Abstract
Parasites with complex life cycles are expected to manipulate the behaviour of their intermediate hosts (IHs), which increase their predation rate and facilitate the transmission to definitive hosts (DHs). This ability, however, is a double-edged sword when the parasite can also be transmitted vertically in the IH. In this situation, as the manipulation of the IH behaviour increases the IH death rate, it conflicts with vertical transmission, which requires healthy and reproducing IHs. The protozoan Toxoplasma gondii, a widespread pathogen, combines both trophic and vertical transmission strategies. Is parasite manipulation of host behaviour still adaptive in this situation? We model the evolution of the IH manipulation by T. gondii to study the conflict between these two routes of transmission under different epidemiological situations. Model outputs show that manipulation is particularly advantageous for virulent strains and in epidemic situations, and that different levels of manipulation may evolve depending on the sex of the IH and the transmission routes considered. These results may help to understand the variability of strain characteristics encountered for T. gondii and may extend to other trophically transmitted parasites.
Keywords: trophic transmission, behavioural manipulation, vertical transmission, adaptive dynamics, population genetics approach, virulence
1. Introduction
Trophically transmitted parasites often have the ability to manipulate the behaviour of their intermediate hosts (IHs), which facilitates transmission to definitive hosts (DHs) through predation [1–3]. The potential adaptive value of indirect transmission via IHs has been explored in several studies [4–7]. This evolutionary perspective helped identify conditions most favourable for the evolution of parasite manipulation under the assumption that the parasite is transmitted exclusively through predation. For example, an increase in the probability of predation of infected prey by DH hosts could help sustain parasite transmission when the density of DH hosts is low [8]. However, the adaptive nature of manipulation has also been questioned, especially when associated with high fitness costs [9–11]. These costs may be due to the physiological constraints associated with manipulation itself [12,13] or to a higher probability of early death (because of predation by a non-host species, for example) [14,15]. In addition, other constraints may act on the evolution of host manipulation if the pathogen can be transmitted by alternative transmission routes that conflict with trophic transmission, especially transmission routes that need the host to survive.
In this study, we explore how vertical transmission, defined as the transmission of a pathogen from a mother to its offspring before or around the time of birth, can interfere with the epidemiology and the evolution of trophically transmitted parasites. On the one hand, by enhancing the prevalence of the infection in IHs, and thus the probability of predation of infected IHs by DHs, vertical transmission increases the probability to complete the parasite life cycle. On the other hand, a potential conflict may emerge between vertical and trophic routes of transmission, because vertical transmission needs a healthy host to be efficient, whereas trophic transmission relies on the death of infected IHs through predation. This conflict has been demonstrated experimentally in a biological system where two parasite species, the microsporidia Dictyocoela sp. (roeselum) and the Acanthocephala Polymorphus minutus, which use different routes of transmission, are exploiting the same host: the amphipod Gammarus roeseli. Haine et al. [16] showed that the vertically transmitted microsporidia downregulate the manipulation induced by the trophically transmitted Acanthocephala. The protection conferred by the vertically transmitted pathogen can evolve as a consequence of the conflict arising between horizontal and vertical routes of transmission [17]. The situation, however, is less clear when the same parasite species can transmit both vertically and trophically.
Among parasites that exhibit both trophic and vertical transmission stand common species such as several helminths [18], or protozoa such as Neospora caninum [19] and Toxoplasma gondii [20]. The latter, being one of the most widespread parasites in the world, raises important health issues as a zoonosis. The strong geographical variation observed in the risk of people [21] suggests that the transmission dynamics of T. gondii varies spatially. In particular, distinct strains are found in different areas of the world, which suggests some variation in selection pressures among these environments. Toxoplasma gondii mainly spreads through a complex life cycle, with a trophic transmission from prey IHs—including rodents—to DHs (i.e. felids; mainly domestic cats, Felis catus).
Toxoplasma gondii infection is reported to alter behaviour in rodents [22,23]. In particular, the innate aversion of rodents to cat odours is turned into an attraction [24,25]. Interestingly, some studies showed that this modification of the perception of cat odours does not alter the behavioural responses associated with other predator and non-predator odours [24–26]. As a consequence, behavioural manipulation in rodents could be expected to specifically increase predation of infected rodents by cats. However, Worth et al. [27] highlighted inconsistencies between effects of T. gondii behavioural manipulation: while some studies reported behavioural modifications specific to cat stimuli [24–26], others showed unspecific modification in activity and anxiety levels [22,23], with sometimes conflicting results. Moreover, Kannan et al. [28] showed differences in the duration of the behavioural modification in mice between two T. gondii strains. These inconsistencies could result from the characteristics of host used in experiments, and the relevance of laboratory experiments regarding the behaviour of wild-living hosts may be questioned. Nevertheless, they show that manipulation can vary in its intensity and expression; thus it is possible that differences in selective pressures for manipulation occur among strain/host systems. Moreover, the magnitude of the impact of modification of IH behaviour on parasite spread in different epidemiological situations has never been measured.
Toxoplasma gondii may also be transmitted through vertical transmission in IHs. The effect of vertical transmission on the overall spread of the parasite is not clear as its magnitude may depend on the host species or strain, as well as on the parasite strain. In captive and chronically infected house mice Mus musculus and field mice Apodemus sylvaticus, more than 80% of pups were infected [29]. In a natural population of house mice, vertical transmission occurred in 75% of pregnancies [30]. On the contrary, in BALB/c laboratory mice, and in other species such as rats Rattus sp. and hamsters Mesocricetus auratus, vertical transmission occurs essentially when infection is acquired during pregnancy, but is less likely in chronically infected individuals [31–33]. Also, a recent study showed that the parasite can be sexually transmitted in rats from males to females [34]. If this route of transmission proves to be common, then enhanced predation via behavioural manipulation could also conflict with sexual transmission. Finally, T. gondii also exhibits different levels of virulence in IHs. Virulence in laboratory mice varies according to parasite strain: with highly virulent strains, a single oocyst may kill a mouse within 10 days (LD100 = one parasite), whereas the 50% lethal doses are above 102 parasites for strains with low virulence [35,36]. Notably, the virulence observed in laboratory mice may not apply to other IH species: some mice-virulent strains may be avirulent for rats [35].
