Abstract
Social animals encountering natural dangers face decisions such as whether to freeze, flee or harass the threat. The American crow, Corvus brachyrhynchos, conspicuously mobs dangers. We used positron emission tomography to test the hypothesis that distinct neuronal substrates underlie the crow's consistent behavioural response to different dangers. We found that crows activated brain regions associated with attention and arousal (nucleus isthmo-opticus/locus coeruleus), and with motor response (arcopallium), as they fixed their gaze on a threat. However, despite this consistent behavioural and neural response, the sight of a person who previously captured the crow, a person holding a dead crow and a taxidermy-mounted hawk activated distinct forebrain regions (amygdala, hippocampus and portion of the caudal nidopallium, respectively). We suggest that aspects of mobbing behaviour are guided by unique neural circuits that respond to differences in mental processing—learning, memory formation and multisensory discrimination—required to appropriately nuance a risky behaviour to specific dangers.
Keywords: fear, memory, whole brain image
1. Introduction
The behaviour of animals is profoundly shaped by encounters with danger. Small birds utter high-pitched calls, freeze and hide at the sight of a hawk [1]. Whether hunted by sharks or wolves, grazing animals adjust their foraging locations, eating behaviour and vigilance to the presence of predators [2]. Herding and flocking animals gather in coordinated masses as a predator closes the chase [3]. In contrast to such self-preserving behaviours, social animals also engage in risky behaviour at the sight of danger. Some deer and antelope conspicuously prance and flag their tails as they flee from a lion [4]. Vervet monkeys inspect threats before warning their troop members with predator-specific vocalizations [5]. Social birds known for their brashness and intellect, such as the crow, attack would-be predators. When a crow discovers a predatory hawk perched, it rushes headlong towards the hunter, climbing high, then diving at the raptor who flinches just as the crow pulls up to rocket skyward and dive again. As the crow harasses, it gives harsh scolding vocalizations that attract other crows to join the mob [6]. The predator occasionally kills the aggressive crow, but more often it leaves the area, affording crows time for essential behaviours that increase survival [7]. Other natural dangers are met with the same crow response. The intensity varies, but not the fundamental aspects of the behaviour [6,8]. A person who has wronged a crow in the past is instantly recognized, scolded and mobbed [8,9]. Even a dead crow attracts a scolding mob [10,11]. Antipredator behaviour, even the paradoxical risking of life by a mobbing crow, is understood from a behavioural, ecological and evolutionary perspective [12–14]. However, the way in which the brain integrates diverse sensations, context, history and emotion into a unique set of behavioural responses, such as the mobbing behaviour of a crow, is unknown [15].
Neuroscientists have produced a general understanding of the way in which neuronal firing patterns affect regional brain activity, and the way distinct regions of the brain network to evoke a great diversity of vertebrate behaviours [16]. We know, for example, in birds that midbrain vocal centres control scolding [17,18], and in birds and mammals that the amygdala is involved in learned fear responses [19,20], including those of a crow encountering a person known to be dangerous [21]. However, because a great variety of stimuli elicit the same diving, calling and rallying behaviour during mobbing, the crow's stereotypical actions are the final message from a neural network that must guide innate reflexive responses, enable rapid associative and spatial learning, and weigh the costs and benefits of risky actions.
Functional imaging studies complement more traditional cell recording, stimulation, lesion, gene expression and tracing studies to increase our understanding of how animal brains work [22]. Imaging enables researchers to visualize the brain's activity during the performance of a task, but its application to animals other than humans has been limited by the need to restrain or sedate subjects during scanning [22,23]. We have overcome this with the relatively non-invasive F-18 fluorodeoxyglucose positron emission tomography (FDG-PET [24]; figure 1) and have begun to image the brains of wild crows as they assess natural challenges [21].
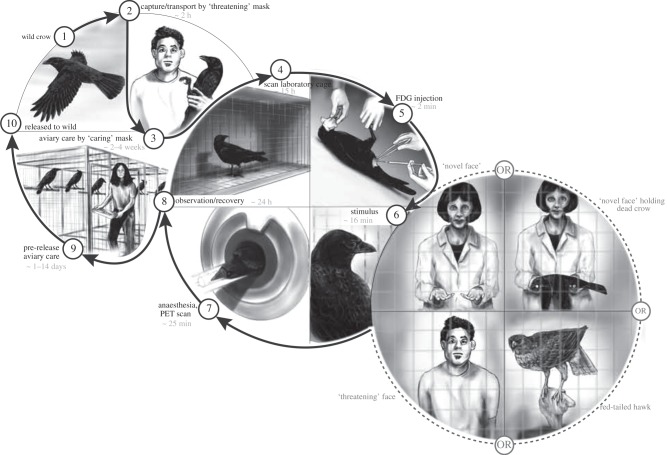
Figure 1.
Sequence of steps in the experimental protocol. Crows learned two faces during their approximately one month long tenure in captivity: the face of the person who captured them (threatening face) and the face of the person who fed and cared for them (caring face). During stimulation, as crows metabolized a previous interperitoneal injection of (F-18) fluorodeoxyglucose (FDG), they saw these faces, another person never seen before (novel) holding or not holding a taxidermy-mounted crow in prone (dead) position, or a taxidermy-mounted red-tailed hawk whose head moved. Rubber masks molded from actual people were used to create faces so that the same face could be randomly assigned as either threatening, caring or novel for each crow. Each of the 25 crows only saw one of the six possible stimuli (n = 5 threatening face, n = 4 caring face, n = 5 novel face, n = 4 novel face holding dead crow, n = 4 hawk, n = 3 room without any person or taxidermy mount). After stimulation, crows were anaesthetized, and the distribution and relative concentration of FDG throughout the whole brain was assessed. After sufficient radioactive decay (24 h), crows were returned to the wild.
