Abstract
In 1942, Walls described the concept of a ‘nocturnal bottleneck’ in placental mammals, where these species could survive only by avoiding daytime activity during times in which dinosaurs were the dominant taxon. Walls based this concept of a longer episode of nocturnality in early eutherian mammals by comparing the visual systems of reptiles, birds and all three extant taxa of the mammalian lineage, namely the monotremes, marsupials (now included in the metatherians) and placentals (included in the eutherians). This review describes the status of what has become known as the nocturnal bottleneck hypothesis, giving an overview of the chronobiological patterns of activity. We review the ecological plausibility that the activity patterns of (early) eutherian mammals were restricted to the night, based on arguments relating to endothermia, energy balance, foraging and predation, taking into account recent palaeontological information. We also assess genes, relating to light detection (visual and non-visual systems) and the photolyase DNA protection system that were lost in the eutherian mammalian lineage. Our conclusion presently is that arguments in favour of the nocturnal bottleneck hypothesis in eutherians prevail.
Keywords: eutherian, bottleneck, activity, vision, photolyases, endothermia
A more likely view is that the placental mammals had an early history of strict nocturnality.
…what was the retina like in these strictly nocturnal ‘bottle-neck’ insectivores? [1, p. 687]
1. Introduction
A general view that early mammals were small insectivores, living on trees and being only night active, has become commonplace in textbooks, with some articles devoted specifically to vision [1,2], whereas others are more general in nature [3,4]. Menaker and co-workers formulated a specific ‘nocturnal bottleneck hypothesis’ [5–7], although some researchers have suggested the involvement of a period of mesopia (medium-light levels) in the evolution of eyes in response to light-restricted habitats [8], as observed in other species (e.g. birds; [9]). The main focus of this review is the ‘nocturnal bottleneck’ conjecture that was inspired by the fact that mammals have only one (retinal) photic input pathway to their circadian pacemaker system, whereas non-mammalian species have several parallel retinal and extra-retinal circadian photic input systems (e.g. hypothalamus, pineal gland, parietal eye). Further evidence to support the hypothesis arose from consistent findings in mammalian evolution, including the evolution of endothermia [3,10] and the adaptation of photosensory systems (e.g. the loss of photoreceptor pigments: [7,8]).
The nocturnal bottleneck hypothesis suggests that early eutherian mammals faced competition with diurnal reptiles (e.g. dinosaurs) during the Mesozoic era: [1,6,11,12]. Thought to be mainly ectothermic, these reptiles would have had to restrict their activity to the daytime because solar radiation was essential to increase their body temperature to operational levels [13]. Predation pressure and inter-species competition are thought to have stimulated the development of endothermia, a major adaptive change that enabled early mammals to become nocturnal and independent of solar radiation and environmental temperature. Nocturnal activity, in turn, might have led to dramatic changes in photoreception, including the loss of underused photoreceptor structures and ultraviolet (UV) protection mechanisms in the light-exposed tissues such as the skin and the eye. With the addition of interesting differences between the evolution of methaterians (including extant marsupials) and eutherians (including extant placentals) and recent palaeontological findings, we are forced to reconsider the evolutionary pressures that dictated the mammalian radiation. While it remains to be seen whether we will ever be able to reconstruct early predation patterns, new insights have been obtained concerning the evolution of endothermia [13]. Genetic analysis also offers comparative perspectives about changes in anatomy and physiology, which could shed new light on the bottleneck hypothesis. We will discuss these topics within the context of the existing literature and evaluate the overall validity of the nocturnal bottleneck hypothesis.
2. Mesozoic radiation of mammalian taxa: a palaeontological timeline
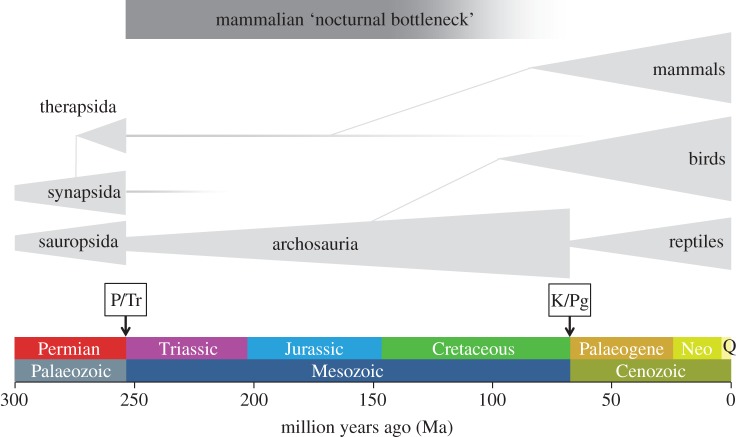
Which taxa should be included in the mammalian clade is the subject of a long and ongoing debate [14]. There is general agreement that this clade appeared in the Mesozoic era (figure 1), around 250 million years ago (Ma), after a massive extinction of plants and animals during the Permian–Triassic or P/Tr event: this catastrophe is described as a ‘greenhouse with lethal temperatures’ and is also known as the Great Dying [16]. The precise causes of this global warming are under debate, as are the causes of another mass extinction at the end of the Mesozoic, the Cretaceous–Palaeogene event (figure 1), 65.5 Ma (K/Pg, also known as the Cretaceous–Tertiary or K/T; e.g. see the discussion raised by [17]). The general climate of the Mesozoic era can be described as warm, with relatively small surfaces covered with ice, if at all [18]. Patterns in temperature and humidity will have correlated strongly with large geological events such as the split of the supercontinent Pangaea into Laurasia and Gondwana during mid-Mesozoic (about 190 Ma), followed by further fragmentation into the southern hemispheric continents (185–80 Ma). This process continued, resulting in the final separation of South America, Antarctic, Australia and the bridging of South and North America in the Cenozoic era. This era has become known as the era of the mammals, in contrast to the preceding Mesozoic era of the dinosaurs.
Figure 1.
Evolutionary timeline indicating the sequence of events concerning the mammalian nocturnal bottleneck. The black/grey box indicates the time frame of the nocturnal bottleneck that is the period when mammals and archosaurs co-existed. Grey lines and triangle shapes indicate species radiation and roughly indicate the number of species present [14,15]. Coloured bars along the timeline indicate geological time intervals (Q, Quaternary). P/Tr indicates the Permian/Triassic extinction event (‘the Great Dying’), K/Pg indicates the Cretaceous/Palaeogene extinction event.
Mammal-like reptiles survived the P/Tr event and gave rise to what Kemp [14] describes as the ‘Mesozoic mammals’. Their development over a long time span of about 145 myr is remarkable, with 10–20 major groups being discerned. All the Mesozoic mammalian groups showed characteristics of small, insectivorous animals [14], although a few exceptions do exist [19]. During the same era, at least 1000 terrestrial dinosaur species evolved in a large range of body sizes.
With the disappearance of the dinosaurs after the K/Pg event, the radiation of mammalian species exploded. For the eutherian mammals, the debate seems undecided whether the Early Cenozoic radiation of mammals was based on an earlier (short fuse model) or later (long fuse model) Mesozoic mammalian divergence (molecular-based analyses [20]). A third option would be an explosive model, based on morphological analyses of fossil and living mammals, suggesting a Cenozoic divergence [21]. In the latter view, all Mesozoic eutherian findings are placed outside the placental crown group. Based on molecular analyses, the Mesozoic eutherian (placental)/metatherian (marsupial) divergence has been placed at 170 Ma [22]: this date predates that suggested by palaeontological findings of an early metatherian mammal Sinodelphis in China (125 Ma; [23]), but is consistent with the discovery of an earliest eutherian species Juramaia (160 Ma, [24]) also from China. Marsupial radiation in the Mesozoic, observed in North America, was nearly eliminated in the K/T event, and Cenozoic radiation occurred mainly in South America and subsequently in Australia (oldest finding 55 Ma). Presently, the persisting crown group of Theria is composed of marsupials (about 265 species) and placentals (about 4400 living species). The position of the third (and earliest) persisting mammalian group of the monotremes (five living species, in three genera) is still very puzzling [14]; however, the finding of Steropodon in Australia (115 Ma; [25]) confirmed their general notion of ‘old’ mammals.
