Abstract
Objective
Depression is a frequent side effect of interferon therapy in patients with chronic hepatitis C (CHC). The aim of this study was to identify baseline and on-treatment predictors of depression in CHC patients receiving peginterferon and ribavirin.
Methods
201 prior nonresponders with advanced fibrosis were treated with peginterferon alfa-2a and ribavirin for 24 weeks in the Hepatitis C Antiviral Long-term Treatment against Cirrhosis trial. Of these, 74 continued on antiviral therapy through week 48. Mood states were assessed with the Beck Depression Inventory II and the Composite International Diagnostic Interview. Plasma cortisol and whole blood serotonin levels were measured in 101 subjects at weeks 0, 4, 24, 48 and 72.
Results
The incidence of interferon-induced depression was 23% and 42% at weeks 24 and 48, respectively. Although 22% of patients had baseline depression, the absence of a week 20 virological response was the only independent predictor of interferon-induced depression at week 24 (p= 0.0009). Plasma cortisol levels did not change during treatment nor correlate with depression. In contrast, whole blood serotonin/platelet levels significantly decreased during treatment but did not correlate with interferon-induced depression through week 24 (p=0.35) nor through week 48 (p = 0.51).
Conclusion
Depression during peginterferon and ribavirin therapy was associated with a lower antiviral response. The significant reduction in whole blood serotonin levels over time suggest that further studies of the serotonergic pathway are warranted to identify the mediators of interferon-induced depression.
Introduction
Commonly reported psychiatric side effects of interferon therapy include depression, anxiety, and irritability although mood changes associated with the underlying medical condition can be difficult to discern from medication effects (1, 2). Peginterferon and ribavirin combination therapy is associated with clinically significant depression in 30% to 50% of patients with chronic hepatitis C (CHC) treated in clinical trials as well as in clinical practice (3,4). Using a battery of objective and validated neuropsychological tests, cognitive function did not worsen during peginterferon and ribavirin treatment of CHC patients with advanced fibrosis (5). However, many CHC patients receiving antiviral therapy complain not only of impaired cognition but also of moderate to severe depression, anxiety and fatigue as measured by self-report and interviewer administered questionnaires (5,6). Since psychiatric side-effects are a leading cause of interferon dose reductions or premature discontinuation, further studies regarding the incidence and pathogenesis of interferon induced mood alterations are warranted.
Selective serotonin reuptake inhibitors (SSRI) can effectively treat and potentially prevent the depression seen in patients receiving interferon (7–10). However, the studies reported to date have identified different and sometimes conflicting predictors of interferon-induced depression including baseline mood status (10–13). Furthermore the mechanism(s) of interferon-induced depression have not been elucidated, although a few studies have suggested a possible role for changes in the hypothalamic-pituitary adrenal (HPA) axis as well as central catecholamine and serotonin systems (14–17). The Hepatitis C Antiviral Long-term Treatment against Cirrhosis trial (HALT-C) is a prospective multi-center study designed to investigate the potential benefit of maintenance peginterferon in reducing the rate of disease progression in prior interferon nonresponders with advanced fibrosis (18,19). During the lead-in phase, all patients received peginterferon alfa-2a and ribavirin for 24 weeks and subjects who remained viremic at week 20 were eligible for randomization to low dose peginterferon or no treatment. The subjects who had undetectable HCV RNA by polymerase chain reaction (PCR) at week 20 continued in the responder arm of the study to complete 48 weeks of antiviral treatment. In this manuscript, the incidence, baseline predictors and potential biomarkers associated with mood disturbances in 201 HALT-C patients are reported. It was hypothesized that depressive symptoms at baseline would predict depression during antiviral therapy and that depression scores would positively correlate with plasma cortisol levels and negatively correlate with whole blood serotonin levels.
Methods
Patient population and study design
The 201 patients enrolled in this ancillary study were recruited from the University of Michigan (UM) and the University of Southern California (USC). Inclusion criteria for the HALT-C trial were detectable serum HCV RNA, a liver biopsy within 12 months of enrollment showing an Ishak fibrosis score of 3 to 6, and a lack of response to previous interferon treatment. Exclusion criteria included any other co-existent liver disorder, a history of hepatic decompensation, and intolerance to interferon (19). Additional exclusion criteria were active use of illicit drugs, ongoing excessive alcohol consumption, a history of suicide attempt or hospitalization for depression within the past 5 years and a history of severe or incapacitating psychiatric condition within the past 6 months. A history of major depression that was not severe or incapacitating at enrollment was not considered an exclusion criterion. All patients provided written informed consent to participate in the HALT-C trial as well as in this ancillary study at UM and USC.
All subjects were treated with peginterferon alfa-2a at a dose of 180 μg per week (Pegasys, Roche Laboratories, Nutley, NJ) and ribavirin in doses of 1.0–1.2 grams per day (Copegus, Roche Laboratories, Nutley NJ). Serum HCV RNA testing was done using the quantitative COBAS Amplicor HCV Monitor test (Roche Molecular Diagnostics, Branchburg, NJ) with a lower detection limit of 600 IU/ml and the qualitative COBAS Amplicor HCV test (Roche Molecular Diagnostics, Branchburg, NJ) with a lower detection limit of 100 IU/ml. Patients who tested HCV RNA negative at week 20 were considered virological responders and continued with combination antiviral therapy for 48 weeks in the responder arm of the study. Patients without detectable HCV RNA at week 72 were considered to have a sustained virological response (SVR). Adherence was defined as taking at least 80% of the prescribed peginterferon and ribavirin dose through week 12 and week 20 (20).
Psychiatric assessment
The Composite International Diagnostic Interview (CIDI) is a standardized instrument for the assessment of mental health disorders according to Diagnostic and Statistical Manual of Mental disorders- IV (DSM-IV) criteria (21). In this study, the computerized lifetime (LT) version of the CIDI that included depression, anxiety, alcohol, and substance abuse modules was self-administered at baseline (week 0). During antiviral therapy, the 12 month version of the depression module alone was administered at weeks 24, 48 and 72.
