Abstract
Introduction
To examine the associations of peripheral atherosclerosis, assessed by the ABI at baseline with the extent of AAC and with CAC measured by MDCT at follow-up examination in the Jackson Heart Study cohort.
Methods
Four categories of ABI: <0.90, 0.90–0.99, 1.00–1.39; >1.40. Presence of CAC/AAC was defined as scoring above the 75th percentile among participants with non-zero CT calcium scores. We conducted multivariable log-binomial models for this analysis examining the relationship between ABI and the presence of CAC or AAC using normal ABI (1.0 ≤ ABI ≤ 1.39) as the reference group. We estimated prevalence ratios adjusted for age, smoking, HTN, DM, BMI, LDL, HDL, CRP, systolic and diastolic blood pressure, and use of lipid-lowering medication.
Results
There were 2,398 patients in this analysis (women: 65%, average age 55 years). AAC scores were not significantly different between sex. CAC scores were significantly higher in males than females regardless of ABI groups. The prevalence of significant AAC was 1.7 times higher for ABI < 0.90 (PR=1.70; 95% CI=1.26–2.28; p=0.0004) and 1.57 times higher for ABI 0.90–0.99 (PR=1.57; 95% CI=1.20–2.03; p=0.0008) than the normal ABI; AAC prevalence did not differ between subjects with ABI > 1.40 compared to those with normal ABI. The prevalence of the significant CAC was higher for ABI <0.90 (PR=1.55; 95% CI=1.12–2.14; p-value=0.0081) and ABI 0.90–0.99 (PR=1.60; 95% CI=1.05–2.46; p=0.0402) compared to normal ABI; CAC prevalence did not differ between subjects with ABI > 1.40 compared to those with normal ABI
Conclusion
Lower ABI was significantly associated with the extent of AAC and CAC in this cohort. ABI can provide clinicians with an inexpensive additional tool to assess vascular health and cardiovascular risk without exposing the patient to ionizing radiation.
Keywords: Coronary Artery Calcium, Abdominal Aortic Calcium, Ankle-Brachial Index, Peripheral Arterial Disease
Introduction
Atherosclerosis is one of the underlying pathophysiologic processes for the leading causes of death in the US and most other countries (1). Peripheral arterial disease (PAD) is a significant manifestation of atherosclerosis in the peripheral circulation, which not only predicts ischemic damage to limbs and other related morbidity, but also has been shown to be an important predictor of coronary and cerebrovascular atherosclerosis and events. (2–6) Coronary calcium as detected by multi-detector computed tomography (MDCT) is highly correlated with coronary heart disease (CHD) events (7). Similarly, calcification of the aorta as detected by radiographs has been shown to be a predictor of intermittent claudication, ischemic stroke, CHD and cerebrovascular disease (CVD) (8,9). Utilizing a simple non-invasive measurement of the peripheral circulation (i.e., the ankle –brachial index [ABI]), we sought to determine the relationship between the presence (or absence) of peripheral vascular compromise and the degree of calcium deposition in the distal aorta and the coronary circulation as determined by MDCT.
Methods
Study Cohort
A description of the study population and objectives of the Jackson Heart Study (JHS) has been previously reported. (10,11) The JHS is a prospective observational study of CVD and its risk factors in 5301 African-Americans residing in the Jackson, Mississippi metropolitan area. The study cohort includes previous Atherosclerosis Risk in Communities Study (ARIC) participants, randomly selected participants and volunteers between 35 and 84 years of age as well as a subset of family members age 21 or older who comprise the family study.(11)
During the initial visit (Exam 1, 2000–2004), ABI was calculated for 4621of the participants. Exam 2 (2005–2008), included MDCT examination of all eligible and consenting participants. The present analysis included data from all participants who returned for Exam 2, except those excluded for a history of prevalent CVD or exclusion in the CT protocol: weight greater than 350lbs, pregnant (pregnancy status unknown), female participant <40 years old, and males <35 years old. There were 2398 participants who met the criteria for inclusion in this analysis (women: 65%, average age 55 years).