Here, we develop and analyse a model of the epidemiology and evolution of T. gondii. First, we study how manipulation of the IHs and vertical transmission affect the epidemiology of this system through their effects on the basic reproductive ratio R0 of T. gondii. Then, we analyse the evolution of manipulation of IH behaviour under different epidemiological scenarios. We show that the selection acting on this trait may vary between an epidemic and an endemic state. We also study the prediction that the manipulation of the IH should be sex-specific, because only females transmit the disease vertically. Males should exhibit higher rates of behavioural manipulation than females, except when sexual transmission from male to female may offer yet another route of transmission for the parasite. We discuss all these theoretical predictions in the light of available experimental and empirical data on T. gondii.
2. Epidemiological model
The model is based on T. gondii transmission, and represents the complex life cycle with trophic transmission and vertical transmission in IHs: felids (DHs) excrete oocysts in the environment through their faeces; rodents (IHs) are contaminated by sporulated oocysts or vertical transmission; DHs get infected by preying upon infected prey (figure 1). The model follows Lélu et al. [37] after modifying the environmental contamination [38] and neglecting the possibility of direct transmission from environment to DHs. The DH population is split into three compartments: S1, I1 and R1 (with N1 = S1 + I1 + R1), representing the numbers of susceptible, infectious and immune DHs, respectively. Within a limited period of time, infectious DHs excrete millions of oocysts that sporulate in the environment and become infectious for IHs. In the environment, E represents the quantity of cat faeces contaminated by oocysts of the parasite. The IH population is divided into two compartments, S2 and I2 (with N2 = S2 + I2), representing the numbers of susceptible and infected prey, respectively.
Figure 1.
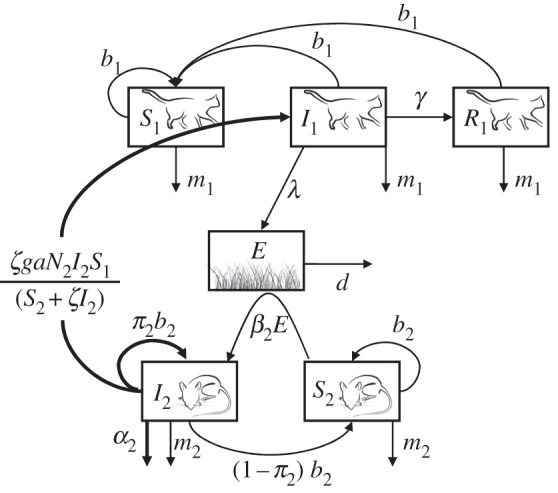
Schematic of the model for the transmission of T. gondii. S1, I1 and R1 stand for the numbers of susceptible, infected and recovered cats, respectively. E represents the density of contaminated faeces in the environment. S2 and I2 stand for the numbers of susceptible and infected prey, respectively. Bold arrows and parameters represent the three traits studied: behavioural manipulation of IHs ζ, vertical transmission π2 and virulence α2.
The total population size of each type of host is denoted by Ni, with i = 1 or 2 for DHs and IHs, respectively. The total population size depends on the birth rate bi, the mortality rate mi, the intrinsic growth rate ri = (bi − mi) and the carrying capacity Ki. The parameter ki measures the intensity of density-dependent competition on mortality ki = ri/Ki. In the prey dynamics, a mortality term owing to predation by cats is added and represented by a Lotka–Volterra (i.e. type I) functional response to predation [39,40]. This term is denoted by aN2, with a being the predation rate. Cats become infected by ingesting an infected prey I2 with a probability of infection g. Infected cats I1 spread oocysts at a rate λ in the environment, and oocysts die at a rate d. A susceptible prey can become infected through contacts with the contaminated environment E, at a rate β2. Infected DHs can recover and reach compartment R1 at a rate γ but do not suffer additional mortality following infection (i.e. no parasite virulence on the DH). As a consequence, the total population size of the DH remains constant and equal to N1 = K1. By contrast, infection of the IH is assumed to induce an increase in the mortality by a constant rate α2, measuring parasite virulence on the IH, and the equilibrium population size of the IH varies as a function of these additional sources of mortality.
Manipulation of IH behaviour is assumed to increase the predation of infected rodents by DHs. As a result, the incidence function that was 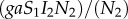 without manipulation [37] becomes
without manipulation [37] becomes 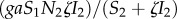 , with ζ being the intensity of manipulation. Vertical transmission is assumed to occur in infected IHs only, and the parameter π2 stands for the proportion of infected offspring produced by an infected IH. The above life cycle yields the following system of ordinary differential equations:
, with ζ being the intensity of manipulation. Vertical transmission is assumed to occur in infected IHs only, and the parameter π2 stands for the proportion of infected offspring produced by an infected IH. The above life cycle yields the following system of ordinary differential equations:
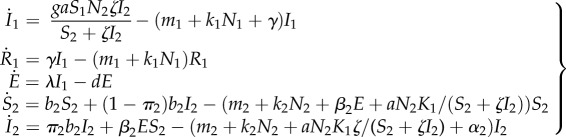 |
2.1 |
with N1 = K1 and S1 = K1 − I1 − R1, assuming cat population has reached its equilibrium (see [37]). Table S1 in the electronic supplementary material lists the different parameters and details their default (or range of) values together with some references where these parameters have been estimated for T. gondii.
3. How does manipulation affect the basic reproductive ratio R0?
The basic reproductive ratio R0 represents the average number of secondary cases caused by a single infectious individual introduced in a fully susceptible population [41]. For multi-host parasites, R0 is thus a synthetic parameter that accounts for the potential of spread of a parasite at the beginning of an epidemic. Here, we analyse how manipulation, vertical transmission and virulence affect this quantity. R0 is a complex but increasing function of two components, R0T and R0V, which are the basic reproductive ratios resulting from trophic transmission and vertical transmission, respectively (see the electronic supplementary material, appendix S2A):
and
where 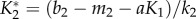 represents the population size of prey at the disease-free equilibrium.
represents the population size of prey at the disease-free equilibrium.