The Beck Depression Inventory (BDI) II, is a 21-item, self-administered questionnaire designed to screen for depressive symptoms over the previous 2 weeks (22). The BDI was administered at weeks 0 (baseline), 4, 12, 24, 36, 48, 60 and 72. A score <10 was considered as no depression, 11–14 as minimal, 15–19 as mild, 20–28 as moderate and >28 as severe depression. Lifetime alcohol consumption was estimated using a modification of the Skinner survey (23). Years of education were coded by highest level completed and occupation was categorized on a scale of 1 to 6 with 1= unskilled/farm labor and 6= professional/technical (24).
Definition of depression
Depression at baseline (week 0) was defined as either a BDI ≥ 11 or fulfilling the DSM-IV criteria for major depression during the previous 12 months on the CIDI. For subjects without baseline depression, interferon-induced depression was defined at each post-baseline time-point as a BDI ≥ 11 or fulfilling DSM-IV criteria for depression via the CIDI. For subjects with baseline depression (i.e. baseline BDI-II ≥ 11 or CIDI criteria for depression), interferon-induced depression was defined at each post-baseline time-point as a BDI score that had increased by 6 points or more compared to baseline. The rationale for this criterion includes prior studies that used a numerical increase in BDI-II scores or other self-rating scales to define Interferon induced depression in subjects with baseline depression (25, 26). Use of antidepressants such as SSRI’s (e.g. fluoxetine) and non-SSRI’s (e.g. nortriptyline) was recorded but was not part of the definition of depression.
Cortisol and serotonin assays
In subjects enrolled at UM, fasting morning blood samples were processed immediately and stored at −70° C. Samples were tested in batches to minimize inter-assay variability. Plasma cortisol was measured at the UM Hospital Chemistry Laboratory by a chemiluminescent immunoassay on an Advia Centaur analyzer (Bayer Diagnostics, Tarrytown, NY). The coefficient of variation for the cortisol assay varied from 4.3% to 8% over the reference range for morning cortisol levels of 7 to 22 ug/dL (25). Whole blood serotonin was measured using a high-pressure liquid chromatography method with florescence detection (Bioanalytical Systems, West Lafayette, IN) (26). The dynamic range of the assay was 1 to 10,000 nmol/L with a coefficient of variation of 3.0%. Samples with low or undetectable whole blood serotonin levels were repeated to verify results. Since platelets are a rich source of serotonin in whole blood and interferon therapy is known to reduce platelet counts, the whole blood serotonin/platelet ratio was analyzed as a biomarker of whole body serotonin stores.
Data analyses
Kaplan-Meier product-moment analysis was used to estimate the cumulative incidence of depression at week 24 and week 48. Cox-proportional hazards analysis was used to identify baseline predictors associated with depression in both the week 24 and week 48 cohorts. In addition, adherence to antiviral medications as well as week 12 virological response (i.e. decline in HCV RNA by > 2 log10 or undetectable) and week 20 virological response (i.e. HCV RNA undetectable) were tested as predictors of depression. The association of each variable with interferon-induced depression was tested alone and a multivariable model was computed that included all variables with p < 0.15 and controlling for baseline depression. The association between the biological markers and baseline BDI-II scores in the UM patients was evaluated by the Pearson correlation coefficient. Change over time in the biological markers was evaluated using repeated measures analysis of variance (ANOVA). The association between changes in biomarkers and development of interferon-induced depression through week 24 and 48 was evaluated using Cox proportional hazard analysis. All analyses were carried out using SAS statistical software version 9.1 (SAS Institute, Inc, Cary, NC).
Results
Study population
The baseline characteristics of the 102 subjects enrolled at UM and the 99 subjects enrolled at USC were comparable in all respects except for the proportion of Hispanics, number of HCV genotype 1 patients, and the duration of HCV infection. Therefore, the data from the 201 subjects were grouped together for analysis (Table 1). At week 24, 185 subjects completed the BDI-II while the remaining 16 subjects had withdrawn from the HALT- C study (n=13), the cognitive study (n=2) or failed to complete the BDI-II (n=1). Seventy-four of the 201 patients (37%) had undetectable HCV RNA at week 20 and continued in the responder arm of the trial through week 48. The remaining 127 subjects with detectable HCV RNA at week 20 had antiviral therapy discontinued at week 24 and were eligible for the randomized phase of HALT-C.
Table 1.
