure 1.
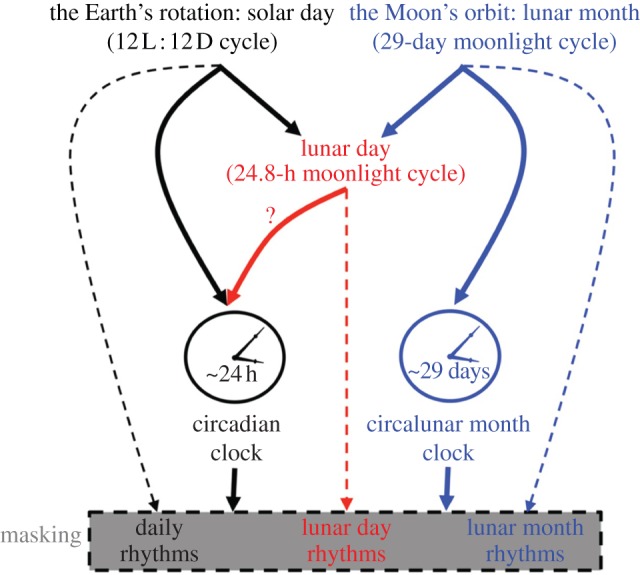
Mechanisms underlying moonlight effects on overt physiological and behavioural rhythms. The lunar month is associated with a 29-day moonlight-dark cycle with a peak during full Moon and a trough during new Moon. The solar day is associated with a 12 L : 12 D cycle. The interaction between the Earth's rotation and the Moon's orbit around the Earth leads to the lunar day, associated with a 28.4-hour moonlight-dark cycle on days in which moonlight is intense enough. The 12 L : 12 D cycle entrains (black solid arrow) circadian clocks that generate circadian rhythms. The lunar day moonlight-dark cycle could potentially entrain (red solid arrow) the circadian clock. The 29-day moonlight-dark cycle entrains (blue solid arrow) circalunar month clocks that generate circalunar month rhythms. Every light environmental cycle exerts masking (dashed arrows) on the expression of biological rhythms.
(i). Invertebrates
In corals, the repeated episodes associated with broadcast spawning year after year is controlled by the environment (Moon phase and moonlight) and an endogenous biological clock. Jokiel et al. [18] experimentally manipulated moonlight phases, which resulted in asynchronous planula release from Pocillopora damicornis colonies when they were maintained in constant full/new Moon conditions. Biophysical evidence shows that corals exhibit photoreception in the blue region of the light spectrum [90] and are extraordinarily sensitive to blue spectra matching blue moonlight irradiance levels [91]. Nevertheless, the molecular elements underpinning the detection and response to the low intensity blue moonlight has remained undescribed for many years, and only recently Levy et al. [92] reported the presence of an ancient family of blue-light-sensing photoreceptors, cryptochromes (CRYs), in the ubiquitous reef-building coral, Acropora millepora. In addition to being CRYs from the simplest eumetazoan described to date, cry2 gene was expressed preferentially during full versus new Moon nights, suggesting a key role in mass coral spawning. cry2 codes for the ‘mammalian type’ CRY that works as transcription factor in the core circadian clock of mammals and is suggested to have similar function in non-Drosophilid insects, but is absent from Drosophila melanogaster.
Recent studies have begun to unravel the genetic basis of the lunar and circadian clock, as well as moonlight photoreception in the marine midge Clunio marinus. Mapping of clock genes and light receptors identified ciliary opsin 2 as a candidate to be involved in both lunar and diurnal timing; cryptochrome 1 (cry1 = ‘Insect type’ cryptochrome; main photoreceptor of the circadian clock in D. melanogaster) as a candidate gene for lunar timing; and two timeless (tim2, tim3) genes as candidate genes for diurnal timing [93]. tim2 is also called timeout and is involved in chromosome stability and light entrainment of the circadian clock in D. melanogaster [94]. The function of tim3 is still unknown, but it has sequence similarities to tim2 and is also present in mosquitoes as Aedes aegypti and Culex quinquefasciatus [93]. The photoreceptor responsible for moonlight detection is most probably located in the larval ocelli [95]. The latter show a lunar-rhythmic change of shielding pigment transparency indicating that they do not only function as moonlight receptors, but that they are also controlled by the circalunar clock itself, hence being primary candidates for tracing input and output pathways of the lunar pacemaker. Additionally, the reversible optical change of shielding pigment transparency in Clunio reveals a mechanism to enhance photosensitivity under the condition of very dim light.
The fruitfly D. melanogaster does not show any lunar periodicity, but its circadian clock has been shown to be very sensitive to light [96,97] . The locomotor activity of fruitflies can be easily entrained to moonlight-dark cycles, and when artificial moonlight is given during the dark period of a 12 L : 12 D cycle, the flies’ usual crepuscular activity patterns turn more nocturnal: the flies shift their activity largely into the night [98]. Fruitflies seems to be active at dim light [99]; thus, part of their nocturnality is due to moonlight-stimulated activity without shifting the circadian clock [100]. Such responses are also called masking effects (see below). Nevertheless, a significant part of the flies’ nocturnal activity is caused by a phase shift of the molecular clock in response to moonlight [98]. Thus, moonlight is capable of shifting the clock and can act as significant zeitgeber in fruitflies. Interestingly, the blue-light photopigment cryptochrome (CRY1) seems to be dispensible for the phase-shifting capability of the clock, since flies without functional CRY1 can still shift activity into the night, whereas eyeless flies cannot [98]. Notably, the effect of moonlight on Drosophila's activity rhythm was entirely studied in the laboratory at constant moderate temperatures. A recent study shows that the flies do not increase activity during full Moon nights in nature [101], most probably because of the lower night-temperatures. The same applies for other nocturnal activities as mating that can be observed under certain conditions in the laboratory [102]. Nevertheless, the laboratory studies showed unequivocally that the fruitflies have the capability to respond to moonlight.
(ii). Mammals
In hamsters, constant night-time illumination at or below moonlight intensities have at least four effects on the circadian system: re-entrainment is accelerated following a shift in the light cycle [103]; reproductive responsiveness to shortened day lengths is enhanced [104]; the propensity to split activity rhythms is augmented [105,106]; the range of entrainment is increased [103]. The authors hypothesized that dim, natural light at night may normally modify the phase relationships between multiple circadian oscillators.
