Abstract
Flowering plants are characterized by striking variation in reproductive systems, and the evolutionary lability of their sexual traits is often considered a major driver of lineage diversification. But, evolutionary transitions in reproductive form and function are never entirely unconstrained and many changes exhibit strong directionality. Here, I consider why this occurs by examining transitions in pollination, mating and sexual systems, some of which have been considered irreversible. Among pollination systems, shifts from bee to hummingbird pollination are rarely reversible, whereas transitions from animal to wind pollination are occasionally reversed. Specialized pollination systems can become destabilized through a loss of pollinator service resulting in a return to generalized pollination, or more commonly a reliance on self-pollination. Homomorphic and heteromorphic self-incompatibility systems have multiple origins but breakdown to self-compatibility occurs much more frequently with little evidence for subsequent gains, at least over short time-spans. Similarly, numerous examples of the shift from outcrossing to predominant self-fertilization are known, but cases of reversal are very limited supporting the view that autogamy usually represents an evolutionary dead-end. The evolution of dioecy from hermaphroditism has also been considered irreversible, although recent evidence indicates that the occurrence of sex inconstancy and hybridization can lead to the origin of derived sexual systems from dioecy. The directionality of many transitions clearly refutes the notion of unconstrained reproductive flexibility, but novel adaptive solutions generally do not retrace earlier patterns of trait evolution.
Keywords: plant mating, pollination, sexual systems, evolutionary transitions, irreversible evolution
1. Introduction
The ecological versatility of angiosperms is the result of their extraordinary morphological, physiological and life-history diversity. Among the numerous traits that influence life history, those that govern reproduction are particularly influential in facilitating adaptive radiation. Mating patterns affect key evolutionary processes, including genetic transmission, selection response, speciation and the evolutionary diversification of lineages. Indeed, numerous authors have attributed the diversity of angiosperm lineages, for example, Orchidaceae [1] and Polemoniaceae [2], to their reproductive versatility. But how flexible are angiosperm reproductive traits and are some evolutionary transitions more likely to occur than others? Although the lability of floral traits has been considered a hallmark of angiosperm evolution, lineage diversification is never entirely unconstrained. Here, I address these issues by examining evolutionary transitions in pollination, mating and sexual systems, and assess to what extent shifts in reproductive mode are constrained, or in some cases are irreversible.
Evolutionary transitions in form and function are fundamental elements of biological diversification, and the identification of major transitions is an important research programme in plant ecology and evolutionary biology [3,4]. Transitions in plant reproductive systems involve changes in reproductive traits driven largely by natural selection. The traits initially appear within populations and, if adaptive, can spread to survive numerous speciation events and ultimately characterize entire lineages, e.g. evolution of wind pollination [5]. Alternatively, some transitions appear repeatedly but are ephemeral, e.g. evolution of selfing [6]. The longevity of reproductive traits and their influence on diversification rates are currently major topics in evolutionary biology [7]. Parallel changes in reproductive characters among unrelated lineages are of special interest as they can provide insights into selective mechanisms and the genetic and developmental basis of trait convergence. Comparative methods [8] are now widely used for investigating the polarity of transitions, whether they are labile, more restricted in one direction than another or are unidirectional.
The concept of irreversibility was codified as ‘Dollo's Law’, which proposes that structures lost are unlikely to be regained in the same form in which they existed in recent ancestors [9,10]. It is usually assumed that the probability of reversal to an ancestral state is inversely proportional to the developmental complexity of the trait. This perspective was used by Stebbins [11] to interpret the likelihood of reversals for several plant traits, some associated with reproduction. For example, he proposed that shifts from the perennial to the annual life history, changes from radially to bilaterally symmetric flowers and the fusion of floral parts from those that are free, can only be reversed with difficulty. By contrast, he suggested that reproductive traits involving the size and number of organs (e.g. flowers per inflorescence) were highly labile. In general, these predictions have been borne out by comparative analyses and studies of artificial selection. Much of the classical literature on irreversible evolution has focused on morphological characters and fossil evidence; however, in an illuminating essay on reproductive and genetic systems, Bull & Charnov [9] identified several cases of irreversibility, including transitions from outcrossing to selfing and from hermaphroditism to dioecy, topics revisited in this article.
Here, I consider current research on the evolution of plant reproductive systems, focusing on examples in which transitions are constrained or irreversible. I use the term ‘reproductive system’ broadly to include diverse aspects of pollination, mating and sexual system, all of which directly or indirectly affect genetic transmission and evolutionary processes. I begin with transitions between pollination syndromes and ask whether particular floral changes are evolutionarily labile or show evidence of directionality. Next, I consider the gain and loss of self-incompatibility, the principal anti-selfing mechanism in angiosperms, and briefly review several issues relevant to the current debate on whether the evolution of selfing from outcrossing represents an evolutionary dead-end. Finally, I examine the evolution of dioecy from hermaphroditism and consider recent evidence indicating that this transition is not always an endpoint of sexual-system evolution.
2. Transitions in floral traits and pollination syndromes
The immobility of plants and their reliance on pollen vectors for mating is the principal cause of the extraordinary diversification of floral traits and pollination systems in angiosperms. Pollinator-mediated selection on reproductive traits is the primary mechanism of floral diversification and can involve different structural adaptations among related species driven by the same type of pollinator, for example, Pedicularis and Bombus [12], to more specialized floral changes that result from shifts between different functional groups of pollinators [13]. There are numerous examples of shifts between pollination syndromes revealed by phylogenetic analyses [14]. Here, I review examples of transitions in pollination syndromes that are characterized by a strong directional tendency and consider why these patterns occur.
(a). Shifts between animal-pollination syndromes
The transition from bee pollination to hummingbird pollination in New World plants is characterized by striking multi-character changes to floral traits. The hummingbird syndrome has originated on numerous occasions, particularly in western North America where it may have occurred more than 100 times [15]. For example, in the clade comprising Penstemon and segregate genera, a minimum estimate of 21 separate transitions are reported [16]. A striking feature of this transition in Penstemon, and in numerous other genera (e.g. Aquilegia, Costus, Ipomoea, Mimulus and Silene), is that it rarely involves reversals to the bee-pollination syndrome. Why should this be so?