Clinical characteristics of overall study population and patients with and without baseline depression
| Total population (n=201) | Baseline Depression (n= 51) | No Depression (n= 150) | P-value | |
|---|---|---|---|---|
| Age (years) | 50.23 (7.8) | 50.49 (7.2) | 50.15 (8.0) | 0.79 |
| % female | 29% | 41% | 25% | 0.0318 |
| Ethnicity | 0.16 | |||
| % white | 71% | 59% | 75% | |
| % black | 14% | 22% | 12% | |
| % Hispanic | 10% | 14% | 9% | |
| % other | 4% | 6% | 3% | |
| Educational level (years) | 13.38 (2.3) | 12.78 (1.6) | 13.58 (2.5) | 0.0096 |
| Occupational codes* | 4.02 (1.6) | 3.71 (1.6) | 4.12 (1.6) | 0.10 |
| % married | 69% | 57% | 73% | 0.0356 |
| Parenteral risk factors | ||||
| Ever received a transfusion, % | 40% | 53% | 35% | 0.0265 |
| Ever experienced needlestick, % | 15% | 20% | 14% | 0.34 |
| Ever used needles for recreational drugs, % | 45% | 41% | 47% | 0.50 |
| None of the above, % | 14% | 8% | 17% | 0.12 |
| Time since last IFN therapy (days) | 814.16 (740.42) | 857.92 (842.7) | 799.29 (704.8) | 0.63 |
| % Ishak 5/6 | 38% | 39% | 37% | 0.81 |
| Log HCV RNA (IU/ml) | 6.51 (0.5) | 6.58 (0.5) | 6.48 (0.5) | 0.22 |
| % HCV genotype 1 | 88% | 90% | 87% | 0.59 |
| Serum ALT (IU/ml) | 2.52 (1.95) | 2.28 (1.63) | 2.60 (2.1) | 0.31 |
| Platelets (103/ml) | 167.43 (61.0) | 166.06 (59.79) | 167.90 (61.6) | 0.85 |
| % diabetics | 25% | 31% | 23% | 0.25 |
| % prior IFN + Ribavirin | 71% | 73% | 71% | 0.80 |
| Lifetime alcohol consumption (drinks/day) | 2.06 | 2.58 | 1.89 | 0.23 |
| BDI - II score | 6.86 (6.5) | 15.39 (6.3) | 3.95 (3.1) | <0.0001 |
| % BDI >=11 | 22% | 86% | 0% | <0.0001 |
| CIDI depression (last 12 Months) | 7% | 29% | 0% | <0.0001 |
| CIDI depression (lifetime) | 15% | 43% | 6% | <0.0001 |
| % on anxiolytic/antidepressants | 29% | 55% | 20% | <0.0001 |
| Plasma cortisol (ug/dl) | 12.41 (4.5) | 12.53 (4.8) | 12.36 (4.5) | 0.87 |
| Whole blood serotonin (nm/l) | 512.73 (371.1) | 463.96 (462.9) | 530.53 (333.3) | 0.50 |
| Whole blood serotonin/platelets (nm/l/103 platel | 3.12 (1.9) | 2.60 (2.3) | 3.31 (1.8) | 0.10 |
| % Week 12 virological response | 58% | 59% | 57% | 0.85 |
| % Week 20 virological response | 37% | 35% | 37% | 0.79 |
| % Sustained virological response | 19% | 16% | 20% | 0.50 |
| % Took > 80% peginteferon through week 12 | 76% | 75% | 76% | 0.83 |
| % Took > 80 % ribavirin through week 12 | 70% | 63% | 72% | 0.21 |
| % Took > 80% peginterferon through week 20 | 67% | 61% | 69% | 0.26 |
| % Took > 80% ribavirin through week 20 | 59% | 49% | 63% | 0.09 |
1= unskilled labor (n=3) 2= Semiskilled, operative, service (n=53) 3= Not in work force > 10 yr (n=21) 4= skilled labor/craftsman/foreman (n=25)
5= Manager/clerical/sales work (n=53) 6= Proessional/technical (n=43)
Data reported as mean (s.d.) or %
The baseline features of the 201 HALT-C patients demonstrated a mean age of 50 years and 71% were male (Table 1). The mean baseline BDI-II score was 6.9 with 22% having a score greater than 11, 5.5% having a score > 20, and 7% meeting DSM-IV criteria for depression in the previous 12 months. The 51 HALT-C patients (25%) with baseline depression were significantly more likely to be female, non-Caucasian, and have lower educational levels compared to the 150 non-depressed patients (p < 0.05). In addition, the depressed subjects were significantly more likely to be receiving an antidepressant or anxiolytic at week 0. Finally, the whole blood serotonin/platelet levels tended to be lower in the patients with baseline depression (p=0.10).
Depression through week 24
The cumulative incidence of interferon-induced depression in the 201 subjects increased from 2% at week 4 to 6% at week 12 and 22% at week 24 (Figure 1). Use of antidepressant/anxiolytic medications at baseline (p=0.054) and failure to achieve a week 12 virological response (p= 0.018) or week 20 virological response (p=0.0023) were associated with interferon-induced depression (Table 2). Since the HALT-C study had called for 24 weeks of antiviral therapy and there was a significant interaction between week 12 and week 20 virological response (kappa= 0.60, p< 0.0001), only week 20 virological response and other variables with p < 0.15 were used in the multivariate model. In multivariable analysis, only the absence of a week 20 virological response remained significantly associated with interferon-induced depression (p=0.0009).
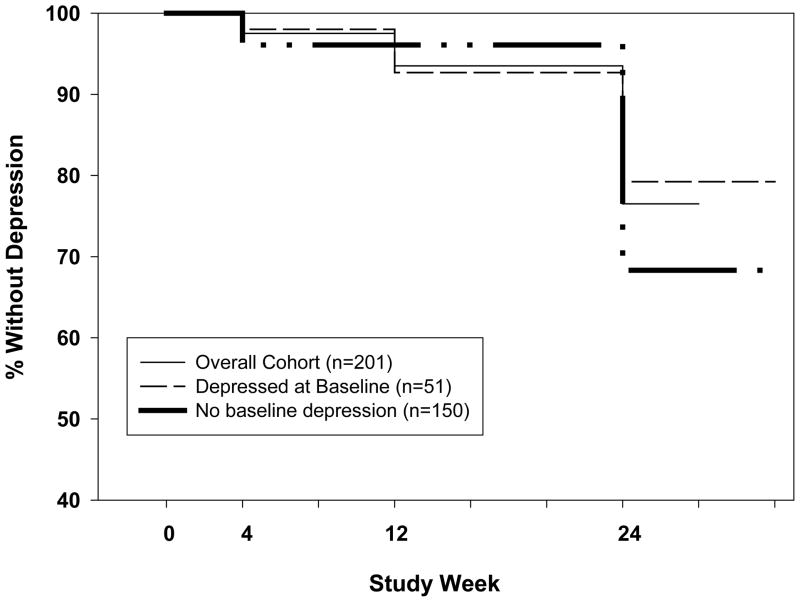
Figure 1.
Interferon-induced depression through week 24 in 201 HALT-C patients. The incidence of interferon-induced depression increased to 23% by week 24. However, the incidence of interferon-induced depression was not significantly different in the 51 subjects with baseline depression compared to the 150 subjects without baseline depression (Hazard ratio = 1.47, 95% CI: 0.79, 2.73, p= 0.22).
Table 2.
