Abstract
Whether movement will enable organisms to alleviate thermal stress is central to the biodiversity implications of climate change. We use the temperature-dependence of ectotherm performance to investigate the fitness consequences of movement. Movement to an optimal location within a 50 km radius will only offset the fitness impacts of climate change by 2100 in 5 per cent of locations globally. Random movement carries an 87 per cent risk of further fitness detriment. Mountainous regions with high temperature seasonality (i.e. temperate areas) not only offer the greatest benefit from optimal movement but also the most severe fitness consequences if an organism moves to the wrong location. Doubling dispersal capacity would provide modest benefit exclusively to directed dispersers in topographically diverse areas. The benefits of movement for escaping climate change are particularly limited in the tropics, where fitness impacts will be most severe. The potential of movement to lessen climate change impacts may have been overestimated.
Keywords: dispersal, fitness, insects, physiology, thermal stress, topography
1. Introduction
Species have moved to different extents and in different directions during past climatic changes [1]. In response to the challenges of projecting these individualistic distribution shifts, several recent analyses have focused on detailed characterizations of the geography of climate change without consideration of biology. These analyses have highlighted the challenges posed to organisms by novel and shifting climates [1–4]. One conclusion stemming from the geography of climate change is that biological impacts will be most severe at temperate and polar latitudes, where the magnitude of climate change is predicted to be the greatest [5,6].
The benefits of movement will depend not only on spatial patterns of climate change but also on the ability of organisms to withstand changes. Incorporating all the aspects of a species biology that determines its response to climate change at broad scales is intractable, but we can consider the implications of gradients in physiology. Limited seasonality in the tropics selects for organisms to specialize their performance over a narrow thermal range [7]. Thus, the latitudinal gradient in thermal performance breadth (greater thermal specialization in the tropics) runs counter to the climate change gradient (greater magnitude of climate change in temperate and polar regions) [8]. The interactions between these two gradients pose a challenge for understanding the biodiversity implications of climate change. A number of recent analyses [8–11] considering the temperature-dependence of fitness and thermal tolerance have concluded that tropical species may be most severely harmed by climate change as they are pushed beyond their narrow thermal safety margin. Latitudinal movements do little to enable tropical species to escape thermal stress owing to the weak latitudinal gradients in temperature [12]. Shifts upward in elevation may offer a refuge for tropical species in topographically diverse regions [13,14]. As many organisms will be unable to disperse to remain in constant thermal niches [15,16], it is essential to understand the fitness consequences of movement within realistic dispersal distances.
Here, we investigate the fitness consequences of population movement to avoid thermal stress. We do so using the temperature-dependence of population growth rates for insects generalized across latitudes by accounting for seasonality by Deutsch et al. [9]. This approach should be broadly indicative for ectotherms and focuses on whether the climate in a colonized cell is suitable for population persistence. We omit other important issues such as whether dispersal ability and landscape connectivity would enable a species to reach the new location, whether the habitat would be suitable in the new location and coupling between cells. We consider the movement of populations as would occur with an established distribution shift rather than with the initial colonization of individuals. Our fitness estimates include density-dependent effects that would not be incurred by the initial colonists and do not account for local adaptation of thermal physiology across a range.
We ask (i) what are the mean and maximum of the fitness change that could be achieved and (ii) what proportion of moves would result in a fitness increase by populations moving in response to climate change? The mean fitness change is indicative of the expectation for a population moving in a random direction to escape thermal stress, whereas the maximum fitness change is indicative of a population able to move to the optimal location. Because populations may persist if even a few individuals move to desirable locations, the maximum fitness advantage may be more informative than the mean. We calculate the proportion of moves that would result in a fitness advantage to assess the expectation that poleward movements can alleviate thermal stress associated with climate change [17]. The mean fitness advantage additionally considers the magnitude of fitness change. We assume two biologically realistic maximum dispersal distances (50 and 100 km) in response to climate changes by 2100. This range corresponds to observed rates of distribution shifts in response to recent climate change (mean of 6.1–17.6 km dec−1) [18,19]. Our analysis accounts for both spatial gradients in physiology and the fitness consequences of annual temperature variation.