Several hypotheses have been proposed to explain the directional bias in favour of transitions from bee to bird pollination. These include selective mechanisms associated with differences between these pollinator groups in pollen-transfer efficiency, and the nature of genetic mutations in floral pigments associated with the two syndromes [15]. Molecular studies of genetic changes to floral pigments, particularly loss-of-function mutations, provide insight on why the shift from bee to bird pollination is hard to reverse [17]. Investigations of mutational changes causing the shift from blue to red flowers in Iochroma have demonstrated that deletion of a gene coding for an enzyme in the anthocyanin pathway is necessary for the transition [18]. Gene inactivation or loss restricts opportunities for subsequent reversion to the ancestral blue-flowered state.
Bird pollination appears to be the endpoint of pollination syndrome evolution in Penstemon and several groups mentioned above. However, comparative analyses of North American species of Aquilegia have revealed a different picture, with two transitions from bumble-bee to hummingbird pollination and five from hummingbird to hawkmoth pollination [19]. Significantly, there is no evidence for reversals indicating strong directionality in the evolution of pollination systems. A key feature of this directionality involves the evolution of increased floral spurs, with length always increasing and never decreasing, accompanying transitions between pollinator groups. The developmental mechanism underlying spur-length evolution involves fine-tuning of cell shape through anisotropic cell expansion, a process that solely accounts for the variation in spur length in the genus [20]. Spur-length variation is much reduced among Eurasian Aquilegia species, all of which are bee-pollinated [21]. Hawkmoths are not uncommon in Eurasia, raising the question of why no Eurasian species have evolved hawkmoth pollination. The absence of hummingbirds in Eurasia—they left prior to the Aquilegia radiation—may have prevented the intermediate floral condition necessary for the evolution of hawkmoth pollination [19,21]. Such historical constraints may explain other examples where transitions appear to have been thwarted.
(b). Evolution from specialization
The evolutionary diversification of pollination syndromes is commonly associated with increased specialization involving a restricted subset of pollinators in a community. The trend from generalization to specialization characterizes most transitions, although reversals are possible. This has occurred in Dalechampia accompanying migration from Africa, where specialized resin-collecting bees are the principal pollinators, to Madagascar where they are absent and are replaced by a diversity of generalist pollen-feeding insects resulting in a secondary diversification [22]. Long-distance dispersal has clearly played a key role in this transition to generalized pollination. It has recently been shown, however, that a single lineage on Madagascar has rapidly evolved secondary specialization involving buzz pollination by carpenter bees Xylocopa spp. [23], illustrating the striking flexibility of pollination systems in Dalechampia.
Specialized systems may take a different route if mutualisms are lost altogether, resulting in transitions to self-pollination. A remarkable example involves Babiana comprising approximately 86 species endemic to the Cape region of South Africa. At least 14 pollinator transitions have occurred in this genus, including two to bird pollination [24]. Babiana ringens (figure 1a) possesses a uniquely specialized bird perch and is pollinated exclusively by malachite sunbirds [25,26]. Field manipulations demonstrated that the perch positions sunbirds during nectar feeding to promote cross-pollination and high seed set [27]. Populations at the eastern edge of the range have smaller flowers and perches, and although malachite sunbirds commonly visit co-occurring species, they ignore the less rewarding B. ringens. Several lines of evidence suggest that because of the lack of bird visitation, these populations have switched to self-pollination, resulting in relaxed selection and partial dissolution of the bird perch [25]. Specialized pollination systems may often be vulnerable to reduced fertility if pollinator service becomes unreliable, and this may help to explain why flowers with highly specialized pollination often self-pollinate as a mechanism of reproductive assurance [28].
Figure 1.
Examples of specialized pollination systems discussed in this article: (a) Babiana ringens (Iridaceae) endemic to the Cape region of South Africa is pollinated exclusively by malachite sunbirds, which use the naked inflorescence axis as a perch when feeding on nectar; (b) Acampe rigida (Orchidaceae), a nectarless species from southern Yunnan Province, China, exhibits structural adaptations to the flower facilitating self-pollination by rain drops (ombrophily). Image courtesy of Jiang-Yun Gao; (c) Tacca chantrieri (Dioscoreaceae) possesses several traits assumed to function in attracting carrion flies (sapromyiophily); however, populations in southern Yunnan Province are highly self-pollinating; (d) unlike most members of the Juncaceae, which are predominantly wind pollinated, Juncus allioides (Juncaceae), a common species of wet grasslands of the Tibetan plateau, China, possesses showy floral displays and is visited by a wide range of generalist pollinators; (e) Bombus lucorum visiting J. allioides. Image courtesy of Shuang-Quan Huang.
An interesting example of a switch to self-pollination involves Acampe rigida (figure 1b), a deceitful tropical orchid with fragrant flowers that offers no floral rewards. Populations flower during the rainy season when pollinator activity is limited, yet unlike most deceitful orchids plants exhibit high fruit set. This paradox was recently resolved by the discovery that pollination is achieved by raindrop-mediated self-pollination, a phenomenon known as ombrophily [29]. Although flowers are self-compatible (SC), they are incapable of autonomous self-pollination in the absence of rain. Deceit pollination is a risky pollination strategy when pollinators are in short supply and a shift to flowering during the rainy season probably exacerbated this problem in A. rigida.
More perplexing are cases of extreme morphological specialization without obvious contemporary function. Tacca, a genus of 15 species, has large pigmented bracts and numerous motile dangling appendages. These elaborate structures were thought to function by deceit, resembling decaying animal material and attracting carrion flies that mediate cross-pollination (sapromyiophily). However, recent studies of Tacca chantrieri (figure 1c), a species with particularly extravagant displays, cast doubt on this hypothesis. Field observations failed to find evidence for sapromyiophily and investigations of mating patterns, pollen-ovule ratios and population genetic structure unexpectedly revealed that populations were highly selfing and not outcrossing [30,31]. Why these elaborate structures are maintained in a selfer remains a mystery, as it is unclear if they have any current utility. Strong developmental constraints and/or limited genetic variation may limit the major structural alterations required for their loss.
(c). Reversions from wind to animal pollination
The shift from animal pollination to wind pollination has occurred at least 65 times among angiosperm families [5]. It is associated with the loss of traits for pollinator attraction, including showy perianth parts, scent, nectaries and adaptations associated with the aerodynamics of pollen dispersal and capture. The reacquisition of floral traits required for attracting and rewarding pollinators has been viewed as a large hurdle, and thus the transition from animal to wind pollination has been considered largely irreversible [32]. Nevertheless, despite these potential constraints, phylogenetic evidence indicates that this transition appears to have occurred within some angiosperm taxa (e.g. Thalictrum, [33]), indicating that although the transition between the two pollination modes is highly asymmetric, it is certainly not unidirectional.
