Abstract
Properties of the circadian and annual timing systems are expected to vary systematically with latitude on the basis of different annual light and temperature patterns at higher latitudes, creating specific selection pressures. We review literature with respect to latitudinal clines in circadian phenotypes as well as in polymorphisms of circadian clock genes and their possible association with annual timing. The use of latitudinal (and altitudinal) clines in identifying selective forces acting on biological rhythms is discussed, and we evaluate how these studies can reveal novel molecular and physiological components of these rhythms.
Keywords: photoperiod, circadian, latitude, circannual, diapause, reproduction
1. Introduction
Two celestial movements of the Earth have deeply impacted the evolution of life: its axial rotation and its elliptical orbit around the sun. They cause daily variation in solar radiation and temperature and, due to the tilt of the Earth's axis, annual variation in the angle of incidence of solar radiation leading to annual variation in light intensity, twilight duration, photoperiod and average daily temperature. Daily and annual variations in solar radiation determine primary production through synergistic effects of temperature and light availability on plant growth. Organisms depending directly or indirectly on primary production and temperature are under selection pressure to optimize annual and daily timing of physiology and behaviour [1]. Daily and annual variation in solar radiation depend on latitude, and this leads to latitude-specific selection pressures in many organisms. We expect that evolution has shaped daily (circadian) and annual (circannual and/or photoperiodic) timing systems as a function of latitude, resulting in phenotypic and genotypic clines yielding information on the underlying mechanisms [2].
2. Latitudinal variation in annual light and temperature patterns: the use of ellipse-like photoperiod–temperature curves
Annual patterns in light and temperature vary with latitude (figure 1). More perpendicular solar transition across the horizon causes shorter twilights at the equator and around the equinoxes (figure 1a). Equatorial day length (civil twilight-based photoperiod) is nearly constant at approximately 13 h d−1. Away from the equator, photoperiod increases during summer and decreases during winter (figure 1b), eventually causing continuous light and continuous darkness near the poles.
Figure 1.
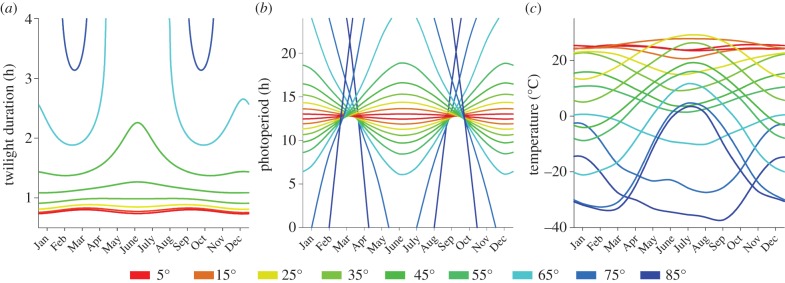
Annual patterns of (a) twilight duration, (b) photoperiod and (c) temperature, for different latitudinal ranges. (a) Twilight duration for Northern Hemisphere, defined as the time between −12° (nautical twilight) and 0° solar altitude (approx. 20–80% of the log light intensity change between midnight and noon). (b) Photoperiod calculation based on civil twilight times at dawn and dusk. Civil twilight (solar altitude 6° below the horizon) is the moment when log light intensity changes most rapidly [3] and is often considered as the time of ‘lights on’ and ‘lights off’ for biological systems. (c) Monthly mean dry bulb air temperatures (mostly between 1960 and 1990) from 873 weather stations around the world obtained from the World Meteorological Organization (http://www.wmo.int), and globally averaged over 10° latitudinal bands. Colours indicate mid-latitude of each band (hemispheres plotted separately).
Throughout the year, the incidence of solar radiation is more perpendicular at the equator than at the poles, causing higher temperatures around the equator. The Earth's axis is slanted relative to the annual orbital path around the sun and together they cause annual variation in solar radiation and temperature, with larger amplitude at the poles than in the tropics (figure 1c). The distribution of land mass and sea currents over the globe affect these patterns and cause quantitative differences between the Southern and Northern Hemisphere, but the general patterns are largely similar.
For understanding latitudinal clines in timing, it is important to understand the correspondence between photoperiod and temperature (figure 1b,c). The temperature capacity of the Earth's surface generates an annual hysteresis, with temperature lagging behind photoperiod. This causes a slanted ellipse-like annual relationship: the photoperiod–temperature (PPT) ellipse (figure 2a). This PPT ellipse changes position, angle and shape with latitude: when moving away from the equator, it shifts to lower temperatures and decompresses asymmetrically in photoperiod and temperature ranges (figure 2a), and eventually becomes more complex in shape approaching the (Ant-) Arctic circles.
Figure 2.
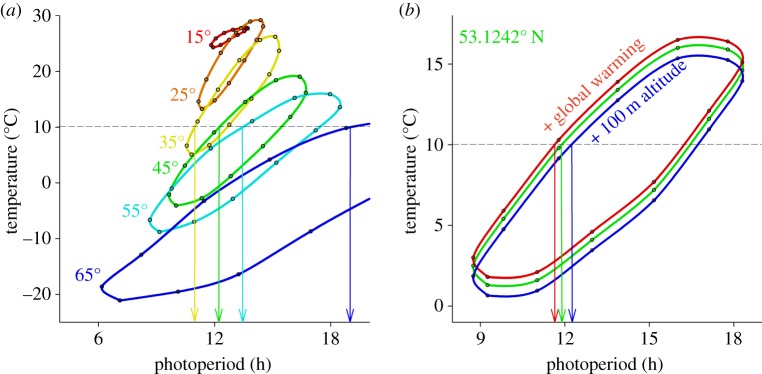
Ellipse-like annual PPT curves predict changing photoperiodic responses with changing latitude and altitude. (a) The annual temperature hysteresis leads to an ellipse-like relationship between temperature and photoperiod, with higher temperatures in autumn than in spring (dots indicate the mid-point of each month). The dotted lines (a,b) indicate a hypothetical threshold temperature at 10 °C at which a certain species starts winter dormancy (e.g. diapause), resulting in a shift towards longer critical photoperiod (CPP) earlier in the year when this species moves north. This fundamental process forms the basis for the expectation that latitudinal clines in photoperiodic response mechanisms may exist in nature. (b) Global warming (by 0.5 °C) will shift PPT ellipses up, whereas altitude will shift PPT ellipses down (by 0.649 °C 100 m−1). The photoperiod–diapause reaction norm (and accompanying CPP) is therefore expected to evolve under these environmental selection pressures (latitude, altitude and global warming).
Temperatures vary considerably between years, but photoperiod remains stable. Organisms therefore use photoperiod as a proximate factor to tune the annual timing of physiology and behaviour to changes in ultimate factors such as temperature [4]. Thus, when many years of temperature data are used for constructing PPT ellipses, they can be useful tools to make evolutionary predictions on photoperiodism (figure 2). For instance, assume an insect at 35° N has optimized its physiology for photoperiodic diapause induction when temperatures drop below 10 °C. The PPT ellipses can be used to illustrate that photoperiods below the critical photoperiod (CPP) should induce diapause (figure 2a, yellow PPT ellipse and arrow). They predict how the CPP will change when this species expands its distribution range north (figure 2a, green, turquoise and blue arrows). The expectation is that CPP will show a positive latitudinal cline, which may have a nonlinear complex shape (figure 2a).