Applying the principles of neuroecology—contrasting the neural responses of a diversity of animals in ecologically salient situations [25]—we are beginning to extend what is known from mammals, including humans, to birds. The amygdala, specifically within the brain's right hemisphere, has thus been implicated as playing a central role in the acquisition of learned fear [26]. By imaging the whole brain of the fearful crow, we revealed a neural network involving telencephalic pallial regions (e.g. nidopallium and mesopallium), subpallial emotional regions (e.g. nucleus taeniae of the amygdala) and premotor regions (e.g. arcopallium), as well as nuclei in the dorsal thalamus and brainstem [21]. Here, we ask how activity within this neural network varies as crows encounter other potential dangers—a predator (red-tailed hawk, Buteo jamaciensis) and a novel person holding a dead crow (figure 1)—that, like the sight of a threatening person, also trigger mobbing and scolding. We expand the use of FDG-PET imaging to test the hypothesis that distinct neuronal substrates underlie the crow's consistent behavioural response to different dangers.
2. Material and methods
We captured 25 adult, probably male crows (based on body size, moult and mouth colour [27]) from large winter roosting aggregations. Capture, handling, housing, diet and general experimental protocols were in accordance with IACUC protocol 3077-01, Washington scientific collection permit 11-359 and US scientific collection permit MB761139-1. Each of these phases of our research is fully described elsewhere ([21], including the associated electronic supplementary material).
Exposure of birds to people was minimal during captivity (holding cages are atop a building accessed only by research staff). At the time of first capture, and when captured from the holding cage for transport to the imaging laboratory, each bird was exposed to a person wearing a particular mask (the threatening face). Daily one person, wearing a different mask (the caring face) fed the birds and washed their cages. During experiments, some crows saw for the first time a person wearing a third, new (novel) face. Because multiple groups of crows were captured over the course of the study, we counter-balanced the masks used: for example, the mask that was learned as threatening by some crows was learned as caring or novel by others.
On the evening prior to an experiment, a single crow was captured from its holding cage by a person wearing the threatening face, placed in a sock to calm it and carried across campus to a small (0.5 × 0.5 × 1 m), wire cage in a fume hood of the imaging laboratory. Water was available, but no food. We draped a blanket across the front of the cage to prevent the bird from seeing out into the laboratory and to keep it calm as it acclimated overnight.
On the day of an experiment, the acclimated crow received an interperitoneal injection of approximately 1 mCi (F-18) FDG (volume range 0.050–0.100 ml). To do so, we removed the crow from the covered cage while covering its face with a hood. All crows remained passive and relaxed during this procedure with no visible signs of stress or struggle. We returned the crow back into the blanket-draped cage and played recorded crow calls (contact kaws, no alarm calls) for 2 min to distract and calm the recently handled subject. We then exposed the crow to one of five stimuli: (i) a person wearing the threatening face, (ii) a person wearing the caring face, (iii) a person wearing the novel face, (iv) a taxidermy-mounted red-tailed hawk that was motorized to enable head movement, and (v) the experimental room without any person or taxidermy mount. Each crow viewed a stimulus during a series of seven, 1 min duration exposures. Between exposures, the blanket was replaced on the cage and the crow allowed to relax out of view of the stimulus for 1 min (our initial tests of the threatening and caring face, and associated empty room control) or 30 s (all tests of hawks, dead conspecifics, novel people and associated controls).
To expose the crow to each stimulus, two researchers were in the laboratory. During exposure to threating and caring human faces, one researcher sat 0.5 m from the cage facing the crow, whereas the second researcher removed the blanket and kneeled next to the sitting person. During exposure to the novel face with and without the dead crow, one masked researcher sat 0.5 m from the cage facing the crow, whereas the second researcher removed the blanket and remained out of sight behind it. During exposure to the taxidermy-mounted red-tailed hawk, the hawk sat 0.5 m from the cage, in the same chair used by masked researchers in all previous tests. As one researcher controlled the blanket to expose the hawk to the crow, the second researcher moved the hawk's head via remote control. The head of the hawk swivelled towards the crow's cage after 15 s and remained fixed on the cage for the rest of the exposure. Between exposures, the hawk was swivelled to initially face to the right or to the left of the crow. During exposures to the hawk, both researchers were out of view of the crow. After seven exposures to a stimulus and associated breaks, we again took the crow out of the cage by reaching under the blanket, covered its eyes with a hood and induced sedation with 3–3.5% isoflurane before placing it in the scanner.
Scanning details [21] are based upon an initial 120 min imaging study we did to determine the time (about 25 min after injection) that FDG activity peaked in the crow brain. Accordingly, we imaged the crow's head using a Siemens Inveon PET system for 16–20 min after exposure to each experimental stimulus. This was followed by an approximately 13 min attenuation scan and then reconstructed using 3D OSEM/MAP (ordered subsets expectation maximization/maximum a posteriori) to an isotropic spatial resolution of 2.5 mm full width at half maximum (FWHM). Finally, an emission image of the crow's torso was taken to verify that there was a clean intraperitoneal injection.
Images were reconstructed for the 10 min time frame starting 27 min after the time of injection. The images were reconstructed using the vendor-supplied 3D OSEM/MAP algorithm with attenuation and scatter correction applied to the data. The image matrix was 128 × 28 × 159. A zoom factor of 1.302 and a beta of 0.25 were used for the MAP smoothing parameter. After the images were reconstructed, they were exported using DICOM for the statistical parametric analysis software.