(b). Masking effect of moonlight
The term ‘masking’ [107] describes an immediate effect of a stimulus that overrides an animal's endogenous clock. The masking effect of light is different in nocturnal and diurnal species: light typically increases activity in diurnal mammals (positive masking) and suppresses it in nocturnal ones (negative masking), while darkness acts in the opposite way [108–111]. Masking has the adaptive value of confining animals to their temporal niche, and may complement the circadian clock in fine-tuning activity patterns in response to environmental stimuli [112]. Although masking is presumably involved in the response to moonlight; in many cases, it is rather difficult to rigorously differentiate its contribution from that of other mechanisms such as circadian or a circalunar clock.
One study showed clearly that ultraviolet light reflected from the Moon acutely increased the amplitude of the circadian rhythm in visual sensitivity of the horseshoe crab [113]. Because these animals use vision to find mates and prefer night-time high tides, moonlight presumably boosts their visual sensitivity as they approach the shallows where UV levels are elevated and competition for mates is high. Owl monkeys (A. azarai boliviensis) and red-fronted lemurs (Eulemur fulvus albifrons) increase nocturnal activity with increased intensities of moonlight in either field studies or seminatural conditions, suggesting a masking effect [87]. Several studies have looked at the effect of clouds covering the Moon [53,114], and even the effect of eclipse of the Moon on activity [89]. These effects suggest that the moonlight-associated activity of these primates is a product of acute stimulatory effects of moonlight on nocturnal activity as evident in the reduced travelled distances in the lemur [115] and reduced activity in the owl monkey [89] during full Moon eclipses and by the effect of clouds covering the Moon on the activity of nightjars [53]. During new Moon nights, owl monkeys become diurnal, compensating for the lack of nocturnal activity. Thus, the switch of the temporal distribution of activity from nocturnal to diurnal in the owl monkeys results from masking of their truly nocturnal circadian system.
A field experiment using open enclosures which tested the effect of two nights 3 h light pulse (2 lux) during new Moon nights on the activity of common and golden spiny mice (Acomys cahirinus and A. russatus, respectively) found that while diurnal golden spiny mice did not respond, nocturnal common spiny mice reduced activity and body temperature in response to the light pulse, suggesting that negative masking was responsible for the observed pattern [116]. Another study of these species found that during full Moon nights foraging activity is low and cortisol metabolite levels in faeces are elevated [70]; possibly mice respond to increased light levels by increasing cortisol levels, which affect their foraging. Moreover, glucocorticoids (GC) may prepare the individual to an expected stressor [117]; GC concentrations may increase in anticipation of a challenge. Light pulses, constant light or dim light during the night may cause elevated GC levels in laboratory rats and mice, and in Nile grass rats [118–123], although in some cases, it was reported to have no effect or even decrease GC release [124,125]. It was recently reported that aberrant light may directly affect mood and cognitive functions in mice [123]; GC treatment was shown to increase anxiety and conditioned fear [126–129], and acute corticosterone elevation enhanced anti-predator behaviours in tree lizard species [130].
GC affects behaviours that may reduce foraging. For example, in a light dark box test, GC treatment resulted in increased latency to leave the dark compartment, and in the elevated plus maze, it increased time spent in the sheltered arms [126–129]. Tree lizards treated with GC responded more quickly to predators and hid longer [130], and in the Adelie penguin (Pygoscelis adeliae) individuals with high pre-foraging corticosterone levels spent less time foraging, and stayed closed to the colony [131]. Interestingly, a recent paper [123] found an increase in corticosterone levels in mice kept under 3.5 L : 3.5 D cycle, but not in melanopsin-knockout mice, which also show impaired masking responses to light [132], supporting the hypothesis that hormonal changes, stress responses and other acute changes form at least part of the mechanism underlying the masking response to moonlight.
6. Physiological and behavioural effects of artificial light at night on animals
We have highlighted the role of natural light at night in shaping the daily activity patterns of animals. In the era of industrialization, artificial illumination is becoming a major force. While the effects of light at night on human health have been the subject of extensive research [133–135], studies on animals are less common and have only begun to accelerate in recent years [136]. Light at night was shown to have negative effects in rodents when chronically administered at intensities comparable with those of street lamps (approx. 5–20 lux): it can suppress immune-function in hamsters [137] and cause obesity in male mice [138]. Even exposure to dim light at night may negatively affect the circadian system of mammals, especially rodents, as evident by changes in clock genes expression patterns [139]. Rotics et al. [140] found reduced nocturnal activity and increased intraspecific encounters in nocturnal common spiny mice (Acomys cahirinus) kept in a field enclosure with artificial illumination [140]. Similarly, nocturnal Santa Rosa beach mice (Peromyscus polionotus l.) fed less and had less foraging success in heavily lit beach patches [141]. In bat colonies roosting inside lit buildings, dusk emergence was significantly delayed and of longer duration, and juveniles were smaller and lighter [142]. Furthermore, the installation of street lights in a previously dark area can shift the timing of activity and alter choice of commuting routes from roost to foraging grounds [143]. Light at night, however, provides some bat species with a good foraging ground as they hunt for insects that are attracted by streetlights [144,145].
Perhaps, one of the most reported effect of light pollution is the nocturnal singing of songbirds. Correlative field studies have documented a significant relationship between the presence of artificial lights and earlier onset of dawn song in several species [146,147]. In addition, female provisioning rate to the chicks during the second part of the nestling phase was increased in great tits (Parus major), suggesting that there might be fitness consequences due to the high workload [148]. Furthermore, besides affecting daily cycles, light pollution has been found to advance the growth of the reproductive system in European blackbirds (Turdus merula) [149] and the time of egg laying in the blue tit (Cyanistes caeruleus) [147] and has therefore been hypothesized to be one of the driving factors responsible for the earlier onset of reproduction in birds thriving in urban areas [150,151].
If recent studies have started to elucidate the effects of artificial light on terrestrial species, very little research has been conducted so far in aquatic ecosystems (but see [152]). Scientists reported reduced overall activity and altered mating behaviour in green frogs [153]. Moreover, artificial light has been extensively used in aquaculture to increase fish growth rates [154]. Nonetheless, negative effects on fish communities have been hypothesized as a consequence of reduced vertical movements of zooplankton in highly lit water bodies [155].