Photoperiodic induction may change over years when global temperatures are changing [5]. This can be described by shifting a PTT ellipse upwards by, for example, 0.5 °C (figure 2b, green curve). This results in an approximately 10 min reduction in CPP (figure 2b, green and red curves and arrows). It is important for evolutionary studies to exploit altitude variation to predict effects of global warming [5,6]. Mean air temperature decreases on average 6.49 °C 1000 m−1 altitude [7]. Thus, 100 m elevation will cause an opposite but comparable shift in PPT and CPP (figure 2b, blue curve and arrow). Sampling at different altitudes may generate a predictable altitudinal cline with a simple linear relationship between CPP and altitude. Indeed, the pitcher plant mosquito Wyeomyia smithii increases its CPP by 11 min for each degree north, which is similar to 100 m altitudinal increase causing 8 min increase in CCP [5,6]. Long-term changes in global air temperatures and altitudinal clines may be important tools to disentangle complex interacting traits in annual and daily timing in physiology and behaviour.
3. Latitudinal clines in photoperiodism
Latitudinal variation in photoperiodism has been investigated mainly in insects in which short photoperiods trigger the induction of diapause, a form of winter dormancy. Diapause can occur in all stages of the life cycle, from embryo to adult, and each species has a genetically determined diapause stage [8]. Insects enter diapause in preparation for winter even when conditions are still favourable [9]. Typically, the developmental stage sensitive to environmental stimuli is earlier than the diapausing stage and sometimes it even occurs in the previous generation, as is the case for larval diapause with maternal induction [10].
Danilevskii [11] was the first to demonstrate a positive correlation between latitude and CPP for diapause induction. He showed that CPP increases by about 1 h every 5–6° latitude in the knot grass moth Acronicta rumicis [11]. Positive correlations between CPP and latitude were later described in many other diapausing insects (figure 3a; reviewed by Tauber et al. [8] and Danks [12]). Latitudinal clines in CPP have further been demonstrated in adult reproductive diapause in the Japanese flower bug Orius [19] and in Finnish malt flies Drosophila montana [21], in pupal diapause in the butterfly Sericinus montelus [13] and in maternally induced larval diapause in the parasitoid wasp Nasonia vitripennis [20]. The combined data reveal a general positive correlation between CPP and latitude, with an increased slope above approximately 40° N (figure 3a).
Figure 3.
Latitudinal clines in photoperiodic and circadian timing mechanisms in insects. Latitude (in ° N) correlates with: (a) critical photoperiod for diapause induction (populations above 800 m altitude were excluded); (b) circadian amplitude of overt rhythms in light--dark (LD) using various indices (see text); (c) phase of entrainment in 3 L : 21 D; (d) circadian period in continuous darkness (DD). (a) Brown, Sericinus montelus (pupae) [13]; black, Wyeomyia smithii (larvae) [14]; purple, Bruchidius dorsalis (larvae) [15]; pink, Chrysopa carnea (adult) [16]; turquoise, Homoeosoma electellum (larvae) [17]; khaki, Tetranychus pueraricola (adult) [18]; cyan, Orius sauteri (adult) [19]; dark blue, Acronicta rumicis (larvae) [11]; red, Nasonia vitripennis (larvae; maternally induced) [20]; green, Drosophila montana (adult) [21]; grey, D. phalerata (adult) [22]; blue, D. transversa (adult) [22]. (b)–(d) Khaki, D. melanogaster (oviposition) [23]; blue, D. subobscura (eclosion) [24]; red, D. littoralis (eclosion) [25]; green, D. auraria (eclosion) [26]. Three equatorial regression data points from Allemand & David [23] were not plotted in (b) for axis consistency.
The latitudinal cline in CPP for diapause induction has adaptive significance related to the seasonal cycles at different latitudes, since CPP is predicted to be longer at higher latitudes (figure 2a). Selection pressure on timing for photoperiodic diapause induction is also evident in studies on alien species recently introduced in new environments. Alien insects can adapt to the seasonal cycles of a newly colonized geographical range by the rapid establishment of latitudinal clines in CPP [27–29]. Rapid change in CPP also occurs in response to global warming (figure 2b), as documented in northern populations of pitcher plant mosquito W. smithii, which shifted their photoperiodic response towards shorter CPP [30].
The latitudinal cline in CPP (figure 3a) leads to the prediction that the proportion of diapausing individuals increases with latitude. This general trend has been found in many species [8,12,31–34]. In some cases, populations at high latitudes evolved obligate diapause where all individuals enter diapause at a certain stage regardless of environmental conditions [35].
In the varied carpet beetle Anthrenus verbasci, natural photoperiodic conditions synchronize the endogenous, self-sustained circannual rhythm of the life cycle with the environment [36,37]. The fact that photoperiod acts as an entrainment factor suggests that properties of circannual rhythmicity might also change with latitude, but this remains to be investigated.
The power of photoperiod as an indicator of seasonal change diminishes towards lower latitudes. At more temperate latitudes, different cues can interact with day length and modify the photoperiodic response. Temperature plays an important role as modulating factor in southern populations of the mosquito Aedes atropalpus [38] and in the fly Calliphora vicina [39], where higher temperatures suppress diapause incidence and shorten the CPP. The larger temperature sensitivity of southern populations allows more flexibility of the photoperiodic response in locations where occasional warm conditions in late summer permit an extension of the reproductive season before winter. Saunders [40] showed that thermoperiod (daily temperature cycles) can alone induce diapause in N. vitripennis, but it is not known whether this thermoperiodic response varies across latitudinal gradients.
Photoperiodic induction of diapause sometimes behaves as a threshold accumulation counter, where individuals must experience a specific number of short days to enter diapause [41]. Beach [38] investigated variation in this trait in relation to latitude. Northern strains require fewer short photoperiods for diapause induction than southern populations, as was found in N. vitripennis [20]. The variation in required number of short days has similar adaptive significance: faster responses are beneficial in northern locations where seasonal change is abrupt (figure 1; [20]).
4. Latitudinal variation in properties of circadian clocks
Endogenous circadian rhythm characteristics may also associate with latitude, because of their possible role in photoperiodism as well as in the timing of daily behaviours [42]. Such variation might reveal important selection pressures on circadian function. Yet, latitudinal variation in circadian rhythms has not been as extensively studied as photoperiodism.
In plants [43], observed free-running periods in circadian leaf movement rhythms of various species were generally shorter further north. For two dandelion (Taraxacum) species, this holds also within the genus. No analyses of latitudinal strains within the same species are known to us apart from a study on leaf movements in Arabidopsis thaliana that, which reports that both the period (τ) in constant conditions and phase angle difference (ψ) in a light--dark (LD) cycle are positively associated with latitude [44].
