Summary
Vegetated biogeomorphic systems (e.g. mangroves, salt marshes, dunes, riparian vegetation) have been intensively studied for the impact of the biota on sediment transport processes and the resulting self‐organization of such landscapes. However, there is a lack of understanding of physical disturbance mechanisms that limit primary colonization in active sedimentary environments.
This study elucidates the effect of sediment disturbance during the seedling stage of pioneer vegetation, using mangroves as a model system. We performed mesocosm experiments that mimicked sediment disturbance as (i) accretion/burial of plants and (ii) erosion/excavation of plants of different magnitudes and temporal distribution in combination with water movement and inundation stress.
Cumulative sediment disturbance reduced seedling survival, with the faster‐growing Avicennia alba showing less mortality than the slower‐growing Sonneratia alba. The presence of the additional stressors (inundation and water movement) predominantly reduced the survival of S. alba.
Non‐lethal accretion treatments increased shoot biomass of the seedlings, whereas non‐lethal erosion treatments increased root biomass allocation. This morphological plasticity in combination with the abiotic disturbance history determined how much maximum erosion the seedlings were able to withstand.
Synthesis and applications. Seedling survival in dynamic sedimentary environments is determined by the frequency and magnitude of sediment accretion or erosion events, with non‐lethal events causing feedbacks to seedling stability. Managers attempting restoration of mangroves, salt marshes, dunes and riparian vegetation should recognize sediment dynamics as a main bottleneck to primary colonization. The temporal distribution of erosion and accretion events has to be evaluated against the ability of the seedlings to outgrow or adjust to disturbances. Our results suggest that selecting fast‐growing pioneer species and measures to enhance seedling growth or temporary reduction in sediment dynamics at the restoration site can aid restoration success for vegetated biogeomorphic ecosystems.
Keywords: Avicennia alba, biogeomorphology, dunes, ecosystem engineer, mangrove, mudflat, restoration, riparian, salt marsh, Sonneratia alba
Short abstract
Seedling survival in dynamic sedimentary environments is determined by the frequency and magnitude of sediment accretion or erosion events, with non‐lethal events causing feedbacks to seedling stability. Managers attempting restoration of mangroves, salt marshes, dunes and riparian vegetation should recognize sediment dynamics as a main bottleneck to primary colonization. The temporal distribution of erosion and accretion events has to be evaluated against the ability of the seedlings to outgrow or adjust to disturbances. Our results suggest that selecting fast‐growing pioneer species and measures to enhance seedling growth or temporary reduction in sediment dynamics at the restoration site can aid restoration success for vegetated biogeomorphic ecosystems.
Introduction
Biogeomorphology, the linkage between geomorphology and ecology, has a strong focus on the impact of vegetation cover on landscape evolution and decay (Thornes 1985; Phillips 2006; Stallins 2006). In the framework of ecosystem engineering, vegetation cover in biogeomorphic systems produces positive feedbacks to the fitness of the engineering species itself and potentially also to other (associated) organisms (Jones, Lawton & Shachak 1994; Corenblit et al. 2007; Hastings et al. 2007). These positive feedbacks (e.g. sediment trapping) result in self‐organized biogeomorphic landscapes (Phillips 1995) such as tidal marshes (Temmerman et al. 2007; Fagherazzi 2008), river floodplains (Tal & Paola 2007) or dunes (Baas 2002). However, plants only change sediment transport to their own favour after they surpass a critical biomass density to interact with the physical processes (Bouma et al. 2009a). Because during primary colonization vegetation density is too low to initiate positive feedbacks, the geomorphic processes exert a one‐directional control (i.e. physics on biota) on the establishment of new seedlings (Corenblit et al. 2007).
The predominant active sedimentary environments that are vegetated by vascular plants (grasses, herbs, shrubs and trees) are mainly characterized by estuarine/coastal (mangrove, tidal marsh), fluvial (flood plain, braided river) or aeolian (dune, nebkha) sediment transport processes. Sediment transport typically results in events of accretion and erosion of small to large magnitude (i.e. periods of high sediment availability or high physical forces), which interrupts more or less stationary periods without significant surface elevation change (e.g. see Deloffre et al. 2006 for tidal flats). Hence, clastic sediments in unvegetated areas of biogeomorphic systems get reworked in an irregular temporal pattern, depending on external forcing. High‐frequency observations of such sediment mixing are scarce and often only the integrated maximum sediment mixing depth is known. For example, sediment mixing on a tidal flat in front of a mangrove can occur down to depths of 30 cm (Smoak & Patchineelam 1999).