Recent experimental studies on Cyperus obtusiflorus and Cyperus sphaerocephalus of the predominantly wind-pollinated sedge family (Cyperaceae) provide convincing evidence of insect pollination [34]. The two species possess showy white or yellow bracts and produce scent that attracts bees, beetles and flies to inflorescences. Insect exclusion studies revealed that pollinators were required for seed set, and wind tunnel experiments demonstrated the low motility of pollen compared with co-occurring wind-pollinated sedges, probably because of the occurrence of pollenkitt, which causes pollen to clump and is closely associated with animal pollination. Insect pollination also occurs in the related and predominantly wind-pollinated rush family (Juncaceae). A particularly striking case involves Juncus allioides (figure 1d,e) from southwest China, which exhibits conspicuous floral displays with inflorescences composed of flowers with large white tepals, and like animal-pollinated members of the Cyperaceae, populations are visited by a wide range of generalist pollinators [35]. Juncaceae are unusual for a wind-pollinated family in possessing pollen tetrads [36], a condition more commonly associated with animal pollination. In both Cyperaceae and Juncaceae, pollen aggregation may have facilitated the transition to animal pollination, perhaps via an intermediate stage involving both wind and animal pollination (ambophily).
3. Directionality of mating and sexual-system transitions
Mating involves the pollination and post-pollination processes resulting in ovule fertilization, including both the maternal and paternal contributions of genes to the next generation. Angiosperms exhibit complex mating patterns because of their immobility, reliance on vectors for pollen dispersal and sexual-system diversity. Next, I consider reproductive adaptations governing mating and sexual-system transitions and review evidence for their directionality.
(a). The gain and loss of self-incompatibility systems
Diverse demographic and environmental factors affect outcrossing rates in plant populations, but whether species are self-incompatible (SI) or SC is particularly influential. Different physiological and molecular mechanisms govern the various types of SI system [37], but collectively they represent the most important means by which plants limit inbreeding depression. Self-incompatibility systems are reported from at least 100 plant families representing approximately 40–60% of all angiosperm species [38], and these are classified using various criteria, including whether the mating types are morphologically distinct (heteromorphic) or not (homomorphic), the genetic mode of action (gametophytic or sporophytic) and the sites in the pistil where self-pollen tubes are arrested (stigma, style and ovary). The widespread phylogenetic distribution of SI and the occurrence of families with variation in SI systems suggest more frequent de novo origins of this character than previously thought. Earlier literature on SI often stressed the complex nature of self-recognition systems and the stringent conditions required for their origin.
Although SI systems show multiple independent origins, the frequency of loss far exceeds the number of gains. Moreover, once SI is lost, there is compelling evidence that it is rarely ever regained, at least in its ancestral form. It is important to add this caveat: although it is unlikely that the more derived sporophytic SI has ever evolved directly from the widespread, ancestral gametophytic SI, given their quite different molecular mechanisms, sporophytic SI may have evolved in SC lineages derived from ancestors that originally possessed gametophytic SI. The RNase-based gametophytic system homologous in Solanaceae, Plantaginaceae and Rosaceae has been lost repeatedly, including a minimum of 60 times in the Solanaceae alone [39]. Evidence from shared ancestral polymorphism of S-allele sequences in Solanaceae indicates that once SI is lost in this family, it apparently never re-establishes [40]. Despite methodological difficulties in unequivocally proving Dollo's Law [10], the transition from SI to SC in Solanaceae probably represents the best example of irreversible evolution of a mating system trait.
Are transitions from SI to SC in other systems always irreversible? Recent molecular genetic characterization of the homomorphic sporophytic SI system in Leavenworthia alabamica (Brassicaceae) suggests that this may not always be the case [41]. The S-locus in this species is composed of two linked loci (LaLal2 and LaSCRL) that appear to have evolved secondarily from paralogues of the well-characterized ancestral SRK and SCR genes governing SI in other members of Brassicaceae, but which are absent in L. alabamica. This may have resulted from neo-functionalization of these genes, which are present in the related Arabidopsis lyrata, but do not function in SI. At this stage, it is not possible to unequivocally determine whether the secondary evolution of an S-locus in Leavenworthia occurred in a lineage that had previously lost SI, or whether SRK and SCR were still present when LaLal2 and LaSCRL took on S-locus function. Nevertheless, these results are exciting and suggest that genetic mechanisms governing SI evolution may be less constrained than often portrayed.
Families that contain different forms of SI are of particular interest for investigating evolutionary relationships among SI systems. Recent surveys indicate that 12–22 families appear to contain more than one SI system [38,39]. The Polemoniaceae is of particular interest as it contains approximately 275 species with four different classes of SI, including: gametophytic SI in Phlox, sporophytic SI in Linanthus, late-acting (ovarian) SI in Ipomopsis and heteromorphic SI in Aliciella (reviewed in [42]). It is not known whether these different types of incompatibility arose independently, whether they are evolutionarily related or whether several forms of SI operate together in governing self-rejection, as has been suggested by Lewis [43] for Brassicaceae and Asteraceae, but not confirmed. Phylogenetic and molecular genetic studies of families containing more than one type of SI system would be valuable to determine whether homologous loci govern self-rejection and the extent to which one system might act in concert with another.
In heteromorphic SI, there are two (distyly) or three (tristyly) mating groups that, in addition to their specific incompatibility relationships, differ in style length, anther height and pollen and stigma polymorphisms. This suite of traits is commonly referred to as the ‘heterostylous syndrome’, and the origin of heterostyly has usually been considered a relatively uncommon event because of the complex evolutionary forces required for assembling the morphological and physiological components of the syndrome, and the restricted range of floral characters that allow the polymorphism to be selected [44,45]. It has been estimated that heterostyly has evolved on at least 23 separate occasions, based on its distribution among 19 orders of angiosperms [45]. However, this number will increase as phylogenetic data become available for more taxa, particularly families that contain very large numbers of heterostylous taxa (e.g. Rubiaceae and Oxalidaceae). Several recent phylogenetic studies (e.g. Linum [46], Lithodora [47], Lithospermum [48] and Nymphoides [49]) have reported multiple origins of heterostyly within each genus. However, uncertainties related to ancestral character state reconstruction owing to limited taxon sampling, and how character transitions should be weighted, have probably led to overestimates in the number of gains of heterostyly in several of these studies. These methodological difficulties highlight the problem in reconstructing the evolutionary history of complex reproductive syndromes.