We obtained structural MRIs of four crow brains using a 3 T MR scanner (Philips Achieva, Philips Healthcare, Andover, MA, USA) and a commercial coil (Philips Healthcare) with T1-weighted magnetization-prepared rapid gradient-echo (TR/TE = 10.8/5.1 ms; Ti = 1000 ms; FA = 9° acquired matrix 512 × 512 mm over 110 slices, voxel 0.2 × 0.2 × 0.6 mm interpolated to 0.1 × 0.1 × 0.3 mm). These MR images were co-registered and averaged to create an anatomical template, which was aligned to a jungle crow (Corvus macrorhynchos) atlas [28].
We stereotaxically aligned PET images to the brain atlas MR template. Nine affine parameters were estimated and applied to images, for consistent stereotactic transformation [29,30]. Alignment precision was estimated to be 1–2 pixels. After normalizing to global values, significant regional differences in cerebral metabolic rate (CMR) were determined using observer-independent voxel-wise subtraction and Z-statistic mapping (NEUROSTAT [24]; program available for download at http://128.95.65.28/~Download/).
The statistical threshold for a given comparison is estimated automatically during processing by algorithms implemented in our software and based on Worsley et al. [31] (hereafter ‘Worsley’) and our three-dimensional version of Friston et al. [32] (hereafter ‘Friston’). We used the following basis for the statistical threshold estimation. The acquired images had a voxel size of 0.667 × 0.667 × 0.796 mm after OSEM reconstruction, which was interpolated to a 0.3488 mm isotropic voxel size after co-registration to the standardized MR atlas template. A three-dimensional Gaussian filter with three-pixel FWHM was applied to reduce small regional variations from standardization to the stereotactic coordinate system. The algorithm then applied a cortical threshold to eliminate voxels associated with white matter, cerebrospinal fluid and extracranial activities. Using the remaining number of voxels (approx. 10 K voxels) and assuming spatial dependence between adjacent voxels with smoothness estimated by the partial derivative of the three-dimensional smoothed surface described earlier [31], the algorithms using both Worsley et al. [31] and Friston et al. [32] equations (two algorithms use different approaches, but results are typically in approximate agreement) estimated that a Z threshold of 3.8 provided a less than 5% probability of making a type 1 statistical error adjusted for multiple voxel comparisons. In addition, we had a strong a priori hypothesis for the activation of the hippocampus, arcopallium (premotor) and amygdala in our stimulation paradigms, so these structures were included at slightly below threshold (lowest Z = 3.63 is Hp). To show the distribution of individual data points (see the electronic supplementary material, table S1) and check for outliers, we applied spherical (r = 0.3488 mm) volumes of interest (VOIs) centred at the coordinates of significant peaks to individual images and report effect size as the percentage increase in activation during stimulation relative to the appropriate baseline condition.
To evaluate the association of blink rate in response to a stimulation with cortical metabolism, we used individual blink rates obtained at the time of PET study in a voxel-wise correlation analysis of the FDG-PET images over conditions in which we were able to assess the rate (all except hawk). Correlation coefficients for each voxel were converted to Z-scores (Fisher transformation) and peak locations of significant correlations were mapped over the entire brain (raw data in electronic supplementary material, table S2). We directly observed blinking at close range during experiments. We derived a single blink rate per crow by averaging the number of flashes of the bird's white nictitating membrane that we counted during each minute of stimulation. We video-recorded laboratory trials, but resolution was insufficient to count blinking. All blink rates (see the electronic supplementary material, table S2) were counted by J.M.M. and I.P. to reduce the effect of variation among observers.
3. Results
In nature, crows fix their gaze as they scold and mob dangerous stimuli [21]. Within the confined space of the small cage used for our experiments, solitary crows cannot fly and, lacking flock mates, they do not vocalize. They do, however, reduce blinking to fix a gaze upon a possible danger. Thus, while we are unable to replicate all aspects of mobbing in the laboratory, we are able to investigate the mental processes associated with one aspect of this behaviour. This behavioural component of mobbing was correlated with peak activity in a small medial region of the rostral nidopallium/mesopallium, the arcopallium and the dorsal brainstem region around the nucleus isthmo-opticus and locus coeruleus (see figure 2a; electronic supplementary material, figure S5). Regional brain activity and infrequent blinking were consistent in crows that viewed two mob-eliciting stimuli: the person who previously captured them and a new person holding a dead crow (figure 2b–d, filled symbols; we could not observe blinking rates during hawk trials). Blinking at the sight of danger was significantly less frequent than when crows viewed the novel person without a dead crow or the face of the person that had cared for them while in captivity (n = 9 threat + dead crow: mean = 26.7 blinks per minute, s.e. = 2.2; n = 9 caring + novel face: mean = 36.3 blinks per minute, s.e. = 2.9; t17d.f. = 2.68, p = 0.02; filled versus open symbols in figure 2b–d). The neural response held in common by crows facing danger probably reflects increased attention (activity in rostral nidopallium/mesopallium and nucleus isthmo-opticus/locus coeruleus) and premotor signalling (arcopallium) [33].