Establishment of seedlings in dynamic biogeomorphic systems is a critical phase in vegetation succession (Corenblit et al. 2007; Friess et al. 2012). Seedlings make use of short disturbance‐free periods to rapidly anchor and gain stability against subsequent disturbances, as shown for mangrove pioneers (‘windows of opportunity’; Balke et al. 2011), and riparian systems (temporal ‘recruitment box’; Mahoney & Rood 1998; Nilsson et al. 2010). After this initial anchorage, remobilization of the sediment can affect seedling survival by (i) surface erosion and gradual excavation of the seedling until uprooted (Balke et al. 2011) and (ii) sediment accretion, which can bury and suffocate the seedling (Thampanya, Vermaat & Terrados 2002). A subsequent series of short‐term mixing events can also develop into a trend of net deposition or net erosion, which may affect mature vegetation. Depending on the force of the fluid motion and the physical structure of the seedling (i.e. the frontal area), self‐scouring can increase the eroded volume and the chance of failure (Bouma et al. 2009b).
Although the effects of many abiotic factors on seedling establishment have been well studied (e.g. Krauss et al. 2008), the effect of physical disturbance originating from sediment dynamics is still poorly understood, even though it has been suggested to form a main bottleneck for the establishment of biogeomorphic ecosystems (Corenblit et al. 2007; Friess et al. 2012). This lack of knowledge might explain why the restoration of biogeomorphic habitats is often problematic (e.g. mangroves, Ellison 2000; Lewis 2005; salt marshes, Hughes & Paramor 2004; dunes, Webb, Oliver & Pik 2000).
This study focuses on the establishment of mangrove pioneer species, which are able to colonize dynamic tidal flats and therefore represent a suitable model system to fill the knowledge gap about physical disturbance as bottlenecks to seedling survival (Ellison 2000; Friess et al. 2012). Due to their high disappearance rate world‐wide, the conservation and restoration of mangroves is a critical need for coastal management (Duke et al. 2007). We question how sediment dynamics (frequency and magnitude of accretion and erosion events) affect seedling survival and performance for two mangrove pioneer species: the fast‐growing Avicennia alba and the slower‐growing Sonneratia alba. Using mesocosms with realistic inundation treatments and wave‐induced water movement (Fig. 1a,b), we addressed the following questions: (i) what is the effect of cumulative small magnitude accretion and erosion events on seedling survival and morphology (Fig. 1c, left) and (ii) how do previous disturbance events affect the maximal erosion event that an individual seedling can withstand (Fig. 1c, right). This study elucidates the effects of sediment disturbance as the principal bottleneck to vegetation establishment in an active sedimentary environment, which has direct implications for restoration practice.
Figure 1.

Photographs of (a) the mesocosms and (b) the experimental pots with Avicennia alba seedlings. (c) Depiction of the cumulative erosion and sediment accretion treatments, and design of critical erosion test.
Materials and methods
Mesocosm setup
Nine fibreglass tanks with inner dimensions of 3 × 1 × 1 m were housed outdoors at the National University of Singapore (Fig. 1a). Each experimental tank was elevated above a second tank that served as a reservoir connected by a pump (Eheim 1260 universal pump, 2400 L h−1; EHEIM GmbH & Co. KG, Deizisau, Germany). Tanks were filled with a mixture of sea‐ and freshwater giving a salinity of 10 to match the salinity of the naturally collected experimental mangrove mud (see below). A timer on the pump was used to automatically fill and drain the experimental tanks, while switching off the pump resulted in complete drainage of the upper tank through the pump back into the reservoir. An overflow at a height of 80 cm inside the experimental tank maintained the water depth when filled.
Three experimental tanks were equipped with pneumatic wave paddles for constant water movement; they were powered by an air compressor and programmed with valve time switches to run during inundation only (cf. La Nafie et al. 2012). The wave paddles extended 100 cm into the middle of the tank with a stroke of 40 cm and a frequency of 0·5 Hz. Mean orbital velocities were measured with a laboratory Acoustic Doppler Velocimeter (Nortek Vectrino ADV; Nortek AS, Rud, Norway) at three central positions at 30 cm water depth: 4·3 cm s−1 at 20 cm, 3·2 cm s−1 at 100 cm and 1·7 cm s−1 at 180 cm distance from the maximum wave paddle extension.
Materials and experimental design
Fresh Avicennia alba (Avicenniaceae, A. alba Blume) propagules and Sonneratia alba (Sonneratiaceae, S. alba J. Smith) fruits were collected from the sediment surface at the Mandai mangrove in Singapore (1°26′22 N, 103°45′51 E). Avicennia propagules that had just shed or still had the seed coat were considered fresh. Whole, intact Sonneratia fruits were collected, which had softened and hence were to soon release the seeds. Avicennia propagules were randomly assigned to experimental treatments, sown in pots filled with mangrove mud and watered with freshwater for 10 days to allow for seedling anchoring (cf. Balke et al. 2011) inside the mesocosm. For Sonneratia, seeds were removed from the fruits, germinated in mangrove mud outside the mesocosms and watered with freshwater for 7 days, after which time the germinated seeds were transferred to randomly assigned pots inside the mesocosm and allowed to adjust for three more days before starting the experiments.