In animals, nearly all latitudinal studies deal with Drosophila (figure 3b–d). Allemand & David [23] established a latitudinal cline in daily rhythms of oviposition in 12 L : 12 D in Drosophila melanogaster from equatorial Africa (approx. 0° N) to Scandinavia (approx. 62° N). The entrained state however precluded assessment of whether the variation is endogenous or due to masking. Lankinen contributed extensively on circadian pupal eclosion rhythms performed in LD and continuous darkness (DD), in Drosophila littoralis [25] from 41.6° N (Caucasus) to 69° N (northern Finland) and in Drosophila subobscura [24] from the Canarian Islands (28° N) into Scandinavia (62° N). Two further studies deal with the eclosion rhythm of Drosophila auraria from Japan [26,45], but these cover only an 8° latitude range (figure 3c,d).
Latitudinal clines in the amplitude of circadian rhythms are summarized for three fruitfly species (figure 3b). The measures of amplitude vary and cannot directly be compared among species. Allemand & David [23] used peak-to-width ratio of the daily peak (in % h−1) of eggs laid. Lankinen [24,25,46] used mean % h−1 in the peak 5 h of daily eclosion in DD . Within species, there is a steep decline in the circadian amplitude with increasing latitude. Pittendrigh & Takamura [26] have argued that the amplitude of the overt rhythm is not the same as that of the circadian pacemaker. They concluded from the northward decrease in amplitude of the phase response curve that the amplitude of the circadian pacemaker increases towards the north. This would also explain the more rapid damping of the eclosion rhythm—slave to the pacemaker—in DD found further north by Lankinen [24] and Pittendrigh et al. [45].
In stable entrainment, the phase angle difference (ψ, defined as time between rhythm and zeitgeber phase markers) increases (i.e. the rhythm advances) as one goes north in two species [23,24]. The data shown (figure 3c) are for 3 L : 21 D. Pittendrigh & Takamura [26] showed, however, that the latitudinal differences in D. auraria vary strongly with photoperiod, and that the variations do not necessarily reflect those of the pacemaker's ψ, where the latitudinal difference is reversed between 14 L : 10 D and 1 L : 23 D. The period τ of the free-running circadian rhythm (figure 3d) decreased from equator to pole in D. littoralis and D. subobscura, but was not associated with latitude—over a smaller range—in D. auraria [26].
It is remarkable that no studies have directly addressed latitudinal variation of circadian organization in vertebrates. Some studies indicate a latitudinal cline in aspects of retinal photoreception, which possibly indicates an adaptation to lower daylight intensities and longer twilight durations at higher latitudes. In fish, the degree of retinal cone contraction is under stronger endogenous control in tropical fish than in polar species [47]. In humans, the frequency of colour deficiency increases from 2 to 10 per cent from the equator to the polar circle [48], a feature that might relate to increasing scotopic light sensitivity [49]. Birds and mammals have been studied at different latitudes to assess the effects of natural zeitgeber properties [3], but not the evolutionary adaptation of their circadian systems to latitude. One study aimed at finding directional selection on circadian clock genes in humans concluded that the genetic variation among world populations was due to genetic drift rather than latitudinal selection [50]. However, another revealed that genetic variation in the hPer2 gene appeared to be under directional selection in non-African populations [51].
We feel that the comparative study of circadian systems adapted to different latitudes is in its infancy. The best studies are based on fruitflies (figure 3). There are clear latitudinal trends in amplitude (negatively), phase (ψ, positively) and free-running period (τ, negatively). Opposite trends in ψ and τ are to be expected, as a longer τ would lead to a smaller positive or more negative ψ. We should, however, be aware of the fact that the observed clines refer to a slave oscillator rather than the pacemaker [26]. Indeed, these authors derive some evidence from their data, in spite of the narrow latitude range, that both amplitude and period of the pacemaker may increase towards the north. Pittendrigh et al. [45] provided a theory on selection pressure leading to increased amplitude and reduced phase response curve (PRC) amplitude as one goes north. As the entraining photoperiod increases, pacemaker amplitude diminishes. Increased pacemaker amplitude towards the poles would help offset the negative—damping—effect of the longer days of summer. The higher pacemaker amplitude might suppress the amplitude of the slave oscillation, which may be responsible for the reversed latitudinal cline observed in overt rhythms. The suggestion is that proper timing is preserved in arctic regions by such adjustments in the pacemaking system, no matter whether the ‘goal’ is photoperiodic time measurement or daily behavioural tuning. These fascinating propositions can be tested in other species with greater north–south spread than D. auraria.
5. Latitudinal clines in clock gene polymorphisms
The first clock gene identified, period (per) in D. melanogaster, encodes a variable length tract of Thr-Gly residues in which the two major variants, (Thr-Gly)17 and (Thr-Gly)20 accounted for 90 per cent of the length variation, and were distributed as a latitudinal cline stretching from the Mediterranean to Scandinavia [52]. A similar weaker cline was also detected in Australia, an observation consistent with the view that natural selection was maintaining the polymorphism [53]. A statistical analysis of linkage disequilibria around the Thr-Gly region confirmed that balancing selection was maintaining the European polymorphism [54]. Further phenotypic studies revealed that the two major European variants showed different patterns of temperature compensation that adapted them to warmer or cooler regions, which paralleled each variant's latitudinal distribution [55]. These studies suggested that the closer the free-running period was to 24 h, the fitter the variant appeared to be, a conclusion that was dramatically demonstrated in competition experiments between pairs of Cyanobacterial clock mutants exposed to different length LD cycles [56].
A second gene, timeless (tim), encodes the major light-sensitive circadian regulator in Drosophila [57,58] and a natural 5′ polymorphism generates two tim alleles through the use of alternative start codons [59]. ls-tim produces a long and short TIM isoform, whereas s-tim generates the shorter isoform only [60]. A latitudinal cline in the frequencies of these allelic variants is observed, so that in the Mediterranean, ls-tim is at levels of 80 per cent, but falls to 20 per cent in Scandinavia [60]. ls-tim flies have lower circadian light-sensitivity than s-tim, and ls-tim females enter reproductive diapause prematurely compared with s-tim, even at long daylengths (diapause is gently photoperiodic in D. melanogaster) [60,61]. Indeed, one could envision this ls-tim ovarian response to reflect reduced photoperiodic light sensitivity, in that these females appear to misinterpret a long day as a short day, mirroring their circadian response to light.
Both of these ls-tim responses thus appear to be adaptive in seasonal environments, particularly in higher latitudes where winter comes earlier when photoperiods are still long. An earlier diapause could be put to good use, and reduced circadian light sensitivity could mitigate the damaging effects on rhythmicity of long summer days. Thus, it was puzzling that ls-tim females were found predominantly in the lower latitudes. The solution to this apparent contradiction was revealed when phylogenetic analysis of tim sequences revealed that ls-tim was relatively new, being, at most, a few thousand years old, and at least, a few hundred years old ([60], V. Zonato and C. P. Kyriacou 2013, unpublished data). Subsequent spatial analyses incorporating more southern European populations revealed that ls-tim population frequencies accurately reflected their distance from southern Italy, suggesting the mutation originated from this region [60]. This was confirmed by neutrality tests that revealed directional, not balancing selection acting on ls-tim. Thus, ls-tim frequencies have increased throughout Europe because of the seasonal nature of the photoperiodic environment, something that is absent in the ancestral homelands of sub-Saharan Africa, where D. melanogaster evolved before migrating to Europe 14 000 years ago.