Despite the growing evidence of the circadian physiological and behavioural effects of light pollution, we have still very limited understanding of its ultimate fitness consequences. Given that the response of a species to artificial lighting is likely to have food web level consequences, it is high time for scientists to elaborate a conceptual framework directed to clarify possible effects of artificial light on predator–prey interactions and ecosystem function.
7. Summary and perspectives
There is no doubt that moonlight confers information that is being used by diverse species as a cue. This influence is only starting to reveal itself, and more study is needed in order to fully understand its role. The mechanisms underlying these responses is even less understood. The study of moonlight chronobiology is extremely important in our era of industrialization, when artificial illumination is becoming widespread while its consequences to humans and ecological systems are poorly understood.
Acknowledgements
This research was supported by THE ISRAEL SCIENCE FOUNDATION (grant no. 93/12).
References
- 1.Tessmar-Raible K, Raible F, Arboleda E. 2011. Another place, another timer: marine species and the rhythms of life. Bioessays 33, 165–172 (doi:10.1002/bies.201000096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De la Iglesia H, Hsu YW. 2010. Biological clocks and rhythms in intertidal crustaceans. Front. Biosci. (Elite Ed) 2, 1394–1404 (doi:10.2741/E200) [DOI] [PubMed] [Google Scholar]
- 3.Brown JS. 1999. Vigilance, patch use and habitat selection: foraging under predation risk. Evol. Ecol. Res. 1, 49–71 [Google Scholar]
- 4.Daly M, Behrends PR, Wilson MI, Jacobs LF. 1992. Behavioral modulation of predation risk—moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys-Merriami. Anim. Behav. 44, 1–9 (doi:10.1016/S0003-3472(05)80748-1) [Google Scholar]
- 5.Stolov HL. 1965. Further investigations of a variation of geomagnetic activity with lunar phase. J. Geophys. Res. 70, 4921–4926 (doi:10.1029/JZ070i019p04921) [Google Scholar]
- 6.Bell B, Defouw RJ. 1966. Dependence of the lunar modulation of geomagnetic activity on the celestial latitude of the moon. J. Geophys. Res. 71, 951–957 (doi:10.1029/JZ071i003p00951) [Google Scholar]
- 7.Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL. 1984. Mass spawning in tropical reef corals. Science 223, 1186–1189 (doi:10.1126/science.223.4641.1186) [DOI] [PubMed] [Google Scholar]
- 8.Willis B, Babcock RC, Harrison PL, Oliver TK. (eds) 1985. Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. In 5th Int. Coral Reef Congress, Tahiti IRSC [Google Scholar]
- 9.Simpson CJ, Cary JL, Masini RJ. 1993. Destruction of corals and other reef animals by coral spawn slicks on ningaloo reef, Western Australia. Coral Reefs 12, 185–191 (doi:10.1007/BF00334478) [Google Scholar]
- 10.Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL. 1986. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier reef. Mar. Biol. 90, 379–394 (doi:10.1007/BF00428562) [Google Scholar]
- 11.Heyward AJ, Babcock RC. 1986. Self-fertilization and cross-fertilization in scleratinian corals. Mar. Biol. 90, 191–195 (doi:10.1007/BF00569127) [Google Scholar]
- 12.Szmant AM. 1986. Reproductive ecology of Caribbean reef corals. Coral Reefs 5, 43–53 (doi:10.1007/BF00302170) [Google Scholar]
- 13.Harrison PAW. 1990. Reproduction, dispersal and recruitment of scleractinian corals. In Ecosystems of the world: coral reefs (ed. Dubinsky Z.), pp. 133–207 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 14.Mendes JM, Woodley JD. 2002. Timing of reproduction in Montastraea annularis: relationship to environmental variables. Mar. Ecol. Progr. Ser. 227, 241–251 (doi:10.3354/meps227241) [Google Scholar]
- 15.Babcock RC, Wills BL, Simpson CJ. 1994. Mass spawning of corals on a high-latitude coral reef. Coral Reefs 13, 161–169 (doi:10.1007/BF00301193) [Google Scholar]
- 16.Orton J. 1920. Sea-temperature, breeding and distribution in marine animals. J. Mar. Biol. Assoc. UK 12, 339–366 (doi:10.1017/S0025315400000102) [Google Scholar]
- 17.Himmelman J. 1980. Synchronization of spawning in marine invertebrates by phytoplankton. In Advances in invertebrate reproduction (ed. Clark WH.), pp. 3–19 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 18.Jokiel PL, Ito RY, Liu PM. 1985. Night irradiance and synchonization of lunar release of planula larvae in the reef coral Pocillopora damicornis. Mar. Biol. 88, 167–174 (doi:10.1007/BF00397164) [Google Scholar]
- 19.Saigusa M. 1985. Tidal timing of larval release activity in non-tidal environment. Jpn J. Ecol. 35, 243–251 [Google Scholar]
- 20.Saigusa M. 1980. Entrainment of the semilunar rhythms by a simulated moonlight cycle in the terrestrial crab, Sesarma haematocheir. Oecologia 46, 38–44 (doi:10.1007/BF00346963) [DOI] [PubMed] [Google Scholar]
- 21.Saigusa M. 1988. Entrainment of tidal and semilunar rhythms by artificial moonlight cycles. Biol. Bull. 174, 126–138 (doi:10.2307/1541779) [Google Scholar]
- 22.Neumann D. 1975. Lunar and tidal rhythms in the development and reproduction of an inetridal organism. In Physiological adaptation to the environment (ed. Vernberg FJ.), pp. 451–463 New York, NY: Intext Education Publishers [Google Scholar]