2. Material and methods
(a). Climate data
We analyse 10′ grid cells, a resolution that balances meaningful climatology and sufficient resolution for investigating the influence of topography. As a climate baseline, we use temperature averages for the late-twentieth century (1950–2000, Climatic Research Unit CL 2.0 high-resolution) [20]. We assume that body temperatures are equal to air temperatures, which is reasonable for small insects but not larger ectotherms. Deutsch et al. [9] confirmed that their initial analysis was robust to this assumption. We model hourly temperatures with a sine curve approximation based on global monthly surface air temperatures and diurnal temperature ranges. We omit the potentially important fitness impacts of weather extremes and variability. We assume that diurnal temperature range will retain its seasonal and geographical patterns into the twenty-first century. We thus model future temperatures by adding anomalies projected for 2070–2100 by a simulation from the Geophysical Fluid Dynamics Laboratory model CM2.1 [21]. These projections are forced by a mid-range (A2) greenhouse gas emissions scenario and included in the intergovernmental panel on climate change fourth assessment report [22]. The large-scale characteristics of the climate model projections relevant to the fitness consequences of climate change, such as a greater magnitude of temperature increase in polar areas, are similar between climate models.
(b). Fitness curves
Deutsch et al. [9] estimated thermal performance curves describing fitness (intrinsic population growth rates, r) as a function of temperature. The asymmetric curves include a Gaussian rise in performance up to the optimal temperature, Topt, and a quadratic decline to zero performance at CTmax [23]:
 |
where σp is a function of the asymmetry of the curve and represents the degree below Topt that fitness declines towards zero. They fitted least-squares estimates of the three parameters (σp, Topt and CTmax) for 46 insect species.
Deutsch et al. [9] then extrapolated the thermal fitness curves from individual locations to the global scale by developing empirical relationships between the three estimate parameters and the seasonal climate variability at individual grid cells. Topt and CTmax were empirically interpolated, and the asymmetry was fixed to a value realistic for insects to interpolate σp.
(c). Movement
For each cell, we identified all cells with centroids within two maximum dispersal distance radii (50 and 100 km). We used the focal cell's fitness curve to calculate fitness following twenty-first century warming in each potential destination cell (figure 1). We calculated the fitness advantage of moving by subtracting fitness in 2100 within the focal cell from that in the destination cell (Pneighbour_2100 − Pfocal_2100). We then calculated the mean and maximum of this quantity across all neighbours along with the proportion of moves that would result in a fitness advantage. Our analysis addresses the relative fitness consequences of population movement rather than whether a population would need to move to persist through climate change. Populations, particularly in temperate areas, may not face thermal stress as a result of climate change even in the absence of thermal adaptation.
Figure 1.
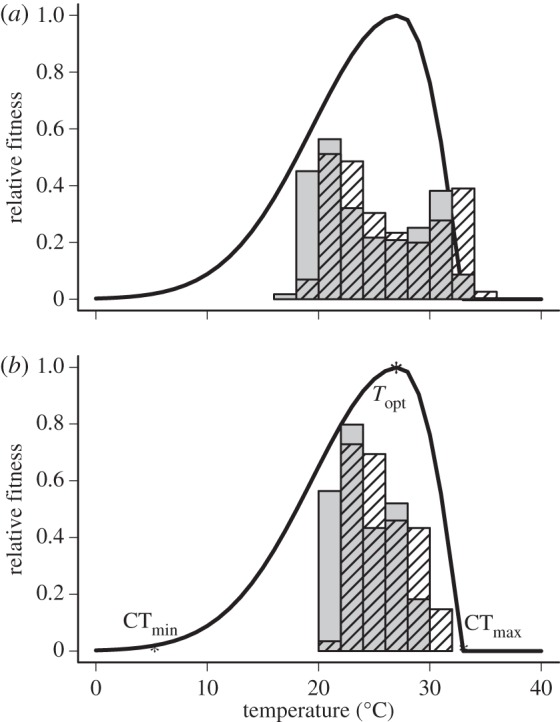
The fitness curve (showing CTmin, Topt and CTmax) for a representative location in coastal Ecuador (1.2496′ S, 80.08366′ W), the tropical location for which directed movement is most advantageous. We show the seasonal and diurnal temperature variation for both the late-twentieth century (1950–1990, grey bars) and for a model simulated climate of the late-twenty-first century (2070–2100, hatched bars). In the (a) focal cell, climate change leads to a fitness detriment as temperatures rise above the insect's CTmax. However, the insect could escape this fitness detriment by moving to the (b) depicted neighbouring cell, where a lesser amount of diurnal temperature variation results in twenty-first century climates not exceeding the insect's CTmax.