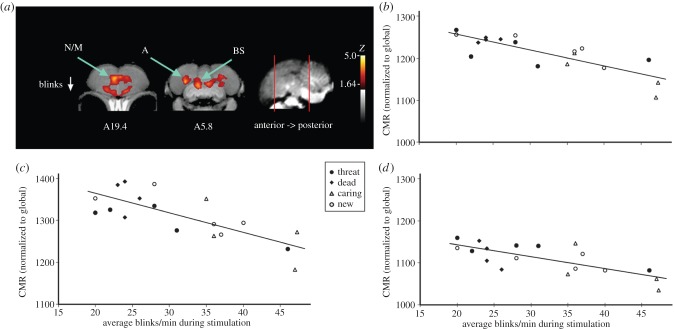
Figure 2.
A fixed stare (reduced blinking), one behavioural response to dangerous situations observed in nature and in the laboratory, was associated with activation of three brain regions. (a) Voxel-wise correlations converted to Z-score maps are superimposed to a composite (n = 4 birds) structural MRI of the crow brain. Voxels with Z > 1.64 are coloured; those with Z > 3.8 (3.6 for small structures hypothesized a priori to be activated by stimulus) are considered significant with associated structures as indicated (BS: dorsal brainstem area possibly, including nucleus isthmo-opticus and locus coeruleus, Z = 4.3; N/M: nidopallium/mesopallium, Z = 3.8; A: arcopallium, Z = 3.7). (b–d) Individual values for normalized (global) uptake in each structure that met the threshold for statistical significance on Z-score voxel-wise mapping. The scatterplots we present describe the individual covariation between neural activity and blinking by all individual crows. Symbol type further describes covariation within and between treatments. Correlation coefficients describe the magnitude of covariation (effect size) between neural activity and behaviour at the brain location where the effect was greatest. (b) Differential activation of the brainstem (BS, r = −0.82) related to subject rate of blinking in each experiment (type is indicated by symbol) where blinking could be observed. (c) Differential activation of the nidopallium/mesopallium (N/M, r = −0.76) in relation to blinking by individual crows. (d) Differential activation of the arcopallium (A, r = −0.74) in relation to blinking by individual crows.
Scolding and mobbing the many dangers encountered by crows in nature is thought to involve learning and assessment of situations with distinctly different costs and benefits [10,11]. Thus, despite a consistent neural response that includes fixing the mobber's gaze, different dangers should activate distinct emotional and cognitive pathways. The sight of a hawk, for example, which is innately feared [34] and consistently dangerous (unlike people), should not activate fear learning (e.g. amygdala), memory or higher order sensory regions of the brain. As expected, and in stark contrast to the pattern of neural activation by crows viewing a person they learned to fear (figure 3a,b), crows that viewed the red-tailed hawk activated their medial hyperpallium, a small lateral portion of the nidopallium/mesopallium, but especially the ventrolateral portion of the caudal nidopallium, which also extended dorsolaterally to encroach the caudolateral nidopallium (NCL; figure 3a,c; electronic supplementary material, tables S3, S5 and figures S2, S4). The strong activity in the ventrolateral portion of the caudal nidopallium, which is well developed in corvids [28] and reported to show a high level of immediate early-gene expression following an exposure to familiar conspecifics [35], suggests that the area may also be involved in discriminating among individuals of other species. This role may further extend to include the dorosolateral portion of the caudal nidopallium, possibly including a multisensory NCL that manages decision-making [36]. There was some evidence of lateralization in response, a bias towards activity in the right hemisphere as in response to the threatening person, although further systematic analysis is warranted to reveal specific roles for each hemisphere.
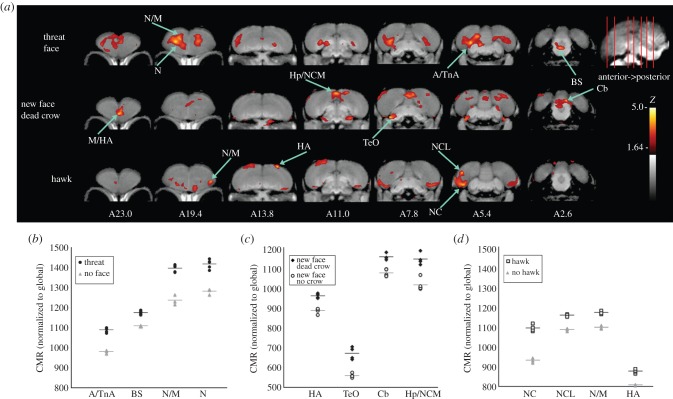
Figure 3.
Differential brain activation patterns of crows show dangerous versus neutral stimuli. (a) Voxel-wise subtractions converted to Z-score maps are superimposed to a composite (n = 4 birds) structural MRI of the crow brain. Top row shows the activation pattern of crows viewing a threatening face previously associated with capture versus a group shown an empty room; second row indicates crows viewing a never before seen person holding a taxidermy mount of a dead crow versus a new person and no crow (electronic supplementary material, figure S1 confirms that regions activated differentially by a person holding a dead crow relative to empty room were the same as those indicated here); bottom row indicates the activation pattern of crows shown a taxidermy-mounted red-tailed hawk versus empty room. Coronal slices, from anterior to posterior, with voxels coloured (Z > 1.64) to indicate heightened activation; those with Z > 3.8 (3.6 for small structures hypothesized a priori to be activated by stimulus) are considered significant with associated structures as indicated. Abbreviations are as defined for (b–d) below. (b–d) Individual values for normalized (global) uptake in each structure that met the threshold for statistical significance on Z-score voxel-wise mapping. Horizontal lines indicate group mean. Individual data points describe variation within and between treatments at VOIs centred on peak activation coordinates. Z-values describe magnitude of the largest differences between treatment and baseline conditions determined during voxel-wise mapping. Percentage increase at each peak in activation describes the greatest average differences in FDG uptake (effect size) between crows that viewed the stimulus and those that viewed the appropriate baseline. (b) Activated structures for threatening face: A/TnA: arcopallium/nucleus taeniae of the amygdala, 11% increased Z = 4.4; BS: dorsal brainstem including nucleus isthmo-opticus and locus coeruleus, 5.9% increased, Z = 4.3; N/M: nidopallium/mesopallium, 12.9% increased, Z = 4.1; N: nidopallium, 10.6% increased, Z = 3.9. (c) Activated structures for new person and dead crow versus new person and no crow: M/HA: mesopallium/apical part of the hyperpallium, 8% increased, Z = 4.0; TeO: optic tectum, 19% increased Z = 3.9; Cb: cerebellum, 7% increased, Z = 3.8; Hp/NCM: hippocampus/caudomedial nidopallium, 13% increased, Z = 3.6 (borderline significance based on a priori prediction). (d) Activated structures for red-tailed hawk: N/M, 7% increased, Z = 4.1; HA, 9% increased, Z = 4.0; NC: caudal nidopallium, 17% increased, Z = 4.2; NCL: caudolateral nidopallium, 7% increased, Z = 4.1.