- 23.Neumann D. 1988. The timing of reproduction to distinct spring tide situations in the intertidal insect Clunio. In Behavioral adaptation to intertidal life (ed. Chelazzi GAV.). New York: Plenum Publishing [Google Scholar]
- 24.Caspers H. 1984. Spawning periodicity and habitat of the Palolo worm Eunice viridis (Polycheta, Eunicidae) in the Samoan islands. Mar. Biol. 79, 229–236 (doi:10.1007/BF00393254) [Google Scholar]
- 25.Fryer G. 1986. Lunar cycles in lake plankton. Nature 322, 306 (doi:10.1038/322306a0) [Google Scholar]
- 26.Corbet PS. 1958. Lunar periodicity of aquatic insects in Lake Victoria. Nature 182, 330–331 (doi:10.1038/182330a0) [Google Scholar]
- 27.Corbet SA, Sellick RD, Willoughby NG. 1974. Notes on the biology of the mayfly Povilla adusta in West Africa. J. Zool. 172, 491–502 (doi:10.1111/j.1469-7998.1974.tb04381.x) [Google Scholar]
- 28.Grant RA, Chadwick EA, Halliday T. 2009. The lunar cycle: a cue for amphibian reproductive phenology? Anim. Behav. 78, 349–357 (doi:10.1016/j.anbehav.2009.05.007) [Google Scholar]
- 29.Rand AS, Bridarolli ME, Dries L, Ryan MJ. 1997. Light levels influence female choice in Tungara frogs: predation risk assessment? Copeia 1997, 447–450 (doi:10.2307/1447770) [Google Scholar]
- 30.Mills AM. 1986. The influence of moonlight on the behavior of goatsuckers (Caprimulgidae). Auk 103, 370–378 [Google Scholar]
- 31.Jackson HD. 1985. Aspects of the breeding biology of the fierynecked nightjar. Ostrich 56, 263–276 (doi:10.1080/00306525.1985.9639598) [Google Scholar]
- 32.Baker GC, Dekker R. 2000. Lunar synchrony in the reproduction of the Moluccan Megapode Megapodius wallacei. Ibis 142, 382–388 (doi:10.1111/j.1474-919X.2000.tb04434.x) [Google Scholar]
- 33.Dixon DR, Dixon LRJ, Bishop JD, Pettifor RA. 2006. Lunar-related reproductive behaviour in the badger (Meles meles). Acta Ethol. 9, 59–63 (doi:10.1007/s10211-006-0016-4) [Google Scholar]
- 34.Penteriani V, Delgado MD, Campioni L, Lourenco R. 2011. Moonlight makes owls more chatty. PLoS ONE 5, e8696 (doi:10.1371/journal.pone.0008696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganey JL. 1990. Calling behavior of spotted owls in northern Arizona. Condor 92, 485–490 (doi:10.2307/1368245) [Google Scholar]
- 36.Mougeot F, Bretagnolle V. 2000. Predation risk and moonlight avoidance in nocturnal seabirds. J. Avian Biol. 31, 376–386 (doi:10.1034/j.1600-048X.2000.310314.x) [Google Scholar]
- 37.Woods CP, Brigham RM. 2008. Common poorwill activity and calling behavior in relation to moonlight and predation. Wilson J. Ornithol. 120, 505–512 (doi:10.1676/06-067.1) [Google Scholar]
- 38.Cooper RJ. 1981. Relative abundance of Georgia caprimulgids based on call counts. Wilson Bull. 93, 363–371 [Google Scholar]
- 39.Bender DJ, Bayne EM, Brigham RM. 1996. Lunar condition influences coyote (Canis latrans) howling. Am. Midland Nat. 136, 413–417 (doi:10.2307/2426745) [Google Scholar]
- 40.Hesse R, Doflein F. 1914. Tierbau und Tierleben, Das Thier als Glied des Naturganzen, vol. 2 Leipzig & Berlin, Germany: B. G. Teubner [Google Scholar]
- 41.Youthed GJ, Moran VC. 1969. The lunar-day activity rhythm of myrmeleontid larvae. J. Insect Physiol. 15, 1259–1271 (doi:10.1016/0022-1910(69)90235-2) [Google Scholar]
- 42.Kerfoot WB. 1967. Lunar periodicity of sphecodogastra texana nocturnal bee (Hymenoptera Halictidae). Anim. Behav. 15, 479 (doi:10.1016/0003-3472(67)90047-4) [DOI] [PubMed] [Google Scholar]
- 43.Oehmke MG. 1973. Lunar periodicity in flight activity of honey bees. J. Interdis. Cycle Res. 4, 319–335 (doi:10.1080/09291017309359395) [Google Scholar]
- 44.Weaver RE. 2011. Effects of simulated moonlight on activity in the desert nightsnake (Hypsiglena chlorophaea). Northwest Sci. 85, 497–500 (doi:10.3955/046.085.0308) [Google Scholar]
- 45.Yamagishi M. 1974. Observation on the nocturnal activity of the habu with special reference to the intensity of moonlight. Snake 6, 37–43 [Google Scholar]
- 46.Campbell SR, Mackessy SP, Clarke JA. 2008. Microhabitat use by brown treesnakes (Boiga irregularis): effects of moonlight and prey. J. Herpetol. 42, 246–250 (doi:10.1670/07-0681.1) [Google Scholar]
- 47.Lillywhite HB, Brischoux F. 2012. Is it better in the moonlight? Nocturnal activity of insular cottonmouth snakes increases with lunar light levels. J. Zool. 286, 194–199 (doi:10.1111/j.1469-7998.2011.00866.x) [Google Scholar]
- 48.Clarke JA, Chopko JT, Mackessy SP. 1996. The effect of moonlight on activity patterns of adult and juvenile prairie rattlesnakes (Crotalus viridis viridis). J. Herpetol. 30, 192–197 (doi:10.2307/1565509) [Google Scholar]
- 49.Penteriani V, Kuparinen A, Delgado MD, Lourenco R, Campioni L. 2011. Individual status, foraging effort and need for conspicuousness shape behavioural responses of a predator to moon phases. Anim. Behav. 82, 413–420 (doi:10.1016/j.anbehav.2011.05.027) [Google Scholar]
- 50.Brigham RM, Gutsell RCA, Wiacek RS, Geiser F. 1999. Foraging behaviour in relation to the lunar cycle by Australian owlet-nightjars Aegotheles cristatus. Emu 99, 253–261 (doi:10.1071/MU99031) [Google Scholar]
- 51.Brigham RM, Barclay RMR. 1992. Lunar influence on foraging and nesting activity of common whippoorwills (Phalaenoptilus nuttalli). Auk 109, 315–320 (doi:10.2307/4088200) [Google Scholar]
- 52.Kortner G, Brigham RM, Geiser F. 2000. Metabolism: winter torpor in a large bird. Nature 407, 318. [DOI] [PubMed] [Google Scholar]