The number of secondary cases resulting from vertical transmission, R0V, always increases with vertical transmission π2, and decreases with manipulation ζ (with ζ ≥ 1) or virulence α2. The number of secondary cases resulting from trophic transmission, R0T, increases with manipulation and decreases with virulence α2. Hence, the basic reproductive ratio always decreases with lower proportions of vertical transmission π2, and with higher parasite virulence α2 (figure 2). The effect of manipulation on R0, however, is less clear, because the parameter ζ affects R0V and R0T differently. For the default parameter values (see the electronic supplementary material, table S1), R0 always increases with the intensity of manipulation (figure 2), except for π2 = 1 and α2 = 0, where R0 decreases very weakly with manipulation (figure 2a, upper curve; see also electronic supplementary material, appendix S1 for other parameter values). Interestingly, although both vertical transmission and manipulation increase R0, the magnitude of the effect of manipulation depends on the levels of virulence and vertical transmission. In simple epidemiological models, evolution often tends to maximize the basic reproductive ratio [42]. In our model, maximizing R0 would lead to the evolution of a maximal rate of manipulation for almost all the parameter values we consider, except for π2 = 1 and α2 = 0. In §4, we will show that these predictions are not correct, and that the understanding of the selective pressures acting on manipulation requires a more detailed description of the interplay between epidemiology and evolution.
Figure 2.
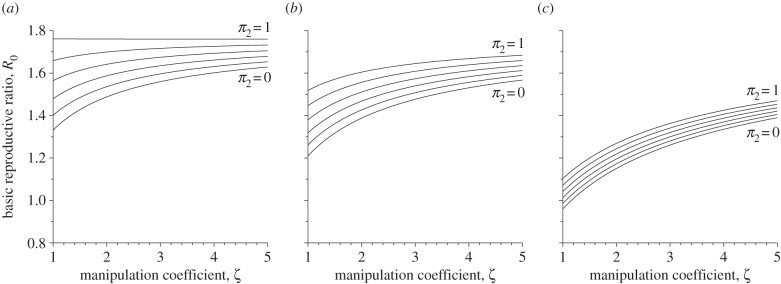
The basic reproductive rate R0 as a function of the behavioural manipulation coefficient, for six values of vertical transmission π2 and three values of virulence α2. The behavioural manipulation coefficient varies from 1 (no manipulation) to 5 (infected prey are five times more likely to be captured than non-infected ones). Vertical transmission ranges from 0 to 1. The virulence values are: (a) 0, no virulence; (b) 2/52, intermediate virulence; and (c) 10/52, high virulence.
4. How does manipulation evolve in interaction with the epidemiological dynamics?
(a). Modelling the variations in frequency of a mutant
In order to better understand how the epidemiological dynamics feed back on the evolutionary dynamics of manipulation, we follow the approach developed by Day & Gandon [43,44] to track the change in frequency of different strains of the pathogen. System (2.1) is modified in order to allow for two pathogen strains, a wild-type (wt) and a mutant m, which only differ in their ability to manipulate IH behaviour (see the electronic supplementary material, appendix S2B). We compute the changes of the frequency of the mutant in the three different compartments of the model, where 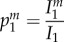 ;
; 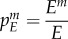 and
and 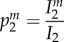 refers to the frequency of the mutant in the DH, E and IH compartments, respectively:
refers to the frequency of the mutant in the DH, E and IH compartments, respectively:
| 4.1a |
| 4.1b |
| 4.1c |
with 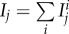 , S1 = K
1− I
1− R1 and where
, S1 = K
1− I
1− R1 and where 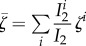 refers to the average manipulation trait value (see the electronic supplementary material, appendix S2B).
refers to the average manipulation trait value (see the electronic supplementary material, appendix S2B).
Equation (4.1a) shows that the change in frequency of the mutant in the DH compartment depends on the intensity of manipulation by the mutant (first term) and on the influx of mutants from the IH compartment (second term). In equation (4.1b), the change in frequency in E only depends on the influx of mutants from the DH compartment. In equation (4.1c), the change in frequency in the IH compartment depends on the influx of mutants from compartment E (first term) and the efflux of mutants that increased as manipulation by the mutant strain increased (second term). All these terms are weighted by the numbers of hosts in the different compartments, and this is where epidemiology feeds back on the evolutionary outcome.
Therefore, increasing manipulation is always costly in the IH compartment, such that if the mutant manipulates more than the wild-type, then 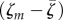 is positive and there will be an efflux of mutant from the IH compartment. This efflux, however, will feed back on equation (4.1a), and later on equation (4.1b) and back on equation (4.1c). The influx of mutant from E to IH may compensate the cost of manipulation in equation (4.1c), but this will depend on the numbers of infected IHs and contaminated faeces (first term in equation (4.1c)). In the following, we show how this impact of epidemiology on evolution may explain variations in evolutionary trajectories during and after an epidemic.
is positive and there will be an efflux of mutant from the IH compartment. This efflux, however, will feed back on equation (4.1a), and later on equation (4.1b) and back on equation (4.1c). The influx of mutant from E to IH may compensate the cost of manipulation in equation (4.1c), but this will depend on the numbers of infected IHs and contaminated faeces (first term in equation (4.1c)). In the following, we show how this impact of epidemiology on evolution may explain variations in evolutionary trajectories during and after an epidemic.
