Abstract
The ability of prey to observe and learn to recognize potential predators from the behaviour of nearby individuals can dramatically increase survival and, not surprisingly, is widespread across animal taxa. A range of sensory modalities are available for this learning, with visual and chemical cues being well-established modes of transmission in aquatic systems. The use of other sensory cues in mediating social learning in fishes, including mechano-sensory cues, remains unexplored. Here, we examine the role of different sensory cues in social learning of predator recognition, using juvenile damselfish (Amphiprion percula). Specifically, we show that a predator-naive observer can socially learn to recognize a novel predator when paired with a predator-experienced conspecific in total darkness. Furthermore, this study demonstrates that when threatened, individuals release chemical cues (known as disturbance cues) into the water. These cues induce an anti-predator response in nearby individuals; however, they do not facilitate learnt recognition of the predator. As such, another sensory modality, probably mechano-sensory in origin, is responsible for information transfer in the dark. This study highlights the diversity of sensory cues used by coral reef fishes in a social learning context.
Keywords: social learning, predator recognition, coral reef fishes, disturbance cues, visual cues
1. Introduction
To counter the threat of predation, prey individuals have evolved sophisticated mechanisms to assess risk using visual, olfactory, tactile and auditory cues [1,2]. However, obtaining first-hand information on local predators is dangerous since prey are required to be in close proximity to potential predators. By contrast, information obtained indirectly allows prey to gain knowledge about predators without the risk associated with direct experiences. Animals that live in close proximity to one another have ample opportunity to acquire information by observing nearby conspecifics; not surprisingly, this phenomenon is commonplace among animal taxa [2]. The process whereby less experienced prey (observers) learn from experienced individuals (demonstrators) using social cues is known as social learning [3]. According to reviews by Crane & Ferrari [2] and Griffin [4], social cues are defined as any cue emitted (voluntarily or otherwise) by a conspecific, with well-known cues including the mobbing calls of birds and alarm calls in mammals. Changes in the behaviour of the demonstrator, including fleeing or hiding responses, also constitute social cues, just as would chemical cues released by conspecifics that are injured (alarm cues) or disturbed (disturbance cues) by predators [5].
Social learning of predator recognition has been observed in a range of taxa [4,6,7]; however, little work has attempted to identify the reliance on or diversity of sensory modes used in the acquisition of information. Studies have shown that social learning can occur through visual mobbing displays and overt anti-predator responses (mammals, birds, amphibians, fishes and insects), auditory cues such as alarm calls (mammals and birds), and chemical cues such as injury-released conspecific cues (amphibians and fishes; reviewed in [2]). It is possible that the range of cues being used reflects environmental constraints on information transfer. In aquatic systems, the use of both visual and chemical cues is well documented in fishes [8,9]. Areas with high visibility and low structural complexity are ideal conditions for reliance on visual cues; however, in areas where vision is obstructed, such as low light conditions, high turbidity and topographically complex environments, organisms are likely to demonstrate a well-developed response to chemical cues. Therefore, variation is likely to exist within a habitat, and individuals may acquire information using more than one sensory system or social cue type.
In the majority of studies on social learning in fishes, the demonstrators and observers were placed in the same observation tank [10–16]. While these studies have been important in identifying the ability for organisms to socially transmit information about predators, including their level of threat, research now needs to be expanded to isolate the sensory mechanism responsible for the transfer of social information. A study by Ferrari et al. [17] demonstrated learned predator recognition by fishes that observed conspecifics responding to a threat in an adjacent tank. This study was the first to show that the transmission of information could occur in the absence of all but visual cues. For visually oriented animals such as humans, this result seems rather intuitive. However, there is huge potential for other sensory systems to be used for recognition as well. Information on sensory cues responsible for social learning are required to better understand the factors that can potentially affect information transfer, especially in the light of environmental stressors affecting both visual (turbidity [18]) and chemical (ocean acidification and acid rain [19,20]) properties of aquatic ecosystems.
Further evidence for the importance of non-visual cues is that most reef fishes settle from plankton to benthic habitats at night [21]. These site-attached juveniles must quickly learn the identity of local predators as mortality levels are extremely high within the first 48 h [22]. As such, individuals are faced with a myriad of potential predators and non-predators, necessitating the need to identify those that represent a threat and those that do not. Responding to non-predators wastes valuable time and energy, but failing to respond to predators could cost the prey its life. Under these conditions, it is not surprising that coral reef fishes rely heavily on social information to recognize predators. The importance of social learning in mediating survival was highlighted in a study by Manassa & McCormick [15], where it was documented that fish that directly learnt the predator odour and those that acquired the information through social learning survived at least five times better during predator encounters than naive individuals. As predator detection is an important process, where a mistake can equate to death, individuals are likely to use all cues available to them.
The present study examines the role of different sensory cues in social learning of predator recognition, using juvenile damselfish, Amphiprion percula, as test subjects. The first part of the study investigates whether a predator-naive observer can socially learn to recognize a novel predator when paired with a predator-experienced conspecific in total darkness. The second part of the study aims to isolate potential sensory cues mediating learning in complete darkness. Specifically, we test whether demonstrators release chemical cues (disturbance cues) upon detecting a threat, and whether these cues mediate social learning in the dark. Disturbance cues are ammonia compounds released by ‘disturbed’ prey either through the urogenital system or gills [23–25]. These cues are known to increase vigilance when detected by conspecifics, but to date studies have failed to demonstrate the role of these cues in learning (reviewed in [5]).