Experimental pots were PVC pipes cut to 30 cm length and with a 20 cm diameter, lined with plastic bags, and filled with mud collected from the top 10 cm of a mud flat in front of a mangrove near Lim Chu Kang in northwest Singapore (1°26′52 N, 103°42′35 E). Salinity of the mud was monitored over the course of the experiments (PCSTestr35, Eutech Instruments Pte Ltd, Singapore), and remained between 9 and 10; organic matter of the mud was below 4% as determined by loss on ignition. Dry bulk density of the mud was 1·3 g cm−3. Redox potential in the pots (E h) at 15 cm depth was between −100 and −300 mV over the course of the experiments (Platinum electrode according to Mansfeldt), whereas soil pore water pH was on average 6·8.
Mesocosm experiments lasting for 53 days were carried out for Avicennia in December 2010–January 2011 and for Sonneratia in March–April 2011. Temperatures and daylight hours in Singapore are fairly constant throughout the year, and the mesocoms were covered by a clear roof to prevent rain from diluting the saltwater and minimize shading, so that any seasonality effects were minimized. In both experiments, the nine mesocosms were divided into three groups with different inundation treatments: (i) short inundation of 5 h day−1, semi‐diurnal (ii) long inundation of 10 h day−1, semi‐diurnal and (iii) long inundation of 10 h day−1 with continuous water movement by wave paddles (see Van Loon, Dijksma & van Mensvoort 2007 for typical inundation duration in mangroves).
To mimic sediment erosion or accretion, sediment was added or removed from the PVC pots (Fig. 1c). Erosion was mimicked by placing discs underneath the plastic bag and pushing the sediment above the rim of the pipe. Excess sediment was then gently scraped away from the top without harming the plant. Sediment accretion was mimicked by removing discs that were placed at the start of the experiment from underneath the plastic bag, so that the sediment bag would sink into the pipe and mud was added to level it with the rim (Fig. 1c).
Erosion and accretion treatments were applied on days 23, 33 and 43 of the experiments. Avicennia treatment groups were 2 cm (erosion and accretion), 1 cm (erosion and accretion), or 0 cm (control). For Sonneratia experiments, treatment groups were 1 cm (erosion and accretion) or 0 cm (control) treatments, because seedlings were expected to grow slower than Avicennia. In total, 270 Avicennia seedlings (18 replicates, five sediment treatments, three inundation treatments) and 162 Sonneratia seedlings (18 replicates, three sediment treatments, three inundation treatments) were used. All seedlings for each sediment treatment were equally distributed across three mesocosms. The seedlings were monitored throughout the experiment for survival and height every 3rd day.
At day 53, all surviving seedlings were subjected to a final erosion treatment to determine the critical erosion depth, defined as the depth of erosion at which the seedlings toppled under a predetermined lateral force. This final erosion treatment was used to mimic storm events that rapidly erode layers of sediment by wave action. To determine the appropriate lateral force, we used a wave flume to measure the drag forces experienced by seedlings of 5–25 cm under typical waves. Twelve seedlings were submerged in 30 cm water, attached at the base to a force transducer, which measured the drag exerted on the seedlings (see Appendix S1, Supporting information). Each seedling was exposed to an 8 cm high regular wave (i.e. the maximum wave height before the wave would break in the flume at this water depth). Measured drag forces were translated into the weight needed to be attached to seedlings of different lengths (Fig. 1c), to mimic natural drag: length <5 cm = 15 g, 5–15 cm = 20 g, 15–25 cm = 25 g, >25 cm = 30 g) (see Appendix S1, Supporting information).
The critical erosion depth represented the disturbance magnitude needed in addition to the previous cumulative sediment treatments to achieve 100% failure. This was measured by imposing a 1 cm incremental erosion treatment, following the protocol above, and pulling the seedling using the defined weight attached by clips at 25% and 75% of the seedling's height (Fig. 1c). The critical erosion depth was therefore the number of discs needed until the seedling toppled under lateral force. Toppled seedlings were removed with the sediment and cleaned, so that length and diameter of roots and shoots could be recorded next to fresh weight. Finally, seedlings were oven‐dried at 80 °C for 48 h, or until constant weight was reached, and weighed to determine dry biomass.
