Abstract
More than 100 papers have been published on the rubber hand illusion since its discovery 14 years ago. The illusion has been proposed as a demonstration that the body is distinguished from other objects by its participation in specific forms of intermodal perceptual correlation. Here, we radically challenge this view by claiming that perceptual correlation is not necessary to produce the experience of this body as mine. Each of 15 participants was seated with his/her right arm resting upon a table just below another smaller table. Thus, the real hand was hidden from the participant's view and a life-sized rubber model of a right hand was placed on the small table in front of the participant. The participant observed the experimenter's hand while approaching—without touching—the rubber hand. Phenomenology of the illusion was measured by means of skin conductance response and questionnaire. Both measures indicated that participants experienced the illusion that the experimenter's hand was about to touch their hidden hand rather than the rubber hand, as if the latter replaced their own hand. This did not occur when the rubber hand was rotated by 180° or replaced by a piece of wood. This illusion indicates that our brain does not build a sense of self in a merely reactive way, via perceptual correlations; rather it generates predictions on what may or may not belong to itself.
Keywords: predictive brain, tactile expectation, rubber hand illusion, self
1. Introduction
Usually, we do not question the experience of the body as a part of the self. ‘Body ownership’ is an epistemic definition of the common experience that ‘my body’ belongs to me [1,2], and it is fundamental to self-consciousness [3]. The sense of ownership (SO) is thought to rely on the integration of sensory signals from different modalities [1,4,5]. Illusions manipulating SO, such as the rubber hand illusion (RHI), provide a powerful experimental tool to investigate the individual's bodily self-perception. In the RHI, watching a rubber hand being stroked synchronously with one's own unseen hand causes the rubber hand to be attributed to one's own body, to ‘feel like it is my hand’. This illusion does not occur when the rubber hand is stroked asynchronously with respect to the participant's own hand.
Botvinick & Cohen [5] were the first to show that, after synchronous visuo-tactile stimulation of the rubber hand and the participant's hand, intermanual reaches were displaced towards the rubber hand. In other words, participants perceived the position of their hand to be closer to the rubber hand than it really was. The manipulation of SO with the RHI has been largely confirmed by more than 100 studies (for reviews, see [6,7]). These studies all shared a key research question focused on the necessary and sufficient conditions for inducing a SO. Botvinick & Cohen [5] initially suggested that visuo-tactile integration is both necessary and sufficient for self-attribution of the rubber hand, and that the illusion mainly represents tolerance to discrepancies between the seen and the felt positions of the hand. A more radical proposal then came from Armel & Ramachandran [8], who claimed that, in the RHI, the seen and the felt touch are bound because of their temporal synchrony on the basis of a Bayesian principle of perceptual learning. According to this view, in principle any object could be experienced as part of one's body as a result of a purely bottom-up mechanism. This view was questioned by Tsakiris & Haggard [9], leading to the hypothesis that the brain maintains a coherent sense of one's body by a test-for-fit process, which enables the distinction between corporeal and non-corporeal objects [10]. Indeed, this process would be in charge of comparing the viewed object with a reference model of the body, which contains a reference description of the visual, anatomical and structural properties of one's own body [9–11].
In general, there is now plenty of evidence that intermodal matching is not sufficient for RHI induction and SO [7]. Both, instead, seem to be modulated by visual form [9,12] and anatomical [9,13] and postural [9,11,13–15] congruency between the viewed object and the felt body part. Overall, these findings support the hypothesis that perception of one's body does not only consist of the mere registration of sensory input and SO does not simply rely on stimulus-driven processing. Rather, active processing is also involved, in which top-down influences, originating from the representation of one's own body, play important roles. This hypothesis is in line with more recent approaches to general perception [16]. Differently from classical theories, which look at the brain as a passive device, new approaches emphasize the constructive and active nature of sensory processing. Indeed, there is ample evidence that the processing of perceptual stimuli is controlled by top-down influences that constantly create predictions about forthcoming sensory events and might lead to states of ‘expectancy’ [16]. Expectations about upcoming sensory events can be used to prepare sensory cortices by instantiating a neural context that allows for enhanced processing of the forthcoming event [16]. Specifically, the sight of objects moving towards one's body can originate expectation of a tactile event. Accordingly, bimodal neurons both responsive to tactile stimuli applied to a given body part and selective for the sight of objects moving towards the same body part have been recorded in different macaque monkeys brain regions, such as premotor area F4 [17], posterior parietal [18–20] and temporal cortices [21].
Starting from this evidence, we intend to study whether expectation of touch experience arising at the sight of a human hand approaching a rubber hand is enough to induce an SO over the same rubber hand, that is, even if no physical tactile stimulation is delivered on either the rubber hand or on the real hand.
2. Material and methods
(a). Experiment 1: tactile expectation and rubber hand illusion
(i). Participants
Fifteen right-handed healthy naive participants (mean age 22.5, 13 females), with normal or corrected to normal vision, took part in this study after having provided their written informed consent. The study was approved by the Ethics committee of the ‘G. d'Annunzio’ University, Chieti and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki.