A higher amplitude oscillator to diminish the effects of light at high latitudes has been proposed by Pittendrigh et al. [45]. An alternative mechanism may be to simply diminish the input of light to the clock. In flies, this appears to be resolved by generating a less light-sensitive TIM molecule because the amplitude of the tim mRNA rhythm, which may be a reflection of the oscillator, does not differ between the two tim alleles [61]. Secondly, the tim studies raise the possibility that in insects, circadian clock molecules are also involved in photoperiodism, first suggested by Bünning [62] and confirmed at the molecular level in plants [63]. However, the different isoforms of tim in D. melanogaster affect the levels of diapause, but not the photoperiodic slope of diapause induction. In a number of other insect species, the pitcher plant mosquito W. smithii [64], Chymomyza costata [65], Drosophila triauraria [66], the flesh fly Sarcophaga bullata [67,68] and the bean bug Riptortus pedestris [69–71], clock genes have been directly or indirectly implicated in diapause. This relationship between circadian clock genes and diapause could indicate direct involvement of the circadian clock with diapause, but does not exclude pleiotropic effects of these genes on the diapause mechanism. Taken together, the evidence seems to indicate an involvement of the circadian system in diapause induction, but the mechanisms seem to vary considerably between species [72,73].
While these latter studies do not include latitudinal perspectives, some recent studies have suggested that other non-clock genes may contribute to diapause and are spatially patterned along the latitudinal axis. For example, molecular variation in the couch-potato (cpo) gene in D. melanogaster covaries with diapause levels and shows clinal variation along the East Coast of the USA [74]. In Australia, a similar situation occurs, but the clinal frequencies of the relevant cpo variant is driven by strong linkage disequilibrium with the nearby Payne inversion, which varies significantly with latitude on this continent [74]. A cline for variation in the gene encoding phosphatidylinositol-3-kinase (Dp110), which is involved in insulin signalling, has also been reported in the USA and Canada [75], as has a cline observed for variation in the insulin receptor gene, InR, in both America and Australia [76]. While variation in Dp110 appears to change diapause levels [75], the natural variation in InR has not been studied in terms of its effects on the phenotype. However, InR mutant females do have immature ovaries [77]. Consequently, it appears that the insulin pathway, which is important for lifespan [78], may provide a substrate for natural selection along a latitudinal cline that influences life-extending seasonal phenotypes such as diapause [79]. How clock genes interact, genetically and neuroanatomically, with the insulin pathway to generate the overwintering response remains to be established.
In vertebrates, progress at the molecular level has been slower than in Drosophila. Clock gene polymorphisms in birds have first been described by Fidler & Gwinner [80] in an effort to identify genetic differences between diurnal and nocturnal species. They examined sequences of three components of the core circadian oscillator: BMAL1, CLOCK, and PER2 and found the Clock poly-glutamine (Clock-polyQ) region to be polymorphic within- and among-species. [80]. Populations of both the non-migratory Blue Tit Cyanistes caeruleus and the migratory Bluethroat Luscinia svecica showed considerable polymorphism in Clock-polyQ (nine and seven Clock-polyQ alleles, respectively) and a positive association between Clock-polyQ allele length and breeding latitude (30° range) was found in the blue tit [81]. Corresponding analyses for Bluethroat populations failed to show such a pattern between Clock-polyQ and breeding latitude [81]. Also the swallow genus Tachycineta that breeds across North, South and Central America showed no evidence for an association between Clock-polyQ allele size and breeding latitude across populations from five species [82]. This parallels the lack of latitude Clock-polyQ association in the barn swallow Hirundo rustica [83]. Similarly, studies in Pacific salmon species (genus Oncorhynchus) that overlap in their geographical breeding also generated similar inconsistent patterns of latitudinal clines in Clock-polyQ frequency [84,85]. Thus, the association between breeding latitude and Clock-polyQ length polymorphism is certainly not a general pattern in vertebrates.
6. Discussion
Variation in timing is typically measured in experiments where different strains are exposed to the same controlled environmental conditions. The observed phenotypic differences are based on genetic differences likely to result from selection caused by environmental conditions along latitudinal gradients. Taken together, there is evidence that predicted latitudinal clines indeed exist in circadian and photoperiodic properties. For photoperiodic responses this relationship can be well understood. All insect species investigated show increasing CPP for winter diapause induction in more northern populations (figure 3a), confirming the pioneering work of Danilevskii [11]. This trend is fully consistent with the theoretical prediction derivable from adaptive day length responses (figure 2a), where low autumn temperatures occur earlier in the year and at longer photoperiods when moving away from the equator (figure 2a). Although these clines have almost exclusively been studied in insects, there is no reason why they could not also exist in other organisms. The molecular machinery for photoperiodism in mammals has been well described in laboratory studies [86–88], and it seems timely to take this knowledge to the field [89] where evidence for natural selection acting upon this mechanism can be found by using latitudinal cline approaches.
Few studies address latitudinal variation in circadian properties. The available data in Drosophila suggest three general patterns in latitudinally distributed species: circadian rhythm amplitude of overt rhythms in standard artificial LD cycles decreases in more polar populations (figure 3b), while phase angle difference increases (figure 3c) and, correspondingly, free-running period decreases (figure 3d). The amplitude decrease at higher latitudes might be related to more extreme daily light and temperature cycles. The increase in phase angle difference (i.e. advanced phase in entrainment) may reflect the cline in period (figure 3c,d). We are further aware of the fact that rhythm amplitude does not necessarily reflect pacemaker amplitude, which has been suggested to increase towards the north, as a way to offset the suppressing effect of long photoperiods [26].
A first evolutionary hypothesis is that these latitudinal clines have evolved primarily to support photoperiodic time measurement at different latitudes [90]. Circadian systems are assumed to be involved in photoperiodic time measurement [62,91] and such involvement has been demonstrated in many taxa, including birds, mammals, insects, etc. [1], although there are exceptions [92]. Even if a circadian system is involved, it does not necessarily mean that variation between populations in CPP is reflected in variation in circadian parameters, as shown clearly both in phenotypic analyses in the pitcher plant mosquito [14] and in crossing studies in D. littoralis [46]. Describing geographical, genetic or phenotypic correlations may not clarify the precise mechanism of circadian involvement in photoperiodism, if it exists. The classical debate between external coincidence timing (single circadian oscillator interacting with the LD cycle) and internal coincidence timing (two interacting circadian oscillators) models for circadian involvement has indeed not been settled. Hut & Beersma [2] suggested that predictions for latitudinal clines in circadian period are radically different when assuming an endogenous representation of day length generated by either external or internal coincidence. External coincidence would produce a monotone cline potentially crossing 24 h. Internal coincidence timing would produce a cline that deviates more from 24 h towards the poles, but does not cross 24 h towards the equator. So far, no cline in circadian period was reliably found to cross 24 h, but the cline in D. subobscura approaches 24 h at higher latitudes favouring an external coincidence timing model in Drosophila (figure 3d).