(b). Understanding transient evolution
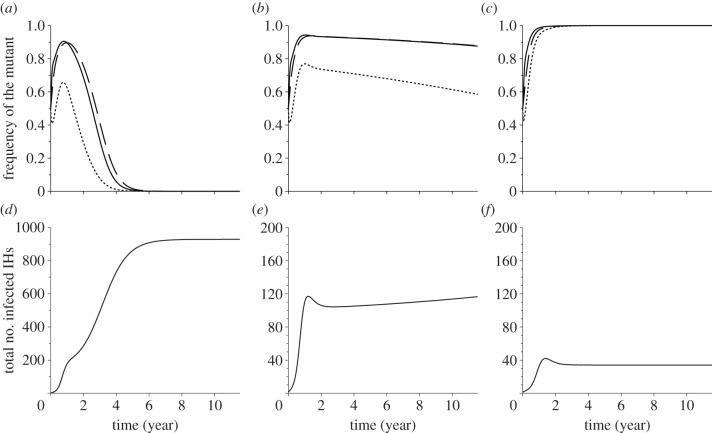
At the early stage of an epidemic, many IHs are uninfected. In this case, the first term of equation (4.1c) is going to be very high. This will induce an increase in manipulation in the short term, whatever the other parameter values of the pathogen (figure 3a–c). However, in the long term, the number of susceptible hosts is going to decrease, and this will reduce the selection for manipulation in the IH compartment. In particular, high rates of vertical transmission yield very high levels of prevalence, and consequently low S2 values. In the extreme case, S2 = 0 and equation (4.1c) becomes 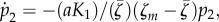 which is always negative when the mutant manipulates more than the wild-type. In other words, although manipulation is favoured at the onset of the epidemic, it will be selected against when the pathogen reaches an endemic state in the host population.
which is always negative when the mutant manipulates more than the wild-type. In other words, although manipulation is favoured at the onset of the epidemic, it will be selected against when the pathogen reaches an endemic state in the host population.
Figure 3.
(a–c) Frequency of the mutant parasite, which alters the IH behaviour (ζm = 5), in each compartment (DH, solid line; E, dashed line; IH, dotted line) when introduced simultaneously at the same frequency with a non-manipulative ‘wt’ strain (ζwt = 1) in a susceptible system, as a function of time, and for three values of virulence α2: (a) 0, no virulence; (b) 2/52, intermediate virulence; and (c) 10/52, high virulence. (d–f) The total number of infected IHs as a function of time for the three virulence rates: (d) no virulence; (e) intermediate virulence; and (f) high virulence. Epidemiological dynamics of the IHs is the only one represented because they are the most affected by behavioural manipulation, vertical transmission and virulence. Parameter π2 = 0.9; initial conditions: I1m = I1wt = Em = Ewt = I2m = I2wt = 1.
The potential complexity of this transient dynamics is obvious when two strains with different abilities to manipulate the IH are in competition: a non-manipulative wild-type strain with ζwt = 1 and a mutant strain with ζm = 5 (figure 3). We considered a case with a high rate of vertical transmission (π2 = 0.9) and explored the effects of different levels of virulence. At the beginning of the simulation, the two strains are introduced at equal frequencies in each compartment at a low number. An epidemic arises because their R0 is higher than 1. The pathogen spreads in each compartment and, later on, reaches a stable endemic equilibrium (figure 3d–f). When the parasite is avirulent (figure 3a) or moderately virulent (figure 3b), the frequency of the mutant transiently increases in all compartments, up to 90% after 1 year. In the long term, however, the manipulating mutant loses the competition with the wild-type. But when the parasite is very virulent (figure 3c), the mutant outcompetes the wild-type and goes to fixation. In other words, at the endemic equilibrium, the evolutionary outcome depends on the pathogen virulence. In the following, we use adaptive dynamics to find the conditions that are most favourable for the evolution of manipulation in the long term.
(c). Predictions at the endemic equilibrium
Here, focus is on the fate of a mutant strain after the wild-type has reached an endemic equilibrium. In this situation, one can use equations (4.1a–c) to determine whether the mutant can invade or not in an endemic equilibrium set by the wild-type. An alternative is to adopt an adaptive dynamics approach that directly derives the ability of the mutant to outcompete the resident strategy (see the electronic supplementary material, appendix S2C). In both cases, if the mutant manipulates more than the wild-type (ζm > ζwt), then it will outcompete the wild-type only if
| 4.2 |
The evolution of higher levels of manipulation thus requires that the rate of vertical transmission is below a threshold set by the mortality rate (including the virulence of the pathogen) and the birth rate of the IH. For the parameter values chosen to model the life cycle of T. gondii, manipulation is always selected for when virulence is above 0.06 and when vertical transmission is lower than 0.5 (figure 4a, below dashed curve). For lower virulence levels, manipulation can be counter-selected if vertical transmission is sufficiently high (figure 4a, above dashed curve).
Figure 4.
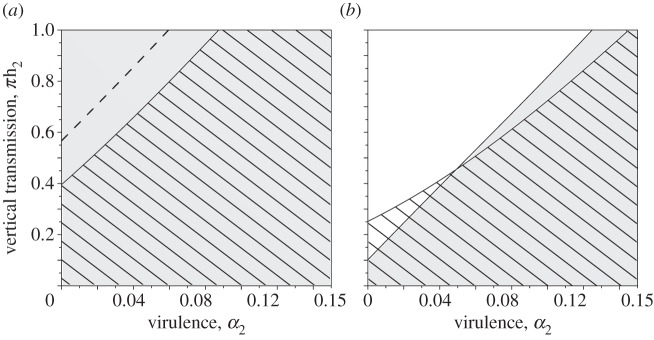
Threshold values of vertical transmission and parasite virulence allowing the evolution of parasite manipulation in the (a) absence or (b) presence of sexual transmission between males and females. Values of vertical transmission and virulence rate for which a mutant parasite manipulating the IHs, ζm > 1, invades a wild-type population without behavioural manipulation at endemic equilibrium, ζwt = 1 (below the dashed line in a), and a mutant parasite manipulating the behaviour of female IHs, ζFm > 1, invades a wild-type population without behavioural manipulation in both male and female IHs at equilibrium, ζMwt = ζFwt = 1, (hatched area in a). In this latter case, behavioural manipulation is always selected in males and is represented by the shaded area in (a). Allowing sexual transmission from male to female in IHs (b) decreases the conditions for the evolution of manipulation in males (shaded area) and in females (hatched area). Note that these latter curves are obtained assuming no manipulation of the IH behaviour in both resident and mutant, i.e. ζMwt = ζFwt = ζMm = ζFm = 1.