(ii). Experimental design
The experimental design was 2 × 2 factorial. The two factors were the viewed object (rubber hand versus piece of wood) and the position of the viewed object (congruent versus incongruent) relative to the participant's hidden arm. The rubber hand was a realistic prosthetic hand. The piece of wood was a plain wooden block, pale and beige in colour, with a thumb-like feature and with one end tapered into a wrist-like shape. The four conditions were (i) rubber hand congruent: the rubber hand was aligned to the participant's own hand and the experimenter moved her hand towards the rubber hand; (ii) rubber hand incongruent: the rubber hand was rotated 180° relative the participant's own hand and the experimenter moved her hand towards the rubber hand; (iii) wood congruent: the piece of wood was aligned to the participant's own hand and the experimenter moved her hand towards the wood; (iv) wood incongruent: the piece of wood was rotated 180° relative the participant's own hand and the experimenter moved her hand towards the wood. Stimuli had comparable overall size.
(iii). Procedure
Participants sat in front of a table. The right arm was placed on the table in a relaxed position at a fixed point inside a frame. A smaller table, measuring 80 cm by 30 cm and 20 cm in height, was positioned over the table where the real hand was placed (figure 1). This table was used to both hide the participant’s hand and to support the object (i.e. the rubber hand or the piece of wood). The participant's hand and the viewed object were aligned on the vertical axis and were positioned at 20 cm from each other. The experimenter stood at the participant's right-hand side, hidden behind a black curtain. She moved her hand towards the viewed object (which varied according to the experimental condition, see §2a(ii)) with a velocity of 0.02 m s–1 (±2% as revealed by post hoc analysis on switch timing, see below) starting from a distance of 70 cm from the viewed object and stopping at 15 cm away from the viewed object. The experimenter's hand never touched the viewed object. The experimenter, previously trained, followed audio instructions by earphones to perform controlled movements during the experiment. The consistency of movement direction and speed across trials was monitored by four pairs of switches fixed onto two vertical rods 70 cm in height, enabling the recording of the experimenter's movements, speed and position. Four switches were positioned on each rod at 60, 45, 30 and 15 cm from the object, respectively. A further switch was positioned at the starting point (70 cm). Each switch fed a signal to the PowerLab (ADinstrument) by means of which participants’ skin conductance response (SCR) was recorded, thus allowing time-locking of experimenter's hand movements with SCR.
Figure 1.
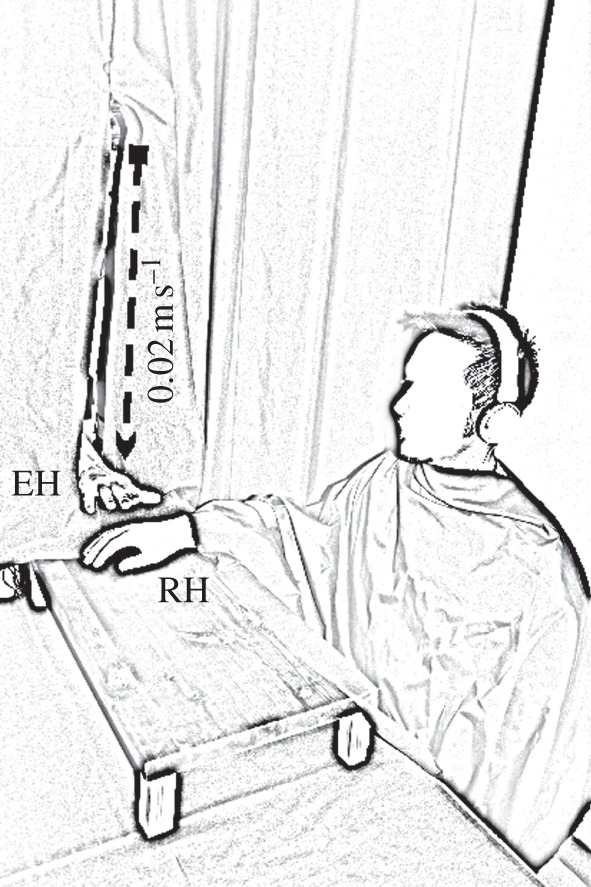
Experiment set-up to evaluate rubber hand illusion. The experimenter moved her hand towards the viewed object (which varied according to the experimental condition, see §2a(ii)) with a velocity of 0.02 m s–1 starting from a distance of 70 cm from the viewed object and stopping at 15 cm away from it. The experimenter's hand never touched the viewed object. White noise was delivered through headphones to conceal any noise made by the switches. EH, experimenter's hand; RH, rubber hand.
The experiment consisted of four blocks, one for each of the four experimental conditions, whose presentation order was counterbalanced between participants. Each block lasted 2 min, during which four approaching movements were performed. At the beginning of the experimental session, participants were instructed to pay attention to the experimenter's hand moving towards either a rubber hand or a piece of wood placed in front of them. After receiving instructions, participants wore earphones through which white noise was delivered for the entire duration of each block. After each block, participants took off their earphones and were required to complete the RHI questionnaire.