2. Material and methods
(a). Fish collection and maintenance
Amphiprion percula larvae were reared from adult breeding pairs collected from the Great Barrier Reef, Australia, and kept at the Marine and Aquaculture Research Facility (MARFU) at James Cook University (ethics permit no. A1595). Pairs were maintained in separate 70 l aquaria and fed INVE Aquaculture Nutrition 12/20 pellets three times daily. A terracotta pot was placed with each breeding pair to allow adequate surface area for egg laying. On the night of hatching (6–8 days post-laying, appearance of embryos indicates readiness to hatch) egg clutches (with terracotta pot) were transferred to separate 70 l aquaria.
Following hatching larvae were reared in a semi-closed system, with the only water flow being a slow flush of filtered UV-sterilized seawater each night, until larvae were competent to settle at 11 days [26]. By using a semi-closed system, larvae were able to feed ad libitum throughout the day, with any unconsumed food removed each night. The larval feeding regime consisted of rotifers (Brachionus sp.) at five individuals per millilitre each morning for the first 3 days and live brine shrimp nauplii (Artemia franciscana) at one individual per millilitre from day 3 onwards. The ratio of brine shrimp to rotifers was increased each day, with five individuals per millilitre fed from day 8.
Cephalopholis argus were collected from the Great Barrier Reef, Australia, and maintained at MARFU in individual 70 l aquaria. Individuals used to produce predator odours were fed twice daily; however, no feeding occurred 24 h prior to collection of predator odours.
(b). Stimulus preparation
Damage-released chemical cues were prepared according to the protocol of McCormick & Manassa [27]. Donors were euthanized by a quick blow to the head, with 25 superficial cuts (minor flesh damage) made with a clean razor blade. Specimens were then rinsed in 15 ml of seawater, previously obtained from each test tank. The 15 ml of damage-released chemical cue was then filtered prior to use, with the cues used no longer than 20 min after preparation. A total of 75 donors (mean standard length, SL ± s.e.: 20.61 ± 2.94 mm) were used during the experiment.
Predator odours (C. argus) were collected in such a way that they were free of possible A. percula damage-released chemical cues. Cephalopholis argus were fed a diet of frozen Marine Food (Fish Fuel Co., South Australia; 46% fish product), which do not contain any damselfish cues. The flow-through aquaria system was turned off 2 h prior to experimentation to ensure the predator odours collected just prior to the experiment were concentrated within the holding tanks.
(c). Experimental set-up
Laboratory experiments were conducted in 7.8 l flow-through observation tanks. Each tank contained an air stone placed at the back corner and an additional piece of plastic tubing fastened alongside the airline for cue injection. Dye trials indicated that it took approximately 12 s for the dye to disperse throughout the tank. On the opposite side of the tank each aquarium contained a shelter of coral rubble. The tanks were covered in black plastic on three sides to block visual cues from adjacent tanks. A single A. percula (mean SL ± s.e.: 20.61 ± 2.94 mm) was placed into each tank and left to acclimate for 48 h prior to experimentation.
(d). Experiment 1: Are visual cues necessary for social learning to occur in fish?
To determine whether social learning of a predator odour requires visual cues, a modified version of the well-established three-stage social learning protocol was used [16]. The modified version uses two light conditions during the social learning stage: normal daylight conditions or total darkness (electronic supplementary material, figure S1).
(i). Stage A: conditioning of naive demonstrators
Chemical alarm cues are known to elicit a strong anti-predator response and mediate predator learning in a similar way to Pavlovian conditioning. Therefore, in this stage, individuals were conditioned to recognize a previously unknown predator by exposing them to either: the novel predator odour paired with chemical alarm cues of conspecifics (true conditioning) or a seawater control (pseudo-conditioning which does not lead to learning). Each demonstrator was conditioned individually in a tank. Prior to the initial observation period, the flow-through system was turned off and 60 ml of tank water drawn up the stimulus injection tube and discarded to remove any stagnant water. A further 120 ml was collected and kept for later use. Immediately prior to the initial observation period, 10 ml of live A. franciscana (approx. 2500 nauplii per tank) was injected into the tube followed by 60 ml of previously collected tank water, to flush the stimulus through the tube. The behaviour of the fish was then recorded for 3 min. After the initial observation, one of two stimuli—60 ml of predator odour paired with either 15 ml of seawater (pseudo-conditioning) or 15 ml of chemical alarm cue (true conditioning)—was injected into the tank, along with a further 10 ml of live A. franciscana. The remaining 60 ml of previously collected tank water was then used to flush the stimuli and food into the tank. This was followed by a final 3 min observation period. The behaviour of 60 fish in each of the two treatments was recorded, with these individuals later used as predator-naive (control) and predator-experienced (experimental) demonstrators in the social learning stage.
(ii). Stage B: social learning stage
Immediately following the final observation period, the demonstrator from stage A was dipped in clean seawater to remove any potential cues, then transferred to another observation tank housing an acclimated naive individual (hereafter, the observer). To differentiate the two fish, the demonstrator was 5 mm smaller or larger than the observer, with fish randomly matched. The two individuals were acclimated for another 2 h before the start of stage B. The learning during this stage was set up under one of two light conditions—light (419–426 lux) or total darkness (0.0–0.1 lux). Experiments took place during daylight hours, with a red light used by the experimenter to navigate around the room when necessary. At the conclusion of the acclimation period, the flow-through system was turned off and 60 ml of tank water was drawn up the stimulus injection tube and discarded, and an additional 60 ml collected and kept for later use. A 60 ml aliquot of predator odour along with 10 ml of live A. franciscana was injected into the tank and flushed with 60 ml of previously collected tank water. No observations were carried out during this stage. We predict that the presence of predator odour in the tank should elicit an anti-predator response from the predator-experienced demonstrators, but not from the predator-naive demonstrators. Observers paired with predator-experienced demonstrators should have an opportunity to learn to recognize the predator odour as risky.