Bivariate and Multivariable predictors of interferon-induced depression through week 24 (n=201 HALT-C patients): Hazard rate ratio, 95% confidence Interval and p-value
| Bivariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value |
| Age | 0.97 | 0.94, 1.01 | 0.11 | 0.97 | 0.94 – 1.01 | 0.22 |
| Female | 1.22 | 0.66, 2.67 | 0.53 | |||
| Ethnicity | 0.52 | |||||
| White | 1.60 | 0.22, 11.63 | ||||
| Black | 2.20 | 0.28, 17.35 | ||||
| Hispanic | 0.91 | 0.10, 8.74 | ||||
| Education level | 1.02 | 0.91, 1.15 | 0.71 | |||
| Occupation | 1.09 | 0.90, 1.32 | 0.36 | |||
| Married | 1.00 | 0.54, 1.86 | 0.99 | |||
| Baseline BDI-II score | 1.01 | 0.96, 1.05 | 0.76 | |||
| Parental risk factors: | ||||||
| Received transfusion | 0.91 | 0.50, 1.66 | 0.75 | |||
| Needle Stick | 1.39 | 0.67, 2.88 | 0.38 | |||
| Recreational drugs | 1.45 | 0.80, 2.60 | 0.22 | |||
| None of above | 0.72 | 0.29, 1.83 | 0.49 | |||
| Time since last IFN | 1.00 | 1.00, 1.00 | 0.68 | |||
| Cirrhosis | 0.86 | 0.46, 1.60 | 0.64 | |||
| Genotype 1 | 1.51 | 0.54, 4.20 | 0.44 | |||
| Antidepressant/Anxiolytic at baseline | 1.79 | 0.99, 3.23 | 0.054 | 1.45 | 0.73, 2.88 | 0.29 |
| Depression at baseline | 1.47 | 0.79, 2.73 | 0.22 | 1.01 | 0.48, 2.14 | 0.98 |
| Diabetes | 0.55 | 0.27, 1.33 | 0.15 | 0.51 | 0.21, 1.22 | 0.13 |
| Prior combination therapy | 1.48 | 0.73, 2.99 | 0.27 | |||
| Total drinks-lifetime | 1.00 | 1.00, 1.00 | 0.10 | 1.00 | 1.00, 1.00 | 0.17 |
| Week 20 virologic response | 0.29 | 0.13, 0.64 | 0.0023 | 0.21 | 0.08, 0.52 | 0.0009 |
| Week 12 virologic response * | 0.79 | 0.27, 0.88 | 0.018 | |||
| Took > 80% peginf through week 20 | 0.76 | 0.42, 1.40 | 0.37 | |||
| Took > 80% ribavirin through week 20 | 1.24 | 0.67, 2.31 | 0.49 | |||
| Took > 80% peginf through week 12 | 0.77 | 0.40, 1.50 | 0.44 | |||
| Took > 80% ribavirin through week 12 | 1.12 | 0.58, 2.16 | 0.75 | |||
Week 12 virological response defined as a decrease in HCV RNA by > 2 log10 IU/ml compared to baseline or undetectable
Amongst the 150 subjects without baseline depression, the cumulative incidence of depression was 2% at week 4, 7% at week 12, and 21% at week 24 (Figure 1). Similarly, in the 51 subjects who met criteria for baseline depression, the cumulative incidence of interferon induced depression was 4% at weeks 4 and 12, and 32% at week 24 (Figure 1). Therefore, the incidence of interferon-induced depression was not significantly different in patients with and without baseline depression (Hazard ratio = 1.47, 95% CI: 0.79, 2.73, p= 0.22).
Depression through week 48
Among the 74 subjects that continued on antiviral therapy through week 48, 64 (86%) had undetectable HCV RNA at week 48 and overall 38 (51%) achieved an SVR at week 72. The actuarial incidence of interferon-induced depression in these subjects increased from 4% at week 4 to 9% at week 24, and 42% at week 48 (Figure 2). However, none of the baseline features including baseline BDI-II scores or adherence to peginterferon or ribavirin were associated with the development of depression through week 48 (Data not shown). Amongst the 66 subjects with available BDI-II scores through week 72, the mean BDI-II scores at week 72 were significantly lower compared to week 24 (4.7 + 0.7 vs 7.8 + 0.7, p = 0.0009) and week 48 (4.7 + 0.7 vs 7.4 + 0.7, p = 0.0006) demonstrating that interferon-induced depression is reversible.
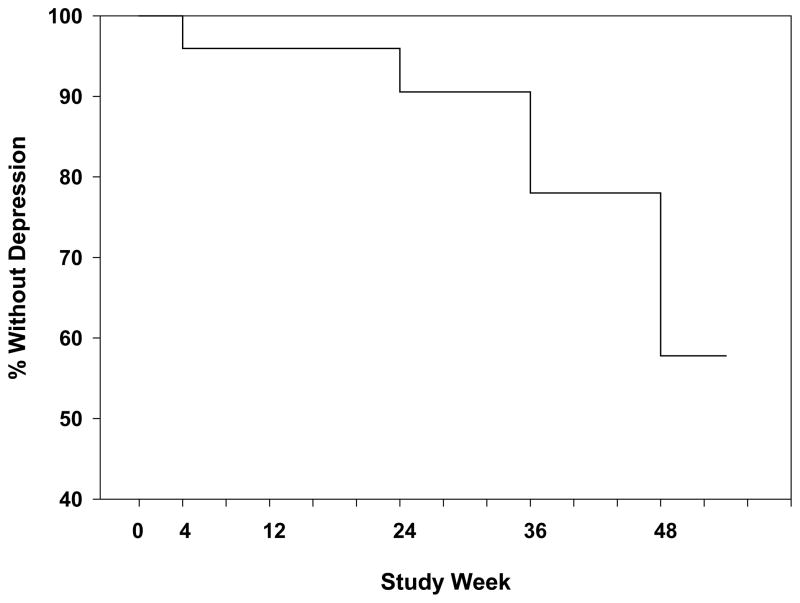
Figure 2.
Interferon- induced depression through week 48 in 74 HALT-C patients. The incidence of interferon-induced depression continued to increase from 9% at week 24 to 42% at week 48.