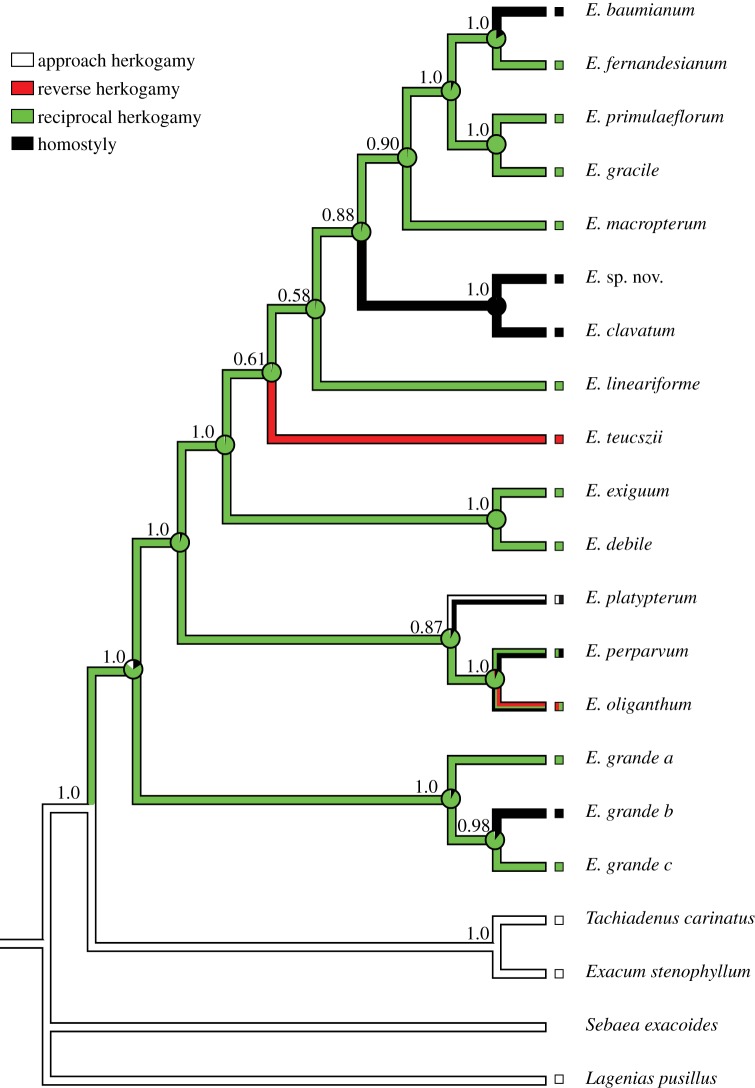
In common with homomorphic SI, losses of heteromorphic SI far exceed the number of gains. In most families, there is evidence for the repeated breakdown of heterostyly resulting in the evolution of homostyly, in which populations are monomorphic for self-pollinating, SC plants with anthers and stigmas of similar height. For example, a recent study of the evolutionary history of Exochaenium [50] documented a single origin of distyly followed by five independent losses, four of which involved transitions to homostyly (figure 2). Selfing variants in heterostylous groups either arise by recombination in the linkage group governing heterostyly or by genetic modifiers of sex-organ position that occur elsewhere in the genome. Homostyle evolution is commonly associated with floral and mating system divergence leading to speciation, and the transition appears to be largely irreversible. Despite numerous cases of the breakdown of heterostyly, there are few convincing examples of the reacquisition of the heterostylous syndrome once it has been lost in a lineage (but see [46]). This is probably because of the complexity of the syndrome and because homostyle evolution is frequently associated with high selfing, the loss of floral traits promoting outcrossing and low levels of genetic diversity in populations. As discussed later, transitions to predominant selfing often appear to be irreversible.
Figure 2.
Reconstruction of the evolutionary history of herkogamy and homostyly in Exochaenium (Gentianaceae) based on a molecular phylogeny of the genus and several outgroups. Three forms of herkogamy (the spatial separation of anthers and stigmas within flowers) are represented in Exochaenium: approach (stigmas above anthers), reverse (stigmas below anthers) and reciprocal herkogamy (polymorphism for approach and reverse herkogamy), also referred to as distyly. In Exochaenium, distyly has a single origin but has broken down to homostyly (stigmas and anthers at similar positions) on four occasions (after Kissling & Barrett [50] with permission from Annals of Botany, Oxford University Press).
(b). Is predominant selfing an evolutionary dead-end?
Stebbins [11] proposed that the evolution of predominant selfing from high levels of outcrossing is an evolutionary dead-end. Since then, there has been sustained interest in the evolutionary fate of selfing populations, and whether transitions from outcrossing to selfing can be reversible. Because this topic has been the subject of two recent reviews [6,51], I discuss it briefly here and focus on unresolved issues and empirical evidence. What is often forgotten in this discussion is the fact that the mating system is a quantitative trait. Selfing rates (s) vary continuously from zero (complete outcrossing) to near 1.00, so that dichotomizing species or populations into selfers and outcrossers can mask considerable complexity. Currently, it is not possible to specify if there is a particular selfing rate beyond which populations are inevitably driven to predominant selfing (e.g. s > 0.9), or for how long a history of selfing is required before populations become incapable of returning to outcrossing. However, it seems likely that in populations with partial selfing (mixed mating), genetic constraints to evolving higher outcrossing may not be especially severe. Populations with mixed mating often possess standing genetic variation for traits promoting outcrossing, e.g. herkogamy, the spatial separation of anthers and stigmas [52], and there is no evidence that they exhibit reduced inbreeding depression compared with predominantly outcrossing species [53]. The purging of deleterious mutations causing inbreeding depression is most commonly viewed as the primary mechanism preventing reversion back to outcrossing, and a sustained history of high selfing is probably required for this to occur. Thus, any discussion of whether selfing represents an evolutionary blind alley should focus primarily on highly autogamous taxa.