5. Should manipulation differ between males and females?
(a). Effect of vertical transmission
Vertical transmission occurs only from mothers to offspring, and thus the conflict between this route of transmission and manipulation may only take place in female IHs. Thus, it is likely that manipulation may evolve in males but not in females. To better formulate this hypothesis, a new version of the model distinguishes between male and female IHs where the quantities S2F, S2M, I2F and I2M refer to the numbers of females and males that are susceptible or infected, respectively. In addition, the manipulation coefficients ζM and ζF are allowed to differ between males and females. The sex ratio at birth is assumed to be equal to 0.5, and the birth rate from female IHs is twice the previous rate, 2 × b2 individuals per female per week, so that we obtain the same endemic equilibrium as the previous model (§4c) if ζM = ζF. The conditions leading to the invasion of a manipulating mutant in a wild-type population at an endemic equilibrium are analysed using again an adaptive dynamics approach (see the electronic supplementary material, appendix S2D). As predicted, it appears that manipulating male IHs is always selected for (figure 4a, shaded area). By contrast, the condition for the spread of a manipulating strain in females depends on the amount of vertical transmission and the mortality rate—as in (4.2)—but also on the level of manipulation of males and the densities of the different types of IHs at the endemic equilibrium (see the electronic supplementary material, appendix S4):
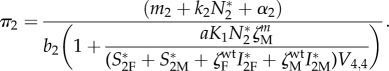 |
4.3 |
with 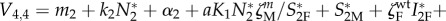
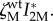
Figure 4 (hatched areas) indicates the values of vertical transmission and virulence that select for manipulation of females IHs, considering a wild-type parasite strain that does not alter the IH behaviour 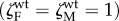 . The conditions for the evolution of manipulation when the trait is not allowed to vary between males and females (unconditional manipulation) are less stringent than when manipulation only affects females (figure 4a, hatched area). As expected, the unconditional manipulation is an intermediate case that falls between the selective pressure acting on males (where maximal levels of manipulations are always selected for) and females (where manipulation is selected for under a lower range of parameter values).
. The conditions for the evolution of manipulation when the trait is not allowed to vary between males and females (unconditional manipulation) are less stringent than when manipulation only affects females (figure 4a, hatched area). As expected, the unconditional manipulation is an intermediate case that falls between the selective pressure acting on males (where maximal levels of manipulations are always selected for) and females (where manipulation is selected for under a lower range of parameter values).
(b). Effect of vertical transmission and sexual transmission
Here, we study how sexual transmission from infected males to females reported in rats [34] could modify our predictions on the evolution of sex-biased levels of manipulation. We allow the pathogen to be transmitted from males to females during mating (transmission in the opposite direction has not been documented) in a new version of our model (see the electronic supplementary material, appendix S2E). Although the thresholds of vertical transmission for males and females are complex expressions of the various parameters of the model (see the electronic supplementary material, appendix S2E, equations (E2) and (E3)), it is notable that the evolution of manipulation in one sex depends on the level of manipulation in the other sex. We numerically identify the situations that select for manipulation in males and females assuming a resident population that does not manipulate females and males (figure 4b). Interestingly, we find that the combination of vertical transmission and sexual transmission decreases the conditions for the evolution of manipulation in both males and females. In fact, with sexual transmission, the conditions for the evolution of manipulation become very similar in males and females. When virulence is very low, females may evolve higher rates of manipulation than males. By contrast, when virulence is very high, females may evolve lower rates of manipulation than males. However, the position of the curves depends on the parameter values; in particular, on the opportunities for sexual transmission (see the electronic supplementary material, appendix S2E for predictions with different mating rates).
6. Discussion
The aim of this study was to investigate the interplay between manipulation of IH behaviour, vertical transmission and virulence at the epidemiological and evolutionary scales. We developed a model, inspired by the life cycle of the parasite T. gondii, which considers trophic transmission from IHs to DHs facilitated by behavioural manipulation, vertical transmission and virulence in the IHs. Note that we assumed no constraint between the different life history traits. In particular, manipulation was considered only to increase predation by the DH predator. The model showed that manipulation was not always selected even in the favourable case where there is no direct physiological cost associated with the evolution of this behavioural modification. The evolution of this trait depends on the epidemiological dynamics, on the level of virulence in the IH and on the existence of other transmission routes (i.e. vertical transmission, sexual transmission) for the pathogen.
We first focused on the interaction between trophic transmission and vertical transmission on the basic reproductive ratio R0 measuring the epidemic potential of a parasite in a fully susceptible host population. A conflict between behavioural manipulation and vertical transmission was observed in R0 analysis: increasing manipulation may increase the contribution of trophic transmission to R0 but decreases the contribution of vertical transmission (see also [38]). With the chosen parameter values, which lie in the range of values estimated from field and experimental conditions (see the electronic supplementary material, table S1), R0 was almost always increased by higher levels of behavioural manipulation (but see electronic supplementary material, appendix S2A).
Then, we studied the evolutionary epidemiology of manipulation. Behavioural manipulation results in a direct fitness cost for the parasite because it increases the IH death rate. But it also indirectly promotes parasite transmission via the DHs, to the environment and back to the IHs. The outcome of the conflict between these direct and indirect effects of behavioural manipulation depends both on the epidemiological dynamics and on the levels of vertical transmission and virulence. At the beginning of an epidemic, increasing behavioural manipulation is always favoured. By contrast, when the parasite has reached an endemic equilibrium, the manipulation of the IH can be selected against. This is particularly true with avirulent parasites that are highly efficient in vertical transmission. In these cases, trophic transmission does not compensate the loss of infected IHs that would have spread the parasite vertically, thus manipulation is of moderate interest. Interestingly, in these situations, decreasing the level of behavioural manipulation below unity could be favoured. Thus, predation avoidance could be favoured not only to enable a parasite to mature in its IHs [6] but also to protect the IHs when vertical transmission is relatively important compared with the horizontal trophic transmission [17,38].
Our model allowed us to study when behavioural manipulation is expected to evolve differentially in male and female IHs. Our analysis shows that, with vertical transmission, behavioural manipulation of male IHs is always selected for, whereas manipulation of females depends on various parameters of the models. This result confirms the hypothesis developed by Duneau & Ebert [45] that parasites could evolve following different strategies in males and females hosts. Interestingly, allowing for sexual transmission from male to female adds a cost of behavioural manipulation in males, because predation of infected males prevents sexual transmission. In this case, the cost of vertical transmission (in females) and the cost of sexual transmission (in males) may balance each other and result in the evolution of similar manipulation strategies in males and females. The intersection of the threshold curves in males and in females (figure 4b) illustrates that our model can favour higher levels of manipulation either in males or in females, depending on the relative strength of vertical and sexual transmission. As the evolution of manipulation in males and females are interdependent (see equation (4.3) in the main text, equations (E2) and (E3) in the electronic supplementary material, appendix S2E), an interesting perspective would be to study the coevolution of these two traits.
(a). Theoretical perspectives
For the sake of simplicity, we assumed that only behavioural manipulation of IH is allowed to evolve in our model. In particular, we did not consider the possible correlated evolutionary response in other life-history traits of the parasite (e.g. vertical transmission, virulence). One may imagine a negative trade-off between these traits: a parasite investing in trophic transmission may allocate less in vertical transmission. Thus, behavioural manipulation (i.e. ζ) that impacts trophic transmission and vertical transmission (i.e. π2) could evolve simultaneously depending on the opportunity for each transmission routes. Other trade-offs could be considered. For example, in mice, highly virulent strains of T. gondii were associated with enhanced migration of the parasite across biological barriers, such as the intestinal epithelium, the placenta and the blood–brain barrier [46–48]. This could suggest that behavioural manipulation, vertical transmission and virulence are positively correlated for T. gondii. In this case, one may expect the evolution of these life-history traits of the parasite towards an intermediate value of behavioural manipulation, vertical transmission and virulence. These different scenarios could be investigated using the general model presented in electronic supplementary material, appendix S2B, which considers behavioural manipulation, vertical transmission and virulence to vary between strains.
(b). Predictions and perspectives applied to Toxoplasma gondii
Our results are relevant for any parasite combining trophic transmission and vertical transmission. But the parameter values were meant to model the life cycle of T. gondii. This parasite exhibits different levels of virulence and vertical transmission according to the host species and parasite strains. Type II strains are usually associated with low virulence in IHs such as mice, and are the most common in Europe and North America [49–51]. Moreover, some strains have demonstrated high vertical transmission rate in house and field mice, with vertical transmission occurring in 75% of pregnancies and above 80% of pups infected [29,30]. Thus, our theoretical analysis predicts that these strains may have evolved towards minimal or absence of behavioural manipulation. By contrast, other type II strains demonstrated vertical transmission rates below 10% in rats, hamsters and BALB/c laboratory mice [31–33]. Our analysis predicts that these latter strains should evolve towards higher levels of behavioural manipulation of the IHs. This prediction remains to be tested, because no study compared the ability of different strains to be transmitted by different routes, using the same host species. Interestingly, the two type II strains PRU and Me-49, with low virulence and low efficiency in vertical transmission [32,33], were reported to manipulate the behaviour of rats and mice [25,26,28].
In South America, recombinant or atypical strains, more virulent for mice, are reported (for reviews, see [35,36]). Our model would predict the evolution of high levels of manipulation in this case. However, manipulation of mice by a highly virulent strain may not be testable, because the infected mice often die from infection within 10 days. Moreover, mice are not the main IHs in South America's ecosystems where the parasite circulates in both domestic and wild systems, with numerous and varied host species, both for IH and DH [52]. Whether these strains are also virulent for wild IHs, and whether they are efficiently transmitted vertically, remain to be tested, and this information would complete our knowledge on the relationship between T. gondii transmission traits. In addition, the variability of host species may play an important role in the evolution of behavioural manipulation, vertical transmission and virulence. According to the physiology of the locally available IH species, different levels of virulence may be selected, and different strategies of transmission could thus be favoured. Further theoretical and experimental studies on the transmission routes and virulence of T. gondii are needed to investigate the potential link between these traits and the level of behavioural manipulation.
Lastly, our model predicts differential manipulation of males and females IHs with strains of low virulence that are efficiently vertically transmitted. Unfortunately, there are little data available to test this hypothesis in T. gondii. Vyas et al. [25] and Kannan et al. [28] found evidence of behavioural manipulation in female laboratory mice infected with this parasite, whereas Webster et al. [23] did not find any difference between male and female behaviour of infected rats. To the best of our knowledge, the study of Xiao et al. [53] is the only one that specifically tested whether male and female IHs are differentially manipulated. The authors showed that females were more attracted by cat odour than males. In our model, the only situation where pathogens evolve higher rates of manipulation in females occurs when the pathogen can transmit sexually from males to females (figure 4b). Interestingly, Xiao et al. [53] used the same PRU strain as Dass et al. [34], who demonstrated the possibility of sexual transmission. It would be particularly interesting to assess experimentally our predictions of male-biased manipulation in other biological systems with no sexual transmission.
Acknowledgements
The authors are grateful to the anonymous reviewers for their valuable comments and suggestions which improved the quality of the paper.
Funding statement
This work has been supported by the Agence De l'Environnement et de la Maitrise de l'Energie (ADEME). S.G. thanks the CNRS and the ERC starting grant no. EVOLEPID 243054 for funding. M.L. conducted this work while a postdoctoral fellow and as a part of the ‘Multiscale modeling of the life cycle of Toxoplasma gondii’ Working Group at the National Institute for Mathematical and Biological Synthesis, sponsored by the National Science Foundation, the US Department of Homeland Security and the US Department of Agriculture through NSF award no. EF-0832858, with additional support from the University of Tennessee, Knoxville.