Changes in serum biomarkers
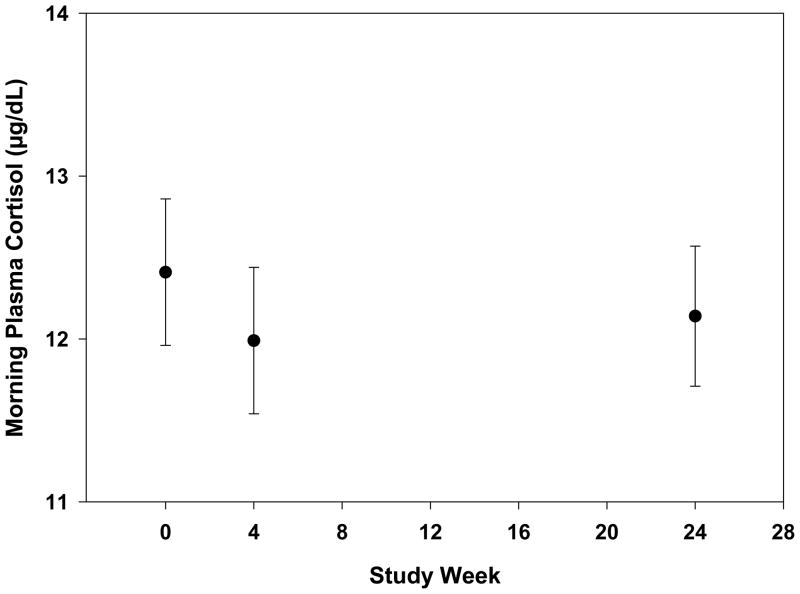
At baseline, plasma cortisol levels ranged from 2.2 to 24.3 ug/dL with only 2% of the patients having cortisol levels that exceeded the normal range. There was no significant correlation between baseline cortisol levels and BDI-II scores. As noted in Figure 3, plasma cortisol levels decreased slightly by week 4 and then remained stable at week 24 with no significant change over time (p= 0.60). Furthermore, there was no significant association between cortisol levels and the development of interferon-induced depression through week 24 (p=0.61) or with BDI-II scores through week 24 (Data not shown).
Figure 3.
Morning plasma cortisol levels through week 24 in 101 HALT-C patients. There was no significant change in the mean plasma cortisol levels over time (p =0.60). Values plotted as mean +/− standard error.
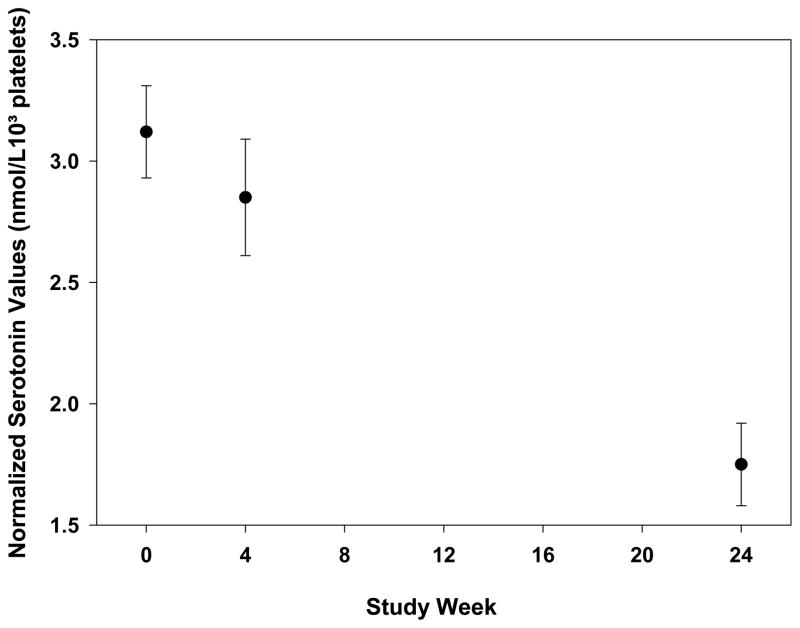
Prior to antiviral therapy, whole blood serotonin levels ranged from 4 to 2023 nmol/L while whole blood serotonin levels normalized to platelet count ranged from 0.02 to 8.80 nmol/L/103 platelets. There were no significant correlations between baseline whole blood or normalized serotonin levels and BDI-II scores (p=0.59 and p=0.23, respectively). The normalized serotonin levels significantly decreased from baseline through week 24 (Figure 4) (p < 0.0001). However, neither changes in normalized serotonin levels nor whole blood serotonin levels correlated with interferon-induced depression through week 24 or week 48 (p=0.35 and p= 0.51, respectively). In addition, there was no significant correlation of these biomarker levels with BDI-II scores (data not shown).
Figure 4.
Normalized whole blood serotonin levels through week 24 in 101 HALT-C patients. The y-axis value was determined by dividing the whole blood serotonin level by the platelet count at each time point and is reported as the mean +/− standard error. The mean whole blood serotonin/platelet level did significantly decline during antiviral therapy (p < 0.0001)
Discussion
Previously published studies of mood states in CHC patients receiving antiviral therapy have been limited by the small numbers of patients enrolled, nonstandardized definitions of depression, and the heterogeneity of antiviral treatment regimens used (2, 27, 28). In this study, 201 CHC patients with advanced fibrosis entering a clinical trial of peginterferon and ribavirin were prospectively assessed for changes in mood status over time using a series of validated instruments. The BDI-II was selected due to its brevity and established utility in detecting depression in patients with medical conditions (22, 29, 30). Furthermore, the BDI-II has been used in previous studies of CHC patients and correlated well with mental health professional assessment of mood disorders (31). The computerized CIDI depression module which is based upon DSM-IV criteria was also used and previously shown to correlate with interviewer administered questionnaires (21). Since the CIDI is self-administered, this instrument may also be more likely to detect depression. Overall, 51 (25%) of the HALT-C patients met criteria for depression before antiviral therapy although the depression was graded as mild in most. The high rate of baseline mood disorders may relate to referral bias, the requirement for previous non-response to interferon and advanced fibrosis to enter the HALT-C study, and the prospective characterization of current and lifetime psychopathology. However, other prospective studies have also identified a 20–30% prevalence of mood disorders in CHC patients prior to antiviral therapy (13, 32)
The cumulative incidence of interferon-induced depression increased to 23% by week 24, a rate that is similar to what has been reported in other studies (Figure 1) (4, 27, 32). In addition, 16 subjects (8%) had to prematurely discontinue therapy by week 24 due to various adverse events. Furthermore, 33% of the treated patients took less than 80% of the prescribed peginterferon dose through week 20 due to a variety of side effects including neuropsychiatric toxicity. Based upon studies of interferon therapy in melanoma and CHC patients, subjects with baseline depression were expected to be at increased risk of interferon-induced depression (10, 13, 33). In support of this, use of anti-depressants/anxiolytics at baseline was associated with interferon-induced depression on univariate analysis (Table 2). Although expected trends were noted for several other baseline variables (i.e. baseline depression (p=0.22), age (p=0.11)), none remained significant in multivariate analysis. The absence of a significant association of baseline depression with interferon-induced depression may have been due to the better tolerability of peginterferon compared to high dose standard interferon used in prior studies and the selection of interferon “tolerant” patients for the HALT-C study (34). It is also possible that the definition of interferon- induced depression used in the current study differs from that used in prior studies. As mentioned, a validated depressive symptom rating scale (i.e. the BDI-II) and the CIDI were used to define interferon-induced depression. To address potential ceiling effects with the BDI-II, an increase of the BDI-II score of > 6 points was required during treatment for subjects with an elevated baseline score which would change the classification of depression from minimal to mild and mild to moderate. In addition, prior studies have suggested that an increase in the BDI-II score of 6 points is clinically significant (25,26). An alternative approach would have been to use a threshold severity construct to define interferon-induced depression (e.g. a BDI-II score > 18). Finally, frequent assessment of patients during antiviral therapy by a mental health professional would be considered the “gold standard” for verifying the presence of a mood disorder. However, this approach was not feasible due to logistical considerations.