Unlike the sight of a person who has threatened a crow in the past, or a hawk, who poses a constant threat, the sight of a dead conspecific associated for the first time with a novel unconditioned stimulus should activate higher-order sensory areas (e.g. nidopallium and mesopallium), associative and spatial learning pathways (e.g. basal ganglia and hippocampus), and, perhaps, if the dead conspecific alone stimulates a change in emotional state, the subpallial limbic network (e.g. amygdala). Consistent with the hypothesized importance of cognition and spatial learning in the response [37,38], the dorsomedial portion of the hippocampus and part of the cerebellum were activated in response to seeing a new person holding a dead crow (figures 3a,d,4; electronic supplementary material, table S4 and figure S3). In contrast to a hypothesized role of emotion [19], the activation of the amygdala was not seen consistently (see the electronic supplementary material, table S3); however, there was distinct hemispheric bias in the response that was parallel to the processing of the threatening face [21]; significant peaks occurred only in the right brain.
Figure 4.
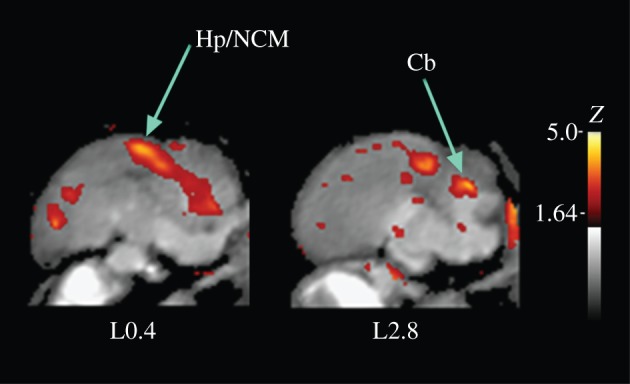
Sagittal view of difference in crow brain activation at the sight of a novel person holding a dead crow versus a novel person without a dead crow. This view illustrates the distinct activation of the region, including the hippocampus and the caudomedial nidopallium (Hp/NCM) and the cerebellum (Cb), that is less apparent in the coronal sections of figure 3a, middle row.
4. Discussion
In the face of danger, despite consistent behaviour and premotor aspects of neural activity (figure 2), crows are not of a like mind. The neural networks activated by dangers that differed in requiring memory (dangerous human), learning (unknown person holding a dead crow) or innate response to a predator that in nature presents multisensory information (the sight and vocalizations of a red-tailed hawk) were distinct in their reliance upon emotional and cognitive pathways (figure 3).
Our results did not provide information about the exact routes through which visual input reached different brain structures to trigger metabolic activity. However, based on the neuroanatomical data on pigeons and songbirds [39], we can speculate about possible visual flows associated with the metabolically active areas (figures 2–4) in the crow brain. These flows travel from the retina to the telencephalon via the tectum (the tectofugal pathway) or via the dorsal thalamus (the thalamofugal pathway; figure 5). The involvement of both visual pathways and the hippocampus increases the complexity of information flow in the brains of crows that viewed a novel person holding the dead crow (figure 5b). Considering the flow of information into the telencephalon and the results from our observation of blinking behaviour, we suggest that visual information associated with sighting a threatening person or a dead crow is sent to the brain stem nuclei controlling muscular responses via two routes: from the entopallium to the brain stem via the arcopallium or the basal ganglia. It is possible that motor output to the brain stem in response to seeing the hawk involved the same pathways or direct output from the hyperpallium to the brain stem (no route is suggested in figure 5c because in this study we were unable to observe crow behavioural responses to hawks).
Figure 5.
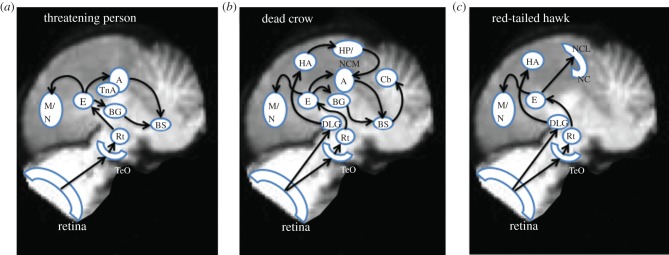
Possible visual input routes to structures in the brain of a crow stimulated by the sight of three dangers: (a) a person who has been learned as threatening, (b) a novel person holding a dead crow, and (c) a hawk that frequently preys on adult and nestling crows. A, arcopallium; BG, basal ganglia; BS, brain stem; Cb, cerebellum; DLG, dorsal lateral geniculate nucleus of the thalamus; E, entopallium; HA, hyperpallium apicale; Hp, hippocampus; M, mesopallium; N, nidopallium; NC, caudal nidopallium; NCL, caudolateral nidopallium; Rt, nucleus rotundus of the thalamus; TeO, optic tectum. (Online version in colour.)