3. Ectothermia in mesozoic reptiles
At the basis of the nocturnal bottleneck hypothesis lies the idea that Mesozoic ectothermic reptiles (including dinosaurs) would be restricted to daytime activity at times when the sun can help to heat up their body to operating temperatures [13]. The general term ‘operating temperatures’ has been usually not only discussed in terms of speed of locomotion but could also potentially extend to digestive efficiency and speed, or even neurobiological function. For these reasons, it might be generally true that Mesozoic reptiles would be ectothermic and hence predominantly diurnal, but evidence is accumulating that some dinosaurs might have possessed partial endothermia based on several arguments (for review, see [26–30]). These arguments include (i) presence of insulating proto-feathers, (ii) fossils of breeding dinosaurs in cold regions, (iii) raised body posture, (iv) fibrolamellar bone structures, (v) presence of nasal turbulate bone structures, (vi) stable growth rate (isotope deposits in bones and teeth), (vii) possible ‘mass homeothermia’ or ‘inert homeothermia’ in large dinosaurs, (viii) the occurrence of small dinosaurs in cold regions and (ix) the existence of potential external thermoregulatory structures in some dinosaur species (e.g. dorsal boney plates in Stegosauria).
Some of the arguments favouring (partial) endothermia in dinosaurs can be debated, but we consider this mostly to be outside of the scope of this paper. One thing may be important to discuss here: the loss of uncoupling protein 1 (UCP1) in Sauropsida (birds and reptiles) [31–35]. UCP1 uncouples the mitochondrial respiratory chain, leading to the production of heat instead of adenosine triphosphate (ATP), the general cellular energy carrier. UCP1 can therefore be seen as one of the components of endothermia. All tetrapods (mammals, birds, reptiles and amphibians) have UCPs, but mitochondrial uncoupling capacity has been confirmed only in UCP1 [31]. Interestingly, the Ucp1 gene was already present in teleost and amphibia and although its precise uncoupling function remains elusive in these species, its conserved state has been dated back to 420 Ma [33,34]. This may lead to the speculation that the potential thermogenic capacity of UCP1 might have been present in a common ancestor of all tetrapods and hence may represent a widespread feature. The loss of this gene in Sauropsida is therefore likely to represent a lack of positive selection pressure for mitochondrial heat generation. This indicates a ‘diurnal bottleneck’ in Sauropsida, with the possibly that their diurnal lifestyle, combined with other forms of heat maintenance, might have reduced the selection pressure for mitochondrial non-shivering thermogenesis. During the Mesozoic, therapod dinosaurs probably regained this feature of mitochondrial uncoupling using the action of adenine nucleotide transferase for non-shivering thermogenesis in the muscles [35]. This saurischian lineage (‘lizard hip’ dinosaurs) included smaller carnivorous proto-feathered and feathered dinosaurs that were probably fast-moving predators (e.g. Velociraptor). This lifestyle probably selected for less dependence on environmental temperatures and saurischians are now generally believed to have had partial endothermia, allowing them to incubate their eggs and inhabit colder regions. This secondary evolutionary step might have been critical in the anatomical development of the musculature of the hind legs for breeding and eventually opened the gate for the evolution of avian flight, which depends primarily on the fore limbs [36]. This evolutionary path, however, does not explain why modern birds generally have a diurnal lifestyle even though they are capable of full endothermic heat production. Flight at night is intrinsically more difficult owing to the significantly lower levels of light; however, highly enhanced scotopic vision (e.g. owls), or other senses (e.g. echolocation in bats), may negate such disadvantages. In this respect, it may be hypothesized that the complex sexual selection mechanisms that have arisen through diverse and vivid feather coloration may be the main drive for a diurnal lifestyle where an acute colour visual sensory system would be an advantage. This, however, will not explain the conservation of avian endothermia and suggests an endothermic advantage in addition to the advantageous capability of being active at night when ambient temperatures are low.
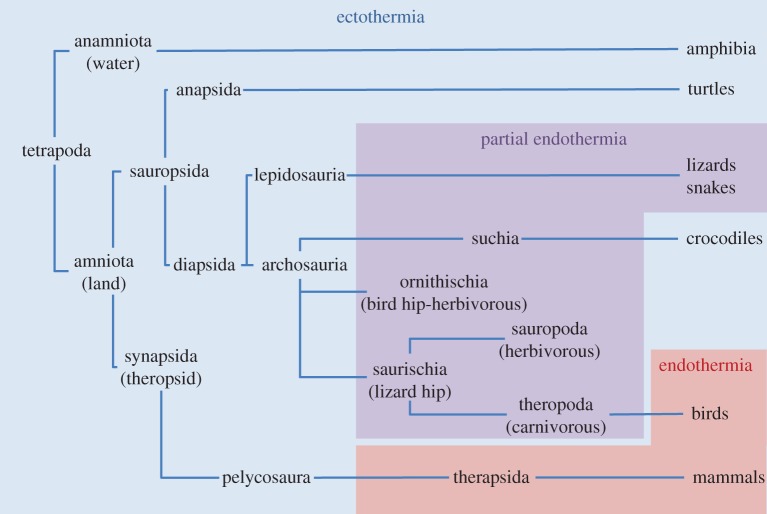
Taken together, it can be concluded that forms of partial endothermia most likely evolved several times during the Mesozoic era in dinosaurs and other reptiles (figure 2). In clades of archosauria (e.g. suchia, ornithischia, saurischia), primitive endothermia likely led to more mature forms of endothermia. It has been suggested that endothermic capacity was lost through secondary evolution in crocodiles [37], whereas evolution of endothermia in therapod lineages eventually led to full endothermia in birds. Independently, parallel evolution in Synapsida led to partial endothermia in therapsid reptiles (250 Ma, Late Permian) and full endothermia in mammals (figure 2).
Figure 2.
Simplified phylogenetic tree of Tetrapoda in which the (inferred) occurrence of ectothermia (blue), partial endothermia (purple) and full endothermia (red) are indicated (based on [26–30,37]).
An important argument for the need of elevated body temperatures in (Mesozoic) reptiles is behavioural regulation of body temperature (selection of warmer environments; sun basking) in extant reptiles. This argument would plea for the existence of a nocturnal bottleneck as it, indeed, ties activity of reptiles to the diurnal niche where warm environments can be selected.
4. Nocturnality as the dominant temporal niche in modern mammals
The Mesozoic is most known for its diversification of reptilian life (the reign of the Archosaurs); however, it is important to note here that, according to some analyses, diversification of mammals (but not Rodentia) might have taken place shortly before the end of the Mesozoic era (250–65.5 Ma: [38]). Based on that assumption, one might expect that most extant mammalian classes may share common features, which originated through directional selection forces that were present during the Mesozoic era when endothermic mammals shared their habitat with ectothermic/partial endothermic dinosaurs.
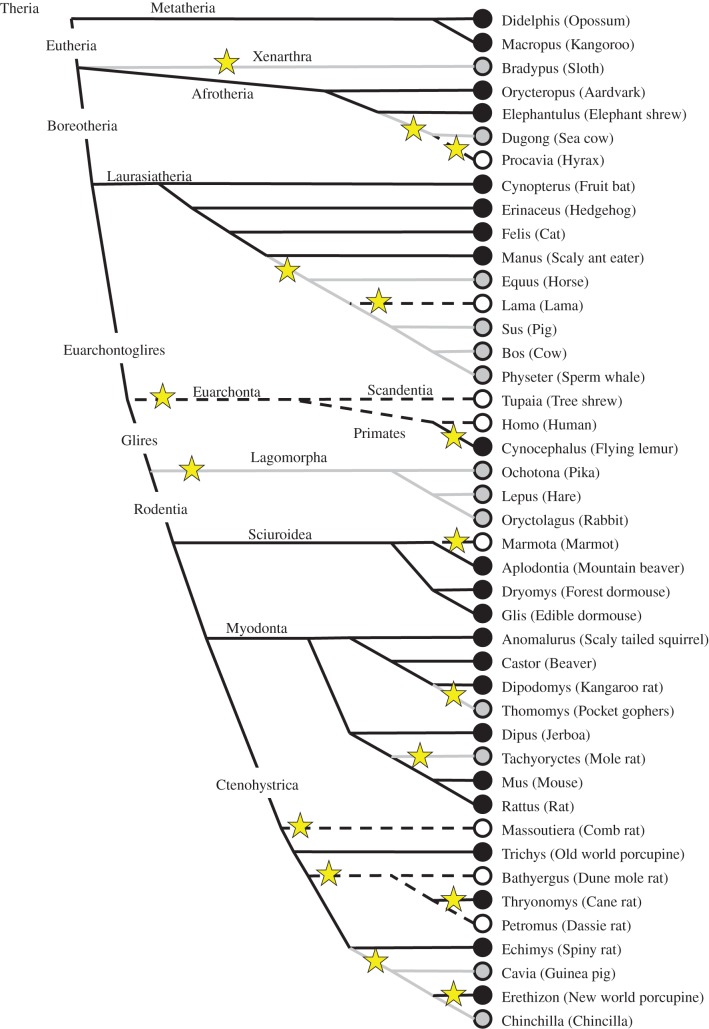
In a large comparative study, Roll et al. [39] divided 700 species into classes of dominant temporal activity niche: nocturnal, diurnal or both (mostly crepuscular). They used an existing phylogenetic construction [38] to reconstruct the evolution of diurnality in rodents, which are thought to have diversified after the K/T boundary [38]. The reconstruction of temporal niche usage within rodents led to the conclusion that rodents shared a nocturnal ancestor that existed before the K/T boundary. Subsequently, diurnality evolved through secondary evolution at least seven times independently in Rodentia [39].