A significant association between failure to clear HCV RNA by week 20 and developing depression during antiviral therapy was observed in the current study. A previous study suggested that CHC patients with more severe psychiatric side effects during treatment were more likely to achieve a virological response (36). However, this association was not found in 4 other studies which demonstrated a negative relationship between fatigue and response to antiviral therapy (37–40). In the current study, there was no significant difference in the BDI-II score at week 4 or week 12 compared to baseline in the week 20 virologic responders versus non-responders (data not shown). Therefore, the association noted in our study may have been due, in part, to the sharing of HCV RNA test results with patients during the study and the expected negative impact that knowledge of persistent viremia could have on a patient’s mood. Future studies that blind patients to virological test results may better discern the biological effect of interferon on mood versus knowledge of the treatment response.
The incidence of interferon-induced depression in the subjects who continued beyond 24 weeks increased from 9% at week 24 to 42% by week 48 (Figure 2). These data suggest that the neuropsychiatric side effects of peginterferon and ribavirin increase over time. Importantly, however, the mean BDI-II scores of subjects returned to pretreatment baseline levels by week 72 demonstrating the reversibility of interferon-induced depression. The lack of a significant association between any of the baseline variables and interferon-induced depression in this subgroup of 74 subjects may, in part, relate to the smaller sample size and the exclusion of non-responders beyond week 24.
In addition to determining the frequency and severity of interferon-induced depression, potential mechanism(s) of neuropsychiatric side-effects were explored in the current study. Previous studies of short-term interferon therapy have demonstrated dramatic changes in the HPA axis in healthy individuals and CHC patients (14, 41, 42). Presumably, the induction of fever and systemic side-effects by interferon can lead to a marked stress response with characteristic increases in adrenocorticotropin releasing hormone (ACTH) and plasma cortisol levels as previously noted in subjects with endogenous depression (1). However, studies of prolonged interferon therapy demonstrate the development of a tolerance to this effect (14). As seen in Figure 3, no significant changes in plasma cortisol levels were noted over time. In addition, baseline plasma cortisol levels did not significantly correlate with baseline BDI-II scores and changes in cortisol levels over time did not track with the appearance of interferon-induced depression or serial BDI-II scores. These data suggest that alterations in the HPA axis as reflected in morning cortisol levels are not associated with interferon-induced depression in CHC patients. However, it remains plausible that more sensitive and specific measurements of subtle changes in the HPA axis such as the ACTH stimulation test or dexamethasone suppression test could have led to different results.
Previous studies have demonstrated that interferon can lead to down-regulation of serotonin synthesis in the brain and depletion of serotonin precursors throughout the body (16, 43). In addition, depletion of whole body serotonin stores via interferon mediated induction of indoleamine-2,3-dioxygenase activity has been postulated as a potential mechanism of interferon-induced depression (44). In the current study, whole blood serotonin levels were measured which includes contributions from platelet-derived serotonin. Since interferon is known to cause thrombocytopenia via direct effects on the bone marrow, changes in normalized whole blood serotonin to platelet levels were also investigated. At baseline, a wide range of whole blood serotonin levels were noted and subjects with baseline depression tended to have lower levels (Table 1). However, neither baseline whole blood nor normalized serotonin levels were associated with baseline BDI-II scores. Nonetheless, significant changes in normalized whole blood serotonin levels were seen during antiviral therapy although the degree of change did not correlate with the occurrence of depression (Figure 4) nor with serial changes in BDI-II scores. The lack of an association between changes in whole blood serotonin levels and interferon-induced depression may have been due to the discordance between whole blood and intracerebral serotonin levels. In addition, the non-specific reduction of platelet count in treated patients from interferon effects on the bone marrow may have obscured a relationship. Finally, the use of SSRI’s in 17% of the HALT-C patients during the lead-in phase and 15% during the responder arm may have confounded a relationship between these parameters. Therefore, investigation of more sensitive markers of serotonin metabolism such as serotonin gene polymorphisms, serotonin receptor expression in peripheral lymphocytes, and serum peptidases induced by interferon would be worthwhile (43–49).
Strengths of the current study include the enrollment of a large number of well-characterized CHC patients at two clinical sites with varying ethnicity, age, and gender. In addition, standardized and objective measures of mood states were used rather than the subjective clinical assessment of a hepatologist. Although formal assessment of suspected mood disorders by a mental health professional would have been preferred, this was not feasible. Other strengths of the current study include the assessment of mood status within a clinical trial wherein all patients were seen at regular and frequent intervals and standardized rules for antiviral dose reductions were utilized. Nevertheless, the results of this study may not be generalizable to other patients with CHC due to the strict inclusion criteria of the HALT-C trial. In particular, the inclusion of patients who had previously tolerated a course of interferon therapy may have excluded patients at risk for severe neuropsychiatric toxicity. In addition, subjects with severe or recent psychopathology at baseline were excluded from enrollment into this study of long-term peginterferon therapy. Finally, as in any study that uses a self-administered questionnaire, some subjects could have overestimated psychiatric symptoms while others may have underestimated their symptoms due to questionnaire fatigue. However, the cumulative incidence of interferon-induced depression at weeks 24 and 48 is similar to what has been reported in other studies of mood disorders in CHC patients (36–40).