Differences in the activation of the amygdala and hippocampus may result from differences in the brain's activity while storing versus recalling fearful information and from differences in the spatial relevance of an observed danger. Lack of significant amygdalar or hippocampal activity (relative to baseline conditions) in response to seeing the innately recognized hawk is consistent with the critical role these structures play in learning and memory. Apparently, the amygdala was activated significantly by stimuli that have previously been learned to be dangerous [40] (electronic supplementary material, table S1). Our results suggest that the amygdala, while critical to the memory of a learned fear, is not critical to the storage of this information, as presumably occurred when crows viewed a new person holding a dead crow. In contrast, it is the hippocampus that is activated during the storage of the learned fears we studied (see the electronic supplementary material, table S2). Additional observation is needed to confirm the limited role of the amygdala in the acquisition of fears that we know to be learned (the identity of a crow's captor), and its limited response to the sight of a dead conspecific. This could be accomplished by imaging FDG uptake by a crow as it is captured or as it views only a dead conspecific, and will be investigated in a future study.
The avian hippocampus is considered to be a homologue of the mammalian counterpart and involved in the formation of spatial memory [41,42]. Our results suggest that the hippocampus may also be involved in fear learning generally, or learning of specific fearful locations where crows encounter reliable indicators of a dangerous place (a dead crow). In contrast, we hypothesize that while the observation of freely moving dangers (a threatening person, a hawk) may also indicate dangerous areas to be avoided, this information is less precise than the location where a kill has occurred, and therefore it may not be overly stimulating to the hippocampus. Imaging crows as they respond to other direct and indirect locational cues could test this idea.
There was strong activation of the rostromedial portion of the hyperpallium, mesopallium and nidopallium at the sight of a person previously learned as threatening, and at the sight of a novel person holding a dead crow (figure 3). This has important implications for understanding the roles of these avian brain areas in the face of different ecological situations. The neural connections of the rostral pallial regions that were activated by the threatening human or unknown person holding a dead crow are not unequivocally known. Part of this region has been suggested to be important to behavioural flexibility [43]. The specific areas activated in our experiments are unlikely to be involved in the sensory processing of any specific modality since they are located far medial to trigeminal or visual regions (e.g. the basorostral pallial nucleus or entopallium) and rostral to auditory regions (e.g. field L). Rather, their column-like organization appears to correspond to the extensive metabolic activity found in zebra finches (Taeniopygia guttata) that were aroused by being chased [44]. Thus, it is possible that the activity of the pallial areas in crows that viewed the association of a novel person with a dead crow, and especially the person whom they had learned was dangerous, is correlated with the general arousal level or specifically heightened attention paid to the sensory environment associated with danger. That the rostral pallial areas were not activated significantly by the sight of the hawk may suggest that the general arousal level in these regions is raised when birds need to learn new information or to use the learned information to initiate immediate and fast reactions, such as assembling a mob around novel dangers.
That the sight of a hawk activated forebrain regions proposed to be critical in decision-making informed by multiple sensory modalities (NCL [36]) was surprising. Perhaps in nature, crows typically attend to the sights and sounds produced by this deadly predator, and adjust their behaviour (to flee or to mob) to the hawk's behaviour (hunting versus perching or eating). Imaging the uptake of FDG by crows attending to visual and vocal stimuli, and a variety of predator actions, would test this hypothesis.
Functional imaging studies, such as the unique application of micro-PET techniques in our study, hold great potential to advance our understanding of the neural basis of animal behaviour because they are amenable to longitudinal studies on the same individuals in distinct situations. We did not take advantage of this strength. Rather, we used a conservative approach that exposed each subject to a single stimulus, thereby minimizing the amount of FDG and anaesthesia each bird received, and eliminating possible carry-over and order effects of dangerous encounters from prior trials. The rapid recovery following testing, consistent responses of individuals and distinct responses of each small experimental group bodes well for the development of future longitudinal studies. In addition, the image-processing and statistical analysis will require further study. For example, although both Worsley and Friston equations (two algorithms that use different approaches, but typically provide results in approximate agreement [31,32]) gave an estimated the Z threshold of 3.8, verification of our estimates for smoothing and grey/white matter ratio in avian brains will require further study to increase robustness of the analysis by including more subjects and a possible within subject design. Crow grey matter in the cortex is thin, and, because of the boundary issue in the stochastic field, the exact type 1 error rate may be higher than the Worsley/Friston estimates.
Our study does facilitate comparative studies across distantly related animals. Comparison, especially as it includes wild animals reacting to natural challenges, enables development of general neuroecological principles and exposure of the evolutionary roots of neural function [25]. Understanding how animals process fear in natural settings increases our understanding of fear's role in structuring vertebrate ecological communities [2], and informs efforts to discourage conflicts between humans and wildlife, as well as conflicts among common and rare species [10].
Acknowledgements
T. R. Birkhead, K. Dial and E. C. Petrie reviewed draft manuscripts. Jack DeLap drafted figure 1.
Funding statement
Funding was provided by the University of Washington's Royalty Research Fund.
