Abstract
Circadian rhythms are ubiquitous in many organisms. Animals that are forced to be active around the clock typically show reduced performance, health and survival. Nevertheless, we review evidence of animals showing prolonged intervals of activity with attenuated or nil overt circadian rhythms and no apparent ill effects. We show that around-the-clock and ultradian activity patterns are more common than is generally appreciated, particularly in herbivores, in animals inhabiting polar regions and habitats with constant physical environments, in animals during specific life-history stages (such as migration or reproduction), and in highly social animals. The underlying mechanisms are diverse, but studies suggest that some circadian pacemakers continue to measure time in animals active around the clock. The prevalence of around-the-clock activity in diverse animals and habitats, and an apparent diversity of underlying mechanisms, are consistent with convergent evolution. We suggest that the basic organizational principles of the circadian system and its complexity encompass the potential for chronobiological plasticity. There may be trade-offs between benefits of persistent daily rhythms versus plasticity, which for reasons still poorly understood make overt daily arrhythmicity functionally adaptive only in selected habitats and for selected lifestyles.
Keywords: circadian, arrhythmic, sleep, plasticity, evolution, ultradian rhythms
1. Introduction
Circadian rhythms are based on endogenous clocks capable of generating oscillations and of imposing this rhythmicity on downstream entities. The core cellular mechanism that generates circadian oscillations involves transcription–translation feedback loops of clock genes and their protein products. Circadian clocks are cellular or multi-cellular entities, and function as pacemakers if, in addition to sustaining their own oscillations, they are also capable of regulating other oscillators [1]. Circadian clocks are key internal regulatory systems sustained in cells of many types, including neurons, fibroblasts and even red blood cells that do not have nuclei [2,3]. In animals, circadian clock cells are organized into multi-cellular pacemakers and, together with the rhythms they regulate, are defined as the ‘circadian system’. The circadian system includes ‘master clocks’ such as the mammalian suprachiasmatic nucleus (SCN) in the brain, and peripheral pacemakers that can regulate rhythmic processes in organs such as liver, heart and ovary. Pacemakers throughout the body and their driven rhythms interact through neuronal, endocrine or paracrine signalling, or are coupled by the action of external synchronizing environmental cues (‘Zeitgeber’) that entrain circadian rhythms to the daily (‘diel’) cycle [2,4].
The pervasiveness of circadian rhythms in life suggests that circadian clocks are functionally adaptive. Indeed, circadian clocks coordinate physiological and biochemical processes in the body, function as a timing reference (e.g. for sun compass orientation and time place memory [5]), allow organisms to anticipate the diel fluctuations in their environments, and are the basis for the transduction of seasonality from photoperiod. Animals that are experimentally subjected to schedules differing profoundly from a day or that have their clocks continuously shifted typically show reduced performance, increased illness and lower survival [6,7]. Genetic data further indicate that natural selection acts on clock-gene variants [8], but the precise fitness benefits of circadian clocks for wild organisms are still largely unclear [4,9].
There is a growing realization that the degree of circadian organization differs widely depending on species' biology. If studied by improved recording techniques or under different environmental conditions, some organisms that were thought to show robust daily rhythms instead showed extended intervals of around-the-clock activity with ultradian rhythmicity (i.e. rhythmic patterns with periodicities that are much shorter than 24 h), or arrhythmicity, without apparent ill effects. Because these observations seem inconsistent with the idea of universal benefits of daily cycles, a closer look could refine our understanding of the mechanisms, functions and adaptive value of circadian clocks in general. This contribution focuses on circumstances wherein animals show prolonged intervals of activity with no circadian or diel rhythms (herein both referred to as circadian rhythms).
2. Evidence for activity around the clock
(a). Polar animals during continuous light or darkness
Polar regions show extreme seasonality driven by rapid changes in day length, culminating each year in several weeks or months of the sun remaining above the horizon in summer or below the horizon in winter. Vertebrate animals experiencing these relatively constant conditions show highly diverse activity patterns ranging from entrained circadian rhythms to arrhythmicity [10–12]. In two well-studied resident arctic herbivore species, the Svalbard reindeer (Rangifer tarandus platyrhynchus) and ptarmigan (Lagopus mutus hyperboreus), rhythmicity persisted throughout the year in low arctic but not high arctic conditions where animals were active around the clock during the constant light in summer and constant dark in winter [13–17]. These patterns have been interpreted as indicating a modified circadian system in highly specialized, herbivorous species that are active throughout the year at high latitudes.
Migratory bird species that occupy high latitudes during the breeding season showed diverse patterns of activity ranging from maintaining daily rhythmicity to switching to around-the-clock activity [10,14,18]; S. S. Steiger, M. Valcu, K. Spoelstra, B. Helm, M. Wikelski & B. Kempenaers 2013, unpublished data). Penguins, which reproduce at Antarctic latitudes, but may migrate to lower latitudes outside the breeding seasons, are also highly plastic in their daily rhythms [11], showing either 24 h entrained or arrhythmic patterns, possibly in association with different vertical migration patterns of their food species.
The circadian system may also be highly plastic in hibernators. In free-living arctic ground squirrels in the low arctic of northern Alaska, circadian rhythms of body temperature and activity are entrained during transitions between seasons and during summer months of 24 h sunlight [19,20]. After ground squirrels become sequestered alone in their dark hibernacula in autumn, circadian rhythms persist, but free-run while animals remain euthermic. However, once they become heterothermic, core body temperatures remain virtually constant (±0.2°C) during torpor bouts lasting 2–3 weeks [20] (figure 1), and arousal episodes do not occur at a predictable time of day. In spring, males return to euthermia, yet remain in their burrows for 2–3 weeks, while they undergo reproductive maturation [21]; under these constantly dark conditions, at high body temperatures, and while feeding from cached food, body temperature rhythms remain arrhythmic until the animals are first exposed to sunlight on emergence [20,22] (figure 1a). Entrained circadian cycles of body temperature and activity resumed within 1–2 days of regular return to the surface and continue under the midnight sunlight (figure 1b).
Figure 1.
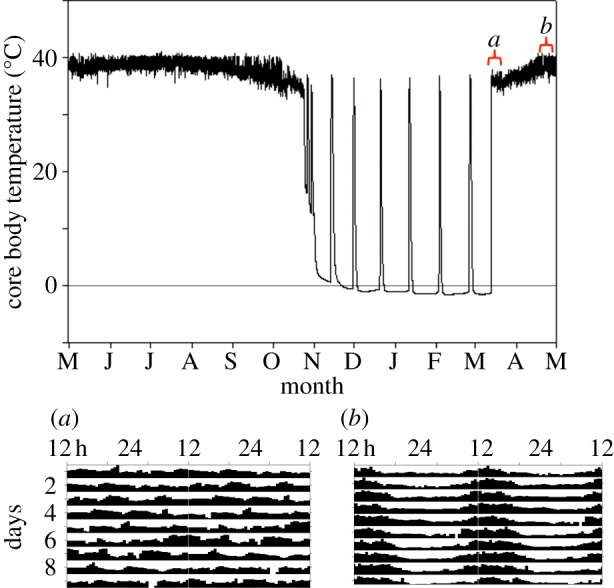
Body temperature of a representative free-living male arctic ground squirrel in northern Alaska, USA over one year (upper panel). Sections of body temperature patterns (lower panels) shown in actogram form (double-plotted positive deviations from 24 h mean, time of day on x-axis, sequential days on y-axis, marked as a and b on upper panel). (a) A 10-day section from March, when the animal remains at high body temperatures within the continuously dark burrow for 2–3 weeks while showing no circadian rhythms. (b) A 10-day section from April, after the animal begins above-ground activity and displays robust, diurnal rhythms in body temperature. Details on methodology are provided in [20]. (Online version in colour.)