In summary, the cumulative incidence of interferon-induced depression in patients receiving peginterferon and ribavirin enrolled in the HALT-C trial was 23% at week 24 and 42% at week 48. Contrary to initial hypotheses, many pretreatment characteristics were not associated with interferon-induced depression including baseline depression, liver disease severity, and lifetime psychiatric and alcohol consumption history. However, as expected receiving an anti-depressant or anxiolytic medication at baseline was associated with depression as was the absence of a virological response at week 12 and week 20. Morning plasma cortisol levels did not change significantly during therapy and were not associated with depression before or during antiviral therapy. In contrast, normalized serotonin levels decreased significantly during antiviral therapy but were not associated with the development of interferon-induced depression. Additional studies of serotonergic pathways in the pathogenesis of mood disorders during antiviral therapy of CHC are warranted to improve our understanding of the biological basis of this common and dose limiting side-effect of treatment.
Study Highlights.
What is current knowledge
Depression is a common and dose limiting side effect of antiviral treatment in hepatitis C patients
The incidence, risk factors, and biological basis for interferon-induced depression are not well established.
Prior studies have been limited by the small number of patients enrolled, non-standardized assessment of depression, and heterogeneous antiviral treatment regimens.
What is new here
The incidence of depression was 23% at week 24 and 42% at week 48 in 201 patients that received pegylated interferon and ribavirin combination therapy in the HALT-C Trial using validated instruments.
Although 22% of patients had mild depression at baseline, the absence of virological clearance at week 20 was the only independent predictor of interferon induced depression.
Plasma cortisol levels did not change during treatment nor correlate with depression.
Significant declines in whole blood serotonin levels were noted during treatment but did not correlate with depression. Additional studies of the seratonergic axis are indicated to further elucidate the mechanism of this common and dose limiting side effect of antiviral treatment in hepatitis C patients.
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
The authors would like to thank Donald Giacherio, PhD, for his assistance with performing the plasma cortisol assays at the University of Michigan Hospital Chemistry Laboratory. In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Carol B. Jones, RN, Susan L. Milstein, RN, and the USC Neuropsychology Technicians
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042) Pamela Richtmyer, BS, LPN, CCRC, Erin Ford, BS, Leslie Giudotti, Emily Anderson.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Teresa M. Curto, MSW, Margaret C. Bell, RN, MS, MPH
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Elizabeth C. Wright, PhD., James E. Everhart, MD, Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
Abbreviations
- ACTH
Adrenocorticotropin releasing hormone
- BDI
Beck depression inventory
- CHC
Chronic hepatitis C
- CIDI
Composite International diagnostic interview
- DSM-IV
Diagnostic and Statistical Manual of Mental disorders, 4th edition
- HALT-C
Hepatitis C Antiviral Long-term Treatment against Cirrhosis
- HPA
Hypothalamic-pituitary- adrenal
- PCR
Polymerase chain reaction
- SSRI
Selective serotonin reuptake inhibitor
- SVR
Sustained virological response
- UM
University of Michigan
- USC
University of Southern California
Footnotes
This is publication number 24 from the HALT-C Trial Group.
Financial disclosures
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: R.J. Fontana is on the speaker’s bureau; K.L. Lindsay is a consultant and receives research support; A.S.F. Lok is a consultant and receives grant support. Authors with no financial relationships related to this project are: Z. Kronfol, L.A. Bieliauskas, C. Back-Madruga, L. Padmanabhan, and A.M. Stoddard.
References
- 1.Kronfol Z. Immune dysregulation in major depression: A critical review of existing evidence. In J Neuropsychopharmacology. 2002;5:333–343. doi: 10.1017/S1461145702003024. [DOI] [PubMed] [Google Scholar]
- 2.Fontana RJ. Neuropsychiatric toxicity of antiviral treatment in chronic hepatitis C. Dig Dis. 2000;18:107–116. doi: 10.1159/000051384. [DOI] [PubMed] [Google Scholar]
- 3.Lee SS, Peltekian K, Krajdens M, et al. Treating chronic hepatitis C with pegylated interferon alfa-2a (40kD) and ribavirin in clinical practice. Aliment Pharmacol Ther. 2006;23:397–408. doi: 10.1111/j.1365-2036.2006.02748.x. [DOI] [PubMed] [Google Scholar]
- 4.Fried MW, Russo MA. Side effects of antiviral therapy for hepatitis C. Gastroenterology. 2003;124:1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 5.Fontana RJ, Bieliauskas LA, Lindsay KL, et al. Cognitive function does not worsen during peginterferon and ribavirin retreatment of chronic hepatitis C. Hepatology. 2007;45:1154–1163. doi: 10.1002/hep.21633. [DOI] [PubMed] [Google Scholar]
- 6.Hilsabeck RC, Hassanein TI, Carlson MD, et al. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. JINS. 2003;9:847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- 7.Gleason OC, Yaters WR, Isbell MD, et al. An open-label trial of citalopram for major depression in patients with hepatitis C. J Clin Psychiatry. 2002;63:194–198. doi: 10.4088/jcp.v63n0304. [DOI] [PubMed] [Google Scholar]
- 8.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 9.Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 10.Musselman DL, Lawson DH, Fumnick JF, et al. Paroxetine for the prevention of depression induced by high dose Interferon-alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 11.Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: Evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 12.Pariante CM, Landau S, Carpiniello B. Interferon alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347:148–9. doi: 10.1056/NEJM200207113470221. [DOI] [PubMed] [Google Scholar]
- 13.Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: Prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon alpha with the development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 15.Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55:1624–30. doi: 10.1136/gut.2005.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaccorso S, Marino V, Puzella A, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-a-based immunotherapy are related to interferon-a-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Cai W, Khaoustove VI, Xie Q, et al. Interferon-a-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Shifffman ML, Di Bisceglie AM, Lindsay KL, et al. Peginterferon Alfa-2a and ribavirin in patients with chronic hepatitis C who failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Lee WM, Dienstag JL, Lindsay KL, et al. Evolution of the HALT-C trial: Pegylated interferon as a maintenance therapy for chronic hepatitis C in previous Interferon nonresponders. Control Clin Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132:103–112. doi: 10.1053/j.gastro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Wittchen HU. Reliability and validity studies of the WHO composite international Diagnostic Interview: A critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. The psychological corporation. San Antonio, TX: Harcourt Brace and Company; 1996. BDI-II manual. [Google Scholar]