Recent molecular studies on mammalian photoperiodism [86–89] have clearly shown an involvement of the circadian system at the level of melatonin regulation and at the hypothalamic induction pathway. This pathway is well conserved across various species studied. Mammals therefore represent an excellent (and virtually unexploited) system to study selection pressures on photoperiodism and circadian organization by using latitudinal clines.
A second, very general, hypothesis is that the systematically shorter circadian periods in Drosophila at higher latitudes are required to enable circadian entrainment under long summer photoperiods. This can be theoretically understood from phase response curves where very long light pulses only generate phase delays, as was demonstrated in mice [93]. This phenomenon would nearly preclude long photoperiod entrainment unless the endogenous circadian period in DD is shorter than 24 h. The long summer photoperiods in the north may select for shorter endogenous periods than the more modest photoperiod lengths in temperate regions. Assessments of clines in other species, specifically small mammals, would be needed to test the generality of this prediction.
Additional selection pressure may be formed by effects of latitude on the phase of circadian entrainment. Twilight duration can be expected to affect phase of circadian entrainment [94] either through zeitgeber strength [94–96], or through species-specific effects of twilight on circadian period under short photoperiods [97,98].
Examination of latitudinal clines in clock gene polymorphisms in free-living vertebrate populations is in its early stages, and does not allow general conclusions. These molecular studies have focused solely on Clock gene polymorphisms, without yielding convincing general patterns. Compensatory mechanisms in the circadian molecular network can readily explain why focusing on a single gene may not be a fruitful approach [99]. The types of neutrality tests that also detect the signatures of natural selection cannot generally be performed on repeated sequences like Clock-polyQ, so alternative methods need to be used [54,100]. Consequently, any connection between clock gene variation, latitude, phenotypes and selection in vertebrates so far remains speculative. Further studies examining multiple gene polymorphisms in more species will be essential to get to grips with the molecular basis of latitudinal clines and their implications for selection. However, the study by Cruciani et al. [51] mentioned earlier is particularly intriguing because these authors observed that in non-African human populations hPER2 showed evidence for positive selection. hPER2, like TIM in Drosophila, is important for circadian light resetting [101]. As humans and flies both emerged out of sub-Saharan Africa, could it be that they were challenged with changing photoperiods at higher latitudes, which led to natural selection on the clock genes underlying circadian photosensitivity?
Despite these caveats, we conclude that establishing latitudinal variation is a powerful tool to study mechanisms underlying circadian organization and photoperiodic responses and to reveal selective forces responsible for daily and seasonal adaptation [2,102]. Further exploitation of latitudinal clines will lead to novel insights into both the mechanism and evolutionary significance of daily and annual biological rhythms. To understand evolution and adaptive significance of daily and annual timing systems, direct selection on circadian entrainment should be disentangled from selection through circadian involvement in photoperiodism. This task asks for studies that combine available knowledge of the underlying molecular mechanisms in daily and annual timing with detailed phenotypic and genotypic description of populations at different latitudes and altitudes.
Funding statement
This work was supported by the Israel Science Foundation. S.P. was supported by EU Marie Curie ITN SPECIATION. C.P.K. thanks BBSRC and NERC for funding the laboratory work. C.P.K. and R.A.H. are supported by EU Marie Curie ITN INsecTIME.
References
- 1.Pittendrigh CS. 1982. Circadian organization and the photoperiodic phenomena. In Biological clocks in seasonal reproductive cycles (eds Follett BK, Follett DE.), pp. 1–35 Bristol, UK: Wright [Google Scholar]
- 2.Hut RA, Beersma DG. 2011. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Phil. Trans. R. Soc. B 366, 2141–2154 (doi:10.1098/rstb.2010.0409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daan S, Aschoff J. 1975. Circadian rhythms of locomotor activity in captive birds and mammals: their variations with season and latitude. Oecologia 18, 269–316 (doi:10.1007/BF00345851) [DOI] [PubMed] [Google Scholar]
- 4.Immelmann K. 1972. Erörterungen zur Definition und Anwendbarkeit der Begriffe ‘ultimate factor’, ‘proximate factor’, und ‘zeitgeber’. Oecologia 9, 259–264 (doi:10.1007/BF00345235) [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw WE, Holzapfel CM. 2010. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 72, 147–166 (doi:10.1146/annurev-physiol-021909-135837) [DOI] [PubMed] [Google Scholar]
- 6.Bradshaw WE. 1976. Geography of photoperiodic response in diapausing mosquito. Nature 262, 384–386 (doi:10.1038/262384b0) [DOI] [PubMed] [Google Scholar]
- 7.International Civil Aviation Organization 1993. Manual of the ICAO standard atmosphere (extended to 80 kilometres (262 500 feet)), Doc 7488-CD, 3rd edn Montréal, Canada: ICAO [Google Scholar]
- 8.Tauber MJ, Tauber CA, Masaki S. 1986. Seasonal adaptations of insects. New York, NY: Oxford University Press [Google Scholar]
- 9.Bale JS, Hayward SA. 2010. Insect overwintering in a changing climate. J. Exp. Biol. 213, 980–994 (doi:10.1242/jeb.037911) [DOI] [PubMed] [Google Scholar]
- 10.Saunders DS. 1965. Larval diapause of maternal origin: induction of diapause in Nasonia vitripennis (Walk.) (Hymenoptera: Pteromalidae). J. Exp. Biol. 42, 495–508 [Google Scholar]
- 11.Danilevskii AS. 1965. Photoperiodism and seasonal development of insects. London, UK: Oliver & Boyd Ltd [Google Scholar]
- 12.Danks HV. 1987. Insect dormancy: an ecological perspective. Ottawa, Canada: Biological Survey of Canada [Google Scholar]
- 13.Wang XP, Yang QS, Dalin P, Zhou XM, Luo ZW, Lei CL. 2011. Geographic variation in photoperiodic dia- pause induction and diapause intensity in Sericinus montelus (Lepidoptera: Papilionidae). Insect Sci. 19, 295–302 (doi:10.1111/j.1744-7917.2011.01473.x) [Google Scholar]
- 14.Bradshaw WE, Quebodeaux MC, Holzapfel CM. 2003. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive response to the photic environment or correlated response to the seasonal environment? Am. Nat. 161, 735–748 (doi:10.1086/374344) [DOI] [PubMed] [Google Scholar]