Using the molecular phylogenetic construction of Huchon et al. [38], we constructed the temporal niche phylogeny within the full therian subclass (figure 3) using the nocturnal, diurnal and crepuscular/arrhythmic classification of Roll et al. [39]. The most parsimonious interpretation leads to a minimum of 16 changes of dominant temporal niche (figure 3). Most subdivisions of Theria are indeed nocturnal, but many changes and reversions occurred, and different interpretations may be possible, especially when higher-quality data on activity patterns in more species become available.
Figure 3.
Phylogenetic reconstruction of dominant temporal niche usage among therian mammals following the molecular phylogeny of Huchon et al. [38]. Symbols indicate the three classifications that were used: nocturnal (black circle, black line), diurnal (open circle, dashed line) and both (crepuscuplar, cathemeral or arrhythmic; grey circle, grey line). Yellow stars indicate phylogenetic changes in temporal niche usage. (Temporal niche data modified after [12,39,50].)
5. Evolution of mammalian endothermia
In mammals, the presence of UCP1, with mitochondrial uncoupling capacity, led to the development of specialized thermogenic, UCP1-expressing, brown adipose tissue (BAT) during the Mesozoic era [31]. BAT has been well studied for its role in thermogenesis during arousal from hibernation and in homeothermic mammals it is stimulated through cold exposure. Interestingly, mammalian thermogenesis in BAT parallels avian thermogenesis in skeletal muscle because both tissues derive from myogenic precursor cells [40]. Uncoupling can therefore be seen as one of the (facultative) features of endothermia to quickly generate heat in extreme situations. It cannot be seen as a general feature of endotherms, because birds have UCP1-independent non-shivering thermogenesis in skeletal muscle instead of BAT [34,35].
The hallmark of heat generation in endotherms is the leakiness of the cellular plasma membrane for ions. The inward flux of sodium is greater in endotherms than in ectotherms, and the sodium pumps therefore need to perform at a higher rate in endothermic cells. The continuous flux of sodium across the membrane will generate heat in every cell of the endothermic body. This leaky membrane hypothesis was mainly proposed by Else & Hulbert [41,42], Else et al. [43] and Hulbert & Else [44], and ingenious experiments in sodium/potassium pump transplantations between mammalian and reptilian membranes showed the interaction of the sodium/potassium pump with the constituents of the plasma membrane in endotherms and ectotherms [42,45].
Apart from differences in plasma membrane ion leak in endotherms, the mitochondrial membrane of endotherms seems to be leaky for protons. This parallels the function of UCP1 and also leads to heat production and less efficient oxidation of nutrient substrates (e.g. glucose) to ATP in the mitochondria [46,47]. As such, the mitochondria in endotherms have approximately five to seven times higher respiration rates compared with similarly sized ectotherms, but much of this higher respiration is translated into heat production (approx. 30%) instead of ATP production [46,47].
In general, metabolism in mammals is regulated through thyroid hormones. Thyroid hormone production in the thyroid gland consists mainly (approx. 80%) of its inactive form (thyroxine, T4). Deiodinases (DIO1 and DIO2) convert inactive T4 into its active form triiodothyronine (T3) locally not only in peripheral tissues but also in the brain. Owing to differences in half-life, T4 levels in plasma are about 40 times higher than T3 levels. Hence, local T3 conversion through DIO1/2 is critical for the regulation of tissue-specific metabolic rates. T3 binds to the nuclear thyroid receptor which, when bound to its ligand, acts as a transcription factor and enhances protein turnover rates of almost all cells in the body. Interestingly, T3 specifically induces production of the sodium/potassium ATPase, the sodium pump that is necessary to restore ion balance over the leaky plasma membranes of the endothermic cell. Hence, thyroid hormone action and leaky membranes in endotherms can be considered to be two sides of the same mammalian endothermic coin and might therefore indicate synergetic evolution.
6. Impact of diurnality on anatomy and physiology in mesozoic reptiles
Of all anatomical features present in animals, the eyes are likely to be adapted to the amount of light to which species are exposed. Therefore, eye anatomy may be an excellent indicator of diurnality or nocturnality [1,12,48–54]. In extant species, a range of adaptations can be measured, such as the short-wavelength-filtering properties of the lens, rod/cone ratios and the retinal irradiance factor (pupil surface area/retinal surface area [12,55,56]. The retinal irradiance factor can be applied in a ‘camera type’ eye, where it describes how much the photon flux through the pupil is spread out over the retinal surface. Animals typically reduce the size of the pupil when exposed to bright light and hence have a low retinal irradiance factor compared with the situation in darkness. This pupil constriction reflex will accommodate higher visual acuity at daytime and protect the retinal photoreceptors from damaging extremes of light intensities, whereas the pupil dilation reflex will allow for higher light penetration onto the retina (increased sensitivity) at the cost of visual acuity. As a result, diurnal animals have evolved large retinae and small pupils (low retinal irradiance factor) compared with nocturnal animals that have smaller retinae and large pupils (hence a high retinal irradiance factor).
In archosaurs, it is possible to approximate such a retinal irradiance factor. Archosaurs possess bony plates in their eyes (scleral ring) surrounding the iris. From these fossilized bony remains, the inner diameter can be obtained as a measure that relates to maximal pupil size, whereas eye diameter, as a proxy for retinal surface, can be obtained from orbital bones ([57–59]; but see also [54]). Interpreting the measures of Schmitz & Motani [59], most herbivorous dinosaurs (ornitischia and Sauropoda) might have had mesopic eyes and could thus be adapted to a diurnal lifestyle as their dominant temporal niche (with less need for high visual acuity compared with predators). Photopic eyes are mainly found in flying dinosaurs (mostly avian theropods but also in Pterosaura). Scotopic dinosaur eyes can mostly be found in carnivorous theropods (but also in Pterosaura), which were apparently more adapted to invade the nocturnal niche. Analyses of more primitive (basal) eyes in the saurischian (Herrerasaurus) and archosaur (Protherosuchus) lineages suggest they were mesopic. Thus, one might conclude that the basal eye shape in primitive archosaurs was mesopic, whereas high acuity eyes (photopic) developed mostly in flying dinosaurs. By contrast, it appears that scotopic eyes evolved later in the Mesozoic era, specifically in predatory theropods. It is therefore conceivable that the early mammals developed nocturnality as a mechanism to minimize predation. Concomitantly, many predatory saurischia may have invaded the nocturnal niche to maximize their food supply partly by predating on these nocturnal mammals. Because the mammalian radiation started before the K/T boundary, it is conceivable that an arms race between sauria and mammals for temporal niche occupation was already underway before the end of both the Mesozoic era and the reign of the dinosaurs.
7. Loss of visual and extra-ocular photoreception and photoreceptor diversity in mammals
The nocturnal bottleneck hypothesis was initially derived from correlating morphological differences of cones and rods in extant species of squamate reptiles and mammals with their ecological niches. Based on this work, Walls proposed his transmutation theory to suggest that some species adapted from diurnality to nocturnality, followed by a reversion to a diurnal lifestyle in many cases [1,48].