(b). Activity around the clock associated with feeding patterns
Lack of overt, daily rhythmicity and ultradian rhythms are often related to feeding behaviour, even at low latitudes [23]. This applies especially to herbivorous animals in which digestive processes impose ultradian cyclic activity patterns [24]. Insectivorous species such as shrews and moles may also show precise ultradian feeding patterns [25,26]. Observations of ultradian rhythms in voles, both in the field and in laboratory conditions, have a particularly long tradition [23]. Vole species such as Microtus arvalis and Microtus agrestis show clear 2–3 h ultradian rhythms; circadian aspects of behaviour seem of secondary importance. In cages equipped with running wheels, one observes a combined circadian and ultradian activity pattern in voles. A pure ultradian activity pattern can be triggered by blocking the running wheel, but spontaneous losses of circadian rhythmicity may be observed even in cages with a running wheel [27].
(c). Eusocial animals
Highly social (eusocial) insects show intervals of activity around the clock in several different contexts. In contrast to most insects, newly emerged honeybees and bumble-bees typically have no circadian rhythms in locomotion or metabolic activity. Endogenous circadian rhythms appear only later in life (reviewed in [28,29]). Older workers typically show plasticity in circadian rhythms that is associated with the division of labour that characterizes insect societies. Studies with honeybees, bumble-bees and ants show that workers that care for (‘nurse’) the brood are active around the clock inside the nest, whereas workers engaged in foraging activities outside the nest show strongly entrained circadian rhythms and consolidated periods of sleep during the night (reviewed in [28,30]). In nursing honeybees, activity around the clock requires direct contact with the brood [31] and intact antennae [32].
Isopteran termites are highly eusocial insects that typically live inside trees, logs or underground, and forage for wood material. Given the complex tunnelling structure of their nest, the sum activity of the nest was recorded in most studies, and results suggest strong diurnal rhythms for foragers yet continuous activity inside the nest. Since the termites were not individually marked, it is unclear whether the in-nest activity is produced by individuals with circadian rhythms differing in their phase of activity (e.g. shift work), or by individual workers that are active around the clock, similar to the pattern in nest honeybees and ants [33–36].
In mammals, eusociality is limited to a few species of mole-rats that live in underground tunnels and are essentially blind. Both solitary and social mole rats typically show clear circadian rhythms and entrainment when monitored individually in the laboratory, indicating that they have a functional circadian clock [37–39]. Telemetry studies suggest that solitary mole-rats also show circadian rhythms under natural field conditions [39,40]. By contrast, eusocial naked mole-rats Heterocephalus gIaber and Damaraland mole-rats (Fukomys damarensis) show attenuated or no circadian rhythms, and are active around the clock when monitored in the social context of the colony [37,41,42].
(d). Activity around the clock associated with reproductive or maternal behaviour
Mammalian mothers may be active around the clock with attenuated circadian rhythms for several weeks during the postpartum period. Offspring in these species are typically born with no apparent circadian rhythms, and their activity and sleep patterns are not consolidated during their first weeks to months of life. In newborn and mother killer whales and bottlenose dolphins, who need to surface regularly to breathe, around-the-clock activity is associated with little or no typical sleep behaviour for the first postpartum month [43]. Adults of these species are also active around the clock but sleep in one brain hemisphere at a time. Newborn humans and other primates also do not have consolidated sleep–wake and activity circadian rhythms for the first weeks postpartum [44–46]. The mothers who tend their infants around the clock may show weak circadian rhythms and fragmented sleep. The attenuated circadian rhythms of the mothers are commonly explained by masking or synchronization with the ultradian rhythms of their infants [47].
Birds during their reproductive season may also show extended intervals of around-the-clock activity [48]. Continuous wakefulness occurs in an arctic-breeding species of wader, the pectoral sandpiper (Calidris melanotos) [18]. In this polygynous species, males are active around the clock during the receptive period of surrounding females. Females are also arrhythmic during their fertile period but shift to entrained daily rhythmicity as soon as they have completed laying of their clutches.
Egg-laying queens of ants [49,50] and honeybees [51,52] are active around the clock with no circadian rhythms. This pattern of activity may be related to the remarkable fecundity of these queens, which are typically not engaged in brood care. By contrast, bumble-bee (Bombus terrestris) queens found annual colonies in early spring and care alone for the first batch of larvae. Colony-founding queens with brood are active around the clock with attenuated circadian rhythms, but virgin queens or queens that lose their brood show activity patterns with strong circadian rhythms. Queens that lose their brood but lay again switched back to activity around the clock. Interestingly, some of these queens switched to around-the-clock activity before having a brood (figure 2). Thus, it appears that around-the-clock activity in B. terrestris is associated with maternal physiology and does not represent simple masking by the brood [53].
Figure 2.
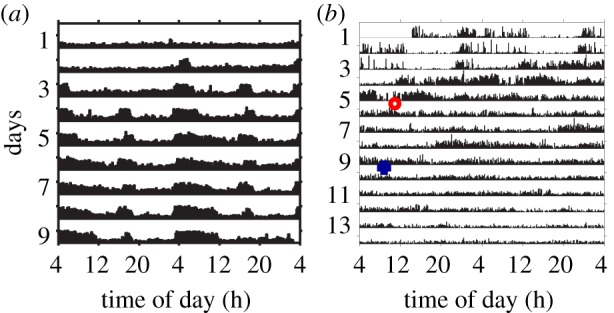
Plasticity in circadian rhythms in locomotor activity in bumble-bee (Bombus terrestris) mother queens. (a) A foundress queen that did not lay eggs after her first batch of brood was removed at day 0 developed statistically significant circadian rhythms on day 3 after egg cell removal. (b) A foundress queen that did lay again after her first batch of eggs was removed. This queen showed weak but statistically significant circadian rhythms in the first 4 days but not in the following days. On day 6 (red ring), the queen's cage was inspected and there were no eggs cells. A new egg cup was detected only at a following inspection on day 10 (blue cross). Thus, this queen switched to activity with no circadian rhythms several days before laying again. Shown are double-plotted actograms (see figure 1). Locomotor activity was monitored with a video-based data acquisition system. Adapted from Eban-Rothschild et al. [53].
(e). Animals during migration
Many birds that are otherwise diurnal assume additional nocturnal activity and travel at night when migrating [54]. Migrating species held in captivity show a corresponding, often dramatic change of circadian activity patterns during migration seasons (figure 3). While continuing to be actively feeding and vigilant during much of the day, they also show nocturnal activity that can last for weeks, termed ‘migratory restlessness’ or ‘Zugunruhe’ [1,55,56]. Onset and termination of migratory restlessness are usually regulated by long-term timekeeping mechanisms, such as circannual rhythms and photoperiodism [57] (figure 3). However, the day-to-day expression of nocturnality is sensitive to additional cues such as food availability and fuel stores: food shortage usually promotes around-the-clock activity, whereas refeeding opportunities promote diurnality until fuel reserves are sufficient to continue migration or Zugunruhe [55,58–61].
Figure 3.