References
- 1.Evans CS, Evans L, Marler P. 1993. On the meaning of alarm calls: functional reference in an avian vocal system. Anim. Behav. 46, 23–38 (doi:10.1006/anbe.1993.1158) [Google Scholar]
- 2.Wirsing AJ, Ripple WJ. 2010. Comparison of shark and wolf research reveals similar behavioral responses by prey. Front. Ecol. Environ. 9, 335–341 (doi:10.1890/090226) [Google Scholar]
- 3.Ioannou CC, Guttal V, Couzin ID. 2012. Predatory fish select for coordinated collective motion in virtual prey. Science 337, 1212–1215 (doi:10.1126/science.1218919) [DOI] [PubMed] [Google Scholar]
- 4.Stankowich T. 2008. Tail-flicking, tail-flagging, and tail position in ungulates with special reference to black-tailed deer. Ethology 114, 875–885 (doi:10.1111/j.1439-0310.2008.01530.x) [Google Scholar]
- 5.Seyfarth RM, Cheney DL, Marler P. 1980. Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim. Behav. 28, 1070–1094 (doi:10.1016/S0003-3472(80)80097-2) [Google Scholar]
- 6.Yorzinski JL, Vehrencamp SL. 2009. The effect of predator type and danger level on the mob calls of the American crow. Condor 111, 159–168 (doi:10.1525/cond.2009.080057) [Google Scholar]
- 7.Pavey CR, Smyth AK. 1998. Effects of avian mobbing on roost use and diet of powerful owls, Nonox strenua. Anim. Behav. 55, 313–318 (doi:10.1006/anbe.1997.0633) [DOI] [PubMed] [Google Scholar]
- 8.Marzluff JM, Walls J, Cornell HN, Withey J, Craig DP. 2010. Lasting recognition of threatening people by wild American crows. Anim. Behav. 79, 699–707 (doi:10.1016/j.anbehav.2009.12.022) [Google Scholar]
- 9.Cornell HN, Marzluff JM, Pecararo S. 2012. Social learning spreads knowledge about dangerous humans among American crows. Proc. R. Soc. B 279, 499–508 (doi:10.1098/rspb.2011.0957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzluff JM, Angell T. 2012. Gifts of the crow. New York, NY: Free Press [Google Scholar]
- 11.Iglesias TL, McElreath R, Patricelli GL. 2012. Western scrub-jay funerals: cacophonous aggregations in response to dead conspecifics. Anim. Behav. 84, 1103–1111 (doi:10.1016/j.anbehav.2012.08.007) [Google Scholar]
- 12.Cheney DL, Seyfarth RM. 1981. Selective forces affecting the predator alarm calls of vervet monkeys. Behaviour 76, 25–61 (doi:10.1163/156853981X00022) [Google Scholar]
- 13.Caro TM, Graham CM, Stoner CJ, Vargas JK. 2004. Adaptive significance of antipredator behaviour in artiodactyls. Anim. Behav. 67, 205–228 (doi:10.1016/j.anbehav.2002.12.007) [Google Scholar]
- 14.Curio E. 1978. Adaptive significance of avian mobbing. 1. Teleonomic hypotheses and predictions. Z. Tierpsychol. 48, 175–183 [Google Scholar]
- 15.Eliasmith C, Steward TC, Choo A, Bekolay T, DeWolf Y, Tang T, Rasmussen D. 2012. A large-scale model of the functioning brain. Science 338, 1202–1205 (doi:10.1126/science.1225266) [DOI] [PubMed] [Google Scholar]
- 16.Goodson JL. 2005. The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav. 48, 11–22 (doi:10.1016/j.yhbeh.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seller TJ. 1981. Midbrain vocalization centres in birds. Trends Neurosci. 12, 301–303 (doi:10.1016/0166-2236(81)90094-1) [Google Scholar]
- 18.Kaplan G. 2008. Alarm calls and referentiality in Australian magpies: between midbrain and forebrain, can a case be made for complex cognition? Brain Res. Bull. 76, 253–263 (doi:10.1016/j.brainresbull.2008.02.006) [DOI] [PubMed] [Google Scholar]
- 19.McGaugh JL. 2004. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Ann. Rev. Neurosci. 27, 1–28 (doi:10.1146/annurev.neuro.27.070203.144157) [DOI] [PubMed] [Google Scholar]
- 20.Saint-Dizier H, Constantin P, Davies DC, Leterrier C, Levy F, Richard S. 2009. Subdivisions of the arcopallium/posterior pallial amygdala complex are differentially involved in the control of fear behaviour in the Japanese quail. Brain Res. Bull. 79, 288–295 (doi:10.1016/j.brainresbull.2009.03.004) [DOI] [PubMed] [Google Scholar]
- 21.Marzluff JM, Miyaoka R, Minoshima S, Cross DJ. 2012. Brain imaging reveals neuronal circuitry underlying the crow's perception of human faces. Proc. Natl Acad. Sci. USA 109, 15 912–15 917 (doi:10.1073/pnas.1206109109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Linden A, Van Meir V, Boumans T, Poirier C, Balthazart J. 2009. MRI in small brains displaying extensive plasticity. Trends Neurosci. 32, 257–266 (doi:10.1016/j.tins.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 23.Berns GS, Brooks AM, Spivak M. 2012. Functional MRI in awake unrestrained dogs. PLoS ONE 7, e38027 (doi:10.1371/journal.pone.0038027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonides J, Smith EE, Koeppe RA, Awh E, Minishima S, Mintun MA. 1993. Spatial working memory in humans as revealed by PET. Nature 363, 623–625 (doi:10.1038/363623a0) [DOI] [PubMed] [Google Scholar]