References
- 1.Barnard CJ, Behnke JM. 1990. Parasitism and host behaviour. London, UK: Taylor and Francis [Google Scholar]
- 2.Combes C. 2001. Parasitism: the ecology and evolution of intimate interactions. Chicago, IL: University of Chicago Press [Google Scholar]
- 3.Moore J. 2002. Parasites and the behaviour of animals. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Choisy M, Brown SP, Lafferty KD, Thomas F. 2003. Evolution of trophic transmission in parasites: why add intermediate hosts? Am. Nat. 162, 172–181 (doi:10.1086/375681) [DOI] [PubMed] [Google Scholar]
- 5.Gandon S. 2004. Evolution of multihost parasites. Evolution 58, 455–469 (doi:10.1111/j.0014-3820.2004.tb01669.x) [PubMed] [Google Scholar]
- 6.Parker GA, Ball MA, Chubb JC, Hammerschmidt K, Milinski M. 2009. When should a trophically transmitted parasite manipulate its host? Evolution 63, 448–458 (doi:10.1111/j.1558-5646.2008.00565.x) [DOI] [PubMed] [Google Scholar]
- 7.Seppälä O, Jokela J. 2008. Host manipulation as a parasite transmission strategy when manipulation is exploited by non-host predators. Biol. Lett. 4, 663–666 (doi:10.1098/rsbl.2008.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vervaeke M, Davis S, Leirs H, Verhagen R. 2006. Implications of increased susceptibility to predation for managing the sylvatic cycle of Echinococcus multilocularis. Parasitology 132, 893–901 (doi:10.1017/S0031182006009838) [DOI] [PubMed] [Google Scholar]
- 9.Cézilly F, Thomas F, Médoc V, Perrot-Minnot MJ. 2010. Host-manipulation by parasites with complex life cycles: adaptive or not? Trends Parasitol. 26, 311–317 (doi:10.1016/j.pt.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 10.Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Stud. Behav. 41, 151–186 (doi:10.1016/s0065-3454(10).41005-0) [Google Scholar]
- 11.Thomas F, Adamo S, Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Process. 68, 185–199 (doi:10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 12.Franceschi N, Bollache L, Cornet S, Bauer A, Motreuil S, Rigaud T. 2010. Co-variation between the intensity of behavioural manipulation and parasite development time in an acanthocephalan–amphipod system. J. Evol. Biol. 23, 2143–2150 (doi:10.1111/j.1420-9101.2010.02076.x) [DOI] [PubMed] [Google Scholar]
- 13.Thompson SN, Kavaliers M. 1994. Physiological bases for parasite-induced alterations of host behaviour. Parasitology 109, S119–S138 (doi:10.1017/S0031182000085139) [DOI] [PubMed] [Google Scholar]
- 14.Mouritsen KN, Poulin R. 2003. Parasite-induced trophic facilitation exploited by a non-host predator: a manipulator's nightmare. Int. J. Parasitol. 33, 1043–1050 (doi:10.1016/S0020-7519(03)00178-4) [DOI] [PubMed] [Google Scholar]
- 15.Seppälä O, Valtonen ET, Benesh DP. 2008. Host manipulation by parasites in the world of dead-end predators: adaptation to enhance transmission? Proc. R. Soc. B 275, 1611–1615 (doi:10.1098/rspb.2008.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haine ER, Boucansaud K, Rigaud T. 2005. Conflict between parasites with different transmission strategies infecting an amphipod host. Proc. R. Soc. B 272, 2505–2510 (doi:10.1098/rspb.2005.3244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones EO, White A, Boots M. 2011. The evolution of host protection by vertically transmitted parasites. Proc. R. Soc. B 278, 863–870 (doi:10.1098/rspb.2010.1397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoop WL. 1991. Vertical transmission of helminths: hypobiosis and amphiparatenesis. Parasitol. Today 7, 51–54 (doi:10.1016/0169-4758(91)90189-U) [DOI] [PubMed] [Google Scholar]
- 19.Dubey JP. 1999. Recent advances in Neospora and neosporosis. Vet. Parasitol. 84, 349–367 (doi:10.1016/S0304-4017(99)00044-8) [DOI] [PubMed] [Google Scholar]
- 20.Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258 (doi:10.1016/S0020-7519(00)00124-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook AJC, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, Foulon W, Semprini AE, Dunn DT, on behalf of the European Research Network on Congenital Toxoplasmosis 2000. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. BMJ 321, 142–147 (doi:10.1136/bmj.321.7254.142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez LE, Rojnik B, Urrea F, Urdaneta H, Petrosino P, Colasante C, Pino S, Hernandez L. 2007. Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats: a behavioural analysis. Behav. Brain Res. 177, 70–79 (doi:10.1016/j.bbr.2006.11.012) [DOI] [PubMed] [Google Scholar]
- 23.Webster JP, Brunton CFA, MacDonald DW. 1994. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown-rats, Rattus norvegicus. Parasitology 109, 37–43 (doi:10.1017/S003118200007774X) [DOI] [PubMed] [Google Scholar]
- 24.Berdoy M, Webster JP, Macdonald DW. 2000. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B 267, 1591–1594 (doi:10.1098/rspb.2000.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. 2007. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odours. Proc. Natl Acad. Sci. USA 104, 6442–6447 (doi:10.1073/pnas.0608310104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamberton PHL, Donnelly CA, Webster JP. 2008. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology 135, 1143–1150 (doi:10.1017/S0031182008004666) [DOI] [PubMed] [Google Scholar]
- 27.Worth AR, Lymbery AJ, Thompson RCA. 2013. Adaptive host manipulation by Toxoplasma gondii: fact or fiction? Trends Parasitol. 29, 150–155 (doi:10.1016/j.pt.2013.01.004) [DOI] [PubMed] [Google Scholar]