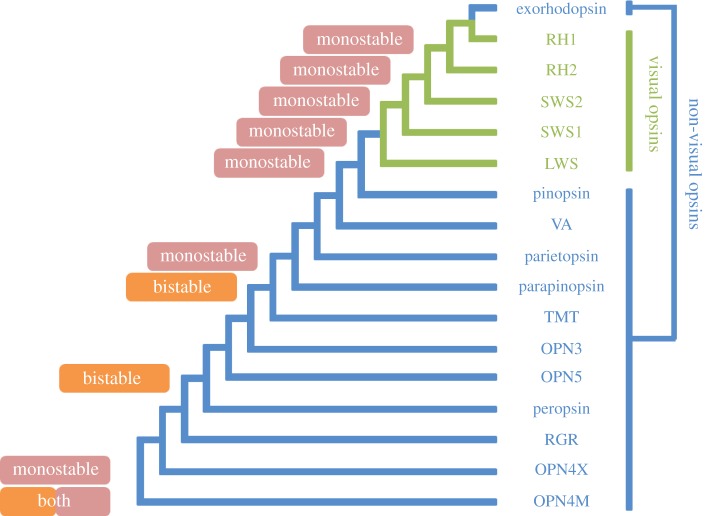
Housed within cone and rod photoreceptors are photopigments that consist of a protein (opsin) linked to a vitamin A-derived light-sensitive retinal chromophore. Visual pigments are classified into five subtypes: a single rod (RH1) opsin and four cone classes (SWS1, SWS2, RH2 and LWS) that are maximally sensitive (known as λmax) to wavelengths ranging from UV to near-red [8,60]; figure 4). Even though it is probable that all five visual pigment genes were present in the reptilian-like mammalian ancestor, a drastic reduction in the number of cone opsin genes occurred with the eutherian radiation [8] (figure 5), a change that appeared to affect all subsequent mammals. First, the RH2 gene was lost, leaving only three other types of cone pigment (trichromacy) in the ancestor to all mammals [61], although the SWS1 gene has recently become non-functional in the monotremes: both the platypus and the echidna possess, therefore, dichromatic colour visual systems [61,62]. This was subsequently followed by the loss of the SWS2 gene, rendering most marsupials (but see [63]) and eutherians as dichromats, with the retinal expression of SWS1 and LWS genes only [8] (figure 5). Thus, it would appear that the loss of cone pigment genes suggests that the visual systems in early mammals experienced lower (scotopic) light intensities.
Figure 4.
A phylogenetic tree showing the evolution of known visual (green) and non-visual (blue) opsin classes in gnathostome vertebrates. The nature of chromophore handling is highlighted, where determined: monostable, the ability of a photopigment to form a stable interaction with either 11-cis retinal or all-trans retinal in a light-dependent manner (pink); bistable, the ability of a photopigment to form stable interactions with both 11-cis retinal and all-trans retinal and the photo-conversion (isomerization) between them (orange). LWS, long-wavelength-sensitive opsin; OPN3, panopsin/encephalopsin; OPN5, neuropsin; OPN4M, mammalian-like melanopsin; OPN4X, xenopus-like melanopsin; RGR, retinal G-protein-coupled receptor; RH1, middle-wavelength-sensitive rhodopsin 1 (rod); RH2, middle-wavelength-sensitive rhodopsin 2 (cone); SWS1, short-wavelength-sensitive opsin 1; SWS2, short-wavelength-sensitive opsin 2; TMT, teleost multiple tissue opsin; VA, vertebrate ancient opsin; pinopsin, pineal gland-specific opsin; parietopsin, parietopsin-expressing opsin; parapinopsin, parapineal gland-expressing opsin; peropsin, retinal pigment epithelial (RPE)-specific rhodopsin homologue (RRH).
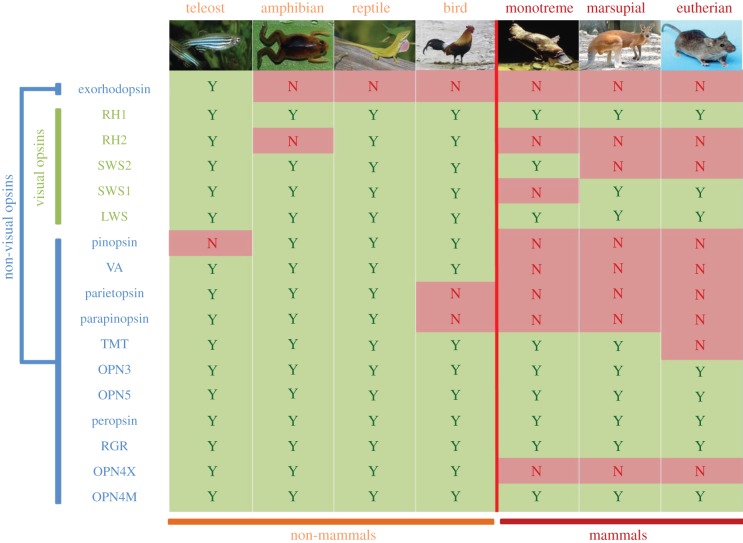
Figure 5.
A summary table showing the presence of opsin orthologues in modern representatives of the main vertebrate classes from bony fishes to mammals. The presence (yes, Y) or absence (no, N) of a particular opsin class is shown, with a vertical red line indicating the reptilian–mammalian boundary. The significant loss of opsin genes during this timeline forms part of the nocturnal bottleneck hypothesis. OPN4M, mammalian-like melanopsin; OPN4X, xenopus-like melanopsin; RGR, retinal G-protein-coupled receptor; peropsin, retinal pigment epithelial (RPE)-specific rhodopsin homologue (RRH); OPN5, neuropsin; OPN3, panopsin/encephalopsin; TMT, teleost multiple tissue opsin; parapinopsin, parapineal gland-expressing opsin; parietopsin, parietopsin-expressing opsin; VA, vertebrate ancient opsin; pinopsin, pineal gland-specific opsin; LWS, long-wavelength-sensitive opsin; SWS1, short-wavelength-sensitive opsin 1; SWS2, short-wavelength-sensitive opsin 2; RH2, middle-wavelength-sensitive rhodopsin 2 (cone); RH1, middle-wavelength-sensitive rhodopsin 1 (rod); exorhodopsin, extra-retinal rod-like opsin.
Although the changes in colour vision throughout the mammalian radiation are regarded as one of the most well-characterized examples of neuroecological adaptation, it is becoming clear that the nocturnal bottleneck also played an important role in shaping non-visual photoreception. Like visual pigments, non-visual pigments also consist of an opsin linked to a retinal chromophore. Their sheer number (figure 4) and distribution, and that of the photoreceptors that contain them (in non-mammalian vertebrates, at least), however, is generally broader, involving not only the eye but many extra-retinal tissues as well, such as the pineal, parapineal and parietal glands, the hypothalamus and the skin [64].
Ocular non-visual photoreception occurs in photosensitive retinal ganglion cells (pRGCs) [65]. Consisting of a subset of the total number of RGCs, pRGCs express the bistable melanopsin (OPN4) photopigment, although this opsin class is not limited to this retinal layer in non-mammalian species [66] and may exist in both bistable and monostable forms [67,68] (figure 4). In mammals, melanopsin-expressing pRGCs are responsible for photoentrainment of circadian rhythms, as well as regulating other responses [66], such as pupillary constriction [69], melatonin suppression [70], sleep induction [71] and contributions to the visual system (e.g. tetrachromacy in the peripheral human retina) [72]. Early in vertebrate evolution [68], the melanopsin gene duplicated to yield two distinct lineages, OPNX and OPN4M [73]. Although both gene orthologues are present in non-mammalian vertebrates, OPN4X was lost early in the mammalian radiation to leave only the OPN4M variant [66,73] (figure 5). It is still not fully understood why the OPN4M gene survived in all extant vertebrates so far studied instead of the OPN4X gene. As there appears to be a correlation between the broad expression of the OPN4X gene and the widespread distribution of non-visual photoreceptors in non-mammals, and the loss of both arms of the irradiance detection system to leave the restricted expression of the OPN4M gene in pRGCs only, it is possible that OPN4X-encoded photopigments detect light for the regulation of other physiological responses (e.g. dark photokinesis [74], changes to body pigmentation [75]) that may not be solely involved in regulating specific ocular-dependent circadian rhythms, for example, sleep or pupil constriction. Given the interplay or neural crosstalk that has been discovered between the parallel systems of colour vision (mediated by cones) and circadian photoentrainment (controlled by OPN4M) [76] and the overlap between the spectral sensitivity of melanopsin (λmax ∼ 480 nm) and those of the cone classes lost during the nocturnal bottleneck, namely SWS2 (λmax = 400–470 nm) and RH2 (λmax = 480–530 nm) [8], it has recently been suggested that redundancy between these spectrally similar pigments may have been the main reason for the loss of two mammalian cone classes and the maintenance of a melanopsin variant under mesopic conditions where both rods and cones are generally active [8]. Furthermore, it has been hypothesized that the nocturnal bottleneck was preceded by an elongated ‘mesopic bottleneck’ [8], a conjecture that is consistent with the adaptive changes in opsin gene complement determined in the early mammalian radiation [60,67] and the order in which the cone classes were sequentially lost [8].