(iv). Rubber hand illusion questionnaire
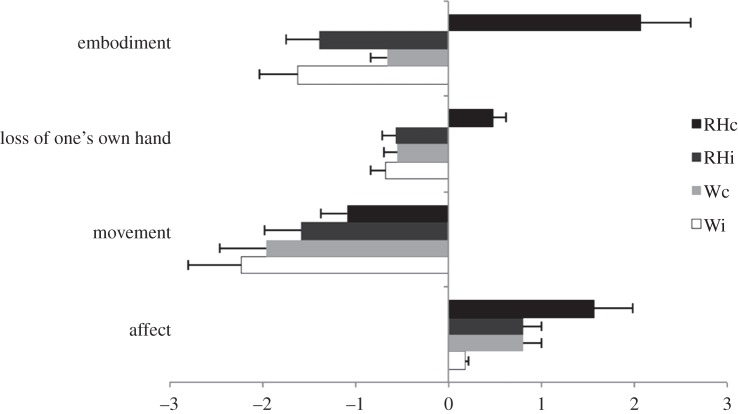
We adopted a total of 21 questions from Longo et al. [22]. The questions referred to four different components of the experience of embodiment during the RHI paradigm: (i) 10 statements referring to the embodiment of rubber hand. These comprised items relating to the feelings that: the rubber hand belonged to the participant, the participant had control over the rubber hand, the rubber hand and real hand were in the same location, and the rubber hand had taken on features of the actual hand. (ii) Five statements referring to the experience of loss of one's hand. These comprised items relating to the feelings of: being unable to move one's hand, one's hand disappearing and one's hand being out of one's control. (iii) Three statements referring to the feeling of movement. These comprised items relating to perceived motion of one's own hand, and to movement of the rubber hand. (iv) Three statements referring to affect. These comprised items relating to the appeal and enjoyment of the experience, and the expected touch being pleasant. Participants completed four versions of the questionnaire, one for each experimental condition. Participants answered each statement by choosing a number from a 7-point Likert Scale, from ‘–3 being strongly in disagreement’ to ‘+3 being strongly in agreement’. The questions appeared in random order.
(v). Skin conductance response
The SCR reflects the activation of the autonomic nervous system. We used SCR as a proxy of the illusion. SCR was recorded using the ADInstruments PowerLab 16/30 system, following standard published guidelines. Silver electrodes were taped to the palmar surface of the participant's left hand II and IV fingers. Recordings of experimenter's hand kinematic and SCR were triggered. The participants wore the electrodes for a few minutes before the recording was initiated to achieve signal stabilization and individual calibration. Data were registered at 1 KHz sample-rate and processed with a Matlab (The Mathworks Inc.) custom program.
(b). Experiment 2: comparing the effects of tactile expectation and only vision of the rubber hand on the rubber hand illusion
One may question whether or not mere exposure to a rubber hand in experiment 1 could have been enough to induce embodiment of the rubber hand [13,23–25]. In this experiment, we compared the contribution of tactile expectation with that of visual exposure with a rubber hand in the RHI.
(i). Participants, experimental design and procedure
Thirty right-handed healthy naive participants (mean age 22.2, 27 females), with normal or corrected to normal vision, took part in this study after having provided their written informed consent. None of them had previously participated in a RHI study and they were naive to the purpose of the study.
Experimental design and procedure were the same as in experiment 1 except for the measured dependent variables. In fact, we did not record SCR and the RHI was assessed only by means of statements referring to the embodiment of the rubber hand. Participants were divided in two groups. The first group was asked to perform the tactile expectation paradigm, as in experiment 1. The second group was asked to perform the vision only paradigm, that is, they were exposed to the same experimental conditions, but in none of them was there an approaching hand.
3. Results
(a). Experiment 1: tactile expectation and rubber hand illusion
(i). Introspective evidence
As described in §2a(iv), participants answered a total of 21 questions for each experimental condition, referring to four different components of the experience of embodiment during the RHI paradigm: (i) embodiment, (ii) loss of one's own hand, (iii) movement, and (iv) affect. The mean ratings for each component of the experience of embodiment were submitted to a 2 × 2 ANOVA with the viewed object (rubber hand versus piece of wood) and the position of the viewed object (congruent versus incongruent) as main factors.
(ii). Embodiment questions
The main effect of the viewed object was significant (F1.14 = 70.4, p < 0.001; 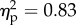 ). The main effect of position of the viewed object was significant (F1.14 = 176.2, p < 0.001;
). The main effect of position of the viewed object was significant (F1.14 = 176.2, p < 0.001; 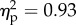 ). The interaction between the two factors was also significant (F1.14 = 19.7, p < 0.001
). The interaction between the two factors was also significant (F1.14 = 19.7, p < 0.001 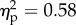 ). Post hoc comparisons with Bonferroni correction showed higher mean rating for the hand congruent (2.1) condition when compared with all the other conditions (−1.4, −0.6 and −1.6 for hand incongruent, wood congruent, and wood incongruent, respectively; all p-values < 0.001; figure 2). None of the other comparisons reached significance (all p-values > 0.18).
). Post hoc comparisons with Bonferroni correction showed higher mean rating for the hand congruent (2.1) condition when compared with all the other conditions (−1.4, −0.6 and −1.6 for hand incongruent, wood congruent, and wood incongruent, respectively; all p-values < 0.001; figure 2). None of the other comparisons reached significance (all p-values > 0.18).
Figure 2.
Mean ratings for the embodiment statements, for the loss of one's hand statements, for the movement statements and for the affect statements. RHc, rubber hand congruent; RHi, rubber hand incongruent; Wc, wood congruent; Wi, wood incongruent. Error bars indicate standard errors.