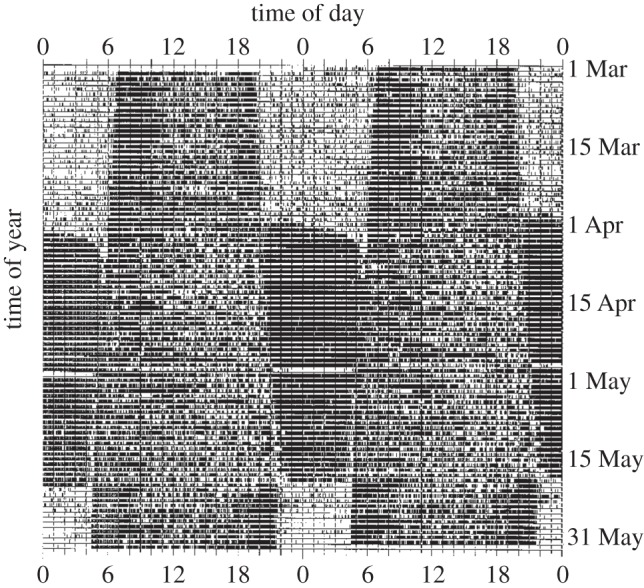
Development of spring migratory restlessness in a songbird, the Siberian stonechat (Saxicola torquata Maura). The figure shows a double plot actogram (see figure 1). Although otherwise strictly diurnal, the bird was almost continuously active from late March until mid-May, when the species migrates under natural conditions. For background information see [55].
(f). Additional animals showing periods of activity around the clock
Sharks and pelagic (open water) teleost fishes, such as tuna, sardines and mackerels, swim and eat around the clock. Many of these continuously swimming fishes are obligate ‘ram gill ventilators’ that would suffocate if they were to cease swimming more than momentarily [62]. Marine mammals are active around the clock because they need to regularly surface to breathe. Reef-dwelling fishes that spend the night among corals perform regular fin motions, perhaps for ventilating the coral area to prevent hypoxia [63]. Cave fish from diverse phylogenetic origins show convergent around-the-clock activity with a reduction in sleep time [64]. Activity around the clock is also common in other blind and cave-dwelling animals [62].
3. The mechanisms underlying activity around the clock
As summarized above, circadian systems of animals are based on multi-level processes, ranging from clock gene loops to neuronal and endocrine communication between tissues. Accordingly, a lack of overt daily rhythmicity can arise in various, mutually non-exclusive ways (figure 4). Even if basic oscillatory mechanisms are undisturbed, overt rhythmicity can be masked by external factors or by uncoupling of motor controlling centres from pacemaker input (figure 4a). Pacemaker cells can be arrested, for example, by pausing of clock-gene transcription (figure 4b), or when the amplitude of pacemaker oscillation is below the threshold needed to drive an overt rhythm of activity (figure 4c). Even if oscillations persist, desynchrony between individual pacemaker cells could dampen the overall pacemaker signal, or different pacemakers could collectively create around-the-clock activity (figure 4d). In addition, strengthening of other periodicities, such as ultradian rhythms with a period shorter than 24 h, could override the daily activity pattern of the circadian pattern and generate activity around the clock.
Figure 4.
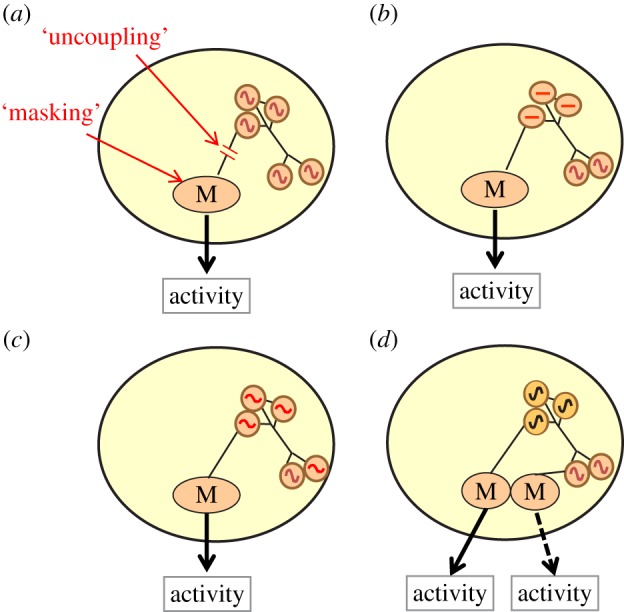
Putative mechanisms underlying around-the-clock activity with no circadian rhythms in animals. (a) The pacemakers controlling circadian cycles such as locomotor activity generate normal rhythms but are uncoupled from motor centres (labelled ‘M’) controlling output (top arrow). The internal rhythms may also be masked by potent external or internal factors (including ultradian rhythms generating pacemakers) that influence circadian output (lower arrow). (b) Some or all pacemaker cells are in an arrested state (marked by a horizontal line). (c) Some or all pacemaker cells have dampened amplitude that is below the threshold needed to drive an overt rhythm. (d) Oscillations persist in individual pacemaker cells, but the phases of these cells are desynchronized (depicted by sine waves with different colours), so that the overall signal is dampened. Alternatively, distinct multicellular pacemakers could interact in ways that promote activity around the clock, for example, if they drive activity at different times of day when they are in anti-phase (depicted by the dashed and solid lines).
(a). The mechanisms of around-the-clock activity in polar animals
Reindeer, which produce melatonin at night during seasonal transitions of the equinox, lose these rhythms during the continuous winter nights of higher latitudes [65]. Lu et al. [13] showed that in R. t. tarandus in autumn, short dark pulses during daytime induce an immediate rise in plasma melatonin that is suppressed by subsequent light exposure. These data suggest that patterns in melatonin production result from positive masking by darkness and are hardly under central circadian control. Cultured fibroblasts from reindeer skin with transgenic constructs of clock gene promoters (mouse Bmal1 or Per2) driving a luciferase reporter were arrhythmic or showed unstable and transient oscillations of bioluminescence. By contrast, using this assay with fibroblasts from mice and hamsters showed clear circadian rhythms [13]. These results are in line with the weak circadian central and peripheral organization in reindeer. It remains enigmatic whether the reindeer SCN cells also show a low amplitude of circadian rhythms at the molecular level (figure 4b,c) or reduced synchronization (figure 4d), in winter and summer [66]. In contrast to the reindeer, in galliform ptarmigans, there are clear daily cycles of melatonin persisting throughout the winter at 70° N latitude [15]. This may indicate that parts of the circadian system remained functional in these birds.
In hibernators, the near 0°C brain and tissue temperatures that prevail during the arctic winter may directly inhibit gene transcription and translation [67,68], and thereby may also prevent the generation of endogenous circadian rhythms (figure 4b). Indeed, a halt of rhythmicity in clock-gene expression in the SCN has been observed in hibernating European hamsters (Cricetus cricetus) [69]. Alternatively, it may be that expression of circadian rhythms is actively regulated to allow hibernators to remain in continuous torpor for weeks at a time.
(b). Mechanisms underlying ultradian activity rhythms in voles and other rodents
The timing of activity bouts in the common vole is under strict ultradian clock control and associated with the temporal organization of foraging activity [70]. Eating and regular gut-fill and emptying times might be expected to produce patterns of body temperature change due to the heat increment of feeding and the increase in metabolism that accompanies digestion. Experimental manipulation of circadian periodicity by photoperiod or deuteriation (D2O) of drinking water (which slows circadian rhythms) affected the phase but not the period length of ultradian patterns [71]. Lesion studies suggest that the ultradian rhythm generation in voles does not depend on the SCN-based circadian system [27,72] (figure 5). In cases where the ultradian rhythm was impaired, the retrochiasmatic area in the medio-basal hypothalamus was damaged, suggesting that this area may play a role in the expression of ultradian rhythms [27]. More recently, in vitro studies have supported this premise by showing ultradian patterns of firing activity in hypothalamic slices obtained from common voles, whereas in the SCN circadian firing activity and oscillations in clock-gene transcript levels prevail. This is also true if voles have access to running wheels and ad libitum food supply, in contrast to liver clock-gene mRNA patterns, which in these circumstances become non-circadian [73].
Figure 5.
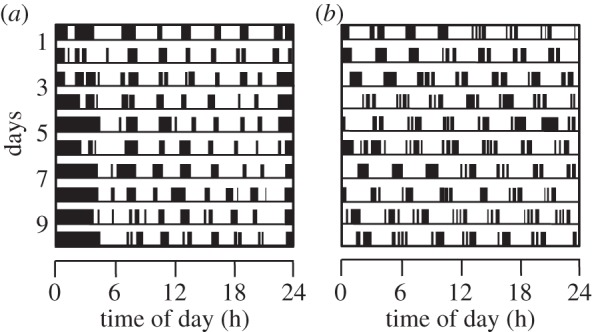
The influence of SCN removal on the locomotor activity in common voles (Microtus arvalis). Single plot actograms showing 10-day records of locomotor activity measured by passive infrared detectors. (a) An intact animal. (b) An SCN-lesioned animal. Adapted from Gerkema & Daan [72].
The ultradian feeding patterns in larger herbivores differ in many aspects from those in voles. Whereas reindeer seem to respond to worse food conditions by lengthening the ultradian pattern, voles do respond to increased food demands and lower food quality by shortening feeding rhythms [24]. Voles have a more derived digestive system than larger ruminants and hindgut digesters, maintaining a strict ultradian pattern of coprophagia [72].
(c). The mechanisms for plasticity in circadian rhythms in bees
In honeybees [74], bumble-bees (Belluci and Bloch, unpublished observations) and ants [75], oscillations in clock-gene expression in the brain are significantly attenuated or absent in nurses active around the clock. This pattern is not consistent with masking or uncoupling, which assume robust oscillations in clock-gene expression (figure 4a). Differences between the environment experienced by rhythmic foragers and nurses active around the clock (e.g. light and temperature) are not likely to account for task-related plasticity in circadian rhythms. Nurse honeybees are active around the clock even when experiencing a light–dark illumination regime, and foragers continue to show strong circadian rhythms under constant conditions (reviewed in [28,30]). Honeybee nurses are typically younger than foragers, but their attenuated rhythms are not due to their circadian system being underdeveloped because they switch to activity with strong circadian rhythms shortly after transfer to the laboratory [31,74]. In addition, in colonies with a severe shortage in nurses, some old foragers with strong circadian rhythms revert to care for the brood and are active around the clock, like nurses in normal colonies [76].
Nurses removed from the hive and monitored individually showed an onset of the morning bout of activity that was correlated with the subjective morning in the hive from which they were collected and not with the time of removal from the hive [31]. These observations are not consistent with the hypothesis that pacemakers in the brain are arrested at a certain phase when in the hive (which would predict a correlation between the onset of activity and the time of removal; figure 4b). Thus, a more probable explanation is that some clock cells that generate strong molecular oscillations in foragers are not oscillating in nurses, or they generate rhythms but the pacemaker cells are not synchronized with each other (figure 4d). Another possibility is that pacemakers in the nurse brain do cycle and are in phase with each other, but their amplitude is low relative to foragers (figure 4c). In bumble-bees the division of labour relates to body size. Small bees are more likely to care for the brood, have weaker circadian rhythms in locomotor activity and have fewer cells that are immunostained with antibodies directed against the circadian neuropeptide PDF compared with brains of large bees, which typically have strong circadian activity rhythms and perform more foraging activities [77,78]. Thus, perhaps there are fewer pacemaker cells in the brain circadian network of smaller bumble-bee workers, which in turn generate weaker circadian rhythms.
(d). Mechanisms for activity around the clock in birds
Diurnal birds that show additional nocturnality (Zugunruhe) during migration seasons maintain attenuated, but still very clear circadian rhythms of plasma melatonin [79]. These data suggest that around-the-clock activity in migratory birds does not imply a halt of the entire circadian system. Instead, several studies support a model of two separate oscillators controlling the daily and nocturnal activities (figure 4d). Presumably, when the birds are diurnal the daytime and night-time oscillators are phase-locked [80,81], and daytime activity suppresses the Zugunruhe oscillator. When phase-coupling weakens, the Zugunruhe oscillator is no longer suppressed and stabilizes in antiphase to daytime activity. Once established, Zugunruhe consists of extensive, flight-like movements that persist even in small cages and can be clearly distinguished from hopping, preening or feeding, which are typically controlled by the diurnal oscillator [80]. When free-running period lengths were estimated separately for the two behaviours, they were shorter for daytime activity than for Zugunruhe, supporting an involvement of two oscillators. In some species, masking of activity at certain times during the year may be a separate or additional way of regulating Zugunruhe (figure 4a) [82].
The two-oscillator model conforms with ideas about a multi-oscillatory pacemaking system [83–86]. The avian clock system consists of several anatomical structures that presumably reinforce each other's rhythmicity, including the pineal, which secretes melatonin with an endogenous rhythm. A high-amplitude melatonin rhythm supports stability and self-sustainment of the circadian clock [57,81,87,88], and its elimination in songbirds disrupts rhythms of both Zugunruhe and daytime activity [89]. During migration seasons melatonin cycles are spontaneously damped, but secretion is still rhythmic, which may suggest the involvement of melatonin in the coupling of the two putative oscillators [79].
4. The adaptive value of activity around the clock
Since circadian rhythms in physiology and behaviour are functionally important, and their disturbance typically compromises cognitive performance, immunity, health and survival, we assume that strong selection pressures are necessary to evolve mechanisms to sustain prolonged activity around the clock without harm. This premise is consistent with the evidence that many of the animals discussed above are adapted to very specific habitats (e.g. polar regions, caves and open sea) or life-history traits (e.g. social cavity-dwelling animals, migratory birds).
For some (but not all) species in polar regions, circadian activity may be disadvantageous because regimented animals may miss ephemeral feeding opportunities that would occur during an animal's inactive phase [17]. Social naked mole-rats may benefit from having active foragers, maintenance workers and vigilant defenders around the clock [41]. Interestingly, many non-social fossorial mole-rats and moles that essentially do not have to leave their subterranean tunnels do show circadian rhythms in their activity patterns. In eusocial bees and ants the pattern of circadian rhythmicity is linked to the division of labour between workers and is thought to improve colony performance. The hypothesis that plasticity in circadian rhythms is functionally significant is supported by the strong link between division of labour and circadian rhythmicity in honeybees [31,76,90], and by comparative studies. There is a similar task-related plasticity in the bumble-bee B. terrestris, in which division of labour is based primarily on size, and in ants, whose division of labour evolved independently of that in honeybees [78,91]. In these cavity-dwelling insects there seems to be little advantage for nest workers to be active with strong circadian rhythms. Moreover, nurses active around the clock are thought to provide better care for the developing larvae [92]. Consistent with this, bumble-bee queens that care alone for the first batch of brood are also active around the clock [53]. Thus, around-the-clock activity with altered sleep pattern in nurse workers of social insects and in mothers caring for the young may have been selected as a mechanism for improved and continuous care, enabling the young to develop more rapidly, or to pass through critical or dangerous periods. Queens of honey bees and ants that do not care for the brood are also active around the clock. Selection in these species could have favoured activity around the clock to increase fecundity. These queens lay hundreds or thousands of eggs per day, allowing rapid growth of their colonies in a short time. Additional specific life-history stages that appear to promote around-the-clock activity include reproduction and migration. Among birds, around-the-clock activity in arctic waders was associated with high reproductive success, such that males that were most active sired the most offspring [18]. Birds during migration are also thought to benefit from their ability to fly at night while feeding during the daytime [93].