Another key example is vertebrate ancient (VA) opsin (figure 4). Discovered originally in teleosts [77], this photopigment is also found in many vertebrate classes [66] (figure 5). Although the function of VA opsin in non-avian species is unknown, there is strong evidence to suggest that this pigment plays a critical role in regulating seasonal breeding in birds via direct hypothalamic photosensitive cells located deep in the brain [78,79]. The correlative loss of both VA opsin [66] (figure 5) and deep-brain opsin-based photoreception in mammals [80,81] not only strengthens the argument for the role of VA opsin in the photoperiodic response of non-mammals but also supports the evolutionary significance of the mesopic/nocturnal bottlenecks.
In addition to OPN4 and VA, many other non-visual opsin classes exist in vertebrates and their phylogenetic relationships are illustrated in figure 4. In the majority of cases, the functional roles of these novel opsins remain to be determined. However, it is interesting to note that some of these genes are conserved in the mammalian lineage (i.e. panopsin (OPN3), neuropsin (OPN5), retinal pigment epithelium (RPE)-specific rhodopsin homologue (RRH; peropsin), retinal G protein-coupled receptor (RGR) and teleost multiple tissue (TMT) opsin, although the latter was subsequently lost in the eutherian mammals), whereas others were not (i.e. pineal opsin (P-opsin), parapinopsin, parietopsin and exorhodopsin; figure 5). By comparing the complement of photopigments present throughout the vertebrate lineage, it is clear that many of the gene loss events occurred with the expansion of mammal diversity and, as such, an epoch of prolonged light restriction may have served to condense the repertoire of both visual and non-visual pigments as their roles became superfluous for survival.
Coupled to other ocular adaptations such as the development of larger eyes, the enlargement of the cornea [49] and pupil [82], a high rod : cone ratio [83], the presence of a tapetum lucidum [84], convergent orbits, larger binocular fields [52], as well as the recently discovered marker of an inverted nuclear architecture in rods of nocturnal species acting as a converging optical lens [85], there is overwhelming evidence to support the argument for a significant reduction in the quality of light at the end of the Mesozoic era, most likely through an extended period of mesopia/nocturnality. As a result, other senses, including improved olfactory sensitivity [86], high-frequency hearing [87] and the development of tactile vibrissae (whiskers) [88], became more acute.
8. Loss of photoprotective mechanisms in mammals
Highly energetic short-wavelength radiation such as UV light is only present in high quantities around noon [55]. Diurnal animals are therefore in need of mechanisms to protect their photoreceptors and their molecular components from photo-oxidative damage caused by high energetic radiation. Maintaining such protective mechanisms is costly and can therefore be expected to face selection pressure. In discussing the evolution of mammalian nocturnality, it therefore deserves attention.
The flavoprotein-based photolyases repair UV-induced DNA damage using visible light in a wide spectrum of organisms [89]. Among the vertebrates, however, this ‘epitome of an error-free process’ [90] could not be shown in eutherian mammals. Painter [90] assumed a loss of this photolyase function after the split of marsupials and eutherian mammals, as deduced from the absence of cyclobutane pyridine dimer (CPD) photolyase activity in man and mice and the presence of such photolyase enzymatic activity systems in the marsupial rat kangaroo Potorous tridactylis [91]. Subsequently, Yasui et al. [92] cloned the CPD photolysase gene in cell-lines from the same marsupial species. Mice overexpressing the marsupial CPD showed improved resistance to UV cellular damage [93]. The P. tridactylis CPD photolyase could even rescue circadian clock function in fibroblasts and liver cells of cryptochrome-deficient mice, underlining a functional connection between the structurally related cryptochromes and photolyases [94]. The supposed difference in photolyase presence between marsupials and eutherians waits for further confirmation in other marsupial species, but would align with an increasing evidence of day active behaviour in marsupials in relation to trichromatic colour vision [63].
Nocturnal mammals have lost the need for protecting their retina from cellular damage by UV light [95,96]. The eye lens of most mammalian species has high transmission for UV light and this feature has been associated with a nocturnal lifestyle [12]. In several species of Sciuridae (squirrels), the lens was found to filter out short-wavelength light (below approx. 450 nm, including UV and blue light) owing to a yellow/orange coloration as observed by the human eye (see images in [55]). The lens coloration is formed by an interaction of crystalline proteins with short-wavelength-filtering compounds, functioning as a low-frequency pass cut-off filter with undisturbed transmission at lower wavelengths. In humans, these compounds are kynurenine (KN) related, including kynurenine; 3-hydroxykynurenine (3OHKN); 3-hydroxykynurenine O-β-d-glucoside (3OHKG); 4-(2-aminophenyl)-4-oxobutanoic acid (AHA); and glutathionyl-kynurenine (GSH-KN; [97]), and all derived from tryptophan [98,99]. Interestingly, the biochemical pathway leading to 3OHKG production in the eye was also found in some (but not all) teleost fishes [100], leading to the conclusion that this photochemical protection mechanism evolved relatively early in vertebrate life (within the gnathostomes) and might have been permissive for the evolutionary radiation of terrestrial vertebrates. Marsupials and eutherian mammals share the same form of a critical enzyme indoleamine 2,3-dioxygenase involved in the tryptophan–kynurenine catabolic pathway, indicating that selection maintained this enzyme throughout mammalian evolution [101]. Interestingly, birds use UV vision for foraging and recognizing plumage coloration and may possess alternative retinal protection mechanisms that do not interfere with UV vision capabilities. This possibility is discussed in the section below.
Nocturnal mammals seem to lack kynurenine-based filtering compounds in their lenses, which allows UV light to penetrate their lenses. In addition, the short-wavelength cone (expressing the SWS1 gene) in many nocturnal mammals is maximally sensitive to UV light (approx. 360 nm), which collectively suggests that UV vision in important in nocturnal mammals [102–104]. This poses an interesting paradox: there seems to be no need for the evolutionary development of retinal UV protection in nocturnal mammals, but UV cone-based vision seems to be used at night when UV light is not present at sufficient quantities to drive photopic vision. This paradox hints towards a more flexible lifestyle in nocturnal mammals and our classification of nocturnal and diurnal species may be too rigid for many ‘nocturnal’ mammalian species. This changeable temporal lifestyle (temporal niche switching) was indeed confirmed in several chronobiological studies in nocturnal mammals [12] and will be discussed in detail below.
A classical case of flexible temporal niche switching was described in the golden spiny mouse (Acomys russatus), which becomes diurnal when its competitor, the common spiny mouse (Acomys cahirinus), is present [105]. An interesting difference was found between these species in that the aqueous humour of the golden spiny mouse eye contained high concentrations of ascorbic acid, a UV-absorbing compound [106]. In general, high levels of ascorbic acid are found in diurnal mammals when compared with nocturnal mammals [95,96,106–108]. Thus, nocturnal mammals may have more plasticity in temporal niche usage than expected [12] and their occasional daytime activity may have ecological benefits, including the use of UV vision, while their retina may be protected for UV damage through high levels of ascorbic acid.
In this respect, it is interesting to discuss UV vision in birds. Although the avian ancestor and many modern clades that comprise the Aves possess cones that are maximally sensitive to violet wavelengths, a few bird species (e.g. a subset of Passeriformes, Psittaciformes, Trogoniformes, Ciconiiformes and Struthioniformes) have reinvented UV vision [8] and indeed lack UV filtering properties of their lens. However, birds also seem to lack high levels of ascorbic levels to protect their retina from UV damage [95,107]. Birds may make use of another compound from the nitrogen catabolic pathway: uric acid, which was also found to have high UV-absorbing properties [107]. Future studies should identify the levels of uric acids in birds and lizards as a possible identifier of ocular adaptation to a diurnal lifestyle.
9. Concluding remarks
Evidence has accumulated that the visual and non-visual systems of photoreception in eutherian mammals in the Mesozoic era showed changes characteristic for a nocturnal lifestyle. The specific, and permanent loss of UV protection (e.g. by photolyases) is an additional argument for an initial restricted exposure to solar radiation in eutherian animals. Endothermy, although not limited to Mesozoic mammals, allowed an active use of the night, in combination with a small body size. Uncertainties in the radiation of eutherian species within or after the Mesozoic era are manifold and questions remain with regard to later developments of regaining diurnal function in behaviour and colour vision. The palaeontological search will continue and reveal new insights, as was the case in the past decade. Further comparative analysis, implicating other tetrapod taxa such as reptiles and birds, and above all marsupial mammals, will be rewarding to further explore the impact of what we see as the very probable occurrence of a Mesozoic nocturnal bottleneck of eutherian mammals.