Statistical analysis
Survival was analysed for toppling and death hazard separately with the Kaplan–Meier method applying the Mantel–Cox log‐rank for treatment comparison (spss 17.0; SPSS Inc., Chicago, IL, USA). Growth rates of shoot height for each 3‐day interval were calculated (GRH = (H 2−H 1)/(t 2−t 1)), with H 1 and H 2 being the stem height (in cm) on day (d) t 1 and t 2 in time. Two‐way and three‐way anovas were performed for analysis of morphological data, that is, comparing root with shoot weight at harvest among the treatment groups and species for all surviving seedlings. A two‐way anova was performed for shoot height at harvest of all surviving seedlings to determine treatment effects on shoot growth within each species.
anova analysis was carried out for the critical erosion threshold, represented by the thickness of the eroded sediment layer to cause toppling at day 53. The amount of seedlings that could be used for critical erosion depth and morphology analysis after harvest was unbalanced because of mortality during the experiment due to the sediment treatments (see Fig 2 for survival). Therefore, the anova analysis only gave an indication of significant differences when comparing survived plants at harvest for morphology and critical erosion thresholds.
Figure 2.
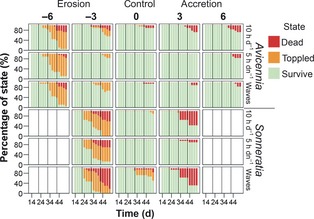
Percentage survival, toppled but alive and death of seedlings of the mangrove pioneer species Avicennia alba (top) and Sonneratia alba (bottom). The vertical lines indicate the moments when cumulative erosion/accretion treatments were applied during the 53 days mesocosm experiments. Inundation treatments in hours per day and water movement treatments by wave paddles (only for 10 h day−1) are indicated on the right side of the figures.
Results
Effects of cumulative accretion/erosion treatments
For both Avicennia and Sonneratia, short inundation (5 h day−1) without sediment treatments resulted in 100% survival throughout the experiments (Fig. 2). In the absence of sediment treatments (i.e. sediment control), longer inundation significantly increased mortality of Sonneratia but had no effect on Avicennia (see Table 1). Sediment treatments caused a significant difference in survival (Kaplan–Meier Mantel Cox, Avicennia: P < 0·2, Sonneratia: P < 0·05) and toppling (Kaplan–Meier Mantel Cox, both species P < 0·001) within the short inundation treatment for both species. Constant water movement by wave paddles within the long inundation treatment increased mortality and toppling for Sonneratia only (Kaplan–Meier Mantel Cox, P < 0·001 and P < 0·05, respectively). A cumulative erosion of 3 × 1 cm throughout the experiments caused significantly greater toppling hazards for Sonneratia than for Avicennia (Kaplan–Meier Mantel–Cox, P < 0·001), as well as a higher mortality (Kaplan–Meier Mantel–Cox, P < 0·001).
Table 1.
Results of Kaplan–Meier Mantel–Cox log‐rank test on survival and toppling of seedlings during the course of the mesocosm experiment. For both species, differences between groups of each variable (inundation treatment and sediment treatment) were tested at the control level of the other variable (5 h day−1 inundation treatment and control sediment treatment)
| Species | Variable | Log‐rank of survival | Log‐rank of toppling | ||||
|---|---|---|---|---|---|---|---|
| χ 2 | d.f. | Sig. | χ 2 | d.f. | Sig. | ||
| Avicennia | Hydro treatment (at control sediment treatment) | 2·09 | 2 | 0·35 | 2·00 | 2 | 0·368 |
| Sediment treatment (at hydro treatment = 5 h day−1) | 7·24 | 4 | 0·12 | 105·41 | 4 | 0·000 | |
| Sonneratia | Hydro treatment (at control sediment treatment) | 8·22 | 2 | 0·02 | 6·04 | 2 | 0·049 |
| Sediment treatment (at hydro treatment = 5 h day−1) | 6·80 | 2 | 0·03 | 43·58 | 2 | 0·000 | |
Growth rates of Avicennia decreased over time but were initially higher than the more constant growth rates of Sonneratia (Fig. 3a). Shoot height at harvest of Avicennia was not significantly different between inundation treatments, but sediment treatment effects were significant at the P < 0·05 level (Table 2). Inundation treatments as well as sediment treatments significantly influenced the final shoot height of Sonneratia (Table 2), where long inundation reduced shoot height but accretion increased shoot height. Differences in root : shoot ratio of biomass (Fig. 3b) of Avicennia and Sonneratia were significantly different between sediment treatment groups (anova, P < 0·001 and anova P < 0·02, respectively) but not between inundation treatments. Burial decreased biomass allocation to below‐ground tissues, whereas erosion increased root biomass relative to the shoot in both species (Fig. 3b).
Figure 3.