Species in temperate regions tend to live in environments with mean temperatures below their thermal optimum. Variable temperate environments combined with the severe fitness declines associated with overheating may account for this pattern [24]. Additionally, if fitness increases in warmer environments (‘hotter-is-better’), then fitness may be maximized when the optimal temperature exceeds environmental temperatures even in constant environments [25]. In current climates, temperate species may increase their fitness by moving according to our model [9]. This may be because we do not account for interannual temperature variability and for fitness declines when temperatures exceed the critical thermal limits. We correct our estimates of the mean fitness advantage of movement by 2100 for this fitness detriment. If there is a fitness advantage of moving in current climates, then we subtract this quantity (Pneighbour_2000 − Pfocal_2000) from our estimate of the mean fitness consequence of movement in response to climate change.
We calculated rugosity as both the average elevation change between the focal cell and neighbouring cells [26] and the coefficient of variation in elevation in the neighbourhood. We calculated temperature seasonality as the coefficient of variation and standard deviation × 100 of monthly temperatures within each cell.
3. Results
Movement within a 50 km radius will not allow populations to escape the fitness impacts of climate change in most areas. Even if a population manages to move to its optimal location, then the fitness advantage of movement is modest (mean and median of maximum fitness advantage = 0.012, 0.008; figure 2). Such optimal movement would offset 3.3–60% of the fitness change owing to climate change (10% and 90% quantiles; mean = 40%; median = 13%) and completely offset the fitness change for only 5 per cent of cells. On average, a population moving randomly in response to climate change will face a further fitness detriment (mean and median of mean fitness advantage = −0.007, −0.002). The mean fitness advantage of movement compensates for the fitness impacts of climate change for only 0.1 per cent of cells (10% and 90% quantiles: −38% and 0.1%; mean = −43%; median = −3%). The proportion of moves that result in a fitness advantage is relatively constant across cells (25% and 75% quantiles: 35% and 57%; 10% and 90% quantiles: 20% and 71%), and the mean (46%) and median (47%) values are similar to the expectation that movement in one direction (often poleward or upward in elevation) is beneficial. We confirmed that our results are robust to the spatial resolution of our analysis by repeating the analysis for a portion of South America at a resolution of 1 km (see the electronic supplementary material, figure S1). While the high-resolution analyses reveal greater spatial variability in the fitness advantages of movement, the results are qualitatively similar.
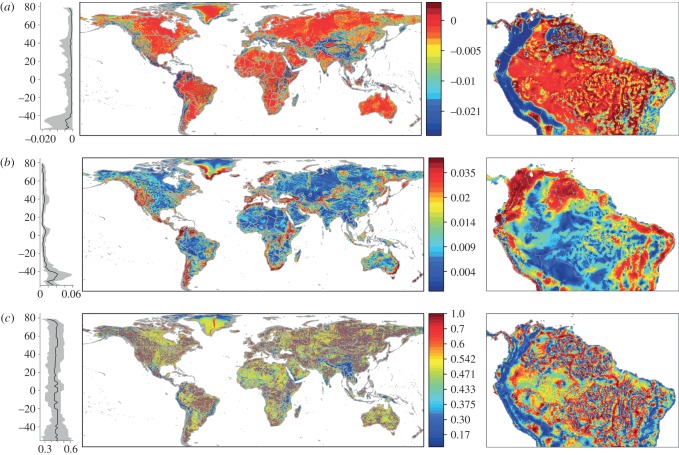
Figure 2.