(iii). Loss of one's hand questions
The main effect of the viewed object was significant (F1.14 = 8.8, p < 0.05; 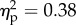 ). The main effect of position of the viewed object was significant (F1.14 = 5.8, p < 0.05;
). The main effect of position of the viewed object was significant (F1.14 = 5.8, p < 0.05; 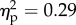 ). The interaction between the two factors was also significant (F1.14 = 5.2, p < 0.05;
). The interaction between the two factors was also significant (F1.14 = 5.2, p < 0.05; 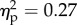 ). Post hoc comparisons with Bonferroni correction showed higher mean rating for the hand congruent (0.49) condition when compared with all the other conditions (−0.56, −0.55 and −0.67 for hand incongruent, wood congruent, and wood incongruent, respectively; all p-values < 0.05; figure 2). None of the other comparisons reached significance (all p-values ≥ 0.9).
). Post hoc comparisons with Bonferroni correction showed higher mean rating for the hand congruent (0.49) condition when compared with all the other conditions (−0.56, −0.55 and −0.67 for hand incongruent, wood congruent, and wood incongruent, respectively; all p-values < 0.05; figure 2). None of the other comparisons reached significance (all p-values ≥ 0.9).
(iv). Movement questions
The main effect of the viewed object was significant (F1.14 = 55.5, p < 0.001; 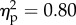 ). The main effect was explained by higher mean rating for the rubber hand (−1.3) condition when compared with the wood condition (−2.1). The position of the viewed object and its interaction were not significant (figure 2).
). The main effect was explained by higher mean rating for the rubber hand (−1.3) condition when compared with the wood condition (−2.1). The position of the viewed object and its interaction were not significant (figure 2).
(v). Affect questions
The main effect of the viewed object was significant (F1.14 = 10.6, p < 0.01; 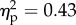 ). The main effect of viewed object was explained by higher mean rating for the rubber hand (1.2) condition when compared with the wood condition (0.5; figure 2). The main effect of position of the viewed object was significant (F1.14 = 14.6, p < 0.01;
). The main effect of viewed object was explained by higher mean rating for the rubber hand (1.2) condition when compared with the wood condition (0.5; figure 2). The main effect of position of the viewed object was significant (F1.14 = 14.6, p < 0.01; 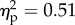 ). This was explained by higher mean rating for the congruent (1.2) condition when compared with incongruent condition (0.5). The interaction between the two factors was not significant (F1.14 = 0.15, p = 0.7).
). This was explained by higher mean rating for the congruent (1.2) condition when compared with incongruent condition (0.5). The interaction between the two factors was not significant (F1.14 = 0.15, p = 0.7).
(vi). Skin conductance response
The raw data were down-sampled by a factor of 100. The down-sampled raw data were bandpass filtered (0.01–0.5 Hz) with a Chebyshev type 2 digital filter in order to cut down slow signal variations and high-frequency noise.
A principal component analysis (PCA) was performed for each of the four distances from the object in each block. This was done in order to find a common timecourse in the four trials of each block, thus lowering the signal noise while extracting signal features related to the approaching movements. We always found that the first PCA component explained most of the variability of the signal (mean 60%, s.d. = 3%) for both the viewed object and distance. Thus, we decided to perform the statistical analysis on the first PCA component over the four trials as this component could be roughly considered the best descriptor of SCR to the approaching movements for each condition.
For further statistical analysis, we considered the maximum value of the first component of the PCA in a 10 s time-interval after each switch onset activated by the experimenter's hand movement (PCA SCR value).
The PCA SCR values in all experimental conditions were submitted to a 2 × 2 × 4 ANOVA with the viewed object (rubber hand versus piece of wood), the position of the viewed object (congruent versus incongruent) and distance (60 versus 45 versus 30 versus 15 cm) as main factors. The main effect of the viewed object was significant (F1.14 = 11.5, p < 0.01; 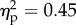 ). The main effect of the position of the viewed object was significant (F1.14 = 18.6, p < 0.01;
). The main effect of the position of the viewed object was significant (F1.14 = 18.6, p < 0.01; 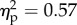 ). The main effect of distance was significant (F3.42 = 5.1, p < 0.01;
). The main effect of distance was significant (F3.42 = 5.1, p < 0.01; 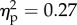 ). Strikingly, the three-way interaction was significant (F3.42 = 3.6, p < 0.05;
). Strikingly, the three-way interaction was significant (F3.42 = 3.6, p < 0.05; 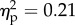 ). The interaction was explained by the concomitant higher SCR values—when compared with all the other experimental conditions—while observing the experimenter's hand approaching the rubber hand in the congruent position at the distance of 30 and 15 cm (p-values < 0.01 for both distances; figure 3). For detailed values, see table 1.
). The interaction was explained by the concomitant higher SCR values—when compared with all the other experimental conditions—while observing the experimenter's hand approaching the rubber hand in the congruent position at the distance of 30 and 15 cm (p-values < 0.01 for both distances; figure 3). For detailed values, see table 1.
Figure 3.

Mean SCR (microsiemens, µS) across viewed objects and distances. RHc, rubber hand congruent; RHi, rubber hand incongruent; Wc, wood congruent; Wi, wood incongruent. Error bars indicate standard errors.
Table 1.