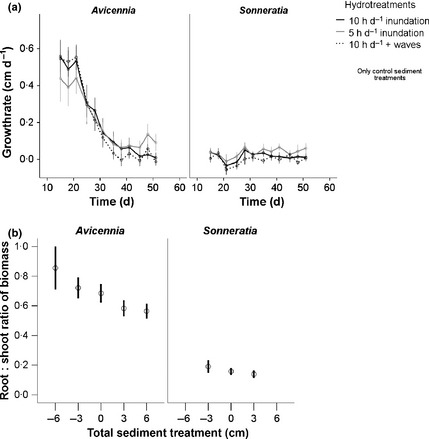
(a) Growth rates (cm day−1) of Avicennia alba and Sonneratia alba seedlings exposed to three hydro treatments over 53 days. Error bars indicate 95% confidence intervals. N varied according to survival (see Fig. 2). (b) Root : shoot biomass ratio at harvest (day 53) for all surviving A. alba and S. alba seedlings of each cumulative sediment treatment group. Inundation treatments are pooled. Error bars indicate 95% confidence intervals, N is variable according to survival (see Fig. 2).
Table 2.
Two‐way anova with between‐subjects effects for shoot height of the survived harvested seedlings of Avicennia alba and Sonneratia alba (including toppled seedlings)
| Type III SS | d.f. | MS | F | Sig. | |
|---|---|---|---|---|---|
| Avicennia | |||||
| Corrected model | 414·51 | 14 | 29·61 | 1·81 | 0·039 |
| Intercept | 41 989·68 | 1 | 41 989·68 | 2566·22 | 0·000 |
| Sediment treatment | 163·26 | 4 | 40·82 | 2·49 | 0·044 |
| Hydro treatment | 50·60 | 2 | 25·30 | 1·55 | 0·215 |
| Sediment treatment*hydro treatment | 217·81 | 8 | 27·23 | 1·66 | 0·109 |
| Error | 3517·93 | 215 | 16·36 | ||
| Sonneratia | |||||
| Corrected model | 43·68 | 8 | 5·46 | 10·64 | 0·000 |
| Intercept | 1249·08 | 1 | 1249·08 | 2433·58 | 0·000 |
| Sediment treatment | 13·10 | 2 | 6·55 | 12·76 | 0·000 |
| Hydro treatment | 19·51 | 2 | 9·75 | 19·00 | 0·000 |
| Sediment treatment*hydro treatment | 0·85 | 4 | 0·21 | 0·41 | 0·798 |
| Error | 49·27 | 96 | 0·51 | ||
Final critical erosion threshold in relation to previous cumulative treatments
For both species, the critical erosion depth of the one‐time erosion event at harvest was significantly affected by previous sediment treatments (anova, P < 0·001). As inundation treatments did not significantly affect the critical erosion depth, all inundation treatments were pooled for further comparative analysis (Fig. 4). The faster‐growing Avicennia seedlings (Fig. 3a) had on average a critical erosion threshold >1 cm higher than Sonneratia (Fig. 4). Accounting for the cumulative treatments by adding the previously eroded layers and deducting the previously accreted layers from the critical erosion threshold (Fig. 4), this net erosion showed the opposite trend as the critical erosion depth at harvest. Cumulative accretion increased critical erosion compared with cumulative erosion treatments and control, but reduced the net erosion for both species. Cumulative erosion reduced critical erosion at harvest compared with the control and accretion treatment, but increased net erosion.
Figure 4.
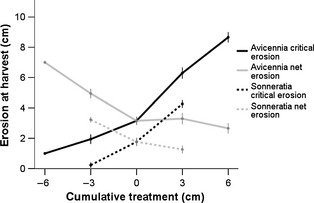
Critical erosion depth at harvest (black lines) determined by the final erosion treatment with a defined drag force. Previous cumulative accretion (positive values on x‐axes) increased critical erosion depth, whereas previous erosion decreased critical erosion depth. Net erosion (grey lines) as calculated by cumulative treatment + final erosion treatment shows the effect of morphological adjustment during the experiments. Seedlings are pooled for all inundation treatments, and error bars represent the 95% confidence interval, N is variable according to survival (Fig. 2).
Discussion
Vegetated biogeomorphic systems have been extensively studied for the interaction of the vegetation cover with geomorphic processes (see e.g. Tal & Paola 2007; Temmerman et al. 2007), whereas the establishment of biogeomorphic systems remains largely understudied. The effect of physical disturbance on seedling establishment has been suggested as a main bottleneck for primary colonization in biogeomorphic systems (Lovelock et al. 2010; Friess et al. 2012) and hence needs to be understood to improve restoration efforts. Our results demonstrate how sediment disturbance (i.e. vertical accretion and erosion) in combination with other environmental stressors (flooding and wave action) affect the growth, survival and stability of mangrove pioneer plants. Our experiments reveal that resistance to sediment disturbance at a given moment in time is determined by the disturbance history and the ability of the seedling to outgrow and adjust to such disturbances.