What empirical evidence is there for reversions from predominant selfing to high outcrossing? The limited data available are meagre at best and hardly conclusive. In Scutellaria angustifolia, there is phylogenetic evidence that a large-flowered outcrossing subspecies is derived from a small-flowered selfing subspecies [54], but the mating systems of these two taxa have not been quantified. Controlled crosses between selfing variants of Eichhornia paniculata resulted in an outcrossing phenotype, owing to genetic complementarity of mating-system modifier alleles; however, the variants originate from widely separated parts of the geographical range and are unlikely to exchange genes [55]. In Medicago, the most parsimonious reconstruction of the evolutionary history of mating patterns suggests that selfing is the ancestral condition, with recurrent transitions to outcrossing; however, other weighting schemes and morphological evidence contradict this conclusion [56]. Perhaps, the most convincing case of a reversion from selfing to outcrossing involves island populations of SC, homostylous Turnera ulmifolia. Homostyly in this group has originated through recombination in the distyly linkage group [57], and these forms have colonized Caribbean islands presumably because of their facility for autonomous self-pollination (Baker's Law). On some islands, the homostyles have evolved various degrees of herkogamy through selection on quantitative genetic variation in stigma–anther separation, and this has resulted in increased outcrossing rates compared with ancestral homostyles [57,58]. Turnera ulmifolia is an allohexaploid and hybridity may have boosted standing genetic variation in floral traits, enabling selection for outcrossing. Also, these events occurred on islands, which are known for many striking examples of adaptive reversals in common evolutionary trends [59]. The future search for examples of the evolution of outcrossing from selfing might profitably examine plant radiations on islands.
(c). Evolution to and from dioecy
Dioecy occurs in only 6–7% of flowering plant species but is represented in close to half of all angiosperm families, originating on at least 100 occasions from hermaphroditism [60,61]. The most widely accepted hypothesis for the evolution of dioecy is that it functions to avoid inbreeding, a frequent cost of hermaphroditism. Dioecy usually evolves from SC rather than SI ancestors, a pattern consistent with this explanation, and several different routes are involved of which the gynodioecy and monoecy pathways are most common [62]. The transition to separate sexes has often been viewed as an endpoint of sexual-system evolution, in part, because reversions to hermaphroditism have seemed unlikely, particularly in animals [9]. Because in flowering plants, dioecious taxa are often at the tips of phylogenies and are significantly lower in species richness than hermaphroditic sister groups, dioecy like selfing has been described as an evolutionary dead-end [63].
Although in many angiosperm taxa, dioecy commonly represents a terminus of sexual-system evolution, there is growing evidence that in some groups this is not the case. Lloyd [64] first documented reversions from dioecy to monoecy in three species of Cotula, and since then several other examples have come to light. For example, phylogenetic reconstructions of the evolutionary history of sexual systems in Momordica indicate seven independent transitions from dioecy to monoecy [65], apparently associated with changes in the ecology and pollination biology of species. In Wurmbea/Iphigenia, long-distance dispersal from Australia to New Zealand is associated with a transition from dioecy to hermaphroditism, a pattern consistent with Baker's Law [66]. In Mercurialis annua, diploid populations are dioecious, whereas derived androdioecious and monoecious populations are polyploid [67]. Most transitions from dioecy to other sexual systems require the establishment of hermaphroditism from unisexuality and depend on the occurrence of standing genetic variation in sex expression in dioecious populations, or the occurrence of hybridization.
Sagittaria latifolia provides a particularly striking example of the evolution of diverse gender strategies from dioecy. Populations are most commonly either monoecious or dioecious, a relatively uncommon condition in most flowering plants [68]. In many dioecious populations of S. latifolia, a low frequency (less than 5%) of male plants produce some female flowers; a common phenomenon among dioecious species known as ‘male sex inconstancy’. At the northern range limit of S. latifolia in eastern North America, a more complex situation occurs involving a wider range of gender strategies, including subdioecious (females, males and hermaphrodites), androdioecious (males and hermaphrodites) and gynodioecious (females and hermaphrodites) populations. Investigations using sexual-system-specific molecular markers indicate that subdioecious populations have arisen by two distinct mechanisms: (i) directly from dioecious populations through increase in the frequency of sex-inconstant males; or (ii) through hybridization between monoecious and dioecious populations ([69]; S. B. Yakimowski & S. C. H. Barrett 2013, unpublished data). Also some monoecious populations in this region have originated directly from subdioecious populations containing sex inconstant males. Their hermaphroditic condition probably enabled them to found colonies following dispersal. Thus, in S. latifolia, both standing genetic variation in sex expression and hybridization play a role in promoting evolution from dioecy, and the routes linking sexual systems involve two-way rather than one-way streets.
4. Conclusions
This review has largely highlighted examples of transitions in which there is strong evidence of directionality. Although angiosperms display remarkable reproductive flexibility, phyletic heritage, developmental and genetic constraints, and the nature of selection acting on pollination and mating can direct changes along particular evolutionary pathways more often than others. Determining the underlying molecular mechanisms responsible for constrained evolution could contribute significantly to our understanding of reproductive transitions.
Selection experiments and studies of experimental evolution offer valuable opportunities to explore the tempo and directionality of reproductive trait evolution. For example, experimental manipulations of stress conditions in cultures of Caenorhabditis elegans resulted in a shift in mating system from predominant selfing to partial outcrossing [70]. Similar types of studies in predominantly autogamous plants could provide useful lessons for assessing the adaptive benefits of recombination and segregation arising from outcrossing. Few studies have investigated the response to selection on levels of autogamy, but where this has been conducted both increased and decreased levels of self-pollination were observed [71]. Although challenging, such experiments can be especially revealing if they include the presence and absence of pollinators, as was recently done to examine the patterns of phenotypic and genetic change associated with the selfing syndrome [72].
Finally, our ability to identify constrained evolution and the occurrence of irreversibility has largely used phylogenetic methods. However, as pointed out by Goldberg & Igic [10], tests of irreversible evolution frequently provide incorrect results owing to a variety of technical problems associated with the models used. Future studies on the problem of irreversible evolution will benefit from the integration of improved phylogenetic methods, selection experiments and the molecular genetic dissection of trait evolution.
Acknowledgements
Part of this article was the Presidential Address to the annual meeting of the Canadian Society for Ecology and Evolution in Ottawa, July 2012. I thank Andrea Case, Aneil Agrawal, Asher Cutter, Sean Graham, Lawrence Harder, Boris Igic, Mark Rausher, Dan Schoen, James Thomson and Stephen Wright for valuable discussion and providing articles and unpublished manuscripts.