References
- 1.Walls GL. 1942. The vertebrate eye and its adaptive radiation. Bloomfield Hills, MI: Cranbrook Institute of Science [Google Scholar]
- 2.Schwab IR. 2012. Evolutions witness: how eyes evolved. New York, NY: Oxford University Press [Google Scholar]
- 3.Young JZ. 1962. The life of vertebrates, 2nd edn pp. 569 New York, NY: Oxford University Press [Google Scholar]
- 4.McFarland WN, Pough FH, Cade TJ, Heiser JB. 1985. Vertebrate Life, 2nd edn New York, NY: Macmillan [Google Scholar]
- 5.Menaker M, Tosini G. 1996. The evolution of vertebrate circadian systems. In Sixth Sapporo Symp. on Biological Rhythms: circadian organization and oscillatory coupling (eds Honma K, Honma S.) pp. 39–52 Sapporo, Japan: Hokkaido University Press [Google Scholar]
- 6.Menaker M, Moreira LF, Tosini G. 1997. Evolution of circadian organization in vertebrates. Brazil J. Med. Biol. Res. 30, 305–313 (doi:10.1590/S0100-879X1997000300003) [DOI] [PubMed] [Google Scholar]
- 7.Foster RG, Menaker M. 1993. Circadian photoreception in mammals and other vertebrates. In Light and biological rhythms in man (ed. Wetterberg L.), pp. 73–91 Oxford, UK: Pergamon Press [Google Scholar]
- 8.Davies WI, Collin SP, Hunt DM. 2012. Molecular ecology and adaptation of visual photopigments in craniates. Mol. Ecol. 21, 3121–3158 (doi:10.1111/j.1365-294X.2012.05617.x) [DOI] [PubMed] [Google Scholar]
- 9.Martin GR. 1982. An owl's eye: schematic optics and visual performance in Strix aluco. J. Comp. Physiol. 145, 341–349 (doi:10.1007/BF00619338) [Google Scholar]
- 10.Crompton AW, Taylor CR, Jagger JA. 1978. Evolution of homeothermy in mammals. Nature 272, 333–336 (doi:10.1038/272333a0) [DOI] [PubMed] [Google Scholar]
- 11.Crompton AW. 1980. Biology of the earliest mammals. In Comparative physiology: primitive mammals (eds Schmidt-Nielsen K, Bolis L, Taylor CR.), pp. 1–12 New York, NY: Cambridge University Press [Google Scholar]
- 12.Hut RA, Kronfeld-Schor N, Van der Vinne V, De la Iglesia HO. 2012. In search of a temporal niche: environmental factors. Prog. Brain Res. 199, 281–304 (doi:10.1016/B978-0-444-59427-3.00017-4) [DOI] [PubMed] [Google Scholar]
- 13.Clark A, Pörtner H-O. 2010. Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 85, 703–727 (doi:10.1111/j.1469-185X.2010.00122.x) [DOI] [PubMed] [Google Scholar]
- 14.Kemp TS. 2005. The origin and evolution of mammals. Oxford, UK: Oxford University Press [Google Scholar]
- 15.dos Reis M, Noue J, Hasegawa M, Asher RJ, Donoghue PC, Yang Z. 2012. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc. R. Soc. B 279, 3491–3501 (doi:10.1098/rspb.2012.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Joachimski MM, Wighall PB, Yan C, Chen Y, Jiang H, Wang L, Lai X. 2012. Lethally hot temperatures during the early Triassic greenhouse. Science 338, 366–370 (doi:10.1126/science.1224126) [DOI] [PubMed] [Google Scholar]
- 17.Schulte P, et al. 2010. The Chicxulub asteroid impact and mass extinction at the Cretaceous–Paleogene boundary. Science 327, 1214–1218 (doi:10.1126/science.1177265) [DOI] [PubMed] [Google Scholar]
- 18.Price GD. 1999. The evidence and implications of polar ice during the Mesozoic. Earth Sci. Rev. 48, 183–210 (doi:10.1016/S0012-8252(99)00048-3) [Google Scholar]
- 19.Hu Y, Meng J, Wang Y, Li C. 2005. Large Mesozoic mammals fed on young dinosaurs. Nature 433, 149–152 (doi:10.1038/nature03102) [DOI] [PubMed] [Google Scholar]
- 20.Bininda-Edmunds ORP, et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 21.O'Leary MA, et al. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339, 662–667 (doi:10.1126/science.1229237) [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Hedges SB. 1998. A molecular timescale for vertebrate evolution. Nature 392, 917–920 (doi:10.1038/31927) [DOI] [PubMed] [Google Scholar]
- 23.Luo ZX, Ji Q, Wible JR, Yuan CX. 2003. An early Cretaceous tribosphenic mammal and etatherian evolution. Science 302, 1934–1940 (doi:10.1126/science.1090718) [DOI] [PubMed] [Google Scholar]
- 24.Luo ZX, Yuan CX, Meng QJ, Ji Q. 2011. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476, 442–445 (doi:10.1038/nature10291) [DOI] [PubMed] [Google Scholar]
- 25.Archer M, Flannery TF, Ritchie A, Molnar RE. 1985. First Mesozoic mammal from Australia, an early Cretaceous monotreme. Nature 318, 363–366 (doi:10.1038/318363a0) [Google Scholar]
- 26.Nespolo RF, Bacigalupe LD, Figueroa CC, Koteja P, Opazo JC. 2011. Using new tools to solve an old problem: the evolution of endothermy in vertebrates. Trends Ecol. Evol. 26, 414–423 (doi:10.1016/j.tree.2011.04.004) [DOI] [PubMed] [Google Scholar]
- 27.Grigg GC, Beard LA, Augee ML. 2004. The evolution of endothermy and its diversity in mammals and birds. Physiol. Biochem. Zool. 77, 982–997 (doi:10.1086/425188) [DOI] [PubMed] [Google Scholar]
- 28.Hayes JP, Garland T. 1995. The evolution of endothermy: testing the aerobic capacity model. Evolution 49, 836–847 (doi:10.2307/2410407) [DOI] [PubMed] [Google Scholar]
- 29.Ruben J. 1995. The evolution of endothermy in mammals and birds: from physiology to fossils. Annu. Rev. Physiol. 57, 69–95 (doi:10.1146/annurev.ph.57.030195.000441) [DOI] [PubMed] [Google Scholar]
- 30.Farmer CG. 2000. Parental care: the key to understanding endothermy and other convergent features in birds and mammals. Am. Nat. 155, 326–334 (doi:10.1086/303323) [DOI] [PubMed] [Google Scholar]
- 31.Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (doi:10.1152/physrev.00015.2003) [DOI] [PubMed] [Google Scholar]
- 32.Hughes J, Criscuolo F. 2008. Evolutionary history of the UCP gene family: gene duplication and selection. BMC Evol. Biol. 8, 306 (doi:10.1186/1471-2148-8-306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jastroch M, Wuertz S, Kloas W, Klingenspor M. 2005. Uncoupling protein 1 in fish uncovers an ancient evolutionary history of mammalian nonshivering thermogenesis. Physiol. Genomics 22, 150–156 (doi:10.1152/physiolgenomics.00070.2005) [DOI] [PubMed] [Google Scholar]
- 34.Tine M, Kuhl H, Jastroch M, Reinhardt R. 2012. Genomic characterization of the European sea bass Dicentrarchus labrax reveals the presence of a novel uncoupling protein (UCP) gene family member in the teleost fish lineage. BMC Evol. Biol. 12, 62 (doi:10.1186/1471-2148-12-62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter I, Seebacher F. 2009. Endothermy in birds: underlying molecular mechanisms. J. Exp. Biol. 212, 2328–2336 (doi:10.1242/jeb.029009) [DOI] [PubMed] [Google Scholar]
- 36.Newman SA. 2011. Thermogenesis, muscle hyperplasia, and the origin of birds. Bioessays 33, 653–656 (doi:10.1002/bies.201100061) [DOI] [PubMed] [Google Scholar]
- 37.Seymour RS, Bennett-Stamper CL, Johnston SD, Carrier DR, Grigg GC. 2004. Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. Physiol. Biochem. Zool. 77, 1051–1067 (doi:10.1086/422766) [DOI] [PubMed] [Google Scholar]
- 38.Huchon D, Madsen O, Sibbald MJ, Ament K, Stanhope MJ, Catzeflis F, de Jong WW, Douzery EJ. 2002. Rodent phylogeny and a timescale for the evolution of Glires: evidence from an extensive taxon sampling using three nuclear genes. Mol. Biol. Evol. 19, 1053–1065 (doi:10.1093/oxfordjournals.molbev.a004164) [DOI] [PubMed] [Google Scholar]