Measurements
Ankle-brachial index
To determine the ankle-brachial index (ABI), ankle and brachial systolic BP were measured by trained technicians using a sphygmomanometer along with an Ultrasonic Doppler Flow Detector, Model 811-B by Parks Medical Electronic-Inc., Aloha, Oregon U.S.A. Technicians followed a standard protocol, using a contour wrapping technique with the midpoint of the bladder over the posterior tibial artery; the lower end of the bladder approximately 3cm above the medial malleolus. This was done bilaterally and systolic BP measurements were taken in each leg with ultrasound measurement of the posterior tibial artery while the participant was in the supine position; the dorsalis pedis artery was used for measurement if the posterior tibial pulse could not be found by palpation or by Doppler pen probe. Measurements of the brachial systolic BP, usually in the right brachial, were taken twice. The first brachial measurement was made prior to leg measurements and the second measurement was made after the leg measurements were completed. Two ABIs (one for the right leg and one for the left leg) were calculated as the average of the two ankle systolic BP measurements divided by the average of the two brachial readings. The lower of the two ABIs were considered the ABI for the participant for the current study.
CT Angiography
The research CT protocol included the heart and lower abdomen using a 16-channel multidetector CT system equipped with cardiac gating (Lightspeed 16 Pro; GE Healthcare, Milwaukee, WI). For each coronary scan, the effective radiation dose is estimated to be 1.5 mSv (150 mrem) for men and 1.9 mSv (190 mrem) for women. The difference is that the breast is irradiated in this scan, and because the breast dose carries a weighting factor of .05 of the total effective dose, the effective dose is higher for women. A similar effective dose was calculated for the abdominal scan used in FHS-SCAN and MESA studies of 2.7 mSv for both women and men is noted since neither gender’s gonads are directly irradiated. The scanning took place at the JHS CT Data Acquisition Center located at the Jackson Medical Mall (Jackson, Mississippi). Quality control and image analysis were performed at a core reading center (Wake Forest University School of Medicine, Winston-Salem, NC). The protocol included scout images, one electrocardiogram-gated series of the entire heart, and a series through the lower abdomen from L3 to S1. The complete methodology for the cardiac gated CT scans of the coronary arteries has been previously reported (12).
CAC and AAC was quantified utilizing the Agatston scoring, modified to account for slice thickness. Calcified artery plaque was computed by multiplying each lesion by a weighted attenuation score on a TeraRecon Aquarius Workstation (TeraRecon, San Mateo, CA); scoring was in Hounsfeld units. The presence of CAC and AAC was defined as Agatston score > 0; the reproducibility in scoring was 0.99.
Statistical Analysis
Statistical analyses were carried out using SAS version 9.1. ABI was categorized as: <0.90 Low ABI, 0.90–0.99 borderline ABI, 1.00–1.39 normal ABI, >1.40 high ABI. The presence of significant CAC/AAC was defined as greater than 75th percentile among participants with valid measurements. In order to examine the optimal threshold value, we used a non-parametric approach using the generalized additive model (GAM). Threshold values were determined based on the value of CAC/AAC corresponding to a log-hazard ratio (HR) of 0 in the GAM curves. The partial prediction plots for “smooth term” demonstrated that having low ABI increased with an increase of CAC and AAC (p=0.001, p=0.002, respectively). The optimal threshold values of CAC and AAC to predict low ABI were very similar to seventy-five percentiles of each variable among participants with any values.
Descriptive analyses were conducted to describe the sample. Analyses of covariance were used to compute the sex-specific age-adjusted means and percentages for the participant characteristics by ABI group. We used linear regression analysis for the linear trend test of relations of ABI as a continuous variable with BMI, systolic and diastolic blood pressure, C-reactive protein, HDL cholesterol, LDL cholesterol, coronary artery calcium score, and abdominal aortic calcium score, controlling for age. Cochran-Armitage trend test was used to assess the linear relations of ABI with smoking, hypertension, diabetes, and use of anti-hyperlipidemic medication. The difference in prevalence of calcification risk among ABI subgroups was compared using a 2 by 2 maximum likelihood estimation and bar charts were provided for the graphical examination of the differences.
We then conducted multivariable log-binomial models to determine the relationship between risk of calcification and ABI. Normal ABI group (1.0 ≤ ABI ≤ 1.39) was treated as the reference group. By using the multivariable log regression, we estimated age-adjusted prevalence ratio (PR) and PRs fully adjusted for age, smoking, hypertension, diabetes mellitus, body mass index, LDL cholesterol, HDL cholesterol, and C-reactive protein, systolic and diastolic blood pressure, and use of anti-hyperlipidemic medication. We used a copy method approach for fitting the log-binomial regