Abstract
Daily rhythms of physiology and behaviour are governed by an endogenous timekeeping mechanism (a circadian ‘clock’). The alternation of environmental light and darkness synchronizes (entrains) these rhythms to the natural day–night cycle, and underlying mechanisms have been investigated using singly housed animals in the laboratory. But, most species ordinarily would not live out their lives in such seclusion; in their natural habitats, they interact with other individuals, and some live in colonies with highly developed social structures requiring temporal synchronization. Social cues may thus be critical to the adaptive function of the circadian system, but elucidating their role and the responsible mechanisms has proven elusive. Here, we highlight three model systems that are now being applied to understanding the biology of socially synchronized circadian oscillators: the fruitfly, with its powerful array of molecular genetic tools; the honeybee, with its complex natural society and clear division of labour; and, at a different level of biological organization, the rodent suprachiasmatic nucleus, site of the brain's circadian clock, with its network of mutually coupled single-cell oscillators. Analyses at the ‘group’ level of circadian organization will likely generate a more complex, but ultimately more comprehensive, view of clocks and rhythms and their contribution to fitness in nature.
Keywords: Drosophila, honeybee, suprachiasmatic nucleus, coupling, social synchronization, entrainment
1. Introduction
In nature, synchronization of daily behaviours of conspecifics should allow for the completion of common goals, such as mating and reproduction, defending against predators, hunting in packs or huddling as a means of energy conservation. How do groups of animals achieve such collective temporal synchrony when the individuals of the group each have circadian clocks with different endogenous periods? Is there a role for social synchronization, in the sense that the rhythms of each animal both influence and are sensitive to the rhythms of others in the ensemble? And do social interactions work by resetting the clock's oscillation or instead by ‘masking’, that is, by circumventing or overriding the clock to directly modify the expression of rhythmic behaviours? Circadian biologists have been asking these questions for decades (an early review [1] was published over 30 years ago), in search of robust, reliable and experimentally tractable animal models for rigorous study.
2. Searching for sync
The literature on the effects of social interactions on circadian rhythmicity, predominately but not exclusively studies of pairs of mammals in the laboratory, has been critically reviewed previously [2–5]. While social stimuli have been reported to alter various rhythm parameters in experimental animals and humans, mutual synchronization—such that the phase relationship between subjects remains constant over time under constant environmental conditions—appears to be unusual. Of course, it is difficult to assess the significance of a failure to synchronize without some knowledge of the behavioural ecology of the studied species in their natural habitat, but this is often unknown. In addition, there have also been conceptual and technical problems with the research: domesticated animals are commonly used whose behaviour and physiology in the laboratory environment may be quite different from that of their counterparts in the wild; and until recently [6], it has not been feasible to record from individuals in direct physical contact for relatively long periods of time.
Oscillator theory can suggest factors that should promote the collective synchronization of oscillators [7], but these have not been routinely incorporated into the design of the animal experiments. For example, the degree of synchronization is expected to increase as the strength of the coupling between oscillators rises above a critical value. Besides the frequency of interactions and their type, the degree of familiarity between animals might also affect their ‘coupling strength’, and this is a variable that has been mostly uncontrolled in the literature (indeed, familiarity does seem to be critical for the apparent rhythm synchronization of common marmosets (Callithrix jacchus jacchus; [8]). Another factor influencing oscillator synchronization is the number of interacting oscillators. For weakly coupled oscillators, the phase of any one oscillator is governed by its own intrinsic properties as well as effects from the field of oscillators in the network; the magnitude of this field effect increases with the number of oscillators, favouring synchronization over time [7]. Interestingly, two reports of likely mutual synchronization, in bats and fish, do involve relatively large animal numbers (rather than pairs). Schneider's roundleaf bats (Hipposideros speoris) individually caged in a cave without access to light synchronize their activity rhythms to local time, in phase with their free-living conspecifics that leave the nest daily around sunset (numbers in the colony not known, but presumably many); a control bat caged in a cave devoid of other bats instead exhibited a free-running rhythm [9]. The circadian activity rhythm of Atlantic killifish (Fundulus heteroclitus) expresses a free-running period of significantly greater precision when the fish are housed as shoals of 25 than as aggregates of five [10]; and while individual white suckers (Catostomus commersoni) become arrhythmic after 15–30 days in constant darkness, shoals of 25 fish show a less variable circadian period and no evidence of arrhythmicity [11].
In this paper, we wish to highlight three powerful models that promise to strongly contribute to a better understanding of mutually synchronized circadian oscillators. They all include comparatively large numbers of coupled oscillators, and individually feature special advantages for elucidating neurobiological mechanisms, including the use of molecular genetic manipulations, knowledge of complex natural societies and application of electrophysiological and computational approaches.
3. Fruitflies (Drosophila melanogaster) and the chemistry of synchronization
In flies, circadian patterns of locomotor activity and mating are influenced by social groups [12–17]. When flies are maintained under a light–dark (LD) cycle and then released into constant darkness, the locomotor activity rhythms of individual flies kept in isolation are less synchronized than those of individuals previously maintained within a group of 40 [12]. When wild-type individuals are housed with arrhythmic mutants, they show less synchronous behavioural activity than wild-type individuals sampled from homogeneous control groups, indicating that the social environment may affect individual patterns of behaviour and group synchrony [12,16]. Couples may influence one another as well. When males or females are maintained in heterosexual pairs, their locomotor activity pattern significantly differs from their same-sex paired counterparts [14]. In addition, their distinct locomotor activity pattern is synchronized with close-proximity encounters and courtship behaviour, suggesting that the night time is an ideal time for mating. Although this study indicates that males seem to drive the timing difference in the mixed couple, another study indicates that strain-specific temporal patterns of mating in groups are determined by females [17]. Whether males or females control the pattern of mating may depend on a difference in the experimental design that evaluates couples on the one hand, and larger groups on the other, but this remains to be tested. Overall, these results indicate that social groups influence the timing of circadian rhythms and that group dynamics may reflect and shape the daily course of events, but they do not prove involvement of a circadian clock.
Circadian clock time in flies is generated by an inherited mechanism that shares homology with many other animals, including humans. The genetic mechanism is expressed in the central nervous system in a well-characterized circuit of neurons that governs behavioural rhythms [18]. There are also clock cells in peripheral tissues [18]. Such clock cells have been associated with chemosensory neurons involved in taste and smell [19,20] and other cells such as the oenocytes that produce cuticular hydrocarbons [15]. Volatile chemical signals emitted by flies can synchronize locomotor activity [12,16], and some cuticular hydrocarbons are known to modulate mating latency [21]. However, it is not known whether this contribution of the hydrocarbons regulates mating over the course of the day. There is a circadian pattern of accumulation of cuticular hydrocarbons on the body surface of flies [15,22]. The social environment influences the expression of pheromones, and a portion of this influence is separable from the circadian regulation of pheromone expression, because it is evident both in arrhythmic mutants and in mixed groups composed of arrhythmic mutants and wild-types [15,22]. Moreover, the social environment influences the expression of circadian clock genes in the brain and oenocytes as well as clock-controlled genes in the oenocytes that influence the production of cuticular pheromones [15]. When wild-type and arrhythmic flies are housed together, clock gene expression in the head and oenocytes of the wild-type flies is reduced in amplitude compared with wild-type patterns in homogeneous groups. These changes in gene expression correspond to altered patterns of pheromone expression as well as to increases in the frequency and temporal distribution of mating [15,23]. Recent work suggests that the relationship between the clock in the central nervous system and the clock in the oenocytes is mediated by the neuropeptide pigment-dispersing factor (PDF) acting as a circulating neurohormone [23]. This suggests that when flies are in groups, chemical signals are processed via olfactory and gustatory channels to influence the central clock, which in turn modulates peripheral clocks, resulting in chemical responses. This neuroendocrine pathway is sensitive to the make-up of the social environment and modulates levels of reproductive behaviour. Taken together, these studies indicate that flies influence one another in groups, that the influence is mediated by chemical signalling and that the consequences of this communication process alter levels as well as the concerted temporal pattern of clock gene expression in multiple tissues, peptidergic signalling within individuals, metabolism of cuticular hydrocarbons and the daily timing of behaviours, including locomotor activity and mating (figure 1).
Figure 1.
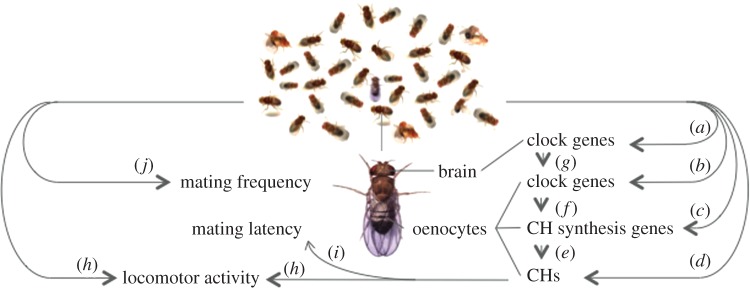
The circadian timing system mediates social synchrony in Drosophila. The diagram depicts various measurements of social influence on individual gene expression and pheromones (arrows on the right) and behaviour (arrows on the left). The social group affects clock gene expression in the head (a) and in peripheral clock cells that make up the oenocytes (b), a tissue where cuticular hydrocarbon pheromones (CHs) are synthesized. The social group also affects CH levels (d) and a metabolic enzyme, desat1 (c), which is regulated by the oenocyte clock (f) [15] and involved in the production of sex pheromones (e). Volatile pheromones affect locomotor activity (h) [12] and the latency to mate (i) [21]. Social synchrony is mediated by the adjustment of clocks within individuals. Our current hypothesis suggests that chemical stimuli from other flies alter circadian pacemaker neurons in the brain (g) which in turn regulate peripheral clocks (f) to influence patterns of communication and behaviour (h–j) [23]. Although effects of light and temperature on clocks are usually discussed in terms of phase, effects of social groups may also influence amplitude of clock gene expression. In addition, an individual's output may feedback onto the group and onto itself; however, to our knowledge, these possibilities have not been addressed in the literature. Note that clocks associated with chemosensation are not shown.
The experiments on social interactions and the clock have mostly relied on the continuous exposure of each fly to others. In one study, cyclic exposure of flies to one another affected the circadian phase of individuals that were already synchronized to an LD cycle, but did not synchronize the flies on its own [24]. This begs a question that has not been addressed in fly laboratory studies: are signals that mediate social synchrony continuous or pulsatile? In addition, although the data suggest that airborne signals generated by flies are sufficient to synchronize locomotor activity rhythms, when flies interact, they engage and communicate with one another using a variety of displays that may include visual postures, motion, touch, taste and smell [25]. Timing signals that rely on smell and taste require multimodal processing at the sensory level, and this requirement certainly extends beyond the chemical senses because taste cannot be separated from touch in the organism as they both involve physical contact. As a result, it is clear that clock mechanisms underlying social signalling integrate cues from multiple senses, including smell and taste, with temporal profiles that can depend on factors, including photoperiod and temperature. Given this complexity, the theory of entrainment that has been crucial for explaining how light signals synchronize biological clocks [26] may require significant revision for determining the role of social timing signals. This is especially true when considering that the organization of circadian rhythms in nature may not be precisely what has been inferred from studies in the laboratory. Perhaps understanding the role of the clock in a real-world ecological context will involve different criteria to assess how individuals and groups are synchronized.
One caveat is that the circadian system has not been implicated as a mechanism for social synchrony in all insects. For example, recent studies on the cockroach Leucophaea maderae failed to find an effect of social interactions on the circadian regulation of locomotor activity [27]. While ‘absence of proof is not proof of absence’, there is a clear note of caution associated with this study because the cockroach is a highly gregarious species.
4. Honeybees (Apis mellifera) and the synchronization of an insect society
Social insects such as honeybees, ants, wasps and termites live in colonies consisting of up to a few million individuals who coordinate almost every aspect of their lives. The temporal coordination of their activities is thought to be important for efficient colony functioning and therefore for colony fitness. The most intuitive aspect of their temporal coordination is synchronizing their phase of activity (‘social entrainment’). Honeybees show a colony-level circadian rhythm that is maintained in constant light and can be phase-shifted as the rhythms of individual animals (for review, see [28]). The free-running circadian period of bees removed from the hive is different from that of the colony. There is a clear after-effect that lasts for several days, during which the bees maintain the colony period before drifting to their own period and phase of activity [29,30]. These observations are consistent with the premise that factors in the colony synchronize the clocks of individual bees.
The synchronization of individuals in insect societies is thought to be functionally significant because it improves colony efficiency. Workers in these complex societies specialize in different tasks that might need tight temporal coordination. For example, honeybee foragers transfer the nectar they collect during the day to a specialized group of ‘nectar receivers’. The nectar receivers accept the nectar load, find empty cells and store the nectar that later will be processed into honey. The nectar receivers typically stay inside the constantly dark and thermoregulated hive but still need to fit their phase of activity to that of the foragers. Synchronizing the clock of nest bees is also needed for other activities; nest bees as young as 3 days of age that typically perform in-nest activities leave the hive for short ‘orientation flights’, which are typically conducted at a certain time during the day [31]. Even ‘nurses’, a group of typically young nest bees that care for the brood around-the-clock with no overt circadian rhythms, show clear circadian rhythms in locomotor activity shortly after removal from the hive. Their phase is synchronized to the ambient day–night cycle [30,32]. Microarray analysis further suggests that more than 150 transcripts are circadianly regulated in the brains of around-the-clock active nurses, suggesting that some brain functions are regulated by entrained pacemakers [33]. Colony defence poses another temporal challenge and demonstrates the potential complexity of the temporal organization of insect societies. The colony is guarded during both day and night, but different foes can be expected at different times. During the day, robbing honeybees from competing colonies, wasps, hornets and bumble-bees may try to enter the hive; during the night, the nest may be attacked by large mammals such as bears and skunks. Is the colony protected by different troops of guards during the day and the night, or by the same guards that are active around-the-clock? There is as yet no answer to this question, but small groups of guards do show circadian rhythms in their aggressiveness towards bumble-bee intruders [34]. Lastly, drones and gynes (young virgin queens) synchronize their mating flights very precisely. Sasaki [35] reported that mutant drones with degenerated compound eyes and no lamina or neural connection to the brain were nevertheless able to precisely time their nuptial flights, which can be explained by extraretinal photoreception or by potent social synchronization.
How might the oscillators of individuals in the colony couple together? A first hypothesis is that the mechanism is not much different from solitary insects that are typically entrained by the strong fluctuations in ambient light and temperature (figure 2a). Foragers and guards spend much of the daytime outside the hive and can be easily entrained by cycles in ambient conditions; nest bees also may experience strong zeitgebers such as light and temperature cycles when occasionally approaching the nest entrance or other parts of the nest in which the microenvironment varies along with ambient conditions [31]. A second hypothesis is that nest bees do not need to experience ambient fluctuations per se because they are entrained by direct contact with foragers or other bees that directly experience ambient day–night cycles (figure 2b). If this hypothesis is supported, then social network theory may be a useful tool for deciphering the temporal organization of insect societies. A third hypothesis is that the sum activity of the foragers changes the hive environment, and these environmental changes in turn reset the clocks of nest bees; in this way, the entire colony is synchronized to the phase of forager activity and thus to the environment (figure 2c). A fourth hypothesis is a pure self-organization model in which the sum activity and metabolism of all individuals in a group are assembled into oscillations in the local microenvironment. These oscillations, even if subtle in the beginning, entrain the clocks of an increasing number of individuals. As additional individuals are entrained to the same phase, the oscillations build up into robust local zeitgebers that are potent enough to entrain the entire colony (figure 2d). An additional possibility is that some individuals in the colony function as a central pacemaker. For example, Moritz & Sakofski [36] showed that a queen from a colony entrained to a phase 8 h apart from that of a group of 150 workers into which she was introduced shifted the group metabolic activity by about 1.4 h towards her colony rhythm; introducing a single worker from the same out-of-phase colony did not have a similar entraining affect. The relevance of these intriguing findings to large colonies consisting of several dozen thousand individuals needs to be confirmed. Of note, the queen typically stays in the dark and thermoregulated parts of the nest and is not exposed to the day–night cycles to which many workers in the colony need to be entrained. Moreover, recent observations suggest that in field colonies, the queen is active around-the-clock with no circadian rhythms [37,38]. The possibility that other bees in the colony form a kind of central pacemaker has not yet, to our knowledge, been investigated.
Figure 2.
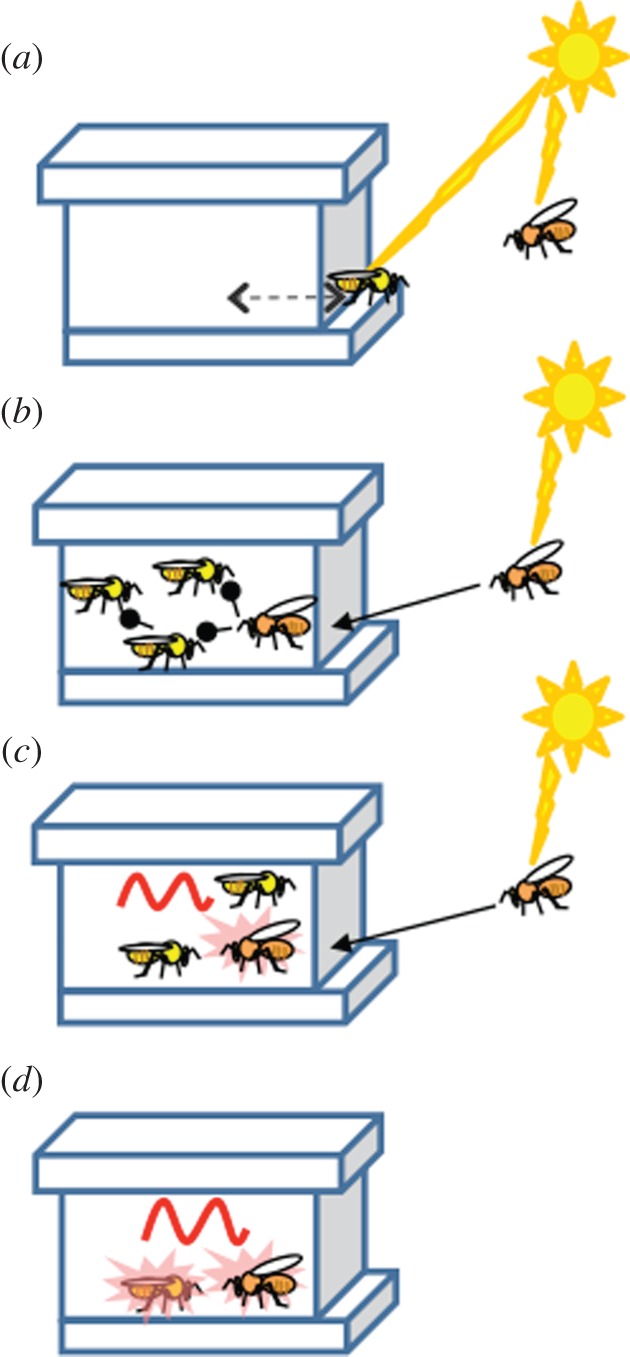
Possible mechanisms for entrainment of circadian rhythms in honeybee colonies. (a) Entrainment by environmental zeitgebers. Both workers performing outside activities, such as foragers and guards (depicted by an orange-coloured bee), and nest workers (depicted by a yellow-coloured bee) are directly entrained by sunlight and temperature cycles. (b) Social entrainment by direct contact. Bees performing outside activities are entrained by environmental zeitgebers, and these individuals entrain nest workers by means of physical or close distance interactions. Entrained nest bees in turn may socially synchronize other nest bees that have not encountered foragers. (c) Entrainment by environmental cycles driven by forager activity. Foragers that are entrained by environmental zeitgebers are more active during the day, and their activity changes the colony microenvironment (pink background), which in turn entrains the clock of other bees in the colony. (d) Self-organized social synchronization. Active bees change their environment, and the sum of the activities of all individuals form weak environmental oscillations that entrain the clocks of an increasing number of bees. The amplitude of these oscillations increases as more and more bees are entrained to the same phase and is eventually strong enough to entrain all the bees in the colony.
Only a few studies, mostly with honeybees, have directly addressed the hypotheses in figure 2. Young nest bees that are physically restricted to the constant environment of the inner dark cavity of the hive still show entrained circadian rhythms in locomotor activity, with higher levels during the day ([39], Eban-Rothschild & Bloch 2011, unpublished data). These findings are inconsistent with the first hypothesis because they suggest that direct exposure to the ambient environment is not necessary for the entrainment of nest bees (figure 2a). Initial studies supported the direct contact hypothesis (figure 2b) because they reported that groups of bees that were entrained to different LD regimes formed a common phase of metabolic activity in experiments allowing for direct contact but not when separated by a double mesh [40]. However, later studies [41] showed weak phase synchronization for two groups of workers that were separated by a thin solid Plexiglas partition; synchronization was improved when the plexiglass division was punched with holes. By colouring the food with dyes, these authors further showed that trophalaxis (mouth-to-mouth food transfer) was not necessary for social phase synchronization. The discrepancies between the two studies were explained by the strong air flows and large gap between the two meshes separating the groups in the first study [40], which were thought to interfere with social synchronization. These experiments suggest that social synchronization of worker circadian activity, as in the case of fruitflies, depends on the variety and timing of physical cues; it can be achieved without direct contact or exchange of air (and volatile odorants), but the synchronization is improved when air exchange is possible. However, since metabolic activity was monitored for a group of bees, it was impossible to distinguish social masking from genuine social entrainment of the endogenous circadian clock. Our unpublished results (Eban-Rothschild & Bloch 2011, unpublished data) show strong phase coherence for 2-day-old bees that were removed from the hive and monitored individually in constant conditions. Similar strong phase coherence was recorded for bees caged in single- or double-mesh enclosures inside the hive, but not for their full-sister bees that were caged individually or in groups of 30 outside the hives. These bees kept a stable phase of circadian rhythms in locomotor activity for one week in the constant conditions of the laboratory, suggesting that factors in the hive environment entrained (rather than masked) the circadian clock of the young bees. The evidence that direct contact is not necessary for the synchronization of bees in the colony is not consistent with the hypothesis depicted in figure 2b.
Temperature is an attractive time-giver for self-organized social synchronization (figure 2d) in honeybees or other cavity- or underground-nesting social insects because it is effective in a constantly dark nest. Moreover, given that in insects, the ambient temperature influences metabolic activity, a feedback loop between temperature and metabolic activity may enforce phase synchronization [41]. There is indeed evidence that the body temperature of bees may increase by 10 °C when they are active [42–45]. Thus, this model can account for the synchronization of bees in a small group. However, its relevance to typical-size free-foraging colonies is not clear because it requires initial entrainment by relatively subtle temperature cycles. Honeybee colonies are tightly thermoregulated, specifically in the brood area [46,47], and in controlled experiments minimal temperature oscillations of 8–10 °C were needed to entrain circadian rhythms in bees [42,43,48]. Other surrogates of bee activity such as humidity, CO2 levels [49], volatile pheromones or comb vibrations may drive a similar self-organization of circadian rhythms. Additional studies are needed to further test this interesting model.
Taken together, the studies with honeybees suggest that the circadian rhythms of individual bees can be synchronized by surrogates of worker activity that build into fluctuations in the microenvironment capable of entraining the rhythms of bees in the colony to a common phase. In free-foraging colonies in the field, it seems that forager activity is the most important driver for oscillations in the colony environment, and since the foragers are entrained by ambient day–night cycles, the entire colony is in phase with the environment (figure 2c). The surrogates of worker activity that are responsible for the entrainment of the nest bees are still to be discovered. Importantly, the hypotheses above are not mutually exclusive; direct contact with the queen or other workers, oscillations in the colony environment and exposure to ambient zeitgebers outside the hive may all influence the phase of circadian rhythms of nest bees.
5. Suprachiasmatic nucleus and the synchrony of cellular circadian oscillators
Behavioural rhythms in insects and mammals appear to be the product of complex brain pacemakers that are composed of multiple individual cellular circadian oscillators. Recent advances in measuring real-time gene expression and electrophysiological activity in cells and tissue slices have begun to reveal how component oscillators are coupled together to create a coherent pacemaker output signal. If cells in circuits and animals in communities share some network design principles for synchronizing their circadian oscillators, then insights made at one level may inform understanding at the other.
In vertebrates, the suprachiasmatic nucleus (SCN) is the master circadian pacemaker that determines daily rhythms in a wide range of behaviours and physiological events. The period of the daily rest–activity cycle has, with few exceptions, been shown to be determined by the SCN in vivo [50–52] or correlated with the period of the SCN in vitro [53–55]. The precision of these behavioural rhythms has been described as remarkably high; for example, the onsets of daily running wheel activity in rodents can have a standard deviation of less than 2 per cent of the average period in the absence of environmental timing cues [26,56,57]. Remarkably, this precision is also present in the rhythms of the isolated SCN [58], indicating that the cells of the SCN determine the period and precision of daily behaviours. Although this has been studied at the cellular level, it is not yet clear if interactions between animals also modulate circadian precision.
How do the cells of the SCN collectively decide on the period they will express? Cell–cell communication synchronizes the periods of the constituent oscillators and establishes the period of the SCN. When the cells of the SCN are prevented from communicating with each other, they express their own cell-autonomous rhythms with periods that range from approximately 16 to 36 h [58,59–62]. Critically, the mean period of the cells depends on those genes that set the circadian period of the behaviour [53–55,63]. When they are allowed to communicate, SCN neurons converge towards an intermediate period [64].
In the absence of cell-to-cell communication, the precision of SCN cellular rhythmicity decreases. For example, cycle-to-cycle variability (standard deviation of their period) increases 10-fold to approximately 2 h [58,62,63]. Although little is known about the source of circadian precision, computational models have predicted that coupling between nonlinear oscillators can synchronize and stabilize the periods of the component oscillators [65–67]. A recent model of isolated SCN cells was able to capture their large changes in circadian period [62]. This model included molecular noise and revealed that small changes in the repression rate of transcription of the ‘clock’ gene Bmal1 could alter a cell's period dramatically. The authors concluded that cell–cell communication could act on the repression of Bmal1 transcription to stabilize circadian cycling. As in the case of fruitflies of mixed genetic backgrounds [12,16], and honeybees in a colony [29], the rhythmic phenotype of an individual can be altered by interaction with other oscillating individuals.
The intact SCN is likely comprised of thousands of circadian cells, with each cell capable of autonomous rhythm generation ([61,62]; but see [68,69]). It is clear that SCN cells are a heterogenous population with distinct oscillatory properties [61,70–72]. Computational models have predicted that inclusion of more damped oscillators or placement of damped oscillators at more highly connected hubs in the network can yield higher and faster synchrony [73–78]. Notably, animals with as little as 25 per cent of SCN cells still show circadian rhythms in locomotor behaviour [79–82]. Taken together, these results indicate that many cells can contribute, but fewer are required, to sustain circadian rhythmicity. One study estimated that the precision of SCN and behavioural cycling could be determined by as few as 25 coupled circadian cells [58]. For the future, it will be intriguing to contrast the number of cells required for synchrony and rhythm precision within the SCN with the number of individuals required within an animal group.
Synchrony among cells in the SCN has been disrupted with genetic knockouts and drugs. For example, blocking neuronal spiking [78,83], synaptic vesicle recycling [84], signalling of vasoactive intestinal polypeptide (VIP) through its cognate receptor (VPAC2) [85–87] or the production of cyclic AMP [88], all result in similar dispersion of the periods and precision of SCN cells. Each of these has resulted also in lower amplitude rhythms in the individual oscillators. In some cases, synchrony can sustain rhythms in otherwise genetically weakened oscillators [63,72] (but see [89]). In the case of the loss of Bmal1, for example, isolated cells show no rhythms in gene expression, but SCN tissue can show oscillations in the range of 24 h [72]. These oscillations were abolished by blocking action potentials or cyclic AMP production, further indicating that cell–cell communication rescued the rhythms in the otherwise arrhythmic cells.
Phase synchrony among SCN cells changes with seasons. The times of peak firing rate and gene expression of single SCN cells spread more during long days compared with short days [90–95]. These changes in the SCN are paralleled by changes in the daily profile of circulating hormones and waveform of locomotor activity (reviewed in [96]). Two similar models have been proposed to underlie this photoperiodic phase dispersion. They propose that long days weaken specific [97] or all [98] connections in the SCN to allow the cells to disperse their phases. In the extreme, constant light can lead to arrhythmic locomotor behaviour and desynchrony among SCN cells [99]. This has been modelled as chronic release of VIP leading to a loss of synchrony [100]. Not yet known is whether the cues among SCN cells must be pulsatile, sinusoidal or constitutive to sustain synchrony among the component oscillators.
Does the degree of phase synchrony have an impact on the collective behaviour of the population? The answer is a definitive ‘yes, but’. Seasonal changes in phase synchrony in the SCN coincide, for example, with changes in response to night-time light; after short days, light at night shifts the SCN more [98,101–103]. Consistent with this, when isolated from animals maintained on short days, the SCN also shifts more to N-methyl-d-aspartate stimulation during the early subjective night [98]. This suggests that photoperiod-induced decreases in phase synchrony reduce the responsivity of the system to perturbation. Interestingly, treatments that drastically reduce synchrony in the SCN appear to increase, rather than decrease, its sensitivity to entraining signals in vitro [104,105]. For example, blocking action potentials or cyclic AMP production increases the SCN's range of entrainment to temperature cycles [104]. This has been modelled as weakened coupling reducing the rhythm amplitude of the individual oscillators. Oscillators with lower amplitude typically shift more to a given stimulus. Thus, reducing synchrony without reducing the amplitude of the component oscillators may allow the SCN to adjust to long days, but at the cost of reduced ability to adjust to changes in the light cycle. Further reducing synchrony to the point of uncoupling actually increases the ability of the system to shift, perhaps because the amplitudes of the component oscillators decrease.
Table 1 summarizes the properties of oscillatory systems that can influence oscillator synchrony and identifies those that have thus far been measured in the SCN.
Table 1.
A summary of properties of oscillatory systems that have been measured in cells of the SCN. Note that many properties are either not yet quantified or we do not yet have the methods to measure them.
| properties of oscillatory systems | measures from SCN cells |
|---|---|
| period | mean over time, cycle-to-cycle variability |
| amplitude | mean magnitude, damping rate |
| synchrony | period distribution, phase distribution (e.g. synchronization index) |
| waveform | not characterized |
| relaxation rate | not available |
| number of nodes | not available |
| number of connections | not available |
| coupling strength | not available |
6. Coda
Here, we have considered the problem of mutual synchronization on the circadian scale—from colony, to organismal, to cellular levels—sharing the common feature that they arise from a network of coupled oscillators that synchronize their periodic behaviours to one another. It is clear that interactions among oscillators can have dramatic effects on the timing (phase and synchrony) and robustness (response to perturbations) of group behaviour, but the underlying mechanisms are not clear. The relative importance of direct contact between interacting individuals versus changes in the common environment may vary between animal species in ways that are currently not understood. Based on the available evidence, we expect to find diverse mechanisms and convergent evolution in multiple species and circuits, with differential roles for entrainment and masking. Future research must include sophisticated analytical methods that, for example, do not demand invariance of cycle-to-cycle period and amplitude over time.
It is intriguing to consider how biological, physical and mathematical oscillators establish synchrony and whether such rules—irrespective of the level of biological organization and evolutionary trajectory—might then relate to shoals of fish, groups of flies, colonies of bees and cells in the SCN. On this we have no answer, other than highlighting some intriguing observations in this review: that individual white suckers and some genetically compromised cells in the SCN are arrhythmic, but rhythmicity can emerge when the fish and the cells are networked as shoals and tissue slices, respectively; that the putative coupling factors, PDF in flies and VIP in mammals, both act via related G-protein-coupled secretin-like receptors that access the pacemaker mechanism in target cells via increases in cyclic AMP; and that robust synchronization in the bee hive, which appears to be largely mediated by oscillations in the colony environment rather than by direct contact, may be analogous to thermal, paracrine and endocrine mechanisms for cellular coupling within an organism.
Temporal niche switching—plasticity of group chronotype—is probably critical to survival in dynamic, challenging environments. Do increases in the number of interacting players, the number of connections or the strength of their interactions (implicated in robust synchronization of cellular oscillators) contribute similarly to social synchronization of animals? What would happen if some of the players differed in their amplitude or precision of oscillation or the way in which they interact with the population? Do social cues modulate responsiveness to other, physical, cues? Could the principles of mutual synchronization of conspecifics be extended to broader ecological contexts such as interactions between predator and prey [106–108] or social thermoregulation during hibernation (as in groups of torpid alpine marmots (Marmota marmota) that exhibit synchronized euthermic arousal bouts [109])? Finally, it is noteworthy that studies on how circadian clocks contribute to biological fitness suggest that they do under conditions of synchrony, but not when they are in an environment devoid of time cues [110,111]. Indeed, when reproductive success is factored into this discussion, it seems worth promoting the hypothesis that social synchrony mediated by circadian clocks contributes to fitness in the natural world.
Funding statement
G.B. is supported by grants from the Israeli Science Foundation (ISF), US–Israel Binational Foundation (BSF), German–Israeli Foundation (GIF) and the US–Israel Binational Agricultural Research and Development Fund (BARD). E.D.H. is supported in part by National Institute of General Medical Sciences (NIGMS) RO1 096873 and National Institute of Mental Health (NIMH) RO1 063104. J.D.L. receives support as a Canada Research Chair and also from Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research. W.J.S. is supported in part by NIGMS R01 GM094109. The contents of this article do not necessarily represent the official views of the NIGMS or NIMH. We thank Jade Atallah for help with figure 1.
References
- 1.Regal PJ, Connolly MS. 1980. Social influences on biological rhythms. Behaviour 72, 171–198 10.1163/156853980X00104 (doi:10.1163/156853980X00104) [DOI] [Google Scholar]
- 2.Davidson AJ, Menaker M. 2003. Birds of a feather clock together—sometimes: social synchronization of circadian rhythms. Curr. Opin. Neurobiol. 13, 765–769 10.1016/j.conb.2003.10.011 (doi:10.1016/j.conb.2003.10.011) [DOI] [PubMed] [Google Scholar]
- 3.Mistlberger RE, Skene DJ. 2004. Social influences on mammalian circadian rhythms: animal and human studies. Biol. Rev. 79, 533–556 10.1017/S1464793103006353 (doi:10.1017/S1464793103006353) [DOI] [PubMed] [Google Scholar]
- 4.Favreau A, Richard-Yris MA, Bertin A, Houdelier C, Lumineau S. 2009. Social influences on circadian behavioural rhythms in vertebrates. Anim. Behav. 77, 983–989 10.1016/j.anbehav.2009.01.004 (doi:10.1016/j.anbehav.2009.01.004) [DOI] [Google Scholar]
- 5.Castillo-Ruiz A, Paul MJ, Schwartz WJ. 2012. In search of a temporal niche: social interactions. In The neurobiology of circadian timing (eds Kalsbeek A, Merrow M, Roenneberg T, Foster RG.), Progress in Brain Research, no. 199, pp. 267–280 Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed] [Google Scholar]
- 6.Paul MJ, Schwartz WJ. 2007. On the chronobiology of cohabitation. Cold Spring Harb. Symp. Quant. Biol. 72, 615–621 10.1101/sqb.2007.72.042 (doi:10.1101/sqb.2007.72.042) [DOI] [PubMed] [Google Scholar]
- 7.Strogatz S. 2003. SYNC: how order emerges from chaos in the universe, nature, and daily life. New York, NY: Hyperion [Google Scholar]
- 8.Erkert HG, Schardt U. 1991. Social entrainment of circadian activity rhythms in common marmosets, Callithrix j. jacchus (primates). Ethology 87, 189–202 10.1111/j.1439-0310.1991.tb00246.x (doi:10.1111/j.1439-0310.1991.tb00246.x) [DOI] [Google Scholar]
- 9.Marimuthu G, Rajan S, Chandrashekaran MK. 1981. Social entrainment of the circadian rhythm in the flight activity of the microchiropteran bat Hipposideros speoris. Behav. Ecol. Sociobiol. 8, 147–150 10.1007/BF00300827 (doi:10.1007/BF00300827) [DOI] [Google Scholar]
- 10.Kavaliers M. 1980. Social groupings and circadian activity of the killifish, Fundulus heteroclitus. Biol. Bull. 158, 69–76 10.2307/1540759 (doi:10.2307/1540759) [DOI] [Google Scholar]
- 11.Kavaliers M. 1980. Circadian activity of the white sucker, Catostomus commersoni: comparison of individual and shoaling fish. Can. J. Zool. 58, 1399–1403 10.1139/z80-192 (doi:10.1139/z80-192) [DOI] [PubMed] [Google Scholar]
- 12.Levine JD, Funes P, Dowse HB, Hall JC. 2002. Resetting the circadian clock by social experience in Drosophila melanogaster. Science 298, 2010–2012 10.1126/science.1076008 (doi:10.1126/science.1076008) [DOI] [PubMed] [Google Scholar]
- 13.Tauber E, Roe H, Costa R, Hennessy JM, Kyriacou CP. 2003. Temporal mating isolation driven a behavioral gene in Drosophila. Curr. Biol. 13, 140–145 10.1016/S0960-9822(03)00004-6 (doi:10.1016/S0960-9822(03)00004-6) [DOI] [PubMed] [Google Scholar]
- 14.Fujii S, Krishnan P, Hardin P, Amrein H. 2007. Nocturnal male sex drive in Drosophila. Curr. Biol. 17, 244–251 10.1016/j.cub.2006.11.049 (doi:10.1016/j.cub.2006.11.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupp JJ, Kent C, Billeter JC, Azanchi R, So AKC, Schonfeld JA, Smith BP, Lucas C, Levine JD. 2008. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383 10.1016/j.cub.2008.07.089 (doi:10.1016/j.cub.2008.07.089) [DOI] [PubMed] [Google Scholar]
- 16.Lone SR, Sharma VK. 2011. Social synchronization of circadian locomotor activity rhythm in the fruit fly Drosophila melanogaster. J. Exp. Biol. 214, 3742–3750 10.1242/jeb.057554 (doi:10.1242/jeb.057554) [DOI] [PubMed] [Google Scholar]
- 17.Billeter JC, Jagadeesh S, Stepek N, Azanchi R, Levine JD. 2012. Drosophila melanogaster females change mating behaviour and offspring production based on social context. Proc. R. Soc. B 279, 2417–2425 10.1098/rspb.2011.2676 (doi:10.1098/rspb.2011.2676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardin PE. 2005. The circadian timekeeping system of Drosophila. Curr. Biol. 15, R714–R722 10.1016/j.cub.2005.08.019 (doi:10.1016/j.cub.2005.08.019) [DOI] [PubMed] [Google Scholar]
- 19.Krishnan B, Dryer SE, Hardin PE. 1999. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400, 375–378 10.1038/22566 (doi:10.1038/22566) [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee A, Tanoue S, Houl JH, Hardin PE. 2010. Regulation of gustatory physiology and appetitive behavior by the Drosophila circadian clock. Curr. Biol. 20, 300–309 10.1016/j.cub.2009.12.055 (doi:10.1016/j.cub.2009.12.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. 2009. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991 10.1038/nature08495 (doi:10.1038/nature08495) [DOI] [PubMed] [Google Scholar]
- 22.Kent C, Azanchi R, Smith B, Formosa A, Levine JD. 2008. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389 10.1016/j.cub.2008.07.088 (doi:10.1016/j.cub.2008.07.088) [DOI] [PubMed] [Google Scholar]
- 23.Krupp JJ, Billeter J-C, Wong A, Choi C, Nitabach MN, Levine JD. Pigment dispersing factor-dependent modulation of pheromone-producing clock cells and mating behavior in Drosophila melanogaster. Neuron. (In press.) 2013 doi: 10.1016/j.neuron.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lone SR, Sadanandappa MK, Sharma VK. 2011. Cyclic presence and absence of conspecifics alters circadian clock phase but does not entrain the locomotor activity rhythm of the fruit fly Drosophila melanogaster. Chronobiol. Int. 28, 497–508 10.3109/07420528.2011.591018 (doi:10.3109/07420528.2011.591018) [DOI] [PubMed] [Google Scholar]
- 25.Billeter J-C, Levine JD. 2012. Who is he and what is he to you? Recognition in Drosophila melanogaster. Curr. Opin. Neurobiol. 23, 17–23 10.1016/j.conb.2012.08.009 (doi:10.1016/j.conb.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 26.Pittendrigh CS, Daan S. 1976. Functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment—pacemaker as clock. J. Comp. Physiol. 106, 291–331 10.1007/BF01417859 (doi:10.1007/BF01417859) [DOI] [Google Scholar]
- 27.Knadler JJ, Page TL. 2009. Social interactions and the circadian rhythm in locomotor activity in the cockroach Leucophaea maderae. Chronobiol. Int. 26, 415–429 10.1080/07420520902876634 (doi:10.1080/07420520902876634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore D. 2001. Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J. Insect Physiol. 47, 843–857 10.1016/S0022-1910(01)00057-9 (doi:10.1016/S0022-1910(01)00057-9) [DOI] [Google Scholar]
- 29.Frisch B, Koeniger N. 1994. Social synchronization of the activity rhythms of honeybees within a colony. Behav. Ecol. Sociobiol. 35, 91–98 10.1007/BF00171498 (doi:10.1007/BF00171498) [DOI] [Google Scholar]
- 30.Shemesh Y, Cohen M, Bloch G. 2007. Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 21, 2304–2311 10.1096/fj.06-8032com (doi:10.1096/fj.06-8032com) [DOI] [PubMed] [Google Scholar]
- 31.Capaldi EA, et al. 2000. Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature 403, 537–540 10.1038/35000564 (doi:10.1038/35000564) [DOI] [PubMed] [Google Scholar]
- 32.Shemesh Y, Eban-Rothschild A, Cohen M, Bloch G. 2010. Molecular dynamics and social regulation of context-dependent plasticity in the circadian clockwork of the honey bee. J. Neurosci. 30, 12 517–12 525 10.1523/JNEUROSCI.1490-10.2010 (doi:10.1523/JNEUROSCI.1490-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Zas SL, Southey BR, Shemesh Y, Rubin EB, Cohen M, Robinson GE, Bloch G. 2012. Microarray analysis of natural socially regulated plasticity in circadian rhythms of honey bees. J. Biol. Rhythms 27, 12–24 10.1177/0748730411431404 (doi:10.1177/0748730411431404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troen H, Dubrovsky I, Tamir R, Bloch G. 2008. Temporal variation in group aggressiveness of honeybee (Apis mellifera) guards. Apidologie 39, 283–291 10.1051/apido:2008005 (doi:10.1051/apido:2008005) [DOI] [Google Scholar]
- 35.Sasaki M. 1990. Photoperiodic regulation of honeybee mating-flight time: exploitation of innately phase-fixed circadian oscillation. In Advances in invertebrate reproduction, vol. 5 (ed. Hoshi MY.), pp. 503–508 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 36.Moritz RFA, Sakofski F. 1991. The role of the queen in circadian rhythms of honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 29, 361–365 10.1007/BF00165961 (doi:10.1007/BF00165961) [DOI] [Google Scholar]
- 37.Harano K, Sasaki M, Sasaki K. 2007. Effects of reproductive state on rhythmicity, locomotor activity and body weight in the European honeybee, Apis mellifera queens (Hymenoptera, Apini). Sociobiology 50, 189–200 [Google Scholar]
- 38.Johnson JN, Hardgrave E, Gill C, Moore D. 2010. Absence of consistent diel rhythmicity in mated honey bee queen behavior. J. Insect Physiol. 56, 761–773 10.1016/j.jinsphys.2010.01.004 (doi:10.1016/j.jinsphys.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 39.Bloch G, Rubinstein CD, Robinson GE. 2004. Period expression in the honey bee brain is developmentally regulated and not affected by light, flight experience, or colony type. Insect Biochem. Mol. Biol. 34, 879–891 10.1016/j.ibmb.2004.05.004 (doi:10.1016/j.ibmb.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 40.Southwick EE, Moritz RFA. 1987. Social synchronization of circadian rhythms of metabolism in honeybees (Apis mellifera). Physiol. Entomol. 12, 209–212 10.1111/j.1365-3032.1987.tb00743.x (doi:10.1111/j.1365-3032.1987.tb00743.x) [DOI] [Google Scholar]
- 41.Moritz RFA, Kryger P. 1994. Self-organization of circadian rhythms in groups of honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 34, 211–215 10.1007/BF00167746 (doi:10.1007/BF00167746) [DOI] [Google Scholar]
- 42.Fuchikawa T, Shimizu I. 2007. Circadian rhythm of locomotor activity in the Japanese honeybee, Apis cerana japonica. Physiol. Entomol. 32, 73–80 10.1111/j.1365-3032.2006.00543.x (doi:10.1111/j.1365-3032.2006.00543.x) [DOI] [PubMed] [Google Scholar]
- 43.Fuchikawa T, Shimizu I. 2007. Effects of temperature on circadian rhythm in the Japanese honeybee, Apis cerana japonica. J. Insect Physiol. 53, 1179–1187 10.1016/j.jinsphys.2007.06.013 (doi:10.1016/j.jinsphys.2007.06.013) [DOI] [PubMed] [Google Scholar]
- 44.Nijland MJM, Hepburn HR. 1985. Ontogeny of a circadian rhythm in the cluster temperature of honey bees. S. Afr. J. Sci. 81, 100–101 [Google Scholar]
- 45.Kaiser W. 1988. Busy bees need rest, too: behavioral and electromyographical sleep signs in honeybees. J. Comp. Physiol. A 163, 565–584 10.1007/BF00603841 (doi:10.1007/BF00603841) [DOI] [Google Scholar]
- 46.Winston ML. 1987. The biology of the honey bee. Cambridge, MA: Harvard University Press [Google Scholar]
- 47.Fahrenholz L, Lamprecht I, Schricker B. 1989. Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat-production of members of different bee castes. J. Comp. Physiol. B 159, 551–560 10.1007/BF00694379 (doi:10.1007/BF00694379) [DOI] [Google Scholar]
- 48.Moore D, Rankin MA. 1993. Light and temperature entrainment of a locomotor rhythm in honey bees. Physiol. Entomol. 18, 271–278 10.1111/j.1365-3032.1993.tb00599.x (doi:10.1111/j.1365-3032.1993.tb00599.x) [DOI] [Google Scholar]
- 49.Ohashi M, Okada R, Kimura T, Ikeno H. 2009. Observation system for the control of the hive environment by the honeybee (Apis mellifera). Behav. Res. Methods 41, 782–786 10.3758/BRM.41.3.782 (doi:10.3758/BRM.41.3.782) [DOI] [PubMed] [Google Scholar]
- 50.Ralph MR, Foster RG, Davis FC, Menaker M. 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978 10.1126/science.2305266 (doi:10.1126/science.2305266) [DOI] [PubMed] [Google Scholar]
- 51.Silver R, LeSauter J, Tresco PA, Lehman MN. 1996. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–813 10.1038/382810a0 (doi:10.1038/382810a0) [DOI] [PubMed] [Google Scholar]
- 52.Sujino M, Masumoto KH, Yamaguchi S, van der Horst GT, Okamura H, Inouye ST. 2003. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr. Biol. 13, 664–668 10.1016/S0960-9822(03)00222-7 (doi:10.1016/S0960-9822(03)00222-7) [DOI] [PubMed] [Google Scholar]
- 53.Liu C, Weaver DR, Strogatz SH, Reppert SM. 1997. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell 91, 855–860 10.1016/S0092-8674(00)80473-0 (doi:10.1016/S0092-8674(00)80473-0) [DOI] [PubMed] [Google Scholar]
- 54.Herzog ED, Takahashi JS, Block GD. 1998. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1, 708–713 10.1038/3708 (doi:10.1038/3708) [DOI] [PubMed] [Google Scholar]
- 55.Nakamura W, Honma S, Shirakawa T, Honma K. 2002. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat. Neurosci. 5, 399–400 10.1038/nn843 (doi:10.1038/nn843) [DOI] [PubMed] [Google Scholar]
- 56.Welsh DK, Engel EM, Richardson GS, Dement WC. 1986. Precision of circadian wake and activity onset timing in the mouse. J. Comp. Physiol. A 158, 827–834 10.1007/BF01324824 (doi:10.1007/BF01324824) [DOI] [PubMed] [Google Scholar]
- 57.Daan S, Oklejewicz M. 2003. The precision of circadian clocks: assessment and analysis in Syrian hamsters. Chronobiol. Int. 20, 209–221 10.1081/CBI-120019309 (doi:10.1081/CBI-120019309) [DOI] [PubMed] [Google Scholar]
- 58.Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. 2004. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J. Biol. Rhythms 19, 35–46 10.1177/0748730403260776 (doi:10.1177/0748730403260776) [DOI] [PubMed] [Google Scholar]
- 59.Welsh DK, Logothetis DE, Meister M, Reppert SM. 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706 10.1016/0896-6273(95)90214-7 (doi:10.1016/0896-6273(95)90214-7) [DOI] [PubMed] [Google Scholar]
- 60.Honma S, Shirakawa T, Katsuno Y, Namihira M, Honma K. 1998. Circadian periods of single suprachiasmatic neurons in rats. Neurosci. Lett. 250, 157–160 10.1016/S0304-3940(98)00464-9 (doi:10.1016/S0304-3940(98)00464-9) [DOI] [PubMed] [Google Scholar]
- 61.Webb AB, Angelo N, Huettner JE, Herzog ED. 2009. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc. Natl Acad. Sci. USA 106, 16 493–16 498 10.1073/pnas.0902768106 (doi:10.1073/pnas.0902768106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meeker K, Harang R, Webb AB, Welsh DK, Doyle FJ, 3rd, Bonnet G, Herzog ED, Petzold LR. 2011. Wavelet measurement suggests cause of period instability in mammalian circadian neurons. J. Biol. Rhythms 26, 353–362 10.1177/0748730411409863 (doi:10.1177/0748730411409863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu AC, et al. 2007. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129, 605–616 10.1016/j.cell.2007.02.047 (doi:10.1016/j.cell.2007.02.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Low-Zeddies SS, Takahashi JS. 2001. Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell 105, 25–42 10.1016/S0092-8674(01)00294-X (doi:10.1016/S0092-8674(01)00294-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winfree AT. 1967. Biological rhythms and the behavior of populations of coupled oscillators. J. Theor. Biol. 16, 15–42 10.1016/0022-5193(67)90051-3 (doi:10.1016/0022-5193(67)90051-3) [DOI] [PubMed] [Google Scholar]
- 66.Enright JT. 1980. Temporal precision in circadian systems: a reliable neuronal clock from unreliable components? Science 209, 1542–1545 10.1126/science.7433976 (doi:10.1126/science.7433976) [DOI] [PubMed] [Google Scholar]
- 67.Strogatz SH, Marcus CM, Westervelt RM, Mirollo RE. 1988. Simple model of collective transport with phase slippage. Phys. Rev. Lett. 61, 2380–2383 10.1103/PhysRevLett.61.2380 (doi:10.1103/PhysRevLett.61.2380) [DOI] [PubMed] [Google Scholar]
- 68.Antle MC, Foley NC, Foley DK, Silver R. 2007. Gates and oscillators II: zeitgebers and the network model of the brain clock. J. Biol. Rhythms 22, 14–25 10.1177/0748730406296319 (doi:10.1177/0748730406296319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foley NC, Tong TY, Foley D, LeSauter J, Welsh DK, Silver R. 2011. Characterization of orderly spatiotemporal patterns of clock gene activation in mammalian suprachiasmatic nucleus. Eur. J. Neurosci. 33, 1851–1865 10.1111/j.1460-9568.2011.07682.x (doi:10.1111/j.1460-9568.2011.07682.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaap J, Albus H, vanderLeest HT, Eilers PH, Détári L, Meijer JH. 2003. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: implications for circadian waveform and photoperiodic encoding. Proc. Natl Acad. Sci. USA 100, 15 994–15 999 10.1073/pnas.2436298100 (doi:10.1073/pnas.2436298100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan L, Karatsoreos I, LeSauter J, Welsh DK, Kay S, Foley D, Silver R. 2007. Exploring spatiotemporal organization of SCN circuits. Cold Spring Harb. Symp. Quant. Biol. 72, 527–541 10.1101/sqb.2007.72.037 (doi:10.1101/sqb.2007.72.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ko CH, et al. 2010. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 8, e1000513. 10.1371/journal.pbio.1000513 (doi:10.1371/journal.pbio.1000513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antle MC, Foley DK, Foley NC, Silver R. 2003. Gates and oscillators: a network model of the brain clock. J. Biol. Rhythms 18, 339–350 10.1177/0748730403253840 (doi:10.1177/0748730403253840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonze D, Bernard S, Waltermann C, Kramer A, Herzel H. 2005. Spontaneous synchronization of coupled circadian oscillators. Biophys. J. 89, 120–129 10.1529/biophysj.104.058388 (doi:10.1529/biophysj.104.058388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernard S, Gonze D, Cajavec B, Herzel H, Kramer A. 2007. Synchronization-induced rhythmicity of circadian oscillators in the suprachiasmatic nucleus. PLoS Comput. Biol. 3, e68. 10.1371/journal.pcbi.0030068 (doi:10.1371/journal.pcbi.0030068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Locke JC, Westermark PO, Kramer A, Herzel H. 2008. Global parameter search reveals design principles of the mammalian circadian clock. BMC Syst. Biol. 2, 22. 10.1186/1752-0509-2-22 (doi:10.1186/1752-0509-2-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vasalou C, Herzog ED, Henson MA. 2011. Multicellular model for intercellular synchronization in circadian neural networks. Biophys. J. 101, 12–20 10.1016/j.bpj.2011.04.051 (doi:10.1016/j.bpj.2011.04.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Webb AB, Taylor SR, Thoroughman KA, Doyle FJ, 3rd, Herzog ED. 2012. Weakly circadian cells improve resynchrony. PLoS Comput. Biol. 8, e1002787. 10.1371/journal.pcbi.1002787 (doi:10.1371/journal.pcbi.1002787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rusak B. 1977. The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster, Mesocricetus auratus. J. Comp. Physiol. A 118, 145–164 10.1007/BF00611819 (doi:10.1007/BF00611819) [DOI] [Google Scholar]
- 80.Davis FC, Gorski RA. 1988. Development of hamster circadian rhythms: role of the maternal suprachiasmatic nucleus. J. Comp. Physiol. A 162, 601–610 10.1007/BF01342635 (doi:10.1007/BF01342635) [DOI] [PubMed] [Google Scholar]
- 81.Harrington ME, Rahmani T, Lee CA. 1993. Effects of damage to SCN neurons and efferent pathways on circadian activity rhythms of hamsters. Brain Res. Bull. 30, 655–669 10.1016/0361-9230(93)90097-U (doi:10.1016/0361-9230(93)90097-U) [DOI] [PubMed] [Google Scholar]
- 82.LeSauter J, Silver R. 1999. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J. Neurosci. 19, 5574–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. 2003. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302, 1408–1412 10.1126/science.1089287 (doi:10.1126/science.1089287) [DOI] [PubMed] [Google Scholar]
- 84.Deery MJ, et al. 2009. Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Curr. Biol. 19, 2031–2036 10.1016/j.cub.2009.10.024 (doi:10.1016/j.cub.2009.10.024) [DOI] [PubMed] [Google Scholar]
- 85.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. 2005. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 8, 476–483 10.1038/nn1419 (doi:10.1038/nn1419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown TM, Hughes AT, Piggins HD. 2005. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J. Neurosci. 25, 11 155–11 164 10.1523/JNEUROSCI.3821-05.2005 (doi:10.1523/JNEUROSCI.3821-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. 2006. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr. Biol. 16, 599–605 10.1016/j.cub.2006.02.023 (doi:10.1016/j.cub.2006.02.023) [DOI] [PubMed] [Google Scholar]
- 88.O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. 2008. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320, 949–953 10.1126/science.1152506 (doi:10.1126/science.1152506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pendergast JS, Friday RC, Yamazaki S. 2009. Endogenous rhythms in Period1 mutant suprachiasmatic nuclei in vitro do not represent circadian behavior. J. Neurosci. 29, 14 681–14 686 10.1523/JNEUROSCI.3261-09.2009 (doi:10.1523/JNEUROSCI.3261-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hazlerigg DG, Ebling FJ, Johnston JD. 2005. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr. Biol. 15, R449–R450 10.1016/j.cub.2005.06.010 (doi:10.1016/j.cub.2005.06.010) [DOI] [PubMed] [Google Scholar]
- 91.vanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. 2007. Seasonal encoding by the circadian pacemaker of the SCN. Curr. Biol. 17, 468–473 10.1016/j.cub.2007.01.048 (doi:10.1016/j.cub.2007.01.048) [DOI] [PubMed] [Google Scholar]
- 92.Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. 2007. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc. Natl Acad. Sci. USA 104, 7664–7669 10.1073/pnas.0607713104 (doi:10.1073/pnas.0607713104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Naito E, Watanabe T, Tei H, Yoshimura T, Ebihara S. 2008. Reorganization of the suprachiasmatic nucleus coding for day length. J. Biol. Rhythms 23, 140–149 10.1177/0748730408314572 (doi:10.1177/0748730408314572) [DOI] [PubMed] [Google Scholar]
- 94.Brown TM, Piggins HD. 2009. Spatiotemporal heterogeneity in the electrical activity of suprachiasmatic nuclei neurons and their response to photoperiod. J. Biol. Rhythms 24, 44–54 10.1177/0748730408327918 (doi:10.1177/0748730408327918) [DOI] [PubMed] [Google Scholar]
- 95.Sosniyenko S, Hut RA, Daan S, Sumová A. 2009. Influence of photoperiod duration and light–dark transitions on entrainment of Per1 and Per2 gene and protein expression in subdivisions of the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 30, 1802–1814 10.1111/j.1460-9568.2009.06945.x (doi:10.1111/j.1460-9568.2009.06945.x) [DOI] [PubMed] [Google Scholar]
- 96.Meijer JH, Colwell CS, Rohling JH, Houben T, Michel S. 2012. Dynamic neuronal network organization of the circadian clock and possible deterioration in disease. In The neurobiology of circadian timing (eds Kalsbeek A, Merrow M, Roenneberg T, Foster RG.), Progress in Brain Research, no. 199, pp. 143–162. Amsterdam, The Netherlands: Elsevier [DOI] [PubMed] [Google Scholar]
- 97.Bodenstein C, Gosak M, Schuster S, Marhl M, Perc M. 2012. Modeling the seasonal adaptation of circadian clocks by changes in the network structure of the suprachiasmatic nucleus. PLoS Comput. Biol. 8, e1002697. 10.1371/journal.pcbi.1002697 (doi:10.1371/journal.pcbi.1002697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.vanderLeest HT, Rohling JH, Michel S, Meijer JH. 2009. Phase shifting capacity of the circadian pacemaker determined by the SCN neuronal network organization. PLoS ONE 4, e4976. 10.1371/journal.pone.0004976 (doi:10.1371/journal.pone.0004976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohta H, Yamazaki S, McMahon DG. 2005. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 8, 267–269 10.1038/nn1395 (doi:10.1038/nn1395) [DOI] [PubMed] [Google Scholar]
- 100.To TL, Henson MA, Herzog ED, Doyle FJ., 3rd 2007. A molecular model for intercellular synchronization in the mammalian circadian clock. Biophys. J. 92, 3792–3803 10.1529/biophysj.106.094086 (doi:10.1529/biophysj.106.094086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pittendrigh CS, Elliott J, Takamura T. 1984. The circadian component in photoperiodic induction. In Photoperiodic regulation of insect and molluscan hormones (eds Porter R, Collins GM.), CIBA Fdtn. Symp. 104, 26–47 [Google Scholar]
- 102.Refinetti R. 2002. Compression and expansion of circadian rhythm in mice under long and short photoperiods. Integr. Physiol. Behav. Sci. 37, 114–127 10.1007/BF02688824 (doi:10.1007/BF02688824) [DOI] [PubMed] [Google Scholar]
- 103.Evans JA, Elliott JA, Gorman MR. 2004. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am. J. Physiol. 286, R539–R546 10.1152/ajpregu.00456.2003 (doi:10.1152/ajpregu.00456.2003) [DOI] [PubMed] [Google Scholar]
- 104.Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. 2010. Coupling governs entrainment range of circadian clocks. Mol. Syst. Biol. 6, 438. 10.1038/msb.2010.92 (doi:10.1038/msb.2010.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buhr ED, Yoo SH, Takahashi JS. 2010. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385 10.1126/science.1195262 (doi:10.1126/science.1195262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fenn MGP, Macdonald DW. 1995. Use of midden by red foxes: risk reverses rhythms of rats. J. Mammal. 76, 130–136 10.2307/1382321 (doi:10.2307/1382321) [DOI] [Google Scholar]
- 107.Swarts HM, Crooks KR, Willits N, Woodroffe R. 2009. Possible contemporary evolution in an endangered species, the Santa Cruz Island fox. Anim. Conserv. 12, 120–127 10.1111/j.1469-1795.2008.00229.x (doi:10.1111/j.1469-1795.2008.00229.x) [DOI] [Google Scholar]
- 108.Hudgens BR, Garcelon DK. 2011. Induced changes in island fox (Urocyon littoralis) activity do not mitigate the extinction threat posed by a novel predator. Oecologia 165, 699–705 10.1007/s00442-010-1761-7 (doi:10.1007/s00442-010-1761-7) [DOI] [PubMed] [Google Scholar]
- 109.Arnold W. 1988. Social thermoregulation during hibernation in alpine marmots (Marmota marmota). J. Comp. Physiol. B 158, 151–156 10.1007/BF01075828 (doi:10.1007/BF01075828) [DOI] [PubMed] [Google Scholar]
- 110.Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR. 2005. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 10.1126/science.1115581 (doi:10.1126/science.1115581) [DOI] [PubMed] [Google Scholar]
- 111.Woelfle MA, Yan OY, Phanvijhitsiri K, Johnson CH. 2004. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr. Biol. 14, 1481–1486 10.1016/j.cub.2004.08.023 (doi:10.1016/j.cub.2004.08.023) [DOI] [PubMed] [Google Scholar]