Comparing the initially faster‐growing Avicennia with slower‐growing Sonneratia (Fig. 2) indicates that rapid growth during the seedling stage enhances the ability to resist sediment disturbance. This finding is particularly relevant in locations where disturbance poses a significant barrier to establishment as mimicked in the experiments. Environmental stressors may be expected to indirectly affect the ability to cope with sediment disturbance when reducing the growth rates. In our experiments, this could indeed be demonstrated for inundation stress, which reduced growth and hence survival during the cumulative treatments for slower‐growing Sonneratia, whereas Avicennia could maintain sufficient growth to survive disturbance. Similarly, constant movement by small waves only caused higher mortality and toppling during cumulative treatments for the slower‐growing Sonneratia (Fig 2). Although both Avicennia and Sonneratia naturally colonize tidal flats (Tomlinson 1986), our experiments showed that faster growth increased survival by outgrowing physical disturbance events. High early growth rates are generally regarded as beneficial for primary colonization (Grime 1977); however, to our knowledge, this has not yet been linked to survival of sediment disturbance.
The present study focuses on the critical erosion threshold after cumulative sediment disturbance; however, the critical accretion threshold at harvest was not measured. Assuming that complete burial of the seedling is needed to cause failure, Avicennia seedlings with average shoot heights of 12·5 cm would be able to resist more burial than Sonneratia with 3·4 cm average shoot height (control sediment treatments at day 53). This threshold would be lower for previously buried seedlings and higher for previously eroded seedlings opposite to the critical erosion threshold (Fig. 4). In general, erosion of the same magnitude causes higher mortality than accretion (Fig. 2).
Natural sediment disturbance is not a gradual process but rather a result of short‐term events interrupting longer stationary periods. Each individual disturbance event alters the critical erosion depth of subsequent events, if the lethal threshold is not surpassed (Fig. 5). Whereas disturbance history influences subsequent events in a purely abiotic way, for example, when previously accreted sediments act as a buffer against erosion, each survived change in bed level also feeds back to the morphology of the seedling and hence alters the critical erosion threshold indirectly as shown in our experiments (Fig. 5). Whereas there is no delay of the abiotic disturbance history effects, the biotic feedback to seedling morphology depends on the growth and plasticity of the seedling and the available time for adjustment.
Figure 5.
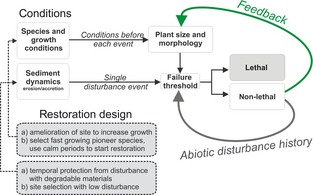
Conceptual framework showing the effects of sediment disturbance on seedling survival and potential restoration applications. Each event of accretion or erosion will feed back to the morphology of the plant if it is below a lethal threshold and influence the failure threshold of subsequent events. This biotic feedback is strongly dependent on the species traits and the local environmental conditions for growth. Disturbance history affects the failure threshold also in a pure abiotic way by, for example, building up layers that thereafter can get eroded again or by filling up previously eroded sediments during accretion events. The restoration design can help reducing seedling mortality, for example, by temporarily reducing the physical disturbance or improving the growth conditions.
Seedlings are able to adjust to repeated small magnitude disturbances when the magnitude–frequency relationship of the disturbance is in balance with the growth conditions and species traits. If there is enough time available between the disturbance events (or plant growth is fast enough), seedlings adjust to burial by an increase in shoot biomass to avoid smothering and to erosion by an increase in the root biomass to avoid toppling (Fig. 3b). This is evident in the present study as plants with faster growth due to higher adaptability or reduced stress were able to withstand higher magnitudes of cumulative disturbance. Decreasing the frequency of the disturbance with the same magnitude would allow more time for the plants to adjust and hence is expected to increase survival. Plasticity of seedlings to environmental conditions is not uncommon but has mainly been related to other abiotic factors such as light or water availability (Sultan 2000). In biogeomorphic systems, sediment accretion has previously only been associated with an increase in shoot growth to outgrow burial (see e.g. Boorman & Boorman 2001; Baas 2002). Thus, the present study fills an important gap by demonstrating both above‐ and below‐ground responses to sediment disturbance, providing a more holistic understanding of morphological plant adaptation after disturbance including sediment erosion (Deloffre et al. 2006; Viles et al. 2008).
This study highlights the importance of incorporating sediment disturbance mechanisms into restoration planning and implementation. For example, using fast‐growing (pioneer) species and/or by temporarily ameliorating the restoration site to improve growth conditions, restoration managers can influence seedling growth and the feedback to seedling morphology, improving seedling stability and maximizing survival. In addition, our results and that of a previous study (Balke et al. 2011) suggest that propagule or seed planting should be conducted immediately prior to, or during, a hydrodynamically and geomorphologically calm period, to minimize seedling dislodgment and/or toppling. Alternatively, a restoration site could require temporal protection from physical disturbance (e.g. using biodegradable materials to stabilize the sediment) to allow seedlings to gain stability in their most vulnerable phase (Fig. 5).