- 39.Roll U, Dayan T, Kronfeld-Schor N. 2006. On the role of phylogeny in determining activity patterns of rodents. Evol. Ecol. 20, 479–490 (doi:10.1007/s10682-006-0015-y) [Google Scholar]
- 40.Seale P, Kajimura S, Spiegelman BM. 2009. Transcriptional control of brown adipocyte development and physiological function—of mice and men. Gene Dev. 23, 788–797 (doi:10.1101/gad.1779209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Else PJ, Hulbert AJ. 1985. An allometric comparison of the mitochondria of mammalian and reptilian tissues: the implications for the evolution of endothermy. J. Comp. Physiol. B 156, 3–11 (doi:10.1007/BF00692920) [DOI] [PubMed] [Google Scholar]
- 42.Else PL, Hulbert AJ. 1987. Evolution of mammalian endothermic metabolism: “leaky” membranes as a source of heat. Am. J. Physiol. 253, R1–R7 [DOI] [PubMed] [Google Scholar]
- 43.Else PL, Turner N, Hulbert AJ. 2004. The evolution of endothermy: role for membranes and molecular activity. Physiol. Biochem. Zool. 77, 950–958 (doi:10.1086/422767) [DOI] [PubMed] [Google Scholar]
- 44.Hulbert AJ, Else PL. 2004. Basal metabolic rate: history, composition, regulation, and usefulness. Physiol. Biochem. Zool. 77, 869–876 (doi:10.1086/422768) [DOI] [PubMed] [Google Scholar]
- 45.Hulbert AJ, Else PL. 1981. Comparison of the “mammal machine” and the “reptile machine”: energy use and thyroid-activity. Am. J. Physiol. 241, R350–R356 [DOI] [PubMed] [Google Scholar]
- 46.Brand MD, Turner N, Ocloo A, Else PL, Hulbert AJ. 2003. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem. J. 376, 741–748 (doi:10.1042/BJ20030984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brand MD, Couture P, Else PL, Withers KW, Hulbert AJ. 1991. Evolution of energy-metabolism. Proton permeability of the inner membrane of liver mitochondria is greater in a mammal than in a reptile. Biochem. J. 275, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walls GL. 1934. The reptilian retina. I. A new concept of visual cell evolution. Am. J. Ophthalmol. 17, 892–915 [Google Scholar]
- 49.Kirk EC. 2004. Comparative morphology of the eye in primates. Anat. Rec. A, Discov. Mol. Cell Evol. Biol. 281, 1095–1103 (doi:10.1002/ar.a.20115) [DOI] [PubMed] [Google Scholar]
- 50.Kirk EC. 2006. Effects of activity pattern on eye size and orbital aperture size in primates. J. Hum. Evol. 51, 159–170 (doi:10.1016/j.jhevol.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 51.Ross CF, Kirk EC. 2007. Evolution of eye size and shape in primates. J. Hum. Evol. 52, 294–313 (doi:10.1016/j.jhevol.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 52.Heesy CP, Hall MI. 2010. The nocturnal bottleneck and the evolution of mammalian vision. Brain Behav. Evol. 75, 195–203 (doi:10.1159/000314278) [DOI] [PubMed] [Google Scholar]
- 53.Corfield JR, Gsell AC, Brunton D, Heesy CP, Hall MI, Acosta ML, Iwaniuk AN, Warrant EJ. 2011. Anatomical specializations for nocturnality in a critically endangered parrot, the kakapo (Strigops habroptilus). PLoS ONE 6, e22945 (doi:10.1371/journal.pone.0022945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall MI, Kamilar JM, Kirk EC. 2012. Eye shape and the nocturnal bottleneck of mammals. Proc. R. Soc. B 279, 4962–4968 (doi:10.1098/rspb.2012.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hut RA, Scheper A, Daan S. 2000. Can the circadian system of a diurnal and a nocturnal rodent entrain to ultraviolet light? J. Comp. Physiol. A 186, 707–715 (doi:10.1007/s003590000124) [DOI] [PubMed] [Google Scholar]
- 56.Hut RA, Oklejewicz M, Rieux C, Cooper HM. 2008. Photic sensitivity ranges of hamster pupillary and circadian phase responses do not overlap. J. Biol. Rhythms 23, 37–48 (doi:10.1177/0748730407311851) [DOI] [PubMed] [Google Scholar]
- 57.Motani R, Schmitz L. 2011. Phylogenetic versus functional signals in the evolution of form–function relationships in terrestrial vision. Evolution 65, 2245–2257 (doi:10.1111/j.1558-5646.2011.01271.x) [DOI] [PubMed] [Google Scholar]
- 58.Schmitz L, Motani R. 2010. Morphological differences between the eyeballs of nocturnal and diurnal amniotes revisited from optical perspectives of visual environments. Vision Res. 50, 936–946 (doi:10.1016/j.visres.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 59.Schmitz L, Motani R. 2011. Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 332, 705–708 (doi:10.1126/science.1200043) [DOI] [PubMed] [Google Scholar]
- 60.Yokoyama S. 2000. Molecular evolution of vertebrate visual pigments. Prog. Retinal Eye Res. 19, 385–419 (doi:10.1016/S1350-9462(00)00002-1) [DOI] [PubMed] [Google Scholar]
- 61.Davies WL, Carvalho LS, Cowing JA, Beazley LD, Hunt DM, Arrese CA. 2007. Visual pigments of the platypus: a novel route to mammalian colour vision. Curr. Biol. 17, R161–R163 (doi:10.1016/j.cub.2007.01.037) [DOI] [PubMed] [Google Scholar]
- 62.Wakefield MJ, Anderson M, Chang E, Wei Ke-jun, Kaul R, Graves JAM, Grützner F, Deeb SS. 2008. Cone visual pigments of monotremes: filling the phylogenetic gap. Visual Neurosci. 25, 257–264 (doi:10.1017/S0952523808080255) [DOI] [PubMed] [Google Scholar]
- 63.Ebeling W, Natoli RC, Hemmi JM. 2010. Diversity of color vision: not all marsupials are trichromatic. PLoS ONE 5, e14231 (doi:10.1371/journal.pone.0014231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster RG, Grace MS, Provencio I, Degrip WJ, Garcia-Fernandez JM. 1994. Identification of vertebrate deep brain photoreceptors. Neurosci. Biobehav. Rev. 18, 541–546 (doi:10.1016/0149-7634(94)90009-4) [DOI] [PubMed] [Google Scholar]
- 65.Foster RG, Hankins MW. 2007. Circadian vision. Curr. Biol. 17, R746–751 (doi:10.1016/j.cub.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 66.Davies WL, Hankins MW, Foster RG. 2010. Vertebrate ancient opsin and melanopsin: divergent irradiance detectors. Photochem. Photobiol. Sci. 9, 1444–1457 (doi:10.1039/c0pp00203h) [DOI] [PubMed] [Google Scholar]
- 67.Davies WI, Zheng L, Hughes S, Tamai TK, Turton M, Halford S, Foster RG, Whitmore D, Hankins MW. 2011. Functional diversity of melanopsins and their global expression in the teleost retina. Cell Mol. Life Sci. 68, 4115–4132 (doi:10.1007/s00018-011-0785-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davies WI, et al. 2012. evolution and functional characterisation of melanopsins in a deep-sea chimaera (elephant shark, Callorhinchus milii). PLoS ONE 7, e51276 (doi:10.1371/journal.pone.0051276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lucas RJ, Douglas RH, Foster RG. 2001. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 4, 621–626 (doi:10.1038/88443) [DOI] [PubMed] [Google Scholar]
- 70.Lucas RJ, Foster RG. 1999. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology 140, 1520–1524 (doi:10.1210/en.140.4.1520) [DOI] [PubMed] [Google Scholar]
- 71.Lupi D, Oster H, Thompson S, Foster RG. 2008. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat. Neurosci. 11, 1068–1073 (doi:10.1038/nn.2179) [DOI] [PubMed] [Google Scholar]