The (a) mean and (b) maximum of the fitness advantage in 2100 of movement within a 50 km radius. We also depict (c) the proportion of moves that would result in a fitness increase. Data are divided into quantiles with red representing higher values and latitudinal patterns (25%, 50% and 75% quantiles) are additionally depicted. The advantages of moving differ in northern South America between the topographically diverse Andes Mountains and the flatter Amazon Basin. (Online version in colour.)
Spatial and seasonal temperature variability interact to determine where populations have the most to gain from movement (see the electronic supplementary material, figure S2). While the mean fitness advantage is highest in topographically uniform areas with high temperature seasonality, the maximum fitness advantage is highest in topographically diverse regions with limited temperature seasonality (figure 3a,b). At a particular value of topographic rugosity, those cells that are more seasonal (further poleward) will experience a greater mean fitness advantage of movement (figure 3a). The proportion of moves resulting in a fitness advantage is both highest and lowest in topographically diverse regions (figure 3c). Overall, mountainous regions offer the most refuge for directed movers, but the most fitness risk if a population moves the wrong direction.
Figure 3.
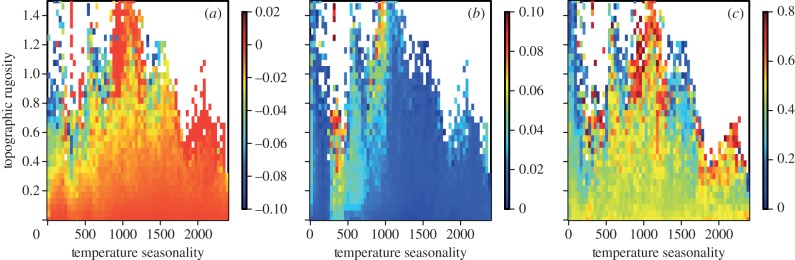
Topographic rugosity (absolute change in elevation (m) between a focal cell and the mean of cells within a 50 km radius) and temperature seasonality (standard deviation of monthly mean temperatures (c) × 100) as predictors of the (a) mean and (b) maximum of the fitness advantage in 2100 of movement within a 50 km radius and (c) the proportion of advantageous moves. (Online version in colour.)
Do these effects result in a latitudinal gradient in the potential to use movement to escape the fitness impacts of climate change? Temperate areas with highly seasonal temperatures will experience the greatest warming, but climate change is expected to increase the fitness of populations in these areas (see the electronic supplementary material, figure S3). The absolute fitness advantage of movement varies little with latitude (figure 2). However, latitudinal patterns exist when examining the relative fitness impacts of movement in relation to the impacts of climate change, because the climate change impacts have the largest magnitude in the tropics (see the electronic supplementary material, figure S4). Movement in temperate areas has the greatest potential to offset climate change impacts, but the fitness consequences are the most severe if species fail to move to an appropriate location. By contrast, movement in the tropics will have little impact on the fitness outcome of climate change in most areas. In temperate areas (20°–60° N and S), the mean of the fitness advantage of movement ranges from −14 per cent to −1 per cent (25% and 75% quantiles) and the maximum ranges from 7% to 30% of the fitness impacts of climate change. In tropical areas (0°–20° N and S), the mean of the fitness advantage of movement ranges from −5 per cent to 0 per cent (25% and 75% quantiles), and the maximum ranges from 3 per cent to 13 per cent. The percentage of fitness increase achieved by optimal movement also varies latitudinally owing to higher fitness in the tropics. In only 7 per cent of locations in the tropics and 18 per cent of locations in temperate areas would a move to an optimal location improve population fitness by 5 per cent. Opportunities to increase fitness are rare on the landscape.