Mean SCR (microsiemens, µS) ±standard errors across viewed objects and distances.
| distance (cm) | rubber hand |
wood |
||
|---|---|---|---|---|
| congruent | incongruent | congruent | incongruent | |
| 60 | 0.41 (±0.12) | 0.28 (±0.07) | 0.22 (±0.06) | 0.25 (±0.08) |
| 45 | 0.46 (±0.09) | 0.24 (±0.06) | 0.23 (±0.06) | 0.27 (±0.08) |
| 30 | 0.64 (±0.13) | 0.26 (±0.06) | 0.24 (±0.06) | 0.29 (±0.09) |
| 15 | 0.88 (±0.20) | 0.26 (±0.07) | 0.27 (±0.08) | 0.29 (±0.11) |
(vii). Correlations between introspective evidence and skin conductance response
We performed Pearson's correlations between the mean scores on embodiment and loss of one's hand questions and the mean SCR change recorded, while observing the experimenter's hand approaching the rubber hand in the congruent position at the distance of 15 cm. Correlations were computed only on embodiment and loss of one's hand questions because those were the only questions revealing the illusion. SCR change was computed by subtracting SCR recorded at the beginning of the trial (i.e. 70 cm) from the SCR at 15 cm. According to the number of comparisons performed, p-value was set at 0.025 (Bonferroni correction). Interestingly, the higher the SCR change, the greater the likelihood of experiencing embodiment of the rubber hand. More precisely, we observed a significant positive correlation between the reported strength of embodiment and the mean SCR change during hand congruent condition (r = 0.58, n = 15, p = 0.024, two–tailed; figure 4), whereas there was no correlation with the reported experience of loss of one's hand and the mean SCR change (r = 0.434, n = 15, p = 0.106, two-tailed).
Figure 4.
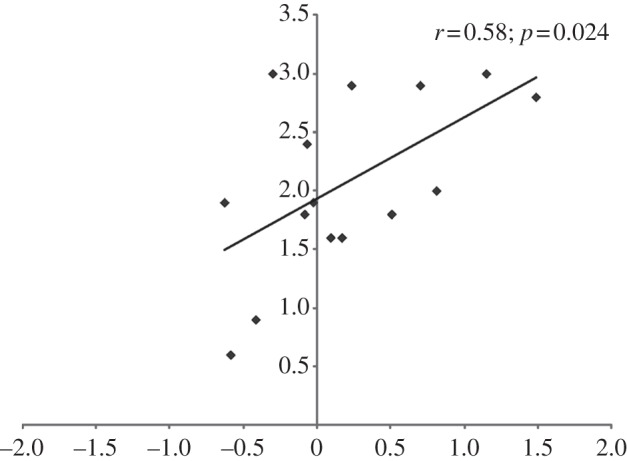
Vividness of the RHI, as revealed by embodiment statements (y-axis) and increase in SCR (x-axis, microsiemens, µS) for all participants across RHI.
(b). Experiment 2: comparing tactile expectation and just vision of the rubber hand on the rubber hand illusion
The mean ratings for the experience of embodiment were submitted to a 2 × 2 × 2 ANOVA with group (tactile expectation versus just vision) as between-subject factor, the viewed object (rubber hand versus piece of wood) and the position of the viewed object (congruent versus incongruent) as within subject factors.
The main effects of viewed object (F1.28 = 46.6, p < 0.001; 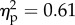 ) and position of the viewed object (F1.28 = 104.6, p < 0.001;
) and position of the viewed object (F1.28 = 104.6, p < 0.001;  ), as well as their interaction (F1.28 = 18.1, p < 0.01;
), as well as their interaction (F1.28 = 18.1, p < 0.01; 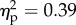 ) were significant. Strikingly, the three-way interaction was also significant (F1.28 = 8.5, p < 0.01;
) were significant. Strikingly, the three-way interaction was also significant (F1.28 = 8.5, p < 0.01; 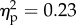 ). Post hoc comparisons showed higher mean rating, in both groups, for the hand congruent condition when compared with all the other conditions (table 2). Importantly, post hoc comparisons also showed higher mean rating for the hand congruent condition (1.7) in the tactile expectation group when compared with the just vision group (0.3; p < 0.01). The other conditions did not differ between groups.
). Post hoc comparisons showed higher mean rating, in both groups, for the hand congruent condition when compared with all the other conditions (table 2). Importantly, post hoc comparisons also showed higher mean rating for the hand congruent condition (1.7) in the tactile expectation group when compared with the just vision group (0.3; p < 0.01). The other conditions did not differ between groups.
Table 2.
Mean Ratings for the embodiment questions (experiment 2).
| rubber hand |
wood |
|||
|---|---|---|---|---|
| congruent | incongruent | congruent | incongruent | |
| tactile expectation | 1.7 (±0.2) | −1.9 (±0.3) | −1.2 (±0.3) | −1.8 (±0.3) |
| just vision | 0.3 (±0.4) | −1.7 (±0.3) | −1.0 (±0.4) | −2.3 (±0.1) |
4. Discussion
The present paper deals with one unanswered empirical question on the sense of body ownership. This question relates to the sufficient and necessary conditions to attribute an external object to our body. The second experiment suggests that mere exposure to the rubber hand is not enough to induce a full-blown embodiment of the RH [24]. We hypothesized that expectations about upcoming sensory events with respect to a rubber hand are sufficient to induce an SO over the same rubber hand, as measured by questionnaire and SCR. Our results suggest two main novel findings: first, actual tactile stimulation is not necessary in order to experience ownership over a non-body object; second, during the RHI, SCR is a reliable measure of the genuine assimilation of the fake hand into the participant's body. Indeed, SCR reveals enhanced autonomic arousal even in the absence of a threat, either to the real or to the fake hand [8,26,27]. But, how is it possible to feel ownership over a dummy hand when tactile stimuli are merely expected rather than actually experienced?