(iii). Stage C: testing the observer for learning
This stage tests the ability of the observer to respond to the predator odour on its own. This stage is necessary to ensure the observer can display the response in the absence of a nearby demonstrator (true learning versus copying behaviour). Immediately following stage B, the observer was rinsed in clean seawater, then transferred to an empty observation tank and acclimated for 2 h. After the acclimation period, the experimental procedures from stage A were repeated, the flow-through system was turned off, and 60 ml of tank water was drawn up the stimulus injection tube and discarded, with a further 120 ml collected and kept. Immediately prior to the initial observation period, 10 ml of A. franciscana was injected into the tube followed by 60 ml of previously collected tank water, to flush the tube. The behaviour of the observer was then recorded for 3 min. After the initial observation, one of two stimuli—a control (60 ml of seawater) or the experimental stimulus (60 ml of predator odour)—was injected into the tank, along with a further 10 ml of live A. franciscana. The remaining 60 ml of previously collected tank water was then used to flush the stimulus through the tube. This was followed by a final 3 min observation period.
We predicted that observers who had successfully learned to recognize the predator odour as risky from their demonstrators should display an anti-predator response when exposed to the predator odour. Those who failed to learn should not respond to the predator odour. A total of 15 observers from each of the four treatments (predator-naive versus predator-experienced demonstrators crossed with an observer exposed to seawater or predator odour) were tested.
(iv). Behavioural assay
A decrease in foraging and activity are two well-established anti-predator responses in damselfish [15,16,27]. Thus, the number of feeding strikes and line crosses during each 3 min observation period were recorded. The observation tanks were divided into four equal vertical and six equal horizontal areas (grid of 4.7 × 4.2 cm rectangles), and every line crossed by the fish was recorded. The number of feeding strikes was recorded regardless of success.
(v). Statistical analysis
Change in behaviour between the initial and final observation periods were used as raw data in the analysis. As activity and feeding are correlated, the two responses were analysed together using a MANOVA approach. The behavioural response of both demonstrators during stage A (true conditioning versus pseudo-conditioning) were compared using a two-way MANOVA. The effect of demonstrator experience (naive versus experienced), light condition during stage B (light versus dark) and stage C cue (control or experimental stimulus) on the anti-predator response of observers was assessed using a 2 × 2 × 2 MANOVA. A series of factorial MANOVAs were then conducted to determine the significance of demonstrator experience (naive versus experienced) on observer behaviour. Inspection of residuals revealed that the data followed parametric assumptions.
(e). Experiment 2: Do juvenile damselfish release disturbance cues and can they be used as social cues to learn the identity of novel predators?
The goal of this experiment was to (i) assess whether damselfish possess disturbance cues, and, if they do, (ii) assess whether these cues can mediate learned predator recognition. The experiment was carried out in four stages (electronic supplementary material, figure S2).
(i). Stage I: conditioning of naive demonstrators
This stage was identical to stage A in experiment 1 and follows the same protocol. A total of 60 demonstrators were conditioned—30 predator-naive and 30 predator-experienced.
(ii). Stage II: collection of disturbance cues
This stage exposed the demonstrators from stage I to predator odour. If detection of the predator odour elicited the release of a disturbance cue from the demonstrators, these cues would be present in the surrounding water. We predict that predator-naive demonstrators would not be ‘disturbed’ by the predator odour, hence would not release disturbance cues. Following stage I demonstrators were dipped in clean seawater to remove any potential cues and transferred individually to clean observation tanks to acclimate for 2 h. The flow-through system was then turned off, and 60 ml of tank water drawn up the stimulus injection tube and discarded, with a further 60 ml collected and kept. A 60 ml aliquot of predator odour was injected into the tank followed by 60 ml of previously collected tank water. After 2 min, 60 ml of tank water containing predator odour and possible disturbance cue was drawn up the stimulus injection tube and retained.
(iii). Stage III: testing disturbance cues on naive individuals
This stage determined whether naive observers respond to the disturbance cues of conspecifics. Naive individuals were exposed to the 60 ml of tank water collected during stage II from predator-naive and predator-experienced demonstrators (n = 15 per treatment). The experimental protocol and behavioural assay for this stage followed that of stage C in experiment 1, with the 60 ml of tank water acting as the stimulus.
(iv). Stage IV: testing if learning occurs following exposure to disturbance cues
The individual from stage III was transferred to another observation tank to investigate whether exposure to disturbance cues paired with predator odour during stage III allowed them to acquire recognition of the novel predator. The observers were exposed to predator odour alone and their anti-predator behaviours were recorded. Again the protocol followed was identical to stage C from experiment 1, with 60 ml of predator odour acting as the stimulus.
(v). Statistical analysis
As for experiment 1, the behavioural response of the demonstrators during stage I (true conditioning versus pseudo-conditioning) was compared using a two-way MANOVA. A two-way repeated-measures MANOVA to test the effect of demonstrator experience (naive versus experienced) and stage (stage III versus stage IV) on the behaviour of observers was conducted. The behaviour of the observers was recorded twice, once during stage III and again during stage IV, thus ‘stage’ was the repeated-measure factor. Inspection of residuals revealed that the data followed parametric assumptions.