- 23.Skinner HA, Sheu WJ. Reliability of alcohol use indices: the lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 24.Fontana RJ, Bieliauskas LA, Back-Madruga C, et al. Cognitive function in hepatitis C patients with advanced fibrosis enrolled in the HALT-C trial. J Hepatol. 2005;43:614–622. doi: 10.1016/j.jhep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Malaguarnera M, DiFazio I, Restuccia S, et al. Interferon-alpha-induced depression in chronic hepatitis C patients: comparison between different types of interferon alpha. Neuropsychobiology. 1998;37:93–97. doi: 10.1159/000026485. [DOI] [PubMed] [Google Scholar]
- 26.Mistler LA, Brunette MF, Marsh BJ, et al. Hepatitis C treatment for people with severe mental illness. Psychosomatics. 2006;47:93–107. doi: 10.1176/appi.psy.47.2.93. [DOI] [PubMed] [Google Scholar]
- 27.Whitley RJ, Meikle AW, Watts NB. Endocrinology, Part VI: Adrenocortical steroids. In: Burtis CA, Ashwood ER, editors. Textbook of Clinical Chemistry. 2. Philadelphia: WB Saunders; 1994. pp. 1822–1825. [Google Scholar]
- 28.Xiao R, Beck O, Hjerndahl P. On the accurate measurement of serotonin in whole blood. Scan J Clin Lab Invest. 1998;58:505–510. doi: 10.1080/00365519850186319. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer M, Schwaiger M, Garkisch AS, et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with hepatitis C. J Hepatol. 2005;42:793–798. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Dan AA, Martin LM, Crone C, et al. Depression, anemia, and health-related quality of life in chronic hepatitis C. J Hepatol. 2006;44:498. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 31.Rutledge T, Reis SE, Olson MB, et al. Depression severity and reported treatment history in the prediction of cardiac risk in women with suspected myocardial ischemia. Arch Gen Psychiatry. 2006;63:874–880. doi: 10.1001/archpsyc.63.8.874. [DOI] [PubMed] [Google Scholar]
- 32.Grothe KB, Dutton GR, Jones GN, et al. Validation of the Beck Depression Inventory-II in a low-income African American sample of medical outpatients. Psychological Assessment. 2005;17(1):110–114. doi: 10.1037/1040-3590.17.1.110. [DOI] [PubMed] [Google Scholar]
- 33.Dieperink E, Ho SB, Thuras P, et al. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–12. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 34.Golden J, O’Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection, and risk factors. Gen Hosp Psychiatry. 2005;27:431–438. doi: 10.1016/j.genhosppsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Capuron L, Raynaud A. Prediction of the depressive effects of interferon-alfa therapy by the patient’s initial affective state. N Engl J Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 36.Rasenack J, Zeuzem S, Feinman SV, et al. Peginterferon alpha-2a (40kD) (Pegasys) improves HR-QOL outcomes compared with unmodified interferona-2a in patients with chronic hepatitis C. Pharmacoeconomics. 2003;21:341–349. doi: 10.2165/00019053-200321050-00005. [DOI] [PubMed] [Google Scholar]
- 37.Kraus MR, Schafer A, Csef H, et al. Compliance with therapy in patients with chronic hepatitis C. Dig Dis Sci. 2001;46:260–65. doi: 10.1023/a:1011973823032. [DOI] [PubMed] [Google Scholar]
- 38.Loftis JM, Socherman RE, Howell CD, et al. Association of interferon-alpha induced depression and improved treatment response in patients with hepatitis C. Neurosci Lett. 2004;365:87–91. doi: 10.1016/j.neulet.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 39.Raison CL, Pariante CM, Capuron L, et al. The association of fatigue with poor virologic response in patients receiving interferon-alpha plus ribavirin for the treatment of hepatitis C. (Abstract) Hepatology. 2005;44:1216. [Google Scholar]
- 40.Raison CL, Broadwell SD, Barisov AS, et al. Depressive symptoms and viral clearance in patients receiving interferon alpha and ribavirin for hepatitis C. Brain Behav Immun. 2005;19:23–7. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Maddock C, Landau S, Barry K, et al. Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry. 2005;10:332–3. doi: 10.1038/sj.mp.4001634. [DOI] [PubMed] [Google Scholar]
- 42.Castera L, Constant A, Henry C, et al. Impact of adherence and sustained virologic response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2006;24:1223–1230. doi: 10.1111/j.1365-2036.2006.03107.x. [DOI] [PubMed] [Google Scholar]
- 43.Muller H, Hiemke C, Hammes E, et al. Sub-acute effects of inferon-a2 on ACTH, cortisol, growth hormone and prolactin in humans. Psychoneuroendocrin. 1992;17:459–465. doi: 10.1016/0306-4530(92)90004-q. [DOI] [PubMed] [Google Scholar]
- 44.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:394–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 45.Lopez JF, Chalmers DT, Little KY, et al. Regulation of serotonin 1A, glucocorticoid, and mineralocorticoid reception in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 46.Maes M, Bonaccorso S. Lower activities of serum peptidases predict higher depressive and anxiety levels following interferon-alpha-based immunotherapy in patients with hepatitis C. Acta Psychiatr Scan. 2004;109:126–131. doi: 10.1046/j.0001-690x.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 47.Schlaak JF, Trippler M, Erim Y, et al. Identification of candidate genes that mediate depressive side effects of pegylated IFN-alpha 2a in patients with chronic hepatitis C. (Abstract) Hepatology. 2006;44:331. [Google Scholar]
- 48.Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuropsychpharmacol Biol Psychiatry. 2004;28:559–576. doi: 10.1016/j.pnpbp.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida K, Alagbe O, et al. Promoter polymorphisms of the Interferon-a receptor gene and development of Interferon-induced depressive symptoms in patients with chronic hepatitis C: Preliminary findings. Neuropsychobiology. 2005;52:55–61. doi: 10.1159/000086605. [DOI] [PubMed] [Google Scholar]
- 50.Wichers MC, Koek GH, Robaeys G, et al. IDO and interferon-a-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 51.Kraus MR, Al-Taie O, Schefer A, et al. Serotonin-1A receptor gene (HTR1A) variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]