- 53.Smit B, Boyles JG, Brigham RM, McKechnie AE. 2011. Torpor in dark times: patterns of heterothermy are associated with the lunar cycle in a nocturnal bird. J. Biol. Rhythms 26, 241–248 (doi:10.1177/0748730411402632) [DOI] [PubMed] [Google Scholar]
- 54.Jetz W, Steffen J, Linsenmair KE. 2003. Effects of light and prey availability on nocturnal, lunar and seasonal activity of tropical nightjars. Oikos 103, 627–639 (doi:10.1034/j.1600-0706.2003.12856.x) [Google Scholar]
- 55.Milsom TP, Rochard JBA, Poole SJ. 1990. Activity patterns of lapwings Vanellus vanellus in relation to the lunar cycle. Ornis Scand. 21, 147–156 (doi:10.2307/3676811) [Google Scholar]
- 56.Pyle P, Nur N, Henderson RP, Desante DF. 1993. The effects of weather and lunar cycle on nocturnal migration of landbirds at Southeast Farallon Island, California. Condor 95, 343–361 (doi:10.2307/1369357) [Google Scholar]
- 57.Pinet P, Jaeger A, Cordier E, Potin G, Le Corre M. 2011. Celestial moderation of tropical seabird behavior. PLoS ONE 6, e27663 (doi:10.1371/journal.pone.0027663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotler BP, Brown JS, Hasson O. 1991. Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72, 2249–2260 (doi:10.2307/1941575) [Google Scholar]
- 59.Longland WS, Price MV. 1991. Direct observations of owls and heteromyid rodents—can predation risk explain microhabitat use. Ecology 72, 2261–2273 (doi:10.2307/1941576) [Google Scholar]
- 60.Price MV, Waser NM, Bass TA. 1984. Effects of moonlight on microhabitat use by desert rodents. J. Mammal. 65, 353–356 (doi:10.2307/1381183) [Google Scholar]
- 61.Theuerkauf J, Jedrzejewski W, Schmidt K, Okarma H, Ruczynski I, Sniezko S, Gula R. 2003. Daily patterns and duration of wolf activity in the Bialowieza Forest, Poland. J. Mammal. 84, 243–253 (doi:10.1644/1545-1542(2003)084<0243:DPADOW>2.0.CO;2) [Google Scholar]
- 62.Clarke JA. 1983. Moonlighs influence on predator–prey interactions between short-eared owls (Adio flammeus) and deermice (Peromyscus maniculatus). Behav. Ecol. Sociobiol. 13, 205–209 (doi:10.1007/BF00299924) [Google Scholar]
- 63.Griffin PC, Griffin SC, Waroquiers C, Mills LS. 2005. Mortality by moonlight: predation risk and the snowshoe hare. Behav. Ecol. 16, 938–944 (doi:10.1093/beheco/ari074) [Google Scholar]
- 64.Eggermann J, Gula R, Pirga B, Theuerkauf J, Tsunoda H, Brzezowska B, Rouys S, Radler S. 2009. Daily and seasonal variation in wolf activity in the Bieszczady Mountains, SE Poland. Mamm. Biol. 74, 159–163 (doi:10.1016/j.mambio.2008.05.010) [Google Scholar]
- 65.Jones M, Mandelik Y, Dayan T. 2001. Coexistence of temporally partitioned spiny mice: roles of habitat structure and foraging behavior. Ecology 82, 2164–2176 (doi:10.1890/0012-9658(2001)082[2164:COTPSM]2.0.CO;2) [Google Scholar]
- 66.Bowers MA. 1988. Seed removal experiments on desert rodents: the microhabitat by moonlight effect. J. Mammal. 69, 201–204 (doi:10.2307/1381778) [Google Scholar]
- 67.Vasquez RA. 1994. Assessment of predation risk via illumination level—facultative central place foraging in the cricetid rodent Phyllotis darwini. Behav. Ecol. Sociobiol. 34, 375–381 (doi:10.1007/BF00197008) [Google Scholar]
- 68.Topping MG, Millar JS, Goddard JA. 1999. The effects of moonlight on nocturnal activity in bushy-tailed wood rats (Neotoma cinerea). Can. J. Zool. 77, 480–485 [Google Scholar]
- 69.Gutman R, Dayan T. 2005. Temporal partitioning: an experiment with two species of spiny mice. Ecology 86, 164–173 (doi:10.1890/03-0369) [Google Scholar]
- 70.Gutman R, Dayan T, Levy O, Schubert I, Kronfeld-Schor N. 2011. The effect of the lunar cycle on fecal cortisol metabolite levels and foraging ecology of nocturnally and diurnally active spiny mice. PLoS ONE 6, e23446 (doi:10.1371/journal.pone.0023446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrison DW. 1978. Lunar phobia in a neotropical fruit bat, Artibeus jamaicensis (Chiroptera Phyllostomidae). Anim. Behav. 26, 852–855 (doi:10.1016/0003-3472(78)90151-3) [Google Scholar]
- 72.Riek A, Kortner G, Geiser F. 2010. Thermobiology, energetics and activity patterns of the Eastern tube-nosed bat (Nyctimene robinsoni) in the Australian tropics: effect of temperature and lunar cycle. J. Exp. Biol. 213, 2557–2564 (doi:10.1242/jeb.043182) [DOI] [PubMed] [Google Scholar]
- 73.Crespo RF, Burns RJ, Mitchell GC, Linhart SB. 1972. Foraging behavior of common vampire bat related to moonlight. J. Mammal. 53, 366 (doi:10.2307/1379175) [Google Scholar]
- 74.Gannon MR, Willig MR. 1997. The effect of lunar illumination on movement and activity of the red fig-eating bat (Stenoderma rufum). Biotropica 29, 525–529 (doi:10.1111/j.1744-7429.1997.tb00048.x) [Google Scholar]
- 75.Rodriguez-Duran A, Vazquez R. 2001. The bat Artibeus jamaicensis in Puerto Rico (West Indies): seasonality of diet, activity, and effect of a hurricane. Acta Chiropterol. 3, 53–61 [Google Scholar]
- 76.Bork KS. 2006. Lunar phobia in the greater fishing bat Noctilio leporinus (Chiroptera : Noctilionidae). Rev. Biol. Trop. 54, 1117–1123 [PubMed] [Google Scholar]
- 77.Ciechanowski M, Zajac T, Bitas A, Dunajski R. 2007. Spatiotemporal variation in activity of bat species differing in hunting tactics: effects of weather, moonlight, food abundance, and structural clutter. Can. J. Zool. 85, 1249–1263 (doi:10.1139/Z07-090) [Google Scholar]