Changes in disturbance characteristics (frequency and magnitude) and growth conditions (temperature and water availability) due to global change potentially affect the functioning of vegetated biogeomorphic systems, especially during the establishment phase. The conceptual framework presented here offers a tool to predict and analyse settling of vegetation in such active sedimentary environments and thereby provides fundamental insights that can be used to improve restoration practice and interpret the effect of changes in disturbance regimes (Gibling & Davies 2012). Understanding the importance of physical processes for seedling establishment in threatened biogeomorphic ecosystems such as mangroves, salt marshes, dunes and riparian vegetation can prevent restoration failure and reduce associated costs.
Supporting information
Appendix S1. Drag force measurements in wave flume.
Acknowledgements
We greatly acknowledge S. Chen, J. Chuah, A. Jain, R. Oh, Y. Wang, R. Webb, S.M. Yaakub and others for their help during the mesocosm experiment. Research was conducted under Singapore NParks permit number NP/RP936‐1. The authors gratefully acknowledge the support & contributions of the Singapore‐Delft Water Alliance (SDWA) Marine 3 research programme, grants R303‐001‐001‐272 and R‐264‐001‐024‐414. STW‐NWO grant 07324 is gratefully acknowledged for funding T.J. Bouma to study the application of salt marsh in coastal defence, which lead to the development of the erosion and accretion pot system.
References
- Baas, A.C.W. (2002) Chaos, fractals and self‐organization in coastal geomorphology: simulating dune landscapes in vegetated environments. Geomorphology, 48, 309–328. [Google Scholar]
- Balke, T. , Bouma, T.J. , Horstman, E.M. , Webb, E.L. , Erftemeijer, P.L.A. & Herman, P.M.J. (2011) Windows of opportunity: thresholds to mangrove seedling establishment on tidal flats. Marine Ecology Progress Series, 440, 1–9. [Google Scholar]
- Boorman, L.A. & Boorman, J.H.M. (2001) The effect of rates of sedimentation and tidal submersion regimes on the growth of salt marsh plants. Continental Shelf Research, 21, 2155–2165. [Google Scholar]
- Bouma, T.J. , Friedrichs, M. , van Wesenbeeck, B.K. , Temmerman, S. , Graf, G. & Herman, P.M.J. (2009a) Density‐dependent linkage of scale‐dependent feedbacks: a flume study on the intertidal macrophyte Spartina anglica . Oikos, 118, 260–268. [Google Scholar]
- Bouma, T.J. , Friedrichs, M. , Klaassen, P. , van Wesenbeeck, B.K. , Brun, F.G. , Temmerman, S. , van Katwijk, M.M. , Graf, G. & Herman, P.M.J. (2009b) Effects of shoot stiffness, shoot size and current velocity on scouring sediment from around seedlings and propagules. Marine Ecology Progress Series, 388, 293–297. [Google Scholar]
- Corenblit, D. , Tabacchi, E. , Steiger, J. & Gurnell, A.M. (2007) Reciprocal interactions and adjustments between fluvial landforms and vegetation dynamics in river corridors: a review of complementary approaches. Earth‐Science Reviews, 84, 56–86. [Google Scholar]
- Deloffre, J. , Lafite, R. , Lesueur, P. , Verney, R. , Lesourd, S. , Cuvilliez, A. & Taylor, J. (2006) Controlling factors of rhythmic sedimentation processes on an intertidal estuarine mudflat — role of the turbidity maximum in the macrotidal Seine estuary, France. Marine Geology, 235, 151–164. [Google Scholar]
- Duke, N.C. , Meyenecke, J.O. , Dittmann, S. , Ellison, A.M. , Anger, A. , Berger, U. , Cannicci, S. , Diele, K. , Ewel, K.C. , Field, C.D. , Koedam, N. , Lee, S.Y. , Marchand, C. , Nordhaus, I. & Dahdouh‐Guebas, F. (2007) A world without mangroves? Science, 317, 41–42. [DOI] [PubMed] [Google Scholar]
- Ellison, A.M. (2000) Mangrove restoration. Do we know enough? Restoration Ecology, 8, 219–229. [Google Scholar]
- Fagherazzi, S. (2008) Self‐organization of tidal deltas. Proceedings of the National Academy of Sciences of the United States of America, 105, 18692–18695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess, D.A. , Krauss, K.W. , Horstman, E.M. , Balke, T. , Bouma, T.J. , Galli, D. & Webb, E.L. (2012) Are all intertidal wetlands created equal? Bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems. Biological Reviews, 87, 346–366. [DOI] [PubMed] [Google Scholar]