3. Results
(a). Experiment 1: social learning in the dark
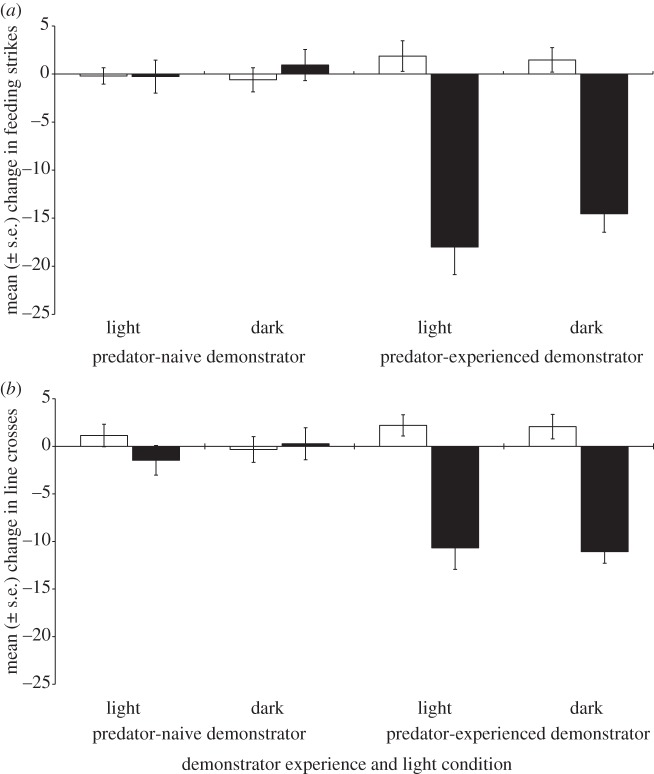
The demonstrators conditioned with chemical alarm cues displayed a significantly stronger anti-predator response than those pseudo-conditioned with seawater (Pillai's trace = 0.68, F2,27 = 28.5, p < 0.001). While cue and demonstrator experience significantly interacted to affect the behaviour of the fish during stage B (social learning stage), light conditions (and any interaction involving light conditions) did not significantly explain the variation in the behaviour of observers, indicating that light conditions did not affect the outcome of learning. The results of the 2 × 2 × 2 MANOVA are presented in table 1. As expected, when observers were paired with naive demonstrators, neither cue (Pillai's trace = 0.02, F2,55 = 0.7, p = 0.5) nor light (Pillai's trace = 0.001, F2,55 = 0.04, p = 0.96), nor any interaction between the two (Pillai's trace = 0.02, F2,55 = 0.6, p = 0.6), affected the behaviour of the fish (figure 1). The observers responded similar to water and predator odour, regardless of light conditions during stage B—in other words, individuals failed to learn the predator odour as risky. Conversely, observers paired with experienced demonstrators subsequently displayed anti-predator responses when exposed to predator odour, but not when exposed to water (Pillai's trace = 0.65, F2,55 = 51.3, p < 0.001). Again, neither light (Pillai's trace = 0.02, F2,55 = 0.44, p = 0.6) nor the light × cue interaction (Pillai's trace = 0.02, F2,55 = 0.6, p = 0.5) affected their response.
Table 1.
Results of the 2 × 2 × 2 way MANOVA testing the effect of demonstrator experience (naive versus experienced), light condition during stage B (light versus dark) and stage C cue (control versus experimental stimulus) on the foraging behaviour and activity level of the observers during stage C (experiment 1).
| source of variance | d.f. | F | significance |
|---|---|---|---|
| demonstrator | 2,111 | 18.6 | <0.001 |
| light | 2,111 | 0.4 | 0.658 |
| cue | 2,111 | 31.7 | <0.001 |
| demonstrator × light | 2,111 | 0.2 | 0.814 |
| demonstrator × cue | 2,111 | 31.8 | <0.001 |
| light × cue | 2,111 | 0.6 | 0.530 |
| demonstrator × light × cue | 2,111 | 0.8 | 0.468 |
Figure 1.
Mean change (±s.e.) in the number of (a) feeding strikes or (b) line crosses between the initial and final observation periods for A. percula observers exposed to seawater (unfilled bars) or predator odour (filled bars). The observers were previously paired with either a predator-naive or predator-experienced demonstrator under light or dark conditions, and exposed to predator odour.