- 25.Sherry DF. 2006. Neuroecology. Ann. Rev. Psychol. 57, 167–197 (doi:10.1146/annurev.psych.56.091103.070324) [DOI] [PubMed] [Google Scholar]
- 26.Haxby JV, Hoffman EA, Gobbini MI. 2000. The distributed human neural system for face perception. Trends Cogn. Sci. 4, 223–233 (doi:10.1016/S1364-6613(00)01482-0) [DOI] [PubMed] [Google Scholar]
- 27.Dos Anjos L, Debus SJS, Madge SC, Marzluff JM. 2009. Family Corvidae. In Handbook of birds of the world (eds del Hoyo J, Eliott A, Christie DA.), pp. 494–641 Barcelona: Lynx Edicions [Google Scholar]
- 28.Izawa E-I, Watanabe S. 2007. A stereotaxic atlas of the brain of the jungle crow (Corvus macrorhynchos). In Integration of comparative neuroanatomy and cognition (eds Hoffman MA, Watanabe S.), pp. 215–273 Tokyo, Japan: Keio University Press [Google Scholar]
- 29.Minoshima S, Koeppe RA, Fessler JA, Mintun MA, Berger KL, Taylor SF, Kuhl DE. 1993. Integrated and automated data analysis method for neuronal activation studies using [O-15] water PET. In Quantification of brain function, tracer kinetics and image analysis in brain PET (eds Uemura K, Lassen NA, Jones T, Kanno I.), pp. 409–415 Tokyo, Japan: Excerpta Medica [Google Scholar]
- 30.Minoshima S, Berger K, Lee K, Mintun M. 1992. An automated method for rotational correction and centering of three-dimensional functional brain images. J. Nucl. Med. 33, 1579–1585 [PubMed] [Google Scholar]
- 31.Worsley KJ, Marrett S, Neelin P, Evans AC. 1996. A unified statistical approach for determining significant signals in location and scale space images of cerebral activation. In Quantification of brain function using PET (eds Myers R, Cunningham V, Bailey D, Jones T.), pp. 327–333 San Diego, CA: Academic Press [Google Scholar]
- 32.Friston K, Frith C, Liddle P, Frackowiak R. 1991. Comparing functional (PET) images: the assessment of significant change. J. Cereb. Blood Flow Metab. 11, 690–699 (doi:10.1038/jcbfm.1991.122) [DOI] [PubMed] [Google Scholar]
- 33.Miceli D, Reperant J, Bertrand C, Rio J-P. 1999. Functional anatomy of the avian centrifugal visual system. Behav. Brain Res. 98, 203–210 (doi:10.1016/S0166-4328(98)00085-0) [DOI] [PubMed] [Google Scholar]
- 34.Cully JF, Ligon JD. 1976. Comparative mobbing behavior of scrub and Mexican jays. The Auk 93, 116–125 [Google Scholar]
- 35.Nishizawa K, Izawa E-I, Wantanabe S. 2011. Neural-activity mapping of memory-based dominance in the crow: neural networks integrating individual discrimination and social behaviour control. Neuroscience 197, 307–319 (doi:10.1016/j.neuroscience.2011.09.001) [DOI] [PubMed] [Google Scholar]
- 36.Güntürkün O. 2005. The avian ‘prefrontal cortex’ and cognition. Curr. Opin. Neurobiol. 15, 686–693 (doi:10.1016/j.conb.2005.10.003) [DOI] [PubMed] [Google Scholar]
- 37.Székely AD. 1999. The avian hippocampal formation: subdivisions and connectivity. Behav. Brain Res. 98, 219–225 (doi:10.1016/S0166-4328(98)00087-4) [DOI] [PubMed] [Google Scholar]
- 38.Spence RD, Zhen Y, White S, Schlinger BA, Day LB. 2009. Recovery of motor and cognitive function after cerebellar lesions in a songbird—role of estrogens. Eur. J. Neurosci. 29, 1225–1234 (doi:10.1111/j.1460-9568.2009.06685.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu T, Watanabe S. 2012. The avian visual system: overview. In How animals see the world: comparative behavior, biology, and evolution of vision (eds Lazareva OF, Shimizu T, Wasserman EA.), pp. 473–482 New York, NY: Oxford University Press [Google Scholar]
- 40.Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. 2001. Activation of the amygdala by cognitive representations of fear. Nat. Neurosci. 4, 437–441 (doi:10.1038/86110) [DOI] [PubMed] [Google Scholar]
- 41.Bingman VP, Hough GE, II, Kahn MC, Siegel JJ. 2003. The homing pigeon hippocampus and space: in search of adaptive specialization. Brain Behav. Evol. 62, 117–127 (doi:10.1159/000072442) [DOI] [PubMed] [Google Scholar]
- 42.Colombo M, Broadbent N. 2000. Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neurosci. Biobehav. Rev. 24, 465–484 (doi:10.1016/S0149-7634(00)00016-6) [DOI] [PubMed] [Google Scholar]
- 43.Timmermans S, Lefebvre L, Boire D, Basu P. 2000. Relative size of the hyperstriatum ventrale is the best predictor of feeding innovation rate in birds. Brain Behav. Evol. 56, 196–203 (doi:10.1159/000047204) [DOI] [PubMed] [Google Scholar]
- 44.Bischof H-J. 2003. Neural mechanisms of sexual imprinting. Anim. Biol. 53, 89–112 (doi:10.1163/157075603769700313) [Google Scholar]