- 78.Lang AB, Kalko EKV, Romer H, Bockholdt C, Dechmann DKN. 2006. Activity levels of bats and katydids in relation to the lunar cycle. Oecologia 146, 659–666 (doi:10.1007/s00442-005-0131-3) [DOI] [PubMed] [Google Scholar]
- 79.Tuttle MD, Taft LK, Ryan MJ. 1982. Evasive behavior of a frog in response to bat predation. Anim. Behav. 30, 393–397 (doi:10.1016/S0003-3472(82)80050-X) [Google Scholar]
- 80.Hecker KR, Brigham RM. 1999. Does moonlight change vertical stratification of activity by forest-dwelling insectivorous bats? J. Mammal. 80, 1196–1201 (doi:10.2307/1383170) [Google Scholar]
- 81.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 82.Vanorsdol KG. 1984. Foraging behavior and hunting success of lions in Queen Elizabeth National Park, Uganda. Afr. J. Ecol. 22, 79–99 (doi:10.1111/j.1365-2028.1984.tb00682.x) [Google Scholar]
- 83.Sabato MAL, de Melo LFB, Magni EMV, Young RJ, Coelho CM. 2006. A note on the effect of the full moon on the activity of wild maned wolves, Chrysocyon brachyurus. Behav. Process. 73, 228–230 (doi:10.1016/j.beproc.2006.05.012) [DOI] [PubMed] [Google Scholar]
- 84.Rasmussen GSA, Macdonald DW. 2011. Masking of the zeitgeber: African wild dogs mitigate persecution by balancing time. J. Zool. 286, 232–242 (doi:10.1111/j.1469-7998.2011.00874.x) [Google Scholar]
- 85.Harmsen BJ, Foster RJ, Silver SC, Ostro LET, Doncaster CP. 2010. Jaguar and puma activity patterns in relation to their main prey. Mamm. Biol. 76, 320–324 (doi:10.1016/j.mambio.2010.08.007) [Google Scholar]
- 86.Gursky S. 2003. Lunar philia in a nocturnal primate. Int. J. Primatol. 24, 351–367 (doi:10.1023/A:1023053301059) [Google Scholar]
- 87.Erkert HG. 2008. Diurnality and nocturnality in nonhuman primates: comparative chronobiological studies in laboratory and nature. Biol. Rhythm Res. 39, 229–267 (doi:10.1080/09291010701683391) [Google Scholar]
- 88.Erkert HG, Fernandez-Duque E, Rotundo M, Scheideler A. 2012. Seasonal variation of temporal niche in wild owl monkeys (Aotus azarai azarai) of the Argentinean chaco: a matter of masking? Chronobiol. Int. 29, 702–714 (doi:10.3109/07420528.2012.673190) [DOI] [PubMed] [Google Scholar]
- 89.Fernandez-Duque E, de la Iglesia H, Erkert HG. 2010. Moonstruck primates: owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS ONE 5, e12572 (doi:10.1371/journal.pone.0012572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levy O, Dubinsky Z, Achituv Y. 2003. Photobehavior of stony corals: responses to light spectra and intensity. J. Exp. Biol. 206, 4041–4049 (doi:10.1242/jeb.00622) [DOI] [PubMed] [Google Scholar]
- 91.Gorbunov MY, Falkowski PG. 2002. Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limnol. Oceanogr. 47, 309–315 (doi:10.4319/lo.2002.47.1.0309) [Google Scholar]
- 92.Levy O, Appelbaum L, Leggat W, Gothlif Y, Hayward DC, Miller DJ, Hoegh-Guldberg O. 2007. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science 318, 467–470 (doi:10.1126/science.1145432) [DOI] [PubMed] [Google Scholar]
- 93.Kaiser TS, Heckel DG. 2012. Genetic architecture of local adaptation in lunar and diurnal emergence times of the marine midge Clunio marinus (Chironomidae, Diptera). PLoS ONE 7 (doi:10.1371/annotation/fe06ff76-bdd0-41d9-be11-07b9646d0ca8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benna C, Bonaccorsi S, Wulbeck C, Helfrich-Forster C, Gatti M, Kyriacou CP, Costa R, Sandrelli F. 2010. Drosophila timeless2 is required for chromosome stability and circadian photoreception. Curr. Biol. 20, 346–352 (doi:10.1016/j.cub.2009.12.048) [DOI] [PubMed] [Google Scholar]
- 95.Fleissner G, Schuchardt K, Neumann D, Bali G, Falkenberg G. 2008. A lunar clock changes shielding pigment transparency in larval ocelli of Clunio marinus. Chronobiol. Int. 25, 17–30 (doi:10.1080/07420520801904008) [DOI] [PubMed] [Google Scholar]
- 96.Helfrich-Forster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. 2002. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J. Neurosci. 22, 9255–9266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirsh J, Riemensperger T, Coulom H, Iche M, Coupar J, Birman S. 2010. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr. Biol. 20, 209–214 (doi:10.1016/j.cub.2009.11.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bachleitner W, Kempinger L, Wulbeck C, Rieger D, Helfrich-Forster C. 2007. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 104, 3538–3543 (doi:10.1073/pnas.0606870104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rieger D, Fraunholz C, Popp J, Bichler D, Dittmann R, Helfrich-Forster C. 2007. The fruit fly Drosophila melangaster favors dim light and times its activity peaks to early dawn and late dusk. J. Biol. Rhythms 22, 387–399 (doi:10.1177/0748730407306198) [DOI] [PubMed] [Google Scholar]
- 100.Kempinger L, Dittmann R, Rieger D, Helfrich-Forster C. 2009. The nocturnal activity of fruit flies exposed to artificial moonlight is partly caused by direct light effects on the activity level that bypass the endogenous clock. Chronobiol. Int. 26, 151–166 (doi:10.1080/07420520902747124) [DOI] [PubMed] [Google Scholar]
- 101.Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP. 2012. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484, 371–375 (doi:10.1038/nature10991) [DOI] [PubMed] [Google Scholar]
- 102.Fujii S, Krishnan P, Hardin P, Amrein H. 2007. Nocturnal male sex drive in Drosophila. Curr. Biol. 17, 244–251 (doi:10.1016/j.cub.2006.11.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gorman MR, Evans JA, Elliott JA. 2006. Potent circadian effects of dim illumination at night in hamsters. Chronobiol. Int. 23, 245–250 (doi:10.1080/07420520500521905) [DOI] [PubMed] [Google Scholar]