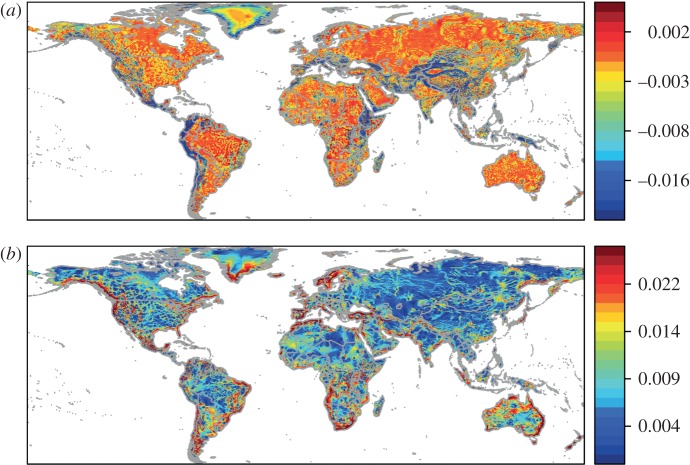
Doubling the movement radius in our analysis primarily benefits directed dispersers in topographically diverse areas (figure 4(b); maximum fitness advantage). By contrast, the benefits of moving further are low for non-directed dispersers in topographically diverse areas (figure 4(a); mean fitness advantage). In only 33 per cent of cells would increasing movement from 50 to 100 km increase the mean fitness. Moving further will have a small impact on the ability of species to compensate for the fitness consequences of climate change. The maximum fitness advantage would offset the fitness impact of climate change for only 10 per cent of cells (see the electronic supplementary material, figure S5). The maximum fitness advantage of such movement ranges from 7 per cent to 101 per cent (10% and 90% quantiles; mean = 122%; median = 25%). The mean fitness advantage of movement compensates for the fitness impacts of climate change for only 0.04 per cent of cells (10% and 90% quantiles: −45% and 8%; mean = −36%; median = −3%).
Figure 4.
The implications of a population doubling its dispersal radius from 50 to 100 km. We subtract the (a) mean and (b) maximum fitness advantage of moving 50 km from the mean and maximum fitness advantage of moving 100 km (quantities in electronic supplementary material, figure S4; quantities in figure 1). The figures present selection for increasing dispersal capabilities. Data are divided into quantiles with red representing higher values. (Online version in colour.)
4. Discussion
Our analysis questions the potential for movement to alleviate the fitness impacts of climate change. Many approaches to predicting the biodiversity implications of climate change that do not explicitly consider fitness suggest that dispersal may be crucial to the maintenance of biodiversity [15]. For example, an analysis using species distribution models [27] estimated that the 21–23% species ‘committed to extinction’ by 2050 would increase to 38–52% without unlimited dispersal. Species distribution models estimate whether a species will remain in its niche and extinction is assumed when an organism looses all or a substantial portion of its range area, whereas our model explicitly examines fitness changes. We argue that our quantification of changes in population growth is relevant and perhaps more informative for estimating extinction risk, because small populations face increased extinction in response to demographic and environmental stochasticity. Our approach addresses the fitness consequences of population movement rather than range shifts per se, although the fitness consequences are related to the viability and consequences of a range shift. Both approaches generally omit barriers to movement. Indeed, dispersal rates may be insufficient for organisms to track their environmental niche [16]. Our analysis, which incorporates spatial gradients in physiology and annual temperature fluctuations, suggests that the ability of migration to mitigate fitness detriment in response to climate change will be modest.
Moving is not always a viable way to reduce the impact of climate change in part because populations are already generally well adapted to local thermal environments, and because there are more ways to reduce fitness than increase fitness through movement (e.g. changes in thermal regimes from one place to another can create scenarios where organisms can move past their peak fitness zones; electronic supplementary material, figure S5). Movement is most advantageous in areas where populations can access locations that have warmed substantially less than the location they are in currently, and where populations can access climates that are currently cooler, but, under warming, will be as warm as current climates (near mountains). Even our modest estimates of the fitness advantages of movement may be overestimates as they are based on temperature alone [17]. The environmental niches available on mountains are likely to experience complex changes as temperature and precipitation shift independently [28,29]. We note that our approach assumes that the temperature-dependence of fitness remains constant over time. Thermal acclimation and adaptation to new mean climate conditions, variability and seasonality over the course of a movement trajectory may augment the viability of movement as a response to climate change. Such mechanisms may account for the prevalence of distribution shifts in response to recent climate change [18].