In herbivore species around-the-clock activity and a lack of circadian rhythms in body temperature may be driven principally by feeding cycles that depend on gut fill and emptying rates, when food is constantly available (animals feeding from caches, grazers) and predation is not a factor (in hibernacula, or on Svalbard). The adaptive significance of post-hibernation arrhythmicity in ground squirrels that remain in constant conditions may be maximizing rates of fat deposition in spring in recovery from winter periods of low food availability [20]. The lack of circadian timing in behaviour in winter and summer has been also interpreted as a cost reduction in the absence of circadian predation pressure or need for synchronized social activity. In voles, the functional significance of around-the-clock activity patterns may be related to the benefits of ultradian rhythms, which are thought to be optimal for the digestive processing of low-quality food in a hind gut system, as may be the case also in ruminants and other herbivore hind gut fermenters. Socially synchronized ultradian rhythms in foraging activity may be also beneficial for reducing predation risk because they allow increased vigilance (more eyes and ears looking for predators) and an effective communication of risk by means of alarm signals [24].
A special challenge for animals active around the clock is the regulation of sleep that is typically tightly linked to the clock-controlled rest–activity cycles. Although there is still no unifying theory for the adaptive significance of sleep, it is well established that sleep deprivation is associated with profound reduction in performance, health and survival. Around-the-clock activity is associated with sensory inputs and is expected to make it difficult for the animal to obtain prolonged restorative bouts of sleep. Thus, animals that are active around the clock are expected to show specific adaptations in their sleep regulation. For example, it has been suggested that constantly active pelagic fishes and sharks function perfectly without sleeping at all [62]. Blind cave fishes need very little sleep [94], and there is also evidence for a reduction in total sleep time without compromising cognitive performance in birds during migration [56]. Behavioural analyses suggest that honeybees active around the clock have intermittent sleep that is distributed throughout the day in association with times of inactivity [95,96].
5. Concluding remarks
The evidence summarized above suggests that around-the-clock activity with attenuated or nil circadian rhythms and without detectable harmful consequences is more common than is generally appreciated. Species showing patterns of activity around the clock are found in taxonomically distant groups, including invertebrates and vertebrates. The selection pressure for evolving the capacity to being active around the clock also appears to be very diverse. The mechanisms underlying around-the-clock activity are not well understood, but the available data suggest that several different mechanisms may lead to this extreme form of chronobiological plasticity. The prevalence of around-the-clock activity in phylogenetically distant species and the diversity of underlying mechanisms are consistent with convergent evolution.
The complexity of the circadian system and its many levels of regulation make it liable to evolutionary modifications that enable plasticity in the expression of overt circadian rhythmicity. The studies that have looked at additional circadian markers suggest a wide range of facilitating mechanisms, ranging from masking to plasticity in the molecular organization of pacemakers. In voles, ultradian cycles of activity are associated with attenuated molecular rhythms in the liver, whereas the central brain SCN clock continues to show normal oscillations in clock-gene expression. Conversely, in hibernators and nurse honeybees around-the-clock activity is associated with attenuated brain clock-gene cycling, suggesting that the central brain pacemakers function differently than when animals show entrained circadian rhythms. In migrating songbirds a circadian pacemaker, the melatonin-secreting pineal gland, remains rhythmic, while the functioning and phase relationship between the oscillators controlling diurnal and nocturnal activity may change. These examples suggest that around-the-clock activity may not imply that all the pacemakers in the body stop generating rhythms. Rather, rhythm attenuation may be limited to only a few pacemakers, including those controlling locomotor activity. In honeybees, there is evidence that at least some brain pacemakers and biochemical processes continue to generate circadian rhythms in nurses active around the clock, in which brain clock-gene oscillations are severely attenuated [31,97].
A view of the circadian system as being evolutionarily dynamic fits well with emerging insights into chronobiology. Accordingly, ideas of multiple, interactive clocks have replaced earlier views of strictly hierarchical organization, and clock regulation is progressively seen as being task-specific [2,4]. The proposed ‘differential pacemaker regulation model’ could also explain the resilience of animals that are active around the clock: vital processes in the brain and other tissues may continue to benefit from circadian regulation even in animals that show no circadian rhythms in their locomotor activity. However, plasticity of the circadian system may counter against benefits of persistent and robust daily rhythmicity [98]. This alleged trade-off may explain why many animal species, including humans, evolved a circadian system with little plasticity, which cannot switch to activity without circadian rhythms without suffering from reduced performance and survival. Comparison of plasticity between different species and its mechanistic basis could thereby indicate which processes in the body, under given environmental conditions, require 24 h rhythmicity. Under some circumstances, when circadian organization may indeed not be critical, ultradian organization of behaviour and physiology could also allow temporal coordination. Approaching these questions may not only shed light on the ecology and evolution of activity patterns of animals, but may also promote answers to fundamental functional and mechanistic questions in chronobiology. We therefore see great value in studying unusual activity patterns of animals with the help of integrative approaches.
Acknowledgements
We thank Taro Fuchikawa and two anonymous reviewers for their helpful comments on a previous version of this manuscript.