- 104.Gorman MR, Elliott JA. 2004. Dim nocturnal illumination alters coupling of circadian pacemakers in Siberian hamsters, Phodopus sungorus. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 190, 631–639 (doi:10.1007/s00359-004-0522-7) [DOI] [PubMed] [Google Scholar]
- 105.Gorman MR, Elliott JA, Evans JA. 2003. Plasticity of hamster circadian entrainment patterns depends on light intensity. Chronobiol. Int. 20, 233–248 (doi:10.1081/CBI-120018576) [DOI] [PubMed] [Google Scholar]
- 106.Evans JA, Elliott JA, Gorman MR. 2005. Circadian entrainment and phase resetting differ markedly under dimly illuminated versus completely dark nights. Behav. Brain Res. 162, 116–126 (doi:10.1016/j.bbr.2005.03.014) [DOI] [PubMed] [Google Scholar]
- 107.Aschoff J. 1960. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb. Symp. Quant. Biol. 25, 11–28 (doi:10.1101/SQB.1960.025.01.004) [DOI] [PubMed] [Google Scholar]
- 108.Aschoff J, Vongoetz C. 1988. Masking of circadian activity rhythms in hamsters by darkness. J. Comp. Physiol. A. Sens. Neural Behav. Physiol. 162, 559–562 (doi:10.1007/BF00612521) [DOI] [PubMed] [Google Scholar]
- 109.Aschoff J, Vongoetz C. 1989. Masking of circadian activity rhythms in canaries by light and dark. J. Biol. Rhythms 4, 29–38 (doi:10.1177/074873048900400102) [DOI] [PubMed] [Google Scholar]
- 110.Redlin U, Hattar S, Mrosovsky N. 2005. The circadian clock mutant mouse, impaired masking response to light. J. Comp. Physiol. A. Sens. Neural Behav. Physiol. 191, 51–59 (doi:10.1007/s00359-004-0570-z) [DOI] [PubMed] [Google Scholar]
- 111.Redlin U, Mrosovsky N. 1999. Masking of locomotor activity in hamsters. J. Comp. Physiol. A. Sens. Neural Behav. Physiol. 184, 429–437 (doi:10.1007/s003590050342) [DOI] [PubMed] [Google Scholar]
- 112.Redlin U. 2001. Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol. Int. 18, 737–758 (doi:10.1081/CBI-100107511) [DOI] [PubMed] [Google Scholar]
- 113.Herzog ED, Barlow RB. 1991. Ultraviolet-light from the nighttime sky enhances retinal sensitivity of limulus. Biol. Bull. 181, 321–322 [DOI] [PubMed] [Google Scholar]
- 114.Erkert HG, Cramer B. 2006. Chronobiological background to cathemerality: circadian rhythms in Eulemur fulvus albifrons (Prosimii) and Aotus azarai boliviensis (Anthropoidea). Folia Primatol. 77, 87–103 (doi:10.1159/000089697) [DOI] [PubMed] [Google Scholar]
- 115.Donati G, Lunardini A, Kappeler PM, Tarli SMB. 2001. Nocturnal activity in the cathemeral red-fronted lemur (Eulemur fulvus rufus), with observations during a lunar eclipse. Am. J. Primatol. 53, 69–78 (doi:10.1002/1098-2345(200102)53:2<69::AID-AJP2>3.0.CO;2-R) [DOI] [PubMed] [Google Scholar]
- 116.Rotics S, Dayan T, Levy O, Kronfeld-Schor N. 2011. Light Masking in the field: an experiment with nocturnal and diurnal spiny mice under semi-natural field conditions. Chronobiol. Int. 28, 70–75 (doi:10.3109/07420528.2010.525674) [DOI] [PubMed] [Google Scholar]
- 117.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 118.Abilio VC, Freitas FM, Dolnikoff MS, Castrucci AML, Frussa R. 1999. Effects of continuous exposure to light on behavioral dopaminergic supersensitivity. Biol. Psychiatry 45, 1622–1629 (doi:10.1016/S0006-3223(98)00305-9) [DOI] [PubMed] [Google Scholar]
- 119.Van der Meer E, Van Loo PLP, Baumans V. 2004. Short-term effects of a disturbed light-dark cycle and environmental enrichment on aggression and stress-related parameters in male mice. Lab. Anim. 38, 376–383 (doi:10.1258/0023677041958972) [DOI] [PubMed] [Google Scholar]
- 120.Mohawk JA, Pargament JM, Lee TM. 2007. Circadian dependence of corticosterone release to light exposure in the rat. Physiol. Behav. 92, 800–806 (doi:10.1016/j.physbeh.2007.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. 2005. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2, 297–307 (doi:10.1016/j.cmet.2005.09.009) [DOI] [PubMed] [Google Scholar]
- 122.Fonken LK, Kitsmiller E, Smale L, Nelson RJ. 2012. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythms 27, 319–327 (doi:10.1177/0748730412448324) [DOI] [PubMed] [Google Scholar]
- 123.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. 2012. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491, 594–598 (doi:101038/nature11673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. 2009. Influence of light at night on murine anxiety- and depressive-like responses. Behav. Brain Res. 205, 349–354 (doi:10.1016/j.bbr.2009.07.001) [DOI] [PubMed] [Google Scholar]
- 125.Buijs RM, Wortel J, van Heerikhuize JJ, Feenstra MGP, Ter Horst GJ, Romijn HJ, Kalsbeek A. 1999. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 11, 1535–1544 (doi:10.1046/j.1460-9568.1999.00575.x) [DOI] [PubMed] [Google Scholar]
- 126.Ardayfio P, Kim KS. 2006. Anxiogenic-like effect of chronic corticosterone in the light–dark emergence task in mice. Behav. Neurosci. 120, 1267 (doi:10.1037/0735-7044.120.6.1267) [DOI] [PubMed] [Google Scholar]
- 127.Conrad CD, MacMillan DD, Tsekhanov S, Wright RL, Baran SE, Fuchs RA. 2004. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiol. Learn Mem. 81, 185–199 (doi:10.1016/j.nlm.2004.01.002) [DOI] [PubMed] [Google Scholar]