Funding statement
My work on plant reproductive systems has been supported by Discovery grants from the Natural Sciences and Engineering Research Council of Canada and funding through the Canada Research Chair's Program.
References
- 1.Dressler RL. 1981. The orchids: natural history and classification. Cambridge, MA: Harvard University Press [Google Scholar]
- 2.Grant V, Grant KA. 1965. Flower pollination in the phlox family. New York, NY: Columbia University Press [Google Scholar]
- 3.Barrett SCH. 2008. Major evolutionary transitions in flowering plant reproduction. Chicago, IL: University of Chicago Press [Google Scholar]
- 4.Feild TS, Edwards EJ. 2012. Major transitions in angiosperm ecology and functional biology. Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Friedman J, Barrett SCH. 2008. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 103, 1515–1527 10.1093/aob/mcp035 (doi:10.1093/aob/mcp035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igic B, Busch JW. 2013. Is self-fertilization an evolutionary dead end? New Phytol. 198, 386–397 10.1111/nph.12182 (doi:10.1111/nph.12182) [DOI] [PubMed] [Google Scholar]
- 7.Goldberg EE, Kohn JR, Lande R, Robertson KA, Smith SA, Igic B. 2010. Species selection maintains self-incompatibility. Science 330, 493–495 10.1126/science.1194513 (doi:10.1126/science.1194513) [DOI] [PubMed] [Google Scholar]
- 8.Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press [Google Scholar]
- 9.Bull JJ, Charnov EL. 1985. On irreversible evolution. Evolution 39, 1149–1155 10.2307/2408742 (doi:10.2307/2408742) [DOI] [PubMed] [Google Scholar]
- 10.Goldberg EE, Igic B. 2008. On phylogenetic tests of irreversible evolution. Evolution 62, 2727–2741 10.1111/j.1558-5646.2008.00505.x (doi:10.1111/j.1558-5646.2008.00505.x) [DOI] [PubMed] [Google Scholar]
- 11.Stebbins GL. 1974. Flowering plants: evolution above the species level. Cambridge, MA: Harvard University Press [Google Scholar]
- 12.Eaton DA, Fenster CB, Hereford J, Huang S-Q, Ree RH. 2012. Floral diversity and community structure in Pedicularis (Orobanchaceae). Ecology 93, S182–S194 10.1890/11-0501.1 (doi:10.1890/11-0501.1) [DOI] [Google Scholar]
- 13.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Ann. Rev. Eco. Evol. Syst. 35, 375–403 10.1146/annurev.ecolsys.34.011802.132347 (doi:10.1146/annurev.ecolsys.34.011802.132347) [DOI] [Google Scholar]
- 14.van der Niet T, Johnson SD. 2012. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends Ecol. Evol. 27, 353–361 10.1016/j.tree.2012.02.002 (doi:10.1016/j.tree.2012.02.002) [DOI] [PubMed] [Google Scholar]
- 15.Thomson JD, Wilson P. 2008. Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence and directionality. Int. J. Plant Sci. 199, 23–28 10.1086/523361 (doi:10.1086/523361) [DOI] [Google Scholar]
- 16.Wilson P, Wolfe AD, Armbruster WS, Thomson JD. 2007. Constrained lability in floral evolution: counting convergent origins of hummingbird pollination in Penstemon and Keckiella. New Phytol. 176, 883–890 10.1111/j.1469-8137.2007.02219.x (doi:10.1111/j.1469-8137.2007.02219.x) [DOI] [PubMed] [Google Scholar]
- 17.Rausher MD. 2008. Evolutionary transitions in floral color. Int. J. Plant Sci. 199, 7–21 10.1086/523358 (doi:10.1086/523358) [DOI] [Google Scholar]
- 18.Smith SD, Rausher MD. 2011. Gene loss and parallel evolution contribute to species difference in flower color. Mol. Biol. Evol. 28, 2799–2810 10.1093/molbev/msr109 (doi:10.1093/molbev/msr109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittall JB, Hodges SA. 2007. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–710 10.1038/nature05857 (doi:10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- 20.Puzey JR, Gerbode SJ, Hodges SA, Kramer EM, Mahadevan L. 2012. Evolution of spur-length diversity in Aquilegia petals is achieved solely through cell-shape anisotropy. Proc. R. Soc. B 279, 1640–1645 10.1098/rspb.2011.1873 (doi:10.1098/rspb.2011.1873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastida JM, Alcántara JM, Rey PJ, Vargas P, Herrera C. 2010. Extended phylogeny of Aquilegia: the biogeographical and ecological patterns of two simultaneous but contrasting radiations. Plant Syst. Evol. 284, 171–185 10.1007/s00606-009-0243-z (doi:10.1007/s00606-009-0243-z) [DOI] [Google Scholar]
- 22.Armbruster WS, Baldwin BG. 1998. Switch from specialized to generalized pollination. Nature 394, 632. 10.1038/29210 (doi:10.1038/29210) [DOI] [Google Scholar]
- 23.Armbruster WS, Lee J, Edwards ME, Baldwin BG. 2013. Floral paedomorphy leads to secondary specialization in pollination of Madasgascar Dalechampia (Euphorbiaceae). Evolution 67, 1196–1203 10.1111/evo.12002 (doi:10.1111/evo.12002) [DOI] [PubMed] [Google Scholar]
- 24.Goldblatt P, Manning JC. 2006. Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. Ann. Bot. 97, 317–344 10.1093/aob/mcj040 (doi:10.1093/aob/mcj040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Waal C, Anderson B, Barrett SCH. 2012. The natural history of pollination and mating in bird-pollinated Babiana (Iridaceae). Ann. Bot. 109, 667–679 10.1093/aob/mcr172 (doi:10.1093/aob/mcr172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Waal C, Barrett SCH, Anderson B. 2012. The effects of mammalian herbivory on inflorescence architecture in ornithophilous Babiana (Iridaceae). Am. J. Bot. 99, 1096–1103 10.3732/ajb.1100295 (doi:10.3732/ajb.1100295) [DOI] [PubMed] [Google Scholar]