A possibility is to look at predictive processing in perception [28]. Broadly speaking, predictive processing refers to any type of psychological or neural process that uses or generates not only information about the past or the present, but also expectations about future states of the body or the environment. Conceptualizations of predictive processing are mainly grounded on Bayesian statistical inference (for reviews, see [29,30]). This method of inference can be used to determine the probability of a certain outcome, given a predetermined assumption, which can then be subsequently updated according to the actual outcome. Concerning the specific domain of perception, assumptions can be either expectations based on previous experiences, or ‘innate’ priors [30]. Both will act as top-down modulators of bottom-up sensory input.
Strikingly, expectation is not bounded within the psychological domain, as it effects the neural level as well. In particular, in sensory cortices, predictive processing has been associated with a reduction in activation thresholds and increase in signal-to-noise ratio, which facilitates subsequent stimulus processing [29,31]. fMRI investigation revealed that anticipation of a sensory stimulus and processing of the somatosensory stimulus itself (i.e. tickling) engage a similar network of activation and deactivation [32]. Moreover, magnetoencephalography investigation showed that expectation of a tactile event involves a pre-stimulus modulation of neuronal oscillations in somatosensory cortex [33].
Here, we demonstrate that expectation of an upcoming sensory event is sufficient to induce SO over a rubber hand, even if no physical tactile stimulation is delivered on either the rubber hand or on the real hand. This finding is in line with theories claiming that predictive processing reflects one of the core principles of brain functioning. According to this view, a fundamental function of the brain is to constantly generate predictions that help to interpret the sensory environment in the most efficient manner, rather than waiting to be activated by incoming stimuli. Such predictions facilitate interactions with external stimuli, conserve effort and ultimately increase the chances of survival. Useful predictions typically do not arise de novo; rather they take advantage of past experiences [30]. Our behavioural and physiological results extend this view from the perception of external environment to the SO. Indeed, they support the hypothesis that our brain does not build a sense of bodily self in a merely reactive way, via perceptual correlations, rather it generates predictions on what could be mine or not. An explanation in terms of predictions and prediction errors for the classic RHI has been very recently provided by Apps & Tsakiris [34]. According to these authors, under synchronous multisensory stimulation between the participant's hand and the rubber hand, touch referral and then ownership would arise as the result of the brain's attempt to minimize the prediction error of seeing and feeling touch at different locations. Conversely, in this study, the change in body ownership is not elicited by the generation of bottom-up prediction errors. Indeed, with our new induction procedure, the RHI arises as an effect of anticipation (i.e. the process of expecting stimuli or events in the spatio-temporal vicinity of the predicted state). Thus, at theoretical level, our results add to the proposal of Apps & Tsakiris [34] that anticipation of touch might be sufficient to elicit changes in sense of body ownership. In other words, the novelty of our proposal would be that violation of expectations about multisensory events is not a necessary condition for RHI to occur. This holds, however, only to the extent that the seen external object resembles the internal body-model.
This is not the whole story, though. Our experiment also revealed that embodiment of the rubber hand only occurred when the approaching stimulus (i.e. experimenter's hand) entered participants’ peripersonal space (PPS, the space around the hand), as shown by the autonomic response. The definition of PPS [35,36] originates from electrophysiological studies based on visual–tactile neurons identified in the premotor area F4 and the ventral intraparietal area (VIP) of the monkey brain [37–39]. The receptive fields of the VIP-F4 neurons are coded in somatic coordinates and anchored to various parts of the body. In particular, the visual receptive fields of F4 neurons around the hand extend from 5 to 35 cm from the tactile receptive fields [17]. Such extension increases in depth when the speed of an approaching stimulus also increases [17]. The advantage of this dynamic mapping of space, which seems also to be a characteristic of humans [40–42], is quite obvious: it enables an efficient mapping of what is really near, thus permitting us either to take advantage of an opportunity or to avoid a threat. Thus, PPS should not be understood only as a particular region surrounding the body that acts as an interface between the body and the environment. Because of its pointing to the environment and the body, PPS cuts across any dichotomy between internal and external milieu. This would be in line with the idea of a ‘body-matrix’ [43], conceived as a dynamic neural representation of the body that extends beyond its surface to integrate visual and auditory inputs arising from the surrounding space with tactile and proprioceptive inputs.
To sum up, using a new induction procedure of the RHI, we showed that anticipation of touch is sufficient to induce, proactively rather than reactively, an SO over a dummy hand. Crucially, for tactile expectation to exert this effect, the approaching stimulus (i.e. experimenter's hand) must fall within the participant's PPS. We speculate that such proactive sense of self, allowed by predictive processing in the brain, plays a role in maintaining a coherent and unitary sense of bodily self.
Data accessibility
Funding statement
This study was supported by a grant from BIAL foundation to M.C. and F.F. and by the EU grant Towards an Embodied Science of InterSubjectivity (TESIS) to V.G. M.C. was also supported by Volkswagen foundation.