- 128.Mitra R, Sapolsky RM. 2008. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl Acad. Sci. USA 105, 5573–5578 (doi:10.1073/pnas.0705615105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Skorzewska A, Bidzinski A, Lehner M, Turzynska D, Sobolewska A, Hamed A, Szyndler J, Maciejak P, Plaznik A. 2007. The effects of acute corticosterone administration on anxiety, endogenous corticosterone, and c-Fos expression in the rat brain. Horm. Behav. 52, 317–325 (doi:10.1016/j.yhbeh.2007.05.007) [DOI] [PubMed] [Google Scholar]
- 130.Thaker M, Lima SL, Hews DK. 2009. Acute corticosterone elevation enhances antipredator behaviors in male tree lizard morphs. Horm. Behav. 56, 51–57 (doi:10.1016/j.yhbeh.2009.02.009) [DOI] [PubMed] [Google Scholar]
- 131.Angelier F, Bost CA, Giraudeau M, Bouteloup G, Dano S, Chastel O. 2008. Corticosterone and foraging behavior in a diving seabird: the Adelie penguin, Pygoscelis adeliae. Gen. Comp. Endocr. 156, 134–144 (doi:10.1016/j.ygcen.2007.12.001) [DOI] [PubMed] [Google Scholar]
- 132.Mrosovsky N, Hattar S. 2003. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol. Int. 20, 989–999 (doi:10.1081/CBI-120026043) [DOI] [PubMed] [Google Scholar]
- 133.Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224 (doi:10.1111/j.1600-079X.2007.00473.x) [DOI] [PubMed] [Google Scholar]
- 134.Kantermann T, Roenneberg T. 2009. Is light-at-night a health risk factor or a health risk predictor? Chronobiol. Int. 26, 1069–1074 [DOI] [PubMed] [Google Scholar]
- 135.Fonken LKA, Nelson RJ. 2011. Illuminating the deleterious effects of light at night. F1000 Med. Rep. 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198 (doi:10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [Google Scholar]
- 137.Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. 2010. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 7, 468–471 (doi:10.1098/rsbl.2010.1108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 7, 18 664–18 669 (doi:10.1073/pnas.1008734107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shuboni D, Yan L. 2012. Nighttime dim light exposure alters the responses of the circadian system. Neuroscience 170, 1172–1178 (doi:10.1016/j.neuroscience.2010.08.009) [DOI] [PubMed] [Google Scholar]
- 140.Rotics S, Dayan T, Kronfeld-Schor N. 2011. Effect of artificial night lighting on temporally partitioned spiny mice. J. Mammal. 92, 159–168 (doi:10.1644/10-MAMM-A-112.1) [Google Scholar]
- 141.Bird BL, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 18, 1435–1439 (doi:10.1111/j.1523-1739.2004.00349.x) [Google Scholar]
- 142.Boldogh S, Dobrosi D, Samu P. 2007. The effects of the illumination of buildings on house-dwelling bats and its conservation consequences. Acta Chiropterol. 9, 527–534 (doi:10.3161/1733-5329(2007)9[527:TEOTIO]2.0.CO;2) [Google Scholar]
- 143.Stone EL, Jones G, Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123–1127 (doi:10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 144.Rydell J. 1992. Exploitation of insects around streetlamps by bats in Sweden. Funct. Ecol. 6, 744–750 (doi:10.2307/2389972) [Google Scholar]
- 145.Frank KD. 1998. Impact of outdoor lighting on moths: an assessment. J. Lepidopterists Soc. 42, 63–93 [Google Scholar]
- 146.Miller MW. 2006. Apparent effects of light pollution on singing behavior of American robins. Condor 108, 130–139 (doi:10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2) [Google Scholar]
- 147.Kempenaers B, Borgstrom P, Loes P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739 (doi:10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 148.Titulaer M, Spoelstra K, Lange C, Visser ME. 2012. Activity patterns during food provisioning are affected by artificial light in free living great tits (Parus major). PLoS ONE 7, e37377 (doi:10.1371/journal.pone.0037377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dominoni D, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 (doi:101098/rspb20123017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Partecke J, Van't Hof T, Gwinner E. 2004. Differences in the timing of reproduction between urban and forest European blackbirds (Turdus merula): result of phenotypic flexibility or genetic differences? Proc. R. Soc. Lond. B 271, 1995–2001 (doi:10.1098/rspb.2004.2821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Helm B, Ben-Schlomo R, Sheriff M, Hut RA, Foster R, Barnes BM, Dominoni DM. Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc. R. Soc. B 280, 20130016 (doi:10.1098/rspb.2013.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nightingale B, Longcore T, Simenstad CA. 2006. Artificial night lighting and fishes. In Ecological consequences of artificial night lighting (eds Longcore T, Rich C.), pp. 257–276 Washington, DC: Island Press [Google Scholar]
- 153.Baker BJ, Richardson JML. 2006. The effect of artificial light on male breeding-season behaviour in green frogs, Rana clamitans melanota. Can. J. Zool. 84, 1528–1532 (doi:10.1139/z06-142) [Google Scholar]
- 154.Boeuf G, Le Bail PY. 1999. Does light have an influence on fish growth? Aquaculture 177, 129–152 (doi:10.1016/S0044-8486(99)00074-5) [Google Scholar]
- 155.Moore MV, Kohler SJ, Cheers MS. 2006. Artificial light at night in freshwater habitats and its potential ecological effects. In Ecological consequences of artificial night lighting (eds Longcore T, Rich C.) pp. 365–384 Washington, DC: Island Press [Google Scholar]