- 15.Kurota H, Shimada M. 2003. Geographical variation in photoperiodic induction of larval diapause in the bruchid beetle, Bruchidius dorsalis: polymorphism in overwintering stages. Entomol. Exp. Appl. 107, 11–18 (doi:10.1046/j.1570-7458.2003.00033.x) [Google Scholar]
- 16.Tauber MJ, Tauber CA. 1972. Geographic variation in critical photoperiod and in diapause intensity of Chrysopa carnea (Neuroptera). J. Insect Physiol. 18, 25–29 (doi:10.1016/0022-1910(72)90061-3) [Google Scholar]
- 17.Kikukawa S, Chippendale GM. 1984. Seasonal adaptations of different geographical populations of the sunflower moth, Homoeosoma electellum. J. Insect Physiol. 30, 451–455 (doi:10.1016/0022-1910(84)90024-6) [Google Scholar]
- 18.Suwa A, Gotoh T. 2006. Geographic variation in diapause induction and mode of diapause inheritance in Tetranychus pueraricola. J. Appl. Entomol. 130, 329–335 (doi:10.1111/j.1439-0418.2006.01050.x) [Google Scholar]
- 19.Shimizu T, Kawasaki K. 2001. Geographic variability in diapause response of Japanese Orius species. Entomol. Exp. Appl. 98, 303–316 (doi:10.1046/j.1570-7458.2001.00787.x) [Google Scholar]
- 20.Paolucci S, van de Zande L, Beukeboom LW. 2013. Adaptive latitudinal cline of photoperiodic diapause induction in the parasitoid Nasonia vitripennis in Europe. J. Evol. Biol. 26, 705–718 (doi:10.1111/jeb.12113) [DOI] [PubMed] [Google Scholar]
- 21.Tyukmaeva VI, Salminen TS, Kankare M, Knott KE, Hoikkala A. 2011. Adaptation to a seasonally varying environment: a strong latitudinal cline in reproductive diapause combined with high gene flow in Drosophila montana. Ecol. Evol. 1, 160–168 (doi:10.1002/ece3.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muona D, Lumme J. 1981. Geographical variation in the reproductive cycle and photoperiodic diapause of Drosophila phalerata and D. transversa (Drosophilidae: Diptera). Evolution 35, 158–167 (doi:10.2307/2407949) [DOI] [PubMed] [Google Scholar]
- 23.Allemand R, David JR. 1976. The circadian rhythm of oviposition in Drosophila melanogaster: a genetic latitudinal cline in wild populations. Experientia 32, 1403–1405 (doi:10.1007/BF01937401) [Google Scholar]
- 24.Lankinen P. 1993. North–south differences in circadian eclosion rhythm in European populations of Drosophila subobscura. Heredity 71, 210–218 (doi:10.1038/hdy.1993.126) [Google Scholar]
- 25.Lankinen P. 1986. Geographical variation in circadian eclosion rhythm and photoperiodic adult diapause in Drosophila littoralis. J. Comp. Physiol. A 159, 123–142 (doi:10.1007/BF00612503) [DOI] [PubMed] [Google Scholar]
- 26.Pittendrigh CS, Takamura T. 1989. Latitudinal clines in the properties of a circadian pacemaker. J. Biol. Rhythms 4, 217–235 (doi:10.1177/074873048900400209) [PubMed] [Google Scholar]
- 27.Bean DW, Dalin P, Dudley TL. 2012. Evolution of critical day length for diapause induction enables range expansion of Diorhabda carinulata, a biological control agent against tamarisk (Tamarix spp.). Evol. Appl. 5, 511–523 (doi:10.1111/j.1752-4571.2012.00262.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomi T. 2007. Seasonal adaptations of the fall webworm Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) following its invasion of Japan. Ecol. Res. 22, 855–861 (doi:10.1007/s11284-006-0327-y) [Google Scholar]
- 29.Sadakiyo S, Ishihara M. 2011. Rapid seasonal adaptation of an alien bruchid after introduction: geographic variation in life cycle synchronization and critical photoperiod for diapause induction. Entomol. Exp. Appl. 140, 69–76 (doi:10.1111/j.1570-7458.2011.01136.x) [Google Scholar]
- 30.Bradshaw WE, Holzapfel CM. 2001. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl Acad. Sci. USA 98, 14 509–14 511 (doi:10.1073/pnas.241391498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demont M, Blanckenhorn WU, Hosken DJ, Garner TW. 2008. Molecular and quantitative genetic differentiation across Europe in yellow dung flies. J. Evol. Biol. 21, 1492–1503 (doi:10.1111/j.1420-9101.2008.01615.x) [DOI] [PubMed] [Google Scholar]
- 32.Leisnham PT, Towler L, Juliano SA. 2011. Geographic variation of photoperiodic diapause but not adult survival or reproduction of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in North America. Ann. Entomol. Soc. Am. 104, 1309–1318 (doi:10.1603/AN11032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masaki S. 1999. Seasonal adaptations of insects as revealed by latitudinal diapause clines. Entomol. Sci. 2, 539–542 [Google Scholar]
- 34.Schmidt PS, Matzkin L, Ippolito M, Eanes WF. 2005. Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution 59, 1721–1732 (doi:10.1554/05-115.1) [PubMed] [Google Scholar]
- 35.Winterhalter WE, Mousseau TA. 2007. Patterns of phenotypic and genetic variation for the plasticity of diapause incidence. Evolution 61, 1520–1531 (doi:10.1111/j.1558-5646.2007.00127.x) [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki Y, Nisimura T, Numata H. 2005. A phase response curve for circannual rhythm in the varied carpet beetle Anthrenus verbasci. J. Comp. Physiol. A 191, 883–887 (doi:10.1007/s00359-005-0012-6) [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki Y, Nisimura T, Numata H. 2012. Circannual rhythm in the varied carpet beetle, Anthrenus verbasci. Prog. Brain Res. 199, 439–456 (doi:10.1016/B978-0-444-59427-3.00025-3) [DOI] [PubMed] [Google Scholar]
- 38.Beach R. 1978. The required day number and timely induction of diapause in geographic strains of mosquito Aedes atropalpus. J. Insect Physiol. 24, 449–455 (doi:10.1016/0022-1910(78)90088-4) [Google Scholar]
- 39.McWatters HG, Saunders DS. 1998. Maternal temperature has different effects on the photoperiodic response and duration of larval diapause in blow fly (Calliphora vicina) strains collected at two latitudes. Physiol. Entomol. 23, 369–375 (doi:10.1046/j.1365-3032.1998.00101.x) [Google Scholar]
- 40.Saunders DS. 1973. Thermoperiodic control of diapause in an insect: theory of internal coincidence. Science 181, 358–360 (doi:10.1126/science.181.4097.358) [DOI] [PubMed] [Google Scholar]
- 41.Saunders DS. 2002. Insect clocks, 3rd edn Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 42.Goto SG. 2013. Roles of circadian clock genes in insect photoperiodism. Entomol. Sci. 16, 1–16 (doi:10.1111/ens.12000) [Google Scholar]
- 43.Mayer W. 1966. Besonderheiten der circadianen Rhythmik bei Pflanzen verschiedener geographischer Breiten. Planta 70, 237–256 (doi:10.1007/BF00396490) [DOI] [PubMed] [Google Scholar]
- 44.Michael TP, Salome PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, Alonso JM, Ecker JR, McClung CR. 2003. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302, 1049–1053 (doi:10.1126/science.1082971) [DOI] [PubMed] [Google Scholar]