References
- 1.Foster RG, Kreitzman L. 2005. Rhythms of life: the biological clocks that control the daily lives of every living thing. New Haven, CT: Yale University Press [Google Scholar]
- 2.Herzog ED. 2007. Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 8, 790–802 (doi:10.1038/nrn2215) [DOI] [PubMed] [Google Scholar]
- 3.O'Neill JS, Reddy AB. 2011. Circadian clocks in human red blood cells. Nature 469, 498–503 (doi:10.1038/nature09702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menaker M. 2006. Circadian organization in the real world. Proc. Natl Acad. Sci. USA 103, 3015–3016 (doi:10.1073/pnas.0600360103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Zee EA, Havekes R, Barf RP, Hut RA, Nijholt IM, Jacobs EH, Gerkema MP. 2008. Circadian time-place learning in mice depends on Cry genes. Curr. Biol. 18, 844–848 (doi:10.1016/j.cub.2008.04.077) [DOI] [PubMed] [Google Scholar]
- 6.Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. 1998. Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl Acad. Sci. USA 95, 8660–8664 (doi:10.1073/pnas.95.15.8660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martino T, et al. 2008. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, 1675–1683 (doi:10.1152/ajpregu.00829.2007) [DOI] [PubMed] [Google Scholar]
- 8.Tauber E, et al. 2007. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316, 1895–1898 (doi:10.1126/science.1138412) [DOI] [PubMed] [Google Scholar]
- 9.De Coursey P. 2004. The behavioral ecology and evolution of biological timing systems. In Chronobiology: biological timekeeping (eds Dunlap JC, Loros JJ, Decoursey P.), pp. 27–65 Sunderland, MA: Sinauer [Google Scholar]
- 10.Ashley NT, Schwabl I, Goymann W, Buck CL. 2012. Keeping time under the midnight sun: behavioral and plasma melatonin profiles of free-living lapland longspurs (Calcarius lapponicus) during the Arctic Summer. J. Exp. Zool. A Ecol. Genet. Physiol. 319, 1–13 [DOI] [PubMed] [Google Scholar]
- 11.Cockrem JF. 1990. Circadian rhythms in Antarctic penguins. In Penguin biology (eds Davis LS, Darby JT.), pp. 319–344 San Diego, CA: Academic Press [Google Scholar]
- 12.Swade RH, Pittendrigh CS. 1967. Circadian locomotor rhythms of rodents in the Arctic. Am. Nat. 101, 431–466 (doi:10.1086/282510) [Google Scholar]
- 13.Lu W, Meng QJ, Tyler NJ, Stokkan KA, Loudon AS. 2010. A circadian clock is not required in an arctic mammal. Curr. Biol. 20, 533–537 (doi:10.1016/j.cub.2010.01.042) [DOI] [PubMed] [Google Scholar]
- 14.Reierth E, Stokkan K-A. 2002. Biological rhythms in arctic animals. In Biological rhythms (ed. Kumar V.), pp. 216–223 New Delhi, India: Narosa [Google Scholar]
- 15.Reierth E, Van't Hof TJ, Stokkan K-A. 1999. Seasonal and daily variations in plasma melatonin in the high-arctic svalbard ptarmigan (Lagopus mutus hyperboreus). J. Biol. Rhythms 14, 314–319 (doi:10.1177/074873099129000731) [DOI] [PubMed] [Google Scholar]
- 16.van Oort B, Tyler N, Gerkema MP, Folkow L, Blys AS, Stokkan K-A. 2005. Circadian organization in reindeer. Nature 438, 1095–1096 (doi:10.1038/4381095a) [DOI] [PubMed] [Google Scholar]
- 17.Van Oort BEH, Tyler NJC, Gerkema MP, Folkow L, Stokkan KA. 2007. Where clocks are redundant: weak circadian mechanisms in reindeer living under polar photic conditions. Naturwissenschaften 94, 183–194 (doi:10.1007/s00114-006-0174-2) [DOI] [PubMed] [Google Scholar]
- 18.Lesku JA, Rattenborg NC, Valcu M, Vyssotski AL, Kuhn S, Kuemmeth F, Heidrich W, Kempenaers B. 2012. Adaptive sleep loss in polygynous pectoral sandpipers. Science 337, 1654–1658 (doi:10.1126/science.1220939) [DOI] [PubMed] [Google Scholar]
- 19.Williams CT, Barnes BM, Buck CL. 2012. Daily body temperature rhythms persist under the midnight sun but are absent during hibernation in free-living arctic ground squirrels. Biol. Lett. 8, 31–34 (doi:10.1098/rsbl.2011.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CT, Barnes BM, Richter M, Buck CL. 2012. Hibernation and circadian rhythms of body temperature in free-living Arctic ground squirrels. Physiol. Biochem. Zool. 85, 397–404 (doi:10.1086/666509) [DOI] [PubMed] [Google Scholar]
- 21.Barnes BM. 1996. Relationships between hibernation and reproduction in male ground squirrels. In Adaptations to the cold: tenth International hibernation symposium (eds Geiser F, Hulbert AJ, Nicol SC.), pp. 71–80 Armidale, Australia: University of New England Press [Google Scholar]
- 22.Hut RA, Barnes BM, Daan S. 2002. Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J. Comp. Physiol. B 172, 47–58 (doi:10.1007/s003600100226) [DOI] [PubMed] [Google Scholar]
- 23.Daan S, Aschoff J. 1981. Short-term rhythms in activity. In Handbook of behavioral neurobiology, vol. 4 (ed. Aschoff J.), pp. 491–498 New York, NY: Plenum [Google Scholar]
- 24.Gerkema MP. 2002. Ultradian rhythms. In Biological rhythms (ed. Kumar V.), pp. 207–215 New Delhi, India: Narosa [Google Scholar]
- 25.Crowcroft P. 1954. The daily cycle of activity in British shrews. Proc. Zool. Soc. London 123, 715–730 (doi:10.1111/j.1096-3642.1954.tb00197.x) [Google Scholar]
- 26.Godfrey GK. 1955. A field study of the activity of the mole (T. europaea L.). Ecology 36, 678–685 (doi:10.2307/1931306) [Google Scholar]
- 27.Gerkema MP, Groos GA, Daan S. 1990. Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole Microtus arvalis. J. Biol. Rhythms 5, 81–95 (doi:10.1177/074873049000500201) [DOI] [PubMed] [Google Scholar]
- 28.Bloch G. 2009. Socially mediated plasticity in the circadian clock of social insects. In Organization of insect societies—from genome to sociocomplexity (eds Gadau J, Fewell J.), pp. 402–431 Cambridge, MA: Harvard University Press [Google Scholar]
- 29.Moore D. 2001. Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J. Insect Physiol. 47, 843–857 (doi:10.1016/S0022-1910(01)00057-9) [Google Scholar]
- 30.Eban-Rothschild A, Bloch G. 2012. Circadian rhythms and sleep in honey bees. In Honeybee neurobiology and behavior (eds Galizia CG, Eisenhardt D, Giurfa M.), pp. 31–45 Dordrecht, The Netherlands: Springer [Google Scholar]
- 31.Shemesh Y, Eban-Rothschild A, Cohen M, Bloch G. 2010. Molecular dynamics and social regulation of context-dependent plasticity in the circadian clockwork of the honey bee. J. Neurosci. 30, 12 517–12 525 (doi:10.1523/JNEUROSCI.1490-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagari M, Bloch G. 2012. The involvement of the antennae in mediating the brood influence on circadian rhythms in ‘nurse’ honey bee (Apis mellifera) workers. J. Insect Physiol. 58, 1096–1103 (doi:10.1016/j.jinsphys.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 33.Hebrant F. 1970. Circadian rhythm of respiratory metabolism in whole colonies of termite, Cubitermes exiguus. J. Insect Physiol. 16, 1229–1235 (doi:10.1016/0022-1910(70)90211-8) [DOI] [PubMed] [Google Scholar]
- 34.Hinze B, Leuthold RH. 1999. Age related polyethism and activity rhythms in the nest of the termite Macrotermes bellicosus (Isoptera, Termitidae). Insectes Sociaux 46, 392–397 (doi:10.1007/s000400050162). [Google Scholar]