- Gibling, M.R. & Davies, N.S. (2012) Palaeozoic landscapes shaped by plant evolution. Nature Geoscience, 5, 99–105. [Google Scholar]
- Grime, J.P. (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist, 111, 1169–1194. [Google Scholar]
- Hastings, A. , Byers, J.E. , Crooks, K. , Cuddington, J.A. , Jones, C.G. , Lambrinos, J.G. , Talley, T.S. & Wilson, W.G. (2007) Ecosysem engineering in space and time. Ecology Letters, 10, 153–164. [DOI] [PubMed] [Google Scholar]
- Hughes, R.G. & Paramor, O.A.L. (2004) On the loss of saltmarshes in south‐east England and methods for their restoration. Journal of Applied Ecology, 41, 440–448. [Google Scholar]
- Jones, C.G. , Lawton, J.H. & Shachak, M. (1994) Organisms as ecosystem engineers. Oikos, 69, 373–386. [Google Scholar]
- Krauss, K.W. , Lovelock, C.E. , McKee, K.L. , Lopéz‐Hoffman, L. , Ewe, S.M.L. & Sousa, W.P. (2008) Environmental drivers in mangrove establishment and early development: a review. Aquatic Botany, 89, 105–127. [Google Scholar]
- La Nafie, Y.A. , de los Santos, C.B. , Brun, F.G. , van Katwijk, M.M. & Bouma, T.J. (2012) Waves and high nutrient loads jointly decrease survival and separately affect morphological and biomechanical properties in the seagrass Zostera noltii . Limnology and Oceanography, 57, 1664–1672. [Google Scholar]
- Lewis, R.R. III (2005) Ecological engineering for successful management and restoration of mangrove forests. Ecological Engineering, 24, 403–418. [Google Scholar]
- Lovelock, C.E. , Sorrell, B.K. , Hancock, N. , Hua, Q. & Swales, A. (2010) Mangrove forest and soil development on a rapidly accreting shore in New Zealand. Ecosystems, 13, 437–451. [Google Scholar]
- Mahoney, J.M. & Rood, S.B. (1998) Streamflow requirements for cottonwood seedling recruitment: an integrative model. Wetlands, 16, 634–645. [Google Scholar]
- Nilsson, C. , Brown, R.L. , Jansson, R. & Merritt, D.M. (2010) The role of hydrochory in structuring riparian and wetland vegetation. Biological Reviews, 85, 837–858. [DOI] [PubMed] [Google Scholar]
- Phillips, J.D. (1995) Biogeomorphology and landscape evolution: the problem of scale. Geomorphology, 13, 337–347. [Google Scholar]
- Phillips, J.D. (2006) Evolutionary geomorphology: thresholds and nonlinearity in landform response to environmental change. Hydrology and Earth System Sciences, 10, 731–742. [Google Scholar]
- Smoak, J.M. & Patchineelam, S.R. (1999) Sediment mixing and accumulation in a mangrove ecosystem: evidence from 210Pb, 234Th and 7Be. Mangroves and Salt Marshes, 3, 17–27. [Google Scholar]
- Stallins, J.A. (2006) Geomorphology and ecology: unifying themes for complex systems in biogeomorphology. Geomorphology, 77, 207–216. [Google Scholar]
- Sultan, S.E. (2000) Phenotypic plasticity for plant development, function and life history. Trends in Plant Science, 5, 537–542. [DOI] [PubMed] [Google Scholar]
- Tal, M. & Paola, C. (2007) Dynamic single‐thread channels maintained by the interaction of flow and vegetation. Geology, 35, 347–350. [Google Scholar]
- Temmerman, S. , Bouma, T.J. , van de Koppel, J. , van der Wal, D. , de Vries, M.B. & Herman, P.M.J. (2007) Vegetation causes channel erosion in a tidal landscape. Geology, 35, 631–634. [Google Scholar]
- Thampanya, U. , Vermaat, J.E. & Terrados, J. (2002) The effect of increasing sediment accretion on the seedlings of three common Thai mangrove species. Aquatic Botany, 74, 315–325. [Google Scholar]
- Thornes, J.B. (1985) The ecology of erosion. Geography, 70, 222–235. [Google Scholar]
- Tomlinson, P. (1986) The Botany of Mangroves. Cambridge University Press, Cambridge. [Google Scholar]
- Van Loon, A.F. , Dijksma, R. & van Mensvoort, M.E.F. (2007) Hydrological classification in mangrove areas: a case study in Can Gio, Vietnam. Aquatic Botany, 87, 80–82. [Google Scholar]
- Viles, H.A. , Naylor, L.A. , Carter, N.E. & Chaput, D. (2008) Biogeomorphological disturbance regimes: progress in linking ecological and geomorphological systems. Earth Surface Processes and Landforms, 33, 1419–1435. [Google Scholar]
- Webb, C.E. , Oliver, I. & Pik, A.J. (2000) Does coastal foredune stabilization with Ammophila arenaria restore plant and arthropod communities in Southeastern Australia? Restoration Ecology, 8, 283–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Drag force measurements in wave flume.