(b). Experiment 2: disturbance cues
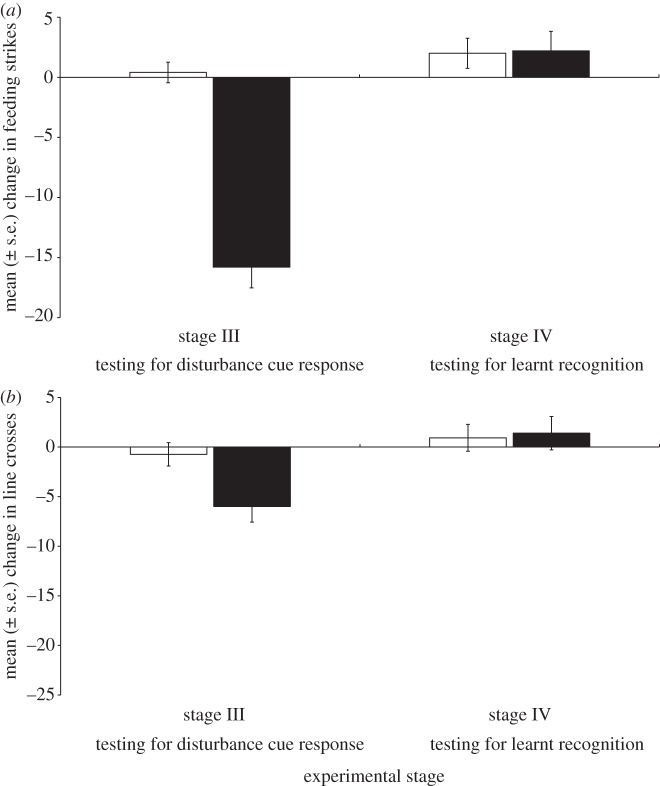
A stronger anti-predator response was observed from demonstrators conditioned with chemical alarm cues compared with those pseudo-conditioned with water (Pillai's trace = 0.69, F2,27 = 29.8, p < 0.001). The two-way repeated-measures MANOVA revealed a significant interaction between demonstrator experience and stage (Pillai's trace = 0.32, F2,27 = 18.7, p < 0.001; figure 2) on the behavioural response of the observers. During stage III, observers exposed to predator-experienced demonstrators displayed significant anti-predator behaviour, while those exposed to cues from predator-naive demonstrators did not (Pillai's trace = 0.7, F2,27 = 34.2, p < 0.001). This indicated that disturbance cues were released by predator-experienced demonstrators exposed to predator odour, with no such cues released by predator-naive demonstrators. However, during stage IV, demonstrator experience did not explain variation in the behaviour of the observers (Pillai's trace = 0.003, F2,27 = 0.04, p = 0.9). Neither group responded to the predator odour.
Figure 2.
Mean change (±s.e.) in the number of (a) feeding strikes or (b) line crosses between the initial and final observation periods for A. percula individuals exposed to potential disturbance cues from predator-naive (unfilled bars) and predator-experienced (filled bars) demonstrators. Following exposure to potential disturbance cues, observers were exposed to predator odour to determine whether learnt recognition occurred.
4. Discussion
Our results reveal that, in the absence of light, social learning of predator recognition can still occur in damselfish. This indicates that visual information—the sight of a frightened conspecific—is not a necessary cue to mediate learning. While previous studies have shown that visual information is enough to elicit learning [17], this is the first study to demonstrate that visual cues are not the only sensory stimuli relied upon for information transfer. It is possible that disturbance cues, released by prey that have detected a predator, could provide the necessary social cues to allow for learning. Our study also shows that disturbance cues elicit an anti-predator response in conspecifics; however, they do not facilitate learnt recognition of a predator.
If vision is compromised, organisms need to rely on other modes of information transmission. Studies on amphibians and freshwater fishes have shown that individuals release a disturbance cue when ‘stressed’, resulting in increased vigilance and anti-predator behaviour in nearby conspecifics [28–33]. Ferrari et al. [34] found that disturbance cues had an additive effect on the response of fishes to damage-released chemical cues. If individuals had been pre-exposed to disturbance cues, greater response intensity to damage-released chemical cues was observed, suggesting that disturbance cues heighten an individual's sense of awareness. However, the few studies that have examined the use of disturbance cues in predator learning have failed to provide support for this mechanism [34,35]. The results of the present study are similar to previous findings from freshwater fishes [34,35]: damselfish possess disturbance cues that are able to elicit increased anti-predator behaviour when detected; however, these cues do not mediate learned predator recognition. Some have argued that the absence of learning is due to the context in which disturbance cues are released. These cues may be released in response to predators; however, they indicate a ‘disturbed’ individual, suggesting that release may also occur as a means of aggression or territorial behaviour [25]. Thus, the lack of learned association between disturbance cues and a novel stimulus may be adaptive, by decreasing the opportunities for learning of irrelevant stimuli.
If social learning of predator recognition can occur in the absence of visual or chemical cues, other senses (mechanical or electrical) must come into play. Damselfish lack electroreceptors present in other fish species, making this sense an unlikely candidate. This leaves mechanical cues as the remaining sense responsible for the ability of individuals to transmit information about risk. Prey fish can detect mechanical disturbances in the water using their lateral line organs, with studies demonstrating the use of this sensory system during nocturnal predation [36]. It is possible that the damselfish are learning the identity of predator by the burst of activity of a nearby frightened conspecific; however, this does not have to be the case. Indeed, it could be a reduction rather than a burst of activity that facilitates learning. Ferrari et al. [34] showed that tadpoles socially transmit recognition of predators among conspecifics and other species with which they co-occur. Predator-experienced demonstrators reduce activity upon detection of known predator odours, and nearby individuals that were naive to the predator used the reduction in activity as an indication of danger, subsequently learning to reduce their activity upon detecting the predator odour. When the ratio of experienced to naive demonstrators increased, there was a greater reduction in nearby activity, and hence greater information transfer to naive individuals. The same mechanism could be operating in damselfish, as they often reduce activity upon exposure to predators. An alternative to fish responding to changes in mechanical disturbance is that they may be responding to sounds (possibly alarm calls) emitted by experienced demonstrators. The ability of coral reef fish to use auditory cues has been extensively studied, with the majority conducted on damselfish species [37–45]. As sound has a low attenuation in water, allowing acoustic signals to propagate quickly over large distances [46], auditory cues are suggested to be important at night or in poor light conditions when visual cues are limited [37]. With the use of mechanical and auditory cues being common among coral reef fishes (discussed in [47]), additional experiments that examine the use of these sensory modes are essential to our understanding of social learning.