- 35.Lewis V, Leighton S, Tabuchi R, Haverty M. 2011. Seasonal and daily patterns in activity of the western drywood termite, Incisitermes minor (Hagen). Insects 2, 555–563 (doi:10.3390/insects2040555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchikawa T, Matsubara K, Miyatake T, Matsuura K. 2012. Acoustic emission monitoring of the effect of temperature on activity rhythms of the subterranean termite Reticulitermes speratus. Physiol. Entomol. 37, 303–308 (doi:10.1111/j.1365-3032.2012.00841.x) [Google Scholar]
- 37.Riccio AP, Goldman BD. 2000. Circadian rhythms of locomotor activity in naked mole-rats (Heterocephalus glaber). Physiol. Behav. 71, 1–13 (doi:10.1016/s0031-9384(00)00281-x) [DOI] [PubMed] [Google Scholar]
- 38.Oosthuizen MK, Cooper HM, Bennett NC. 2003. Circadian rhythms of locomotor activity in solitary and social species of African mole-rats (Family: Bathyergidae). J. Biol. Rhythms 18, 481–490 (doi:10.1177/0748730403259109) [DOI] [PubMed] [Google Scholar]
- 39.Rado R, Shanas U, Zuri I, Terkel J. 1993. Seasonal activity in the blind mole-rat (Spalax ehrenbergi). Can. J. Zool.-Revue Canadienne de Zoologie 71, 1733–1737 (doi:10.1139/z93-245) [Google Scholar]
- 40.Skilba J, Sumbera R, Chitaukali WN, Burda H. 2007. Determinants of daily activity patterns in a free-living Afrotropical solitary subterranean rodent. J. Mammal. 88, 1009–1016 (doi:10.1644/06-MAMM-A-235R1.1) [Google Scholar]
- 41.Davis-Walton J, Sherman PW. 1994. Sleep arrhythmia in the eusocial naked mole-rat. Naturwissenschaften 81, 272–275 (doi:10.1007/BF01131581) [DOI] [PubMed] [Google Scholar]
- 42.Streicher S, Boyles JG, Oosthuizen MK, Bennett NC. 2011. Body Temperature patterns and rhythmicity in free-ranging subterranean Damaraland mole-rats, Fukomys damarensis. PLoS ONE 6, e26346 (doi:10.1371/journal.pone.0026346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyamin O, Pryaslova J, Lance V, Siegel J. 2005. Continuous activity in cetaceans after birth. Nature 435, 1177 (doi:10.1038/4351177a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleitman N, Engelmann TG. 1953. Sleep characteristics of infants. J. Appl. Physiol. 7, 269–282 [DOI] [PubMed] [Google Scholar]
- 45.McGraw K, Hoffmann R, Harker C, Herman JH. 1999. The development of circadian rhythms in a human infant. Sleep 22, 303–310 [DOI] [PubMed] [Google Scholar]
- 46.Rivkees SA. 2003. Developing circadian rhythmicity in infants. Pediatrics 112, 373–381 (doi:10.1542/peds.112.2.373) [DOI] [PubMed] [Google Scholar]
- 47.Nishihara K, Horiuchi S, Eto H, Uchida S. 2002. The development of infants’ circadian rest–activity rhythm and mothers’ rhythm. Physiol. Behav. 77, 91–98 (doi:10.1016/s0031-9384(02)00846-6) [DOI] [PubMed] [Google Scholar]
- 48.Martin GR. 1990. Birds at night. London, UK: Poyser [Google Scholar]
- 49.McCluskey ES. 1967. Circadian rhythms in female ants, and loss after mating flight. Comp. Biochem. Physiol. 23, 665–677 (doi:10.1016/0010-406X(67)90418-5) [DOI] [PubMed] [Google Scholar]
- 50.Sharma VK, Lone SR, Goel A. 2004. Clocks for sex: loss of circadian rhythms in ants after mating? Die Naturwissenschaften 91, 334–337 (doi:10.1007/s00114-004-0526-8) [DOI] [PubMed] [Google Scholar]
- 51.Harano K, Sasaki M, Sasaki K. 2007. Effects of reproductive state on rhythmicity, locomotor activity and body weight in the European honeybee, Apis mellifera queens (Hymenoptera, Apini). Sociobiology 50, 189–200 [Google Scholar]
- 52.Johnson JN, Hardgrave E, Gill C, Moore D. 2010. Absence of consistent diel rhythmicity in mated honey bee queen behavior. J. Insect Physiol. 56, 761–773 (doi:10.1016/j.jinsphys.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 53.Eban-Rothschild A, Belluci S, Bloch G. 2011. Maternity-related plasticity in circadian rhythms of bumble-bee queens. Proc. R. Soc. B 278, 3510–3516 (doi:10.1098/rspb.2011.0579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battley PF. 2006. Consistent annual schedules in a migratory shorebird. Biol. Lett. 2, 517–520 (doi:10.1098/rsbl.2006.0535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helm B. 2006. Zugunruhe of migratory and non-migratory birds in a circannual context. J. Avian Biol. 37, 533–540 (doi:10.1111/j.2006.0908-8857.03947.x) [Google Scholar]
- 56.Rattenborg NC, Mandt BH, Obermeyer WH, Winsauer PJ, Huber R, Wikelski M, Benca RM. 2004. Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol. 2, 924–936 (doi:10.1371/journal.pbio.0020212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwinner E, Helm B. 2003. Circannual and circadian contributions to the timing of avian migration. In Avian migration (eds Berthold PG, Gwinner E, Sonnenschein E.), pp. 81–95 Heidelberg, Germany: Springer [Google Scholar]
- 58.Fusani L, Cardinale M, Schwabl I, Goymann W. 2011. Food availability but not melatonin affects nocturnal restlessness in a wild migrating passerine. Hormones Behav. 59, 187–192 (doi:10.1016/j.yhbeh.2010.11.013). [DOI] [PubMed] [Google Scholar]
- 59.Terrill SB. 1987. Social dominance and migratory restlessness in the dark-eyed junco (Junco hyemalis). Behav. Ecol. Sociobiol. 21, 1–11 (doi:10.1007/BF00324429) [Google Scholar]
- 60.Fusani L, Cardinale M, Carere C, Goymann W. 2009. Stopover decision during migration: physiological conditions predict nocturnal restlessness in wild passerines. Biol. Lett. 5, 302–305 (doi:10.1098/rsbl.2008.0755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goymann W, Spina F, Ferri A, Fusani L. 2010. Body fat influences departure from stopover sites in migratory birds: evidence from whole-island telemetry. Biol. Lett. 6, 478–481 (doi:10.1098/rsbl.2009.1028). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kavanau JL. 1998. Vertebrates that never sleep: implications for sleep's basic function. Brain Res. Bull. 46, 269–279 (doi:10.1016/S0361-9230(98)00018-5) [DOI] [PubMed] [Google Scholar]
- 63.Goldshmid R, Holzman R, Weihs D, Genin A. 2004. Aeration of corals by sleep-swimming fish. Limnol. Oceanogr. 49, 1832–1839 (doi:10.4319/lo.2004.49.5.1832) [Google Scholar]
- 64.Duboué ER, Keene AC, Borowsky RL. 2011. Evolutionary convergence on sleep loss in cavefish populations. Curr. Biol. 21, 671–676 (doi:10.1016/j.cub.2011.03.020) [DOI] [PubMed] [Google Scholar]
- 65.Stokkan KA, van Oort BE, Tyler NJ, Loudon AS. 2007. Adaptations for life in the Arctic: evidence that melatonin rhythms in reindeer are not driven by a circadian oscillator but remain acutely sensitive to environmental photoperiod. J. Pineal. Res. 43, 289–293 (doi:10.1111/j.1600-079X.2007.00476.x) [DOI] [PubMed] [Google Scholar]
- 66.Paul MJ, Schwartz WJ. 2010. Circadian rhythms: how does a reindeer tell time?. Curr. Biol. 20, R280–R282 (doi:10.1016/j.cub.2010.02.008) [DOI] [PubMed] [Google Scholar]