References
- 1.Ehrsson HH. 2012. The concept of body ownership and its relation to multisensory integration. Cambridge, MA: MIT Press [Google Scholar]
- 2.Gallagher S. 2005. How the body shapes the mind. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Tsakiris M, Schutz-Bosbach S, Gallagher S. 2007. On agency and body-ownership: phenomenological and neurocognitive reflections. Conscious. Cogn. 16, 645–660 10.1016/j.concog.2007.05.012 (doi:10.1016/j.concog.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 4.Botvinick M. 2004. Probing the neural basis of body ownership. Science 305, 782–783 10.1126/science.1101836 (doi:10.1126/science.1101836) [DOI] [PubMed] [Google Scholar]
- 5.Botvinick M, Cohen J. 1998. Rubber hands ‘feel’ touch that eyes see. Nature 391, 756. 10.1038/35784 (doi:10.1038/35784) [DOI] [PubMed] [Google Scholar]
- 6.Makin TR, Holmes NP, Ehrsson HH. 2008. On the other hand: dummy hands and peripersonal space. Behav. Brain Res. 191, 1–10 10.1016/j.bbr.2008.02.041 (doi:10.1016/j.bbr.2008.02.041) [DOI] [PubMed] [Google Scholar]
- 7.Tsakiris M. 2010. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia 48, 703–712 10.1016/j.neuropsychologia.2009.09.034 (doi:10.1016/j.neuropsychologia.2009.09.034) [DOI] [PubMed] [Google Scholar]
- 8.Armel KC, Ramachandran VS. 2003. Projecting sensations to external objects: evidence from skin conductance response. Proc. R. Soc. Lond. B 270, 1499–1506 10.1098/rspb.2003.2364 (doi:10.1098/rspb.2003.2364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsakiris M, Haggard P. 2005. The rubber hand illusion revisited: visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform 31, 80–91 10.1037/0096-1523.31.1.80 (doi:10.1037/0096-1523.31.1.80) [DOI] [PubMed] [Google Scholar]
- 10.Tsakiris M, Costantini M, Haggard P. 2008. The role of the right temporo-parietal junction in maintaining a coherent sense of one's body. Neuropsychologia 46, 3014–3018 10.1016/j.neuropsychologia.2008.06.004 (doi:10.1016/j.neuropsychologia.2008.06.004) [DOI] [PubMed] [Google Scholar]
- 11.Costantini M, Haggard P. 2007. The rubber hand illusion: sensitivity and reference frame for body ownership. Conscious. Cogn. 16, 229–240 10.1016/j.concog.2007.01.001 (doi:10.1016/j.concog.2007.01.001) [DOI] [PubMed] [Google Scholar]
- 12.Haans A, Ijsselsteijn WA, de Kort YAW. 2008. The effect of similarities in skin texture and hand shape on perceived ownership of a fake limb. Body Image 5, 389–394 10.1016/j.bodyim.2008.04.003 (doi:10.1016/j.bodyim.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 13.Pavani F, Spence C, Driver J. 2000. Visual capture of touch: out-of-the-body experiences with rubber gloves. Psychol. Sci. 11, 353–359 10.1111/1467-9280.00270 (doi:10.1111/1467-9280.00270) [DOI] [PubMed] [Google Scholar]
- 14.Austen EL, Soto-Faraco S, Enns JT, Kingstone A. 2004. Mislocalizations of touch to a fake hand. Cogn. Affect Behav. Neurosci. 4, 170–181 10.3758/CABN.4.2.170 (doi:10.3758/CABN.4.2.170) [DOI] [PubMed] [Google Scholar]
- 15.Ehrsson HH, Spence C, Passingham RE. 2004. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305, 875–877 10.1126/science.1097011 (doi:10.1126/science.1097011) [DOI] [PubMed] [Google Scholar]
- 16.Engel AK, Fries P, Singer W. 2001. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716 10.1038/35094565 (doi:10.1038/35094565) [DOI] [PubMed] [Google Scholar]
- 17.Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. 1996. Coding of peripersonal space in inferior premotor cortex (area F4). J. Neurophysiol. 76, 141–157 [DOI] [PubMed] [Google Scholar]
- 18.MacKay WA, Crammond DJ. 1987. Neuronal correlates in posterior parietal lobe of the expectation of events. Behav. Brain Res. 24, 167–179 10.1016/0166-4328(87)90055-6 (doi:10.1016/0166-4328(87)90055-6) [DOI] [PubMed] [Google Scholar]
- 19.Duhamel JR, Colby CL, Goldberg ME. 1998. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J. Neurophysiol. 79, 126–136 [DOI] [PubMed] [Google Scholar]
- 20.Hyvärinen J. 1982. Posterior parietal lobe of the primate brain. Physiol. Rev. 62, 1060–1129 [DOI] [PubMed] [Google Scholar]
- 21.Mistlin AJ, Perrett DI. 1990. Visual and somatosensory processing in the macaque temporal cortex: the role of ‘expectation’. Exp. Brain Res. 82, 437–450 10.1007/bf00231263 (doi:10.1007/bf00231263) [DOI] [PubMed] [Google Scholar]
- 22.Longo MR, Schüür F, Kammers MPM, Tsakiris M, Haggard P. 2008. What is embodiment? A psychometric approach. Cognition 107, 978–998 10.1016/j.cognition.2007.12.004 (doi:10.1016/j.cognition.2007.12.004) [DOI] [PubMed] [Google Scholar]
- 23.Farne A, Pavani F, Meneghello F, Ladavas E. 2000. Left tactile extinction following visual stimulation of a rubber hand. Brain 123, 2350–2360 10.1093/brain/123.11.2350 (doi:10.1093/brain/123.11.2350) [DOI] [PubMed] [Google Scholar]