As predation levels change with life-stage and environmental conditions, prey are required to continuously learn the identity of new predators and modify the risk rating of those that no longer represent a threat [48]. Under these conditions, it is not surprising that individuals respond to the social cues of others. Given the risks associated with incorrect predator detection (e.g. death), prey are most likely to rely on multiple sensory systems for predator recognition. As such, it is likely that cue choice is context-dependent, with the spatial and temporal limitations of each sensory modality taken into account prior to use. If however, one or more sensory cue is unavailable, as occurred in this study (i.e. visual cues), prey may be capable of switching to another, less reliable sensory cue in order to avoid capture.
Coral reefs are often thought of as clear-water environments where visual cues are heavily relied upon. However, the structural complexity of reefs may limit the transmission of visual information. Moreover, for an average of 12 h a day reefs are blanketed by darkness, a fact that further reduces the use of visual information and hence the utility of visual cues in predator learning. Chemosensory cues are therefore likely to be of benefit under these conditions. However, chemosensory cues can be limited by water currents, chemicals released by other organisms (e.g. bleaching corals [49]) and the associated impacts of climate change (e.g. ability of individuals to respond to chemical alarm cues [5,19]). This study suggests that mechano-sensory and/or auditory cues can act as social cues enabling individuals to learn the identity of predators in the absence of visual or chemical information. Therefore, it is likely that fish simultaneously use information from multiple cues to learn about predators, and possess the flexibility to choose the most appropriate and informative sensory modality when one or more are unavailable, highlighting the importance of predation as a pervasive selective force.
Acknowledgements
Special thanks to D. Dixson, J. Donelson and B. Devine for laboratory assistance. Thanks to D. Dixson for providing useful comments on a draft of the manuscript, and to P. Manassa for providing artistic support.
Funding statement
This study was funded through the Australian Research Council Centre of Excellence for Coral Reef Studies under the ethics approval guidelines of James Cook University, approval A1067.
References
- 1.Kelley JL, Magurran AE. 2003. Learned predator recognition and antipredator response in fishes. Fish Fish. 4, 216–226 10.1046/j.1467-2979.2003.00126.x (doi:10.1046/j.1467-2979.2003.00126.x) [DOI] [Google Scholar]
- 2.Crane AL, Ferrari MCO. 2013. Social learning of predation risk: a review and prospectus. In Social learning theory: phylogenetic considerations across animal, plant, and microbial taxa (ed. Clark K.), pp. 53–82 New York, NY: Nova Science [Google Scholar]
- 3.Chapman BB, Ward AJW, Krause J. 2008. Schooling and learning: early social environment predicts social learning ability in the guppy, Poecilia reticulata. Anim. Behav. 76, 923–929 10.1016/j.anbehav.2008.03.022 (doi:10.1016/j.anbehav.2008.03.022) [DOI] [Google Scholar]
- 4.Griffin AS. 2004. Social learning about predators: a review and prospectus. Learn. Behav. 32, 131–140 10.3758/BF03196014 (doi:10.3758/BF03196014) [DOI] [PubMed] [Google Scholar]
- 5.Ferrari MCO, Lysak KR, Chivers DP. 2010. Turbidity as an ecological constraint on learned predator recognition and generalization in a prey fish. Anim. Behav. 79, 515–519 10.1016/j.anbehav.2009.12.006 (doi:10.1016/j.anbehav.2009.12.006) [DOI] [Google Scholar]
- 6.Brown C, Laland K. 2001. Social learning and life skills training for hatchery reared fish. J. Fish Biol. 59, 471–493 10.1111/j.1095-8649.2001.tb02354.x (doi:10.1111/j.1095-8649.2001.tb02354.x) [DOI] [Google Scholar]
- 7.Ferrari MCO, Chivers DP. 2008. Cultural learning of predator recognition in mixed-species assemblages of frogs: the effect of tutor-to-observer ratio. Anim. Behav. 75, 1921–1925 10.1016/j.anbehav.2007.10.037 (doi:10.1016/j.anbehav.2007.10.037) [DOI] [Google Scholar]
- 8.Brown GE, Chivers DP. 2006. Learning about danger: chemical alarm cues and predation risk assessment by fishes. In Fish cognition and behaviour (eds Brown C, Laland K, Krause J.), pp. 49–69 Oxford, UK: Blackwell Science Publishing [Google Scholar]
- 9.Brown GE. 2003. Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 4, 227–234 10.1046/j.1467-2979.2003.00132.x (doi:10.1046/j.1467-2979.2003.00132.x) [DOI] [Google Scholar]
- 10.Suboski MD, Bain S, Carty AE, McQuoid LM, Seelen MI, Seifert M. 1990. Alarm reaction in acquisition and social transmission of simulated predator recognition by zebra danio fish (Brachydanio rerio). J. Comp. Psychol. 104, 101–112 10.1037/0735-7036.104.1.101 (doi:10.1037/0735-7036.104.1.101) [DOI] [Google Scholar]