- 72.Horiguchi H, Winawer J, Dougherty RF, Wandell BA. 2012. Human trichromacy revisited. Proc. Natl Acad. Sci. USA 110, E260–E269 (doi:10.1073/pnas.1214240110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellingham J, et al. 2006. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 4, e254 (doi:10.1371/journal.pbio.0040254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandes AM, Fero K, Arrenberg AB, Bergeron SA, Driever W, Burgess HA. 2012. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr. Biol. 22, 2042–2047 (doi:10.1016/j.cub.2012.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiraki T, Kojima D, Fukada Y. 2010. Light-induced body color change in developing zebrafish. Photochem. Photobiol. Sci. 9, 1498–1504 (doi:10.1039/c0pp00199f) [DOI] [PubMed] [Google Scholar]
- 76.van Oosterhout F, et al. 2012. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr. Biol. 22, 1397–1402 (doi:10.1016/j.cub.2012.05.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soni BG, Philp AR, Knox BE, Foster RG. 1998. Novel retinal photoreceptors. Nature 394, 27–28 (doi:10.1038/27794) [DOI] [PubMed] [Google Scholar]
- 78.Halford S, et al. 2009. VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 19, 1396–1402 (doi:10.1016/j.cub.2009.06.066) [DOI] [PubMed] [Google Scholar]
- 79.Davies WI, Turton M, Peirson SN, Follett BK, Halford S, Garcia-Fernandez JM, Sharp PJ, Hankins MW, Foster RG. 2012. Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol. Lett. 8, 291–294 (doi:10.1098/rsbl.2011.0864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hazlerigg D. 2012. The evolutionary physiology of photoperiodism in vertebrates. Prog. Brain Res. 199, 413422 (doi:10.1016/B978-0-444-59427-3.00023-X) [DOI] [PubMed] [Google Scholar]
- 81.Davies WI, Tay BH, Zheng L, Danks JA, Brenner S, Foster RG, Collin SP, Hankins MW, Venkatesh B, Hunt DM. 2012. Evolution and functional characterisation of Melanopsins in a deep-sea chimaera (elephantshark, Callorhinchus milii). PLoS ONE 7, e51276 (doi:10.1371/journal.pone.0051276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kay RF, Cartmill M. 1977. Cranial morphology and adaptations of Palaechthon nacimienti and other Paromomyidae (Plesiadapoidea, Primates), with a description of a new genus and species. J. Hum. Evol. 6, 19–53 (doi:10.1016/S0047-2484(77)80040-7) [Google Scholar]
- 83.Wikler KC, Rakic P. 1990. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J. Neurosci. 10, 3390–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nicol JAC. 1981. Tapeta lucida of vertebrates. In Vertebrate photoreceptor optics (eds Enoch JM, Tobey FL., Jr), pp. 401–431 Berlin, Germany: Springer [Google Scholar]
- 85.Solovei I, Kreysing M, Lanctôt C, Kösem S, Peichl L, Cremer T, Guck J, Joffe B. 2009. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137, 356–368 (doi:10.1016/j.cell.2009.01.052) [DOI] [PubMed] [Google Scholar]
- 86.Streidter GF. 2005. Principles of brain evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 87.Coleman MN, Boyer DM. 2012. Inner ear evolution in primates through the Cenozoic: implications for the evolution of hearing. Anat. Rec. (Hoboken) 295, 615–631 (doi:10.1002/ar.22422) [DOI] [PubMed] [Google Scholar]
- 88.Muchlinski MN. 2010. A comparative analysis of vibrissa count and infraorbital foramen area in primates and other mammals. J. Hum. Evol. 58, 447–473 (doi:10.1016/j.jhevol.2010.01.012) [DOI] [PubMed] [Google Scholar]
- 89.Menck CFM. 2002. Shining light on photolyases. Nat. Genet. 32, 338–339 (doi:10.1038/ng1102-338) [DOI] [PubMed] [Google Scholar]
- 90.Painter RB. 1974. DNA damage and repair in eukaryotic cells. Genetics 78, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cook JS, Regan JD. 1969. Photoreactivation and photoreactivating enzyme activity in on order of mammals (Marsupialia). Nature 223, 1066–1067 (doi:10.1038/2231066a0) [DOI] [PubMed] [Google Scholar]
- 92.Yasui A, Eker AP, Yasuhira S, Yajima H, Kobayashi T, Takao M, Oikawa A. 1994. A new class of DNA photolyases present in various organisms including aplacental mammals. EMBO J. 13, 6143–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schul W, et al. 2002. Enhanced repair of cyclobutane pyrimidine dimers and improved UV resistance in photolyase transgenic mice. EMBO J. 21, 4719–4729 (doi:10.1093/emboj/cdf456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaves I, et al. 2011. The potorous CPD photolyase rescues a cryptochrome-deficient mammalian circadian clock. PLoS ONE 6, e23447 (doi:10.1371/journal.pone.0023447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ringvold A. 1980. Aqueous humour and ultraviolet radiation. Acta Ophthalmol. 58, 69–82 (doi:10.1111/j.1755-3768.1980.tb04567.x) [DOI] [PubMed] [Google Scholar]
- 96.Ringvold A, Anderssen E, Kjönniksen I. 2000. Distribution of ascorbate in the anterior bovine eye. Invest. Ophthalmol. Vis. Sci. 41, 20–23 [PubMed] [Google Scholar]
- 97.Tsentalovich YP, Sherin PS, Kopylova LV, Cherepanov IV, Grilj J, Vauthey E. 2011. Photochemical properties of UV filter molecules of the human eye. Invest. Ophthalmol. Vis. Sci. 52, 7687–7696 (doi:10.1167/iovs.11-8120) [DOI] [PubMed] [Google Scholar]
- 98.Truscott RJ, Wood AM, Carver JA, Sheil MM, Stutchbury GM, Zhu J, Kilby GW. 1994. A new UV-filter compound in human lenses. FEBS Lett. 348, 173–176 (doi:10.1016/0014-5793(94)00601-6) [DOI] [PubMed] [Google Scholar]
- 99.Wood AM, Truscott RJ. 1994. Ultraviolet filter compounds in human lenses: 3-hydroxykynurenine glucoside formation. Vision Res. 34, 1369–1374 (doi:10.1016/0042-6989(94)90135-X) [DOI] [PubMed] [Google Scholar]
- 100.Truscott RJ, Wood AM. 1994. Gourami lenses convert tryptophan into 3-hydroxykynurenine. Ophthalmic Res. 26, 214–218 (doi:10.1159/000267474) [DOI] [PubMed] [Google Scholar]
- 101.Yuasa HJ, Takubo M, Takahashi A, Hasegawa T, Noma H, Suzuki T. 2007. Evolution of vertebrate indoleamine 2,3-dioxygenases. J. Mol. Evol. 65, 705–714 (doi:10.1007/s00239-007-9049-1) [DOI] [PubMed] [Google Scholar]
- 102.Jacobs GH, Neitz J, Deegan JF. 1991. Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature 353, 655–656 (doi:10.1038/353655a0) [DOI] [PubMed] [Google Scholar]
- 103.Jacobs GH. 1992. Ultraviolet vision in vertebrates. Am. Zool. 32, 544–554 [Google Scholar]
- 104.Shi Y, Radlwimmer FB, Yokoyama S. 2001. Molecular genetics and the evolution of ultraviolet vision in vertebrates. Proc. Natl Acad. Sci. USA 98, 11 731–11 736 (doi:10.1073/pnas.201257398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shkolnik A. 1971. Diurnal activity in a small desert rodent. Int. J. Biometeorol. 15, 115–120 (doi:10.1007/BF01803884) [DOI] [PubMed] [Google Scholar]
- 106.Koskela TK, Reiss GR, Brubaker RF, Ellefson RD. 1989. Is the high concentration of ascorbic acid in the eye an adaptation to intense solar irradiation? Invest. Ophthalmol. Vis. Sci. 30, 2265–2267 [PubMed] [Google Scholar]
- 107.Ringvold A, Anderssen E, Kjönniksen I. 2000. UV absorption by uric acid in diurnal bird aqueous humor. Invest. Ophthalmol. Vis. Sci. 41, 2067–2069 [PubMed] [Google Scholar]
- 108.Reiss GR, Werness PG, Zollman PE, Brubaker RF. 1986. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch. Ophthalmol. 104, 753–755 (doi:10.1001/archopht.1986.01050170143039) [DOI] [PubMed] [Google Scholar]