- 27.Anderson B, Cole WW, Barrett SCH. 2005. Specialized bird perch aids in cross-pollination. Nature 435, 41–42 10.1038/435041a (doi:10.1038/435041a) [DOI] [PubMed] [Google Scholar]
- 28.Fenster CB, Martén-Rodríguez M. 2007. Reproductive assurance and the evolution of pollinator specialization. Int. J. Plant Sci. 168, 215–228 10.1086/509647 (doi:10.1086/509647) [DOI] [Google Scholar]
- 29.Fan X-L, Barrett SCH, Lin H, Chen L-L, Zhou X, Gao JY. 2012. Rain pollination provides reproductive assurance in a deceptive orchid. Ann. Bot. 110, 953–958 10.1093/aob/mcs165 (doi:10.1093/aob/mcs165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Barrett SCH, Gao J-Y, Chen J, Cole WW, Liu Y, Bai ZL, Li Q-J. 2005. Predicting mating patterns from pollination syndromes: the case of ‘sapromyiophily’ in Tacca chantrieri (Taccaceae). Am. J. Bot. 92, 517–524 10.3732/ajb.92.3.517 (doi:10.3732/ajb.92.3.517) [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Li Q-J, Li H-T, Chen J, Li D-Z. 2006. Genetic diversity and geographical differentiation in Tacca chantrieri (Taccaceae): an autonomous selfing plant with showy floral display. Ann. Bot. 98, 449–457 10.1093/aob/mcl123 (doi:10.1093/aob/mcl123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox PA, Grubb PJ. 1991. An evolutionary escape for animal-pollinated angiosperms. Phil. Trans. R. Soc. Lond. B 333, 217–224 10.1098/rstb.1991.0070 (doi:10.1098/rstb.1991.0070) [DOI] [Google Scholar]
- 33.Soza VL, Brunet J, Liston A, Smith PS, Di Stilio VS. 2012. Phylogenetic insights into the correlates of dioecy in meadow rues (Thalictrum, Ranuculaceae). Mol. Phyl. Evol. 63, 180–192 10.1016/j.ympev.2012.01.009 (doi:10.1016/j.ympev.2012.01.009) [DOI] [PubMed] [Google Scholar]
- 34.Wragg PD, Johnson SD. 2011. Transition from wind pollination to insect pollination in sedges: experimental evidence and functional traits. New Phytol. 191, 1128–1140 10.1111/j.1469-8137.2011.03762.x (doi:10.1111/j.1469-8137.2011.03762.x) [DOI] [PubMed] [Google Scholar]
- 35.Huang S-Q, Xiong Y-Z, Barrett SCH. In press Experimental evidence of insect pollination in Juncaceae, a primarily wind-pollinated family. Int. J. Plant Sci. [Google Scholar]
- 36.Michalski SG, Durka W. 2010. Pollen and ovule production in wind-pollinated species with special reference to Juncus. Plant Syst. Evol. 286, 191–197 10.1007/s00606-010-0299-9 (doi:10.1007/s00606-010-0299-9) [DOI] [Google Scholar]
- 37.Franklin-Tong VE. 2008. Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Berlin, Germany: Springer [Google Scholar]
- 38.Ferrer MM, Good SV. 2012. Self-sterility in flowering plants: preventing self-fertilization increases family diversification rates. Ann. Bot. 110, 535–553 10.1093/aob/mcs124 (doi:10.1093/aob/mcs124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igic B, Lande R, Kohn R. 2008. Loss of self-incompatibility and its evolutionary consequences. Int. J. Plant Sci. 169, 93–104 10.1086/523362 (doi:10.1086/523362) [DOI] [Google Scholar]
- 40.Igic B, Bohs L, Kohn JR. 2006. Ancient polymorphism reveals unidirectional breeding system shifts. Proc. Natl Acad. Sci. USA 103, 1359–1363. 10.1073/pnas.0506283103 (doi:10.1073/pnas.0506283103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chantha S-C, Herman AC, Platts A, Vekemans X, Schoen DJ. 2013. Secondary evolution of a self-incompatibility locus in the Brassicaceae genus Leavenworthia. PLoS Biol. 11, e1001560. 10.1371/journal.pbio.1001560 (doi:10.1371/journal.pbio.1001560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaDoux T, Friar EA. 2006. Late-acting self-incompatibility in Ipomopsis tenuifolia (Gray) V. Grant (Polemoniaceae). Int. J. Plant Sci. 167, 463–471 10.1086/500985 (doi:10.1086/500985) [DOI] [Google Scholar]
- 43.Lewis D. 1994. Gametophytic–sporophytic incompatibility. In Genetic control of self-incompatibility and reproductive development in flowering plants (eds Williams EG, Clarke AE, Knox RB.), pp. 88–101 Dordrecht, The Netherlands: Kluwer [Google Scholar]
- 44.Barrett SCH, Shore JS. 2008. New insights on heterostyly: comparative biology, ecology and genetics. In Self-incompatibility in flowering plants: evolution, diversity, and mechanisms (ed. Franklin-Tong VE.), pp. 3–32 Berlin, Germany: Springer [Google Scholar]
- 45.Lloyd DG, Webb CJ. 1992. The evolution of heterostyly. In Evolution and function of heterostyly (ed. Barrett SCH.), pp. 151–178 Berlin, Germany: Springer [Google Scholar]
- 46.McDill J, Repplinger M, Simpson BB, Kadereit JW. 2009. The phylogeny of Linum subfamily Linoideae, with implications for their systematics, biogeography, and evolution of heterostyly. Syst. Bot. 34, 386–405 10.1600/036364409788606244 (doi:10.1600/036364409788606244) [DOI] [Google Scholar]
- 47.Ferrero V, Arroyo J, Vargas P, Thompson JD, Navarro L. 2009. Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae). Pers. Plant Ecol. Evol. Syst. 11, 111–125 10.1016/j.ppees.2009.01.004 (doi:10.1016/j.ppees.2009.01.004) [DOI] [Google Scholar]
- 48.Cohen JI. 2011. A phylogenetic analysis of morphological and molecular characters of Lithospermum L. (Boraginaceae) and related taxa: evolutionary relationships and character evolution. Cladistics 27, 559–580 10.1111/j.1096-0031.2011.00352.x (doi:10.1111/j.1096-0031.2011.00352.x) [DOI] [PubMed] [Google Scholar]