- 28.Kannan G, Moldovan K, Xiao JC, Yolken RH, Jones-Brando L, Pletnikov MV. 2010. Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia Parasitol. 57, 151–155 [DOI] [PubMed] [Google Scholar]
- 29.Owen MR, Trees AJ. 1998. Vertical transmission of Toxoplasma gondii from chronically infected house (Mus musculus) and field (Apodemus sylvaticus) mice determined by polymerase chain reaction. Parasitology 116, 299–304 (doi:10.1017/S003118209700231X) [DOI] [PubMed] [Google Scholar]
- 30.Marshall PA, Hughes JM, Williams RH, Smith JE, Murphy RG, Hide G. 2004. Detection of high levels of congenital transmission of Toxoplasma gondii in natural urban populations of Mus domesticus. Parasitology 128, 39–42 (doi:10.1017/S0031182003004189) [DOI] [PubMed] [Google Scholar]
- 31.Dubey JP, Frenkel JK. 1998. Toxoplasmosis of rats: a review, with considerations of their value as an animal model and their possible role in epidemiology. Vet. Parasitol. 77, 1–32 (doi:10.1016/S0304-4017(97)00227-6) [DOI] [PubMed] [Google Scholar]
- 32.Freyre A, Falcon J, Mendez J, Rodriguez A, Correa L, Gonzalez M. 2006. Refinement of the mouse model of congenital toxoplasmosis. Exp. Parasitol. 113, 154–160 (doi:10.1016/j.exppara.2005.12.019) [DOI] [PubMed] [Google Scholar]
- 33.Freyre A, Fialho CG, Bigatti LE, Araujo FAP, Falcon JD, Mendez J, Gonzalez M. 2009. Toxoplasma gondii: congenital transmission in a hamster model. Exp. Parasitol. 122, 140–144 (doi:10.1016/j.exppara.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 34.Dass SAH, Vasudevan A, Dutta D, Soh LJT, Sapolsky RM, Vyas A. 2011. Protozoan parasite Toxoplasma gondii manipulates mate choice in rats by enhancing attractiveness of males. PLoS ONE 6, e27229 (doi:10.1371/journal.pone.0027229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeij JPJ, Boyle JP, Boothroyd JC. 2005. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 21, 476–481 (doi:10.1016/j.pt.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 36.Dardé ML. 2008. Toxoplasma gondii, ‘new’ genotypes and virulence. Parasite 15, 366–371 (doi:10.1051/parasite/2008153366) [DOI] [PubMed] [Google Scholar]
- 37.Lélu M, Langlais M, Poulle ML, Gilot-Fromont E. 2010. Transmission dynamics of Toxoplasma gondii along an urban rural gradient. Theor. Popul. Biol. 78, 139–147 (doi:10.1016/j.tpb.2010.05.005) [DOI] [PubMed] [Google Scholar]
- 38.Turner M, Lenhart S, Rosenthal B, Sullivan A, Zhao X. 2013. Modeling effective transmission pathways and control of the world's most successful parasite. Theor. Popul. Biol. 86, 50–61 (doi:10.1016/j.tpb.2013.04) [DOI] [PubMed] [Google Scholar]
- 39.Lotka AJ. 1925. Elements of physical biology. London, UK: Williams and Wilkins [Google Scholar]
- 40.Volterra V. 1931. Leçons sur la théorie mathématique de la lutte pour la vie. Paris, France: Gauthier-Villars [Google Scholar]
- 41.Diekmann O, Heesterbeek JAP, Metz JAJ. 1990. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 28, 365–382 (doi:10.1007/BF00178324) [DOI] [PubMed] [Google Scholar]
- 42.Frank SA. 1996. Models of parasite virulence. Q. Rev. Biol. 71, 37–78 (doi:10.1086/419267) [DOI] [PubMed] [Google Scholar]
- 43.Day T, Gandon S. 2006. Insights from Price's equation into evolutionary epidemiology. In Disease evolution: models, concepts, and data analysis (eds Feng Z, Dieckmann U, Levin SA.), pp. 23–44 Providence, RI: American Mathematical Society [Google Scholar]
- 44.Day T, Gandon S. 2007. Applying population-genetic models in theoretical evolutionary epidemiology. Ecol. Lett. 10, 876–888 (doi:10.1111/j.1461-0248.2007.01091.x) [DOI] [PubMed] [Google Scholar]
- 45.Duneau D, Ebert D. 2012. Host sexual dimorphism and parasite adaptation. PLoS Biol. 10, e1001271 (doi:10.1371/journal.pbio.1001271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barragan A, Sibley LD. 2002. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 95, 1625–1633 (doi:10.1084/jem.20020258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barragan A, Sibley LD. 2003. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 11, 426–430 (doi:10.1016/S0966-842X(03)00205-1) [DOI] [PubMed] [Google Scholar]
- 48.Freyre A, Falcon J, Mendez J, Gonzalez M, Venzal JM, Morgades D. 2003. Fetal Toxoplasma infection after oocyst inoculation of pregnant rats. Parasitol. Res. 89, 352–353 (doi:10.1007/s00436-002-0759-4) [DOI] [PubMed] [Google Scholar]
- 49.Aubert D, et al. 2010. Molecular and biological characteristics of Toxoplasma gondii isolates from wildlife in France. Vet. Parasitol. 171, 346–349 (doi:10.1016/j.vetpar.2010.03.033) [DOI] [PubMed] [Google Scholar]
- 50.Dubey JP, et al. 2008. Endemic toxoplasmosis in pigs on a farm in Maryland: isolation and genetic characterization of Toxoplasma gondii. J. Parasitol. 94, 36–41 (doi:10.1645/GE-1312.1) [DOI] [PubMed] [Google Scholar]
- 51.Sibley LD, Khan A, Ajioka JW, Rosenthal BM. 2009. Genetic diversity of Toxoplasma gondii in animals and humans. Phil. Trans. R. Soc. B 364, 2749–2761 (doi:10.1098/rstb.2009.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carme B, Demar M, Ajzenberg D, Dardé ML. 2009. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 15, 656–658 (doi:10.3201/eid1504.081306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao J, Kannan G, Jones-Brando L, Brannock C, Krasnova IN, Cadet JL, Pletnikov M, Yolken RH. 2012. Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice. Neuroscience 206, 39–48 (doi:10.1016/j.neuroscience.2011.12.051) [DOI] [PubMed] [Google Scholar]