- 24.Longo MR, Cardozo S, Haggard P. 2008. Visual enhancement of touch and the bodily self. Conscious. Cogn. 17, 1181–1191 10.1016/j.concog.2008.01.001 (doi:10.1016/j.concog.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 25.Rohde M, Di Luca M, Ernst MO. 2011. The rubber hand illusion: feeling of ownership and proprioceptive drift do not go hand in hand. PLoS ONE 6, e21659. 10.1371/journal.pone.0021659 (doi:10.1371/journal.pone.0021659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guterstam A, Petkova VI, Ehrsson HH. 2011. The illusion of owning a third arm. PLoS ONE 6, e17208. 10.1371/journal.pone.0017208 (doi:10.1371/journal.pone.0017208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petkova VI, Ehrsson HH. 2009. When right feels left: referral of touch and ownership between the hands. PLoS ONE 4, e6933. 10.1371/journal.pone.0006933 (doi:10.1371/journal.pone.0006933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. 2006. Predictive codes for forthcoming perception in the frontal cortex. Science 314, 1311–1314 10.1126/science.1132028 (doi:10.1126/science.1132028) [DOI] [PubMed] [Google Scholar]
- 29.Bubic A, Von Cramon DY, Schubotz RI. 2010. Prediction, cognition and the brain. Front. Hum. Neurosci. 4, 25. 10.3389/fnhum.2010.00025 (doi:10.3389/fnhum.2010.00025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown EC, Brüne M. 2012. The role of prediction in social neuroscience. Front. Hum. Neurosci. 6, 147. 10.3389/fnhum.2012.00147 (doi:10.3389/fnhum.2012.00147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunia CHM. 1999. Neural aspects of anticipatory behavior. Acta Psychol. 101, 213–242 10.1016/S0001-6918(99)00006-2 (doi:10.1016/S0001-6918(99)00006-2) [DOI] [PubMed] [Google Scholar]
- 32.Carlsson K, Petrovic P, Skare S, Petersson KM, Ingvar M. 2000. Tickling expectations: neural processing in anticipation of a sensory stimulus. J. Cogn. Neurosci. 12, 691–703 10.1162/089892900562318 (doi:10.1162/089892900562318) [DOI] [PubMed] [Google Scholar]
- 33.van Ede F, de Lange F, Jensen O, Maris E. 2011. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J. Neurosci. 31, 2016–2024 10.1523/jneurosci.5630-10.2011 (doi:10.1523/jneurosci.5630-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apps MA, Tsakiris M. 2013. The free-energy self: a predictive coding account of self-recognition. Neurosci. Biobehav. Rev. (doi:10.1016/j.neubiorev.2013.01.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. 1981. Afferent properties of periarcuate neurons in macaque monkeys. I. Somatosensory responses. Behav. Brain Res. 2, 125–146 10.1016/0166-4328(81)90052-8 (doi:10.1016/0166-4328(81)90052-8) [DOI] [PubMed] [Google Scholar]
- 36.Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. 1981. Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav. Brain Res. 2, 147–163 10.1016/0166-4328(81)90053-x (doi:10.1016/0166-4328(81)90053-x) [DOI] [PubMed] [Google Scholar]
- 37.Fogassi L, Raos V, Franchi G, Gallese V, Luppino G, Matelli M. 1999. Visual responses in the dorsal premotor area F2 of the macaque monkey. Exp. Brain Res. 128, 194–199 10.1007/s002210050835 (doi:10.1007/s002210050835) [DOI] [PubMed] [Google Scholar]
- 38.Graziano MSA. 2001. A system of multimodal areas in the primate brain. Neuron 29, 4–6 10.1016/s0896-6273(01)00174-x (doi:10.1016/s0896-6273(01)00174-x) [DOI] [PubMed] [Google Scholar]
- 39.Rizzolatti G, Fadiga L, Fogassi L, Gallese V. 1997. The space around us. Science 277, 190–191 10.1126/science.277.5323.190 (doi:10.1126/science.277.5323.190) [DOI] [PubMed] [Google Scholar]
- 40.Chieffi S, Fogassi L, Gallese V, Gentilucci M. 1992. Prehension movements directed to approaching objects: influence of stimulus velocity on the transport and the grasp components. Neuropsychologia 30, 877–897 10.1016/0028-3932(92)90033-i (doi:10.1016/0028-3932(92)90033-i) [DOI] [PubMed] [Google Scholar]
- 41.Brozzoli C, Gentile G, Petkova VI, Ehrsson HH. 2011. fMRI adaptation reveals a cortical mechanism for the coding of space near the hand. J. Neurosci. 31, 9023–9031 10.1523/jneurosci.1172-11.2011 (doi:10.1523/jneurosci.1172-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makin TR, Holmes NP, Zohary E. 2007. Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. J. Neurosci. 27, 731–740 10.1523/jneurosci.3653-06.2007 (doi:10.1523/jneurosci.3653-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moseley GL, Gallace A, Spence C. 2012. Bodily illusions in health and disease: physiological and clinical perspectives and the concept of a cortical ‘body matrix’. Neurosci. Biobehav. Rev. 36, 34–46 10.1016/j.neubiorev.2011.03.013 (doi:10.1016/j.neubiorev.2011.03.013) [DOI] [PubMed] [Google Scholar]