The degree to which individuals are able to use movement to lessen fitness detriment associated with climate change depends strongly on their ability to use directed movement to disperse to new locations with higher fitness [30,31]. However, populations of non-directed dispersers may realize close to the maximum fitness benefit of moving if a substantial fraction of their individuals move in a beneficial direction. Our findings confirm the potential for mountains to offer a refuge for organisms facing thermal stress [13,14]. However, they also highlight that the steep temperature gradients in mountainous areas represent a fitness risk for populations that disperse to suboptimal locations. Resurveys demonstrate that some species have managed to track their thermal niches upslope [32,33]. Others may be prevented from doing so or displaced by competitors [8,34]. Our findings support concerns in the debate over assisted colonization regarding whether researchers can accurately predict appropriate locations to which to move populations [35]. The fitness consequences of choosing a location poorly may be severe.
The shallow spatial and seasonal temperature gradients in the tropics limit the ability of tropical species to escape climate change. Temperate species have the potential to either be substantially harmed or assisted by moving in response to climate change. This is consistent with temperature variability substantially influencing fitness in temperate environments [9,24,25]. Our coarse examination does not address potential fitness hurdles to movement. Janzen proposed that ‘mountain passes are higher in the tropics’, because thermal specialization may prevent dispersal and reduce the potential for tropical organisms to evade climate change impacts via movement [7]. Indeed, greater isolation by distance in tropical species suggests reduced dispersal in the tropics [36]. Some more detailed analyses of tropical elevation gradients offer a more optimistic view of the potential for organisms to escape climate change by moving upslope [14], but others highlight the potential for species to be pushed off mountain tops [12]. These approaches do not explicitly consider fitness.
Our finding that the fitness consequences of moving in response to environmental change are spatially complex is consistent with the individualistic distribution shifts observed in response to past environmental change [1,37]. Indeed, we do not detect a strong latitudinal signal in the fitness consequences of movement. The spatial complexities are certainly underestimated due to our omission of factors inhibiting movement, such as barriers and habitat suitability, and factors that eliminate the need to move, such as local adaptation of thermal physiology. Despite these simplifications, the complex fitness consequences of population movement point to the importance of considering thermal physiology when evaluating environmental niches and predicting the implications of environmental change.
Acknowledgements
This work was conducted as part of the Species Range Dynamics Working Group supported by the National Center for Ecological Analysis and Synthesis (NCEAS), a Center supported by the National Science Foundation (NSF) (grant no. DEB-0553768), the University of California, Santa Barbara, and the State of California and the National Evolutionary Synthesis Center, a centre supported by the NSF (grant no. EF-0423641). We thank working group members, particularly M. Angilletta and J. Kingsolver, for helpful comments and discussions. We also thank members of the Buckley and Kingsolver laboratory groups for constructive comments. This work was supported in part by NSF grants nos. EF-1065638 and DEB-1120062 to L.B.B.