- 11.Smith RJF. 1999. What good is smelly stuff in the skin? Cross function and cross taxa effects in fish ‘alarm substances’. In Advances in chemical signals in vertebrates (eds Johnston RE, Muller-Schwarze D, Sorensen PW.), pp. 475–488 New York, NY: Kluwer Academic [Google Scholar]
- 12.Verheijen FJ. 1956. Transmission of a fright reaction amongst a school of fish and the underlying sensory mechanisms. Experientia 12, 202–204 10.1007/BF02170796 (doi:10.1007/BF02170796) [DOI] [Google Scholar]
- 13.Mathis A, Chivers DP, Smith RJF. 1996. Cultural transmission of predator recognition in fishes: intraspecific and interspecific learning. Anim. Behav. 51, 185–201 10.1006/anbe.1996.0016 (doi:10.1006/anbe.1996.0016) [DOI] [Google Scholar]
- 14.Ferrari MCO, Manassa RP, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP. 2012. Effects of ocean acidification on learning in coral reef fishes. PLoS ONE 7, 1–10 10.1371/journal.pone.0031478 (doi:10.1371/journal.pone.0031478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manassa RP, McCormick MI. 2012. Social learning improves survivorship at a life history transition. Oecologia. 171, 843–852 10.1007/s00442-012-2458-x (doi:10.1007/s00442-012-2458-x) [DOI] [PubMed] [Google Scholar]
- 16.Manassa RP, McCormick MI. 2012. Social learning and acquired recognition of a predator by a marine fish. Anim. Cogn. 15, 559–565 10.1007/s10071-012-0484-z (doi:10.1007/s10071-012-0484-z) [DOI] [PubMed] [Google Scholar]
- 17.Ferrari MCO, Trowell JJ, Brown GE, Chivers DP. 2005. The role of learning in the development of threat-sensitive predator avoidance by fathead minnows. Anim. Behav. 70, 777–784 10.1016/j.anbehav.2005.01.009 (doi:10.1016/j.anbehav.2005.01.009) [DOI] [Google Scholar]
- 18.Chivers DP, Al-Batati F, Brown GE, Ferrari MCO. 2013. The effect of turbidity on the recognition and generalization of predators and non-predators in aquatic ecosystems. Ecol. Evol. 3, 268–277 10.1002/ece3.454 (doi:10.1002/ece3.454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leduc AOHC, Ferrari MCO, Kelly JM, Brown GE. 2004. Learning to recognize novel predators under weakly acidic conditions: the effects of reduced pH on acquired predator recognition by juvenile rainbow trout. Chemoecology 14, 107–112 10.1007/s00049-003-0268-7 (doi:10.1007/s00049-003-0268-7) [DOI] [Google Scholar]
- 20.Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP. 2011. Intrageneric variation in antipredator responses of coral reef fishes affected by ocean acidification: implications for climate change projections on marine communities. Glob. Change Biol. 17, 2980–2986 10.1111/j.1365-2486.2011.02439.x (doi:10.1111/j.1365-2486.2011.02439.x) [DOI] [Google Scholar]
- 21.Dufour V, Galzin R. 1993. Colonization patterns of reef fish larvae to the lagoon at Moorea Island, French Polynesia. Mar. Ecol. Prog. Ser. 102, 143–152 10.3354/meps102143 (doi:10.3354/meps102143) [DOI] [Google Scholar]
- 22.Almany GR, Webster MS. 2006. The predation gauntlet: early post-settlement mortality in reef fishes. Coral reefs 25, 19–22 10.1007/s00338-005-0044-y (doi:10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 23.Kiesecker JM, Chivers DP, Marco A, Quilchanos C, Anderson MT, Blaustein AR. 1999. Identification of a disturbance signal in larval red-legged frogs, Rana aurora. Anim. Behav. 57, 1295–1300 10.1006/anbe.1999.1094 (doi:10.1006/anbe.1999.1094) [DOI] [PubMed] [Google Scholar]
- 24.Hazlett BA. 1990. Source and nature of disturbance-chemical system in crayfish. J. Chem. Ecol. 16, 2263–2275 10.1007/BF01026936 (doi:10.1007/BF01026936) [DOI] [PubMed] [Google Scholar]
- 25.Vavrek MA, Elvidge CK, Decarie R, Belland B, Jackson CD, Brown GE. 2008. Disturbance cues in freshwater prey fishes: do juvenile convict cichlids and rainbow trout respond to ammonium as an ‘early warning’ signal? Chemoecology 18, 255–261 10.1007/s00049-008-0412-5 (doi:10.1007/s00049-008-0412-5) [DOI] [Google Scholar]
- 26.Almany GR, Berumen ML, Thorrold SR, Planes S, Jones GP. 2007. Local replenishment of coral reef fish populations in a marine reserve. Science 316, 742–744 10.1126/science.1140597 (doi:10.1126/science.1140597) [DOI] [PubMed] [Google Scholar]
- 27.McCormick MI, Manassa RP. 2008. Predation risk assessment by olfactory and visual cues in a coral reef fish. Coral Reefs 27, 105–113 10.1007/s00338-007-0296-9 (doi:10.1007/s00338-007-0296-9) [DOI] [Google Scholar]
- 28.Mirza RS, Chivers DP. 2002. Behavioural responses to conspecific disturbance chemicals enhance survival of juvenile brook charr, Salvelinus fontinalis, during encounters with predators. Behaviour 139, 1099–1110 10.1163/15685390260437272 (doi:10.1163/15685390260437272) [DOI] [Google Scholar]