- 45.Pittendrigh CS, Kyner WT, Takamura T. 1991. The amplitude of circadian oscillations: temperature dependence, latitudinal clines, and the photoperiodic time measurement. J. Biol. Rhythms 6, 299–313 (doi:10.1177/074873049100600402) [DOI] [PubMed] [Google Scholar]
- 46.Lankinen P, Forsman P. 2006. Independence of genetic geographical variation between photoperiodic diapause, circadian eclosion rhythm, and Thr-Gly repeat region of the period gene in Drosophila littoralis. J. Biol. Rhythms 21, 3–12 (doi:10.1177/0748730405283418) [DOI] [PubMed] [Google Scholar]
- 47.Yammouni R, Bozzano A, Douglas RH. 2011. A latitudinal cline in the efficacy of endogenous signals: evidence derived from retinal cone contraction in fish. J. Exp. Biol. 214, 501–508 (doi:10.1242/jeb.048538) [DOI] [PubMed] [Google Scholar]
- 48.Reimchen TE. 1987. Human color vision deficiencies and atmospheric twilight. Soc. Biol. 34, 1–11 (doi:10.1080/19485565.1987.9988655) [DOI] [PubMed] [Google Scholar]
- 49.Verhulst S, Maes FW. 1998. Scotopic vision in colour-blinds. Vis. Res. 38, 3387–3390 (doi:10.1016/S0042-6989(97)00339-8) [DOI] [PubMed] [Google Scholar]
- 50.Ciarleglio CM, et al. 2008. Genetic differences in human circadian clock genes among worldwide populations. J. Biol. Rhythms 23, 330–340 (doi:10.1177/0748730408320284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruciani F, Trombetta B, Labuda D, Modiano D, Torroni A, Costa R, Scozzari R. 2008. Genetic diversity patterns at the human clock gene period 2 are suggestive of population-specific positive selection. Eur. J. Hum. Genet. 16, 1526–1534 (doi:10.1038/ejhg.2008.105) [DOI] [PubMed] [Google Scholar]
- 52.Costa R, Peixoto AA, Barbujani G, Kyriacou CP. 1992. A latitudinal cline in a Drosophila clock gene. Proc. R. Soc. Lond. B 250, 43–49 (doi:10.1098/rspb.1992.0128) [DOI] [PubMed] [Google Scholar]
- 53.Sawyer LA, Sandrelli F, Pasetto C, Peixoto AA, Rosato E, Costa R, Kyriacou CP. 2006. The period gene Thr-Gly polymorphism in Australian and African Drosophila melanogaster populations: implications for selection. Genetics 174, 465–480 (doi:10.1534/genetics.106.058792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosato E, Peixoto AA, Costa R, Kyriacou CP. 1997. Linkage disequilibrium, mutational analysis and natural selection in the repetitive region of the clock gene, period, in Drosophila melanogaster. Genet. Res. 69, 89–99 (doi:10.1017/S001667239700267X) [DOI] [PubMed] [Google Scholar]
- 55.Sawyer LA, Hennessy JM, Peixoto AA, Rosato E, Parkinson H, Costa R, Kyriacou CP. 1997. Natural variation in a Drosophila clock gene and temperature compensation. Science 278, 2117–2120 (doi:10.1126/science.278.5346.2117) [DOI] [PubMed] [Google Scholar]
- 56.Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. 1998. Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl Acad. Sci. USA 95, 8660–8664 (doi:10.1073/pnas.95.15.8660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suri V, Qian Z, Hall JC, Rosbash M. 1998. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron 21, 225–234 (doi:10.1016/S0896-6273(00)80529-2) [DOI] [PubMed] [Google Scholar]
- 58.Yang Z, Emerson M, Su HS, Sehgal A. 1998. Response of the timeless protein to light correlates with behavioral entrainment and suggests a nonvisual pathway for circadian photoreception. Neuron 21, 215–223 (doi:10.1016/S0896-6273(00)80528-0) [DOI] [PubMed] [Google Scholar]
- 59.Rosato E, Trevisan A, Sandrelli F, Zordan M, Kyriacou CP, Costa R. 1997. Conceptual translation of timeless reveals alternative initiating methionines in Drosophila. Nucleic Acids Res. 25, 455–458 (doi:10.1093/nar/25.3.455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tauber E, et al. 2007. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316, 1895–1898 (doi:10.1126/science.1138412) [DOI] [PubMed] [Google Scholar]
- 61.Sandrelli F, et al. 2007. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science 316, 1898–1900 (doi:10.1126/science.1138426) [DOI] [PubMed] [Google Scholar]
- 62.Bünning E. 1936. Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber dtsch bot Ges 54, 590–607 [Google Scholar]
- 63.Sawa M, Nusinow DA, Kay SA, Imaizumi T. 2007. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318, 261–265 (doi:10.1126/science.1146994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathias D, Jacky L, Bradshaw WE, Holzapfel CM. 2007. Quantitative trait loci associated with photoperiodic response and stage of diapause in the pitcher-plant mosquito, Wyeomyia smithii. Genetics 176, 391–402 (doi:10.1534/genetics.106.068726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stehlik J, Zavodska R, Shimada K, Sauman I, Kostal V. 2008. Photoperiodic induction of diapause requires regulated transcription of timeless in the larval brain of Chymomyza costata. J. Biol. Rhythms 23, 129–139 (doi:10.1177/0748730407313364) [DOI] [PubMed] [Google Scholar]
- 66.Yamada H, Yamamoto MT. 2011. Association between circadian clock genes and diapause incidence in Drosophila triauraria. PLoS ONE 6, e27493 (doi:10.1371/journal.pone.0027493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han B, Denlinger DL. 2009. Length variation in a specific region of the period gene correlates with differences in pupal diapause incidence in the flesh fly, Sarcophaga bullata. J. Insect Physiol. 55, 415–418 (doi:10.1016/j.jinsphys.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 68.Han B, Denlinger DL. 2009. Mendelian inheritance of pupal diapause in the flesh fly, Sarcophaga bullata. J. Hered. 100, 251–255 (doi:10.1093/jhered/esn082) [DOI] [PubMed] [Google Scholar]
- 69.Ikeno T, Numata H, Goto SG. 2011. Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J. Insect Physiol. 57, 935–938 (doi:10.1016/j.jinsphys.2011.04.006) [DOI] [PubMed] [Google Scholar]
- 70.Ikeno T, Numata H, Goto SG. 2011. Photoperiodic response requires mammalian-type cryptochrome in the bean bug Riptortus pedestris. Biochem. Biophys. Res. Commun. 410, 394–397 (doi:10.1016/j.bbrc.2011.05.142) [DOI] [PubMed] [Google Scholar]
- 71.Ikeno T, Tanaka SI, Numata H, Goto SG. 2010. Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol. 8, 116 (doi:10.1186/1741-7007-8-116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saunders DS, Bertossa RC. 2011. Deciphering time measurement: the role of circadian ‘clock’ genes and formal experimentation in insect photoperiodism. J. Insect Physiol. 57, 557–566 (doi:10.1016/j.jinsphys.2011.01.013) [DOI] [PubMed] [Google Scholar]
- 73.Schiesari L, Kyriacou CP, Costa R. 2011. The hormonal and circadian basis for insect photoperiodic timing. FEBS Lett. 585, 1450–1460 (doi:10.1016/j.febslet.2011.02.026) [DOI] [PubMed] [Google Scholar]