- 67.Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, Hallenbeck JM. 1998. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc. Natl Acad. Sci. USA 95, 14 511–14 516 (doi:10.1073/pnas.95.24.14511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Breukelen F, Martin SL. 2001. Translational initiation is uncoupled from elongation at 18°C during mammalian hibernation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1374–R1379 [DOI] [PubMed] [Google Scholar]
- 69.Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson-Pevet M, Simonneaux V, Saboureau M, Pevet P. 2007. The circadian clock stops ticking during deep hibernation in the European hamster. Proc. Natl Acad. Sci. USA 104, 13 816–13 820 (doi:10.1073/pnas.0704699104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerkema MP, Leest VDF. 1991. Ultradian rhythms in the common vole Microtus arvalis during short deprivations of food, water and rest. J. Comp. Physiol. A 168, 591–597 (doi:10.1007/BF00215081) [DOI] [PubMed] [Google Scholar]
- 71.Gerkema MP, Daan S, Wilbrink M, Hop MW, van der Leest F. 1993. Phase control of ultradian feeding rhythms in the common vole (Microtus arvalis): the roles of light and the circadian system. J. Biol. Rhythms 8, 151–171 (doi:10.1177/074873049300800205) [DOI] [PubMed] [Google Scholar]
- 72.Gerkema MP, Daan S. 1985. Ultradian rhythms in behavior: the case of the common vole (Microtus arvalis). In Ultradian rhythms in physiology and behavior (eds Schulz H, Lavie P.), pp. 11–31 Berlin, Germany: Springer [Google Scholar]
- 73.van der Veen DR, Minh NL, Gos P, Arneric M, Gerkema MP, Schibler U. 2006. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc. Natl Acad. Sci. USA 103, 3393–3398 (doi:10.1073/pnas.0507825103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shemesh Y, Cohen M, Bloch G. 2007. Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 21, 2304–2311 (doi:10.1096/fj.06-8032com) [DOI] [PubMed] [Google Scholar]
- 75.Ingram KK, Kutowoi A, Wurm Y, Shoemaker D, Meier R, Bloch G. 2012. The molecular clockwork of the fire ant Solenopsis invicta. PLoS ONE 7, e45715 (doi:10.1371/journal.pone.0045715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bloch G, Robinson GE. 2001. Reversal of honeybee behavioural rhythms. Nature 410, 1048 (doi:10.1038/35074183) [DOI] [PubMed] [Google Scholar]
- 77.Weiss R, Dov A, Fahrbach SE, Bloch G. 2009. Body size-related variation in pigment dispersing factor—immunoreactivity in the brain of the bumblebee Bombus terrestris (Hymenoptera, Apidae). J. Insect Physiol. 55, 479–487 (doi:10.1016/j.jinsphys.2009.01.016) [DOI] [PubMed] [Google Scholar]
- 78.Yerushalmi S, Bodenhaimer S, Bloch G. 2006. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J. Exp. Biol. 209, 1044–1051 (doi:10.1242/jeb.02125) [DOI] [PubMed] [Google Scholar]
- 79.Gwinner E, Schwabl-Benzinger I, Schwabl H, Dittami J. 1993. Twenty-four hour melatonin profiles in a nocturnally migrating bird during and between migratory seasons. Gen. Comp. Endocrinol. 90, 119–124 (doi:10.1006/gcen.1993.1066) [DOI] [PubMed] [Google Scholar]
- 80.Bartell PA, Gwinner E. 2005. A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J. Biol. Rhythms 20, 538–549 (doi:10.1177/0748730405281826) [DOI] [PubMed] [Google Scholar]
- 81.Helm B, Gwinner E, Koolhaas A, Battley P, Schwabl I, Dekinga A, Piersma T. 2012. Avian migration: temporal multitasking and a case study of melatonin cycles in waders. Prog. Brain Res. 199, 457–479 (doi:10.1016/B978-0-444-59427-3.00026-5) [DOI] [PubMed] [Google Scholar]
- 82.Coverdill AJ, Bentley GE, Ramenofsky M. 2008. Circadian and masking control of migratory restlessness in gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii). J. Biol. Rhythms 23, 59–68 (doi:10.1177/0748730407311456) [DOI] [PubMed] [Google Scholar]
- 83.Brandstätter R, Abraham U. 2003. Hypothalamic circadian organization in birds. I. Anatomy, functional morphology, and terminology of the suprachiasmatic region. Chronobiol. Int. J. Biol. Med. Rhythm Res. 20, 637–655 (doi:10.1081/CBI-120023343) [DOI] [PubMed] [Google Scholar]
- 84.Kumar V, Singh BP, Rani S. 2004. The bird clock: a complex, multi-oscillatory and highly diversified system. Biol. Rhythm Res. 35, 121–144 (doi:10.1080/09291010412331313287) [Google Scholar]
- 85.Gwinner E. 2001. Diversity and complexity of avian circadian systems. In Zeitgebers, entrainment and masking of the circadian system (eds Honma K, Honma S.), pp. 201–213 Sapporo, Japan: Hokkaido University Press [Google Scholar]
- 86.Cassone VM, Paulose JK, Whitfield-Rucker MG, Peters JL. 2009. Time's arrow flies like a bird: two paradoxes for avian circadian biology. Gen. Comp. Endocrinol. 163, 109–116 (doi:10.1016/j.ygcen.2009.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. 2010. Coupling governs entrainment range of circadian clocks. Mol. Syst. Biol. 6, 438 (doi:10.1038/msb.2010.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menaker M, Moreira LF, Tosini G. 1997. Evolution of circadian organization in vertebrates. Braz. J. Med. Biol. Res. 30, 305–313 (doi:10.1590/S0100-879X1997000300003) [DOI] [PubMed] [Google Scholar]
- 89.Gwinner E, Hau M, Heigl S. 1997. Melatonin: generation and modulation of avian circadian rhythms. Brain Res. Bull. 44, 439–444 (doi:10.1016/S0361-9230(97)00224-4) [DOI] [PubMed] [Google Scholar]
- 90.Bloch G, Toma DP, Robinson GE. 2001. Behavioral rhythmicity, age, division of labor and period expression in the honey bee brain. J. Biol. Rhythms 16, 444–456 (doi:10.1177/074873001129002123) [DOI] [PubMed] [Google Scholar]
- 91.Ingram K, Krummey S, LeRoux M. 2009. Expression patterns of a circadian clock gene are associated with age-related polyethism in harvester ants, Pogonomyrmex occidentalis. BMC Ecol. 9, 7 (doi:10.1186/1472-6785-9-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bloch G. 2010. The social clock of the honeybee. J. Biol. Rhythms 25, 307–317 (doi:10.1177/0748730410380149) [DOI] [PubMed] [Google Scholar]
- 93.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press [Google Scholar]
- 94.Cavallari N, et al. 2011. A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol. 9, e1001142 (doi:10.1371/journal.pbio.1001142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klein BA, Olzsowy KM, Klein A, Saunders KM, Seeley TD. 2008. Caste-dependent sleep of worker honey bees. J. Exp. Biol. 211, 3028–3040 (doi:10.1242/jeb.017426) [DOI] [PubMed] [Google Scholar]
- 96.Eban-Rothschild AD, Bloch G. 2008. Differences in the sleep architecture of forager and young honeybees (Apis mellifera). J. Exp. Biol. 211, 2408–2416 (doi:10.1242/jeb.016915) [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez-Zas SL, Southey BR, Shemesh Y, Rubin EB, Cohen M, Robinson GE, Bloch G. 2012. Microarray analysis of natural socially regulated plasticity in circadian rhythms of honey bees. J. Biol. Rhythms 27, 12–24 (doi:10.1177/0748730411431404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erkert HG. 1982. Ecological aspects of bat activity rhythms. In Ecology of bats (ed. Kunz TH.), pp. 201–242 New York, NY: Plenum Press [Google Scholar]