References
- 1.Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–485 (doi:10.1890/070037) [Google Scholar]
- 2.Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742 (doi:10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462, 1052–1055 (doi:10.1038/nature08649) [DOI] [PubMed] [Google Scholar]
- 4.Ackerly DD, Loarie SR, Cornwell WK, Weiss SB, Hamilton H, Branciforte R, Kraft NJB. 2010. The geography of climate change: implications for conservation biogeography. Divers. Distrib. 16, 476–487 (doi:10.1111/j.1472-4642.2010.00654.x) [Google Scholar]
- 5.Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (doi:10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 6.Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872 (doi:10.1111/j.1365-2486.2007.01404.x) [Google Scholar]
- 7.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17 (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 8.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Álvarez Pérez HJ, Garland T. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948 (doi:10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296–1297 (doi:10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 11.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 (doi:10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Sorte FA, Jetz W. 2010. Projected range contractions of montane biodiversity under global warming. Proc. R. Soc. B 277, 3401–3410 (doi:10.1098/rspb.2010.0612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush MB. 2002. Distributional change and conservation on the Andean flank: a palaeoecological perspective. Glob. Ecol. Biogeogr. 11, 463–473 (doi:10.1046/j.1466-822X.2002.00305.x) [Google Scholar]
- 14.Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 (doi:10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- 15.Pearson RG. 2006. Climate change and the migration capacity of species. Trends Ecol. Evol. 21, 111–113 (doi:10.1016/j.tree.2005.11.022) [DOI] [PubMed] [Google Scholar]
- 16.Schloss CA, Nuñez TA, Lawler JJ. 2012. Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proc. Natl Acad. Sci. USA 109, 8606–8611 (doi:10.1073/pnas.1116791109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanDerWal J, Murphy HT, Kutt AS, Perkins GC, Bateman BL, Perry JJ, Reside AE. 2012. Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nat. Clim. Change 3, 239–243 (doi:10.1038/nclimate1688) [Google Scholar]
- 18.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 19.Chen I, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 20.New M, Lister D, Hulme M, Makin I. 2002. A high-resolution data set of surface climate over global land areas. Clim. Res. 21, 1–25 (doi:10.3354/cr021001) [Google Scholar]
- 21.Delworth TL, et al. 2006. GFDL's CM2 global coupled climate models. I. Formulation and simulation characteristics. J. Clim. 19, 643–674 (doi:10.1175/JCLI3629.1) [Google Scholar]
- 22.Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL. 2007. IPCC, 2007: climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. New York, NY: Cambridge University Press [Google Scholar]
- 23.Huey RB, Stevenson RD. 1979. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Integr. Comp. Biol. 19, 357–366 (doi:10.1093/icb/19.1.357) [Google Scholar]
- 24.Martin TL, Huey RB. 2008. Why ‘suboptimal’ is optimal: Jensen's inequality and ectotherm thermal preferences. Am. Nat. 171, 102–118 (doi:10.1086/527502) [DOI] [PubMed] [Google Scholar]
- 25.Kingsolver JG. 2009. The well-temperatured biologist. Am. Nat. 174, 755–768 (doi:10.1086/648310) [DOI] [PubMed] [Google Scholar]
- 26.Riley SJ, DeGloria SD, Elliot R. 1999. A terrain ruggedness index that quantifies topographic heterogeneity. Intermountain J. Sci. 5, 23–27 [Google Scholar]
- 27.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 28.Still CJ, Foster PN, Schneider SH. 1999. Simulating the effects of climate change on tropical montane cloud forests. Nature 398, 608–610 (doi:10.1038/19293) [Google Scholar]
- 29.Crimmins SM, Dobrowski SZ, Greenberg JA, Abatzoglou JT, Mynsberge AR. 2011. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 331, 324–327 (doi:10.1126/science.1199040) [DOI] [PubMed] [Google Scholar]
- 30.Armsworth PR, Roughgarden JE. 2005. The impact of directed versus random movement on population dynamics and biodiversity patterns. Am. Nat. 165, 449–465 (doi:10.1086/428595) [DOI] [PubMed] [Google Scholar]
- 31.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 190 52–190 59 (doi:10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264 (doi:10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- 33.Tingley MW, Monahan WB, Beissinger SR, Moritz C. 2009. Birds track their Grinnellian niche through a century of climate change. Proc. Natl Acad. Sci. USA 106, 19 637–19 643 (doi:10.1073/pnas.0901562106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jankowski JE, Robinson SK, Levey DJ. 2010. Squeezed at the top: interspecific aggression may constrain elevational ranges in tropical birds. Ecology 91, 1877–1884 (doi:10.1890/09-2063.1) [DOI] [PubMed] [Google Scholar]
- 35.Stone R. 2010. Home, home outside the range? Science 329, 1592 (doi:10.1126/science.329.5999.1592) [DOI] [PubMed] [Google Scholar]
- 36.Martin PR, McKay JK. 2004. Latitudinal variation in genetic divergence of populations and the potential for future speciation. Evolution 58, 938–945 [DOI] [PubMed] [Google Scholar]
- 37.Davis MB, Shaw RG. 2001. Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679 (doi:10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]