- 29.Wisenden BD, Chivers DP, Smith RJF. 1995. Early warning in the predation sequence: a disturbance pheromone in Iowa daters (Etheostoma exile). J. Chem. Ecol. 21, 1469–1480 10.1007/BF02035146 (doi:10.1007/BF02035146) [DOI] [PubMed] [Google Scholar]
- 30.Bryer PJ, Mirza RS, Chivers DP. 2001. Chemosensory assessment of predation risk by slimy sculpins (Cottus cognatus): response to alarm, disturbance, and predator cues. J. Chem. Ecol. 27, 533–546 10.1023/A:1010332820944 (doi:10.1023/A:1010332820944) [DOI] [PubMed] [Google Scholar]
- 31.Vavrek MA, Brown GE. 2009. Threat-sensitive response to disturbance cues in juvenile convict cichlids and rainbow trout. Ann. Zool. Fenn. 46, 171–180 10.5735/086.046.0302 (doi:10.5735/086.046.0302) [DOI] [Google Scholar]
- 32.Jordäo LC, Volpato GL. 2000. Chemical transfer of warning information in non-injured fish. Behaviour 137, 681–690 10.1163/156853900502286 (doi:10.1163/156853900502286) [DOI] [Google Scholar]
- 33.Jordäo LC. 2004. Disturbance chemical cues determine changes in spatial occupation by the convict cichlid Archocentrus nigrofasciatus. Behav. Process 67, 453–459 10.1016/j.beproc.2004.07.006 (doi:10.1016/j.beproc.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 34.Ferrari MCO, Vavrek MA, Elvidge CK, Fridman B, Chivers DP, Brown GE. 2008. Sensory complementation and acquisition of predator recognition by salmonid fishes. Behav. Ecol. Sociobiol. 63, 113–121 10.1007/s00265-008-0641-1 (doi:10.1007/s00265-008-0641-1) [DOI] [Google Scholar]
- 35.Mirza RS, Chivers DP. 2000. Predator-recognition training enhances survival of brook trout: evidence from laboratory and field-enclosure studies. Can. J. Zool. 78, 2198–2208 10.1139/z00-164 (doi:10.1139/z00-164) [DOI] [Google Scholar]
- 36.Pohlmann K, Grasso FW, Breithaupt T. 2001. Tracking wakes: the nocturnal predator strategy of piscivorous catfish. Proc. Natl Acad. Sci. USA 98, 7371–7374 10.1073/pnas.121026298 (doi:10.1073/pnas.121026298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radford CA, Stanley JA, Simpson SD, Jeffs AG. 2011. Juvenile coral reef fish use sound to locate habitats. Coral Reefs 30, 295–305 10.1007/s00338-010-0710-6 (doi:10.1007/s00338-010-0710-6) [DOI] [Google Scholar]
- 38.Simpson SD, Meekan MG, McCauley RD, Jeffs AG. 2004. Attraction of settlement-stage coral reef fishes to reef noise. Mar. Ecol. Prog. Ser. 276, 263–268 10.3354/meps276263 (doi:10.3354/meps276263) [DOI] [Google Scholar]
- 39.Simpson SD, Meekan MG, Montgomery JC, McCauley RD, Jeffs AG. 2005. Homeward sound. Science 308, 221. 10.1126/science.1107406 (doi:10.1126/science.1107406) [DOI] [PubMed] [Google Scholar]
- 40.Simpson SD, Meekan MG, Jeffs AG, Montgomery JC, McCauley RD. 2008. Settlement-stage coral reef fishes prefer the higher frequency invertebrate-generated audible component of reef noise. Anim. Behav. 75, 1861–1868 10.1016/j.anbehav.2007.11.004 (doi:10.1016/j.anbehav.2007.11.004) [DOI] [Google Scholar]
- 41.Simpson SD, Meekan MG, Larsen NJ, McCauley RD, Jeffs A. 2010. Behavioural plasticity in larval reef fish: orientation is influenced by recent acoustic experiences. Behav. Ecol. 21, 1098–1105 10.1093/beheco/arq117 (doi:10.1093/beheco/arq117) [DOI] [Google Scholar]
- 42.Simpson SD, Munday PL, Wittenrich ML, Manassa RP, Dixson DL, Gagliano M, Yan HY. 2011. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920 10.1098/rsbl.2011.0293. (doi:10.1098/rsbl.2011.0293.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tolimieri N, Haine O, Jeffs AG, McCauley R, Montgomery JC. 2004. Directional orientation of pomacentrid larvae to ambient reef sounds. Coral Reefs 23, 184–191 10.1007/s00338-004-0383-0 (doi:10.1007/s00338-004-0383-0) [DOI] [Google Scholar]
- 44.Leis JM, Carson-Ewart BM, Cato DH. 2002. Sound detection in situ by the larvae of a coral-reef damselfish (Pomacentridae). Mar. Ecol. Prog. Ser. 232, 259–268 10.3354/meps232259 (doi:10.3354/meps232259) [DOI] [Google Scholar]
- 45.Parmentier E, Colleye O, Mann D. 2009. Hearing ability of three clownfish species. J. Exp. Biol. 212, 2023–2026 10.1242/jeb.030270 (doi:10.1242/jeb.030270) [DOI] [PubMed] [Google Scholar]
- 46.Urick RJ. 1983. Principles of underwater sound. New York, NY: McGraw-Hill [Google Scholar]
- 47.Myrberg AA, Fuiman LA. 2002. The sensory world of coral reef fishes. In Coral reef fishes, dynamics and diversity in a complex ecosystem (ed. Sale PF.), pp. 123–148 Amsterdam, The Netherlands: Elsevier Science [Google Scholar]
- 48.Mitchell MD, McCormick MI, Chivers DP, Ferrari MCO. 2013. Generalization of learned predator recognition in coral reef ecosystems: how cautious are damselfish? Funct. Ecol. 27, 299–304 10.1111/1365-2435.12043 (doi:10.1111/1365-2435.12043) [DOI] [Google Scholar]
- 49.Lönnstedt O, McCormick MI, Chivers DP. 2013. Degraded coral disrupts innate antipredator responses of fish. Ecol. Evol. 3, 38–47 10.1002/ece3.388 (doi:10.1002/ece3.388) [DOI] [PMC free article] [PubMed] [Google Scholar]