- 74.Schmidt PS, Zhu CT, Das J, Batavia M, Yang L, Eanes WF. 2008. An amino acid polymorphism in the couch potato gene forms the basis for climatic adaptation in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 105, 16 207–16 211 (doi:10.1073/pnas.0805485105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams KD, Busto M, Suster ML, So AK, Ben-Shahar Y, Leevers SJ, Sokolowski MB. 2006. Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc. Natl Acad. Sci. USA 103, 15 911–15 915 (doi:10.1073/pnas.0604592103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paaby AB, Blacket MJ, Hoffmann AA, Schmidt PS. 2010. Identification of a candidate adaptive polymorphism for Drosophila life history by parallel independent clines on two continents. Mol. Ecol. 19, 760–774 (doi:10.1111/j.1365-294X.2009.04508.x) [DOI] [PubMed] [Google Scholar]
- 77.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. 2001. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110 (doi:10.1126/science.1057987) [DOI] [PubMed] [Google Scholar]
- 78.Partridge L, Alic N, Bjedov I, Piper MD. 2011. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 46, 376–381 (doi:10.1016/j.exger.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sim C, Denlinger DL. 2008. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl Acad. Sci. USA 105, 6777–6781 (doi:10.1073/pnas.0802067105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fidler AE, Gwinner E. 2003. Comparative analysis of avian BMAL1 and CLOCK protein sequences: a search for features associated with owl nocturnal behaviour. Comp. Biochem. Physiol. B 136, 861–874 (doi:10.1016/S1096-4959(03)00276-8) [DOI] [PubMed] [Google Scholar]
- 81.Johnsen A, et al. 2007. Avian clock gene polymorphism: evidence for a latitudinal cline in allele frequencies. Mol. Ecol. 16, 4867–4880 (doi:10.1111/j.1365-294X.2007.03552.x) [DOI] [PubMed] [Google Scholar]
- 82.Dor R, Cooper CB, Lovette IJ, Massoni V, Bulit F, Liljesthrom M, Winkler DW. 2012. Clock gene variation in Tachycineta swallows. Ecol. Evol. 2, 95–105 (doi:10.1002/ece3.73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dor R, et al. 2011. Low variation in the polymorphic clock gene poly-Q region despite population genetic structure across barn swallow (Hirundo rustica) populations. PLoS ONE 6, e28843 (doi:10.1371/journal.pone.0028843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O'Malley KG, Banks MA. 2008. A latitudinal cline in the Chinook salmon (Oncorhynchus tshawytscha) Clock gene: evidence for selection on PolyQ length variants. Proc. R. Soc. B 275, 2813–2821 (doi:10.1098/rspb.2008.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Malley KG, Ford MJ, Hard JJ. 2010. Clock polymorphism in Pacific salmon: evidence for variable selection along a latitudinal gradient. Proc. R. Soc. B 277, 3703–3714 (doi:10.1098/rspb.2010.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dardente H, Wyse C, Birnie M, Dupré S, Loudon A, Lincoln G, Hazlerigg D. 2010. A molecular switch for photoperiod responsiveness in mammals. Curr. Biol. 20, 2193–2198 (doi:10.1016/j.cub.2010.10.048) [DOI] [PubMed] [Google Scholar]
- 87.Krol E, Douglas A, Dardente H, Birnie MJ, Vinne V, Eijer WG, Gerkema MP, Hazlerigg DG, Hut RA. 2012. Strong pituitary and hypothalamic responses to photoperiod but not to 6-methoxy-2-benzoxazolinone in female common voles (Microtus arvalis). Gen. Comp. Endocrinol. 179, 289–295 (doi:10.1016/j.ygcen.2012.09.004) [DOI] [PubMed] [Google Scholar]
- 88.Masumoto KH, Ukai-Tadenuma M, Kasukawa T, Nagano M, Uno KD, Tsujino K, Horikawa K, Shigeyoshi Y, Ueda HR. 2010. Acute induction of Eya3 by late-night light stimulation triggers TSHß expression in photoperiodism. Curr. Biol. 20, 2199–2206 (doi:10.1016/j.cub.2010.11.038) [DOI] [PubMed] [Google Scholar]
- 89.Hut RA. 2011. Photoperiodism: shall EYA compare thee to a summer's day? Curr. Biol. 21, R22–R25 (doi:10.1016/j.cub.2010.11.060) [DOI] [PubMed] [Google Scholar]
- 90.Pittendrigh CS. 1993. Temporal organisation: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 55, 17–54 (doi:10.1146/annurev.ph.55.030193.000313) [DOI] [PubMed] [Google Scholar]
- 91.Saunders DS. 1997. Insect circadian rhythms and photoperiodism. Invert Neurosci. 3, 155–164 (doi:10.1007/BF02480370) [DOI] [PubMed] [Google Scholar]
- 92.Emerson KJ, Dake SJ, Bradshaw WE, Holzapfel CM. 2009. Evolution of photoperiodic time measurement is independent of the circadian clock in the pitcher-plant mosquito, Wyeomyia smithii. J. Comp. Physiol. A 195, 385–391 (doi:10.1007/s00359-009-0416-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Comas M, Beersma DG, Spoelstra K, Daan S. 2006. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J. Biol. Rhythms 21, 362–372 (doi:10.1177/0748730406292446) [DOI] [PubMed] [Google Scholar]
- 94.Wever RA. 1967. Zum Einfluss der Dämmerung auf die circadiane Periodik. Ztschr f verg Physiol. 55, 255–277 [Google Scholar]
- 95.Boulos Z, Macchi M, Terman M. 1996. Effects of twilights on circadian entrainment patterns and re-entrainment rates in squirrel monkeys. J. Comp. Physiol. A 179, 687–694 (doi:10.1007/BF00216132) [DOI] [PubMed] [Google Scholar]
- 96.Boulos Z, Macchi MM, Terman M. 2002. Twilights widen the range of photic entrainment in hamsters. J. Biol. Rhythms 17, 353–363 (doi:10.1177/074873002129002654) [DOI] [PubMed] [Google Scholar]
- 97.Boulos Z, Macchi MM. 2005. Season- and latitude-dependent effects of simulated twilights on circadian entrainment. J. Biol. Rhythms 20, 132–144 (doi:10.1177/0748730404272907) [DOI] [PubMed] [Google Scholar]
- 98.Comas M, Hut RA. 2009. Twilight and photoperiod affect behavioral entrainment in the house mouse (Mus musculus). J. Biol. Rhythms 24, 403–412 (doi:10.1177/0748730409343873) [DOI] [PubMed] [Google Scholar]
- 99.Baggs JE, Price TS, DiTacchio L, Panda S, FitzGerald GA, Hogenesch JB. 2009. Network features of the mammalian circadian clock. PLoS Biol. 7, e52 (doi:10.1371/journal.pbio.1000052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Brien C, Bradshaw WE, Holzapfel CM. 2011. Testing for causality in covarying traits: genes and latitude in a molecular world. Mol. Ecol. 20, 2471–2476 (doi:10.1111/j.1365-294X.2011.05133.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. 2001. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J. Biol. Rhythms 16, 100–104 (doi:10.1177/074873001129001791) [DOI] [PubMed] [Google Scholar]
- 102.Emerson KJ, Bradshaw WE, Holzapfel CM. 2009. Complications of complexity: integrating environmental, genetic and hormonal control of insect diapause. Trends Genet. 25, 217–225 (doi:10.1016/j.tig.2009.03.009) [DOI] [PubMed] [Google Scholar]