- 49.Tippery NP, Les DH. 2011. Phylogenetic relationships and morphological evolution in Nymphoides (Menyanthaceae). Syst. Bot. 36, 1101–1113 10.1600/036364411X605092 (doi:10.1600/036364411X605092) [DOI] [Google Scholar]
- 50.Kissling J, Barrett SCH. 2013. Variation and evolution of herkogamy in Exochaenium: implications for the evolution of distyly. Ann. Bot. 112, 95–102 10.1093/aob/mct097 (doi:10.1093/aob/mct097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright SI, Kalisz S, Slotte T. 2013. Evolutionary consequences of self-fertilization in plants. Proc. R. Soc. B 280, 20130133. 10.1098/rspb.2013.0133 (doi:10.1098/rspb.2013.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shore JS, Barrett SCH. 1990. Quantitative genetics of floral characters in homostylous Turnera ulmifolia var. angustifolia (Turneraceae). Heredity 64, 105–112 10.1038/hdy.1990.13 (doi:10.1038/hdy.1990.13) [DOI] [Google Scholar]
- 53.Winn AA, et al. 2011. Analysis of inbreeding depression in mixed-mating plants provides evidence for selective interference and stable mixed mating. Evolution 65, 3339–3359 10.1111/j.1558-5646.2011.01462.x (doi:10.1111/j.1558-5646.2011.01462.x) [DOI] [PubMed] [Google Scholar]
- 54.Olmstead RG. 1990. Biological and historical factors influencing genetic diversity in the Scutellaria angustifolia complex (Labiatae). Evolution 44, 54–70 10.2307/2409524 (doi:10.2307/2409524) [DOI] [PubMed] [Google Scholar]
- 55.Fenster CB, Barrett SCH. 1994. Inheritance of mating-system modifier genes in Eichhornia paniculata. Heredity 72, 433–445 10.1038/hdy.1994.62 (doi:10.1038/hdy.1994.62) [DOI] [Google Scholar]
- 56.Bena G, Lejeune B, Prosperi J-M, Olivieri I. 1998. Molecular phylogenetic approach for studying life-history evolution: the ambiguous example of the genus Medicago. Proc. R. Soc. Lond. B 265, 1141–1151 10.1098/rspb.1998.0410 (doi:10.1098/rspb.1998.0410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shore JS, Barrett SCH. 1985. The genetics of distyly and homostyly in Turnera ulmifolia L. (Turneraceae). Heredity 55, 167–174 10.1038/hdy.1985.88 (doi:10.1038/hdy.1985.88) [DOI] [Google Scholar]
- 58.Barrett SCH, Shore JS. 1987. Variation and evolution of breeding systems in the Turnera ulmifolia L. complex (Turneraceae). Evolution 41, 340–354 10.2307/2409143 (doi:10.2307/2409143) [DOI] [PubMed] [Google Scholar]
- 59.Carlquist S. 1974. Island biology. New York, NY: Columbia University Press [Google Scholar]
- 60.Renner SS, Ricklefs RE. 1995. Dioecy and its correlates in the flowering plants. Am. J. Bot. 82, 595–606 10.2307/2445418 (doi:10.2307/2445418) [DOI] [Google Scholar]
- 61.Charlesworth D. 2002. Plant sex determination and sex chromosomes. Heredity 88, 94–101 10.1038/sj.hdy.6800016 (doi:10.1038/sj.hdy.6800016) [DOI] [PubMed] [Google Scholar]
- 62.Barrett SCH. 2002. The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284 10.1038/nrg776 (doi:10.1038/nrg776) [DOI] [PubMed] [Google Scholar]
- 63.Heilbuth J. 2000. Lower species richness in dioecious clades. Am. Nat. 156, 221–241 10.1086/303389 (doi:10.1086/303389) [DOI] [PubMed] [Google Scholar]
- 64.Lloyd DG. 1975. Breeding systems in Cotula. IV. Reversion from dioecy to monoecy. New Phytol. 74, 125–145 10.1111/j.1469-8137.1975.tb01346.x (doi:10.1111/j.1469-8137.1975.tb01346.x) [DOI] [Google Scholar]
- 65.Schaefer H, Renner SS. 2010. A three-genome phylogeny of Momordica (Cucurbitaceae) suggests seven returns from dioecy to monoecy and recent long-distance dispersal to Asia. Mol. Phyl. Evol. 54, 553–560 10.1016/j.ympev.2009.08.006 (doi:10.1016/j.ympev.2009.08.006) [DOI] [PubMed] [Google Scholar]
- 66.Case AL, Graham SG, Macfarlane TD, Barrett SCH. 2008. A phylogenetic study of evolutionary transitions in sexual systems in Australasian Wurmbea (Colchicaceae) . Int. J. Plant Sci. 169, 141–156 10.1086/523368 (doi:10.1086/523368) [DOI] [Google Scholar]
- 67.Obbard DJ, Harris SA, Buggs RJA, Pannell JR. 2006. Hybridization, polyploidy, and the evolution of sexual systems in Mercurialis (Euphorbiaceae). Evolution 60, 1801–1815 10.1111/j.0014-3820.2006.tb00524.x (doi:10.1111/j.0014-3820.2006.tb00524.x) [DOI] [PubMed] [Google Scholar]
- 68.Dorken ME, Friedman J, Barrett SCH. 2002. The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae). Evolution 56, 31–41 10.1111/j.0014-3820.2002.tb00847.x (doi:10.1111/j.0014-3820.2002.tb00847.x) [DOI] [PubMed] [Google Scholar]
- 69.Barrett SCH, Yakimowski SB, Field DL, Pickup M. 2010. Ecological genetics of sex ratios in plant populations. Phil. Trans. R. Soc. B 365, 2549–2557 10.1098/rstb.2010.0002 (doi:10.1098/rstb.2010.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morran LT, Cappy BJ, Anderson JL, Phillips PC. 2009. Sexual partners for the stressed: facultative outcrossing in the self-fertilizing nematode Caenorhabditis elegans. Evolution 63, 1473–1482 10.1111/j.1558-5646.2009.00652.x (doi:10.1111/j.1558-5646.2009.00652.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bixby PJ, Levin DA. 1996. Response to selection on autogamy in Phlox. Evolution 50, 892–899 10.2307/2410860 (doi:10.2307/2410860) [DOI] [PubMed] [Google Scholar]
- 72.Bodbyl Roels SA, Kelly JK. 2011. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution 65, 2541–2552 10.1111/j.1558-5646.2011.01326.x (doi:10.1111/j.1558-5646.2011.01326.x) [DOI] [PMC free article] [PubMed] [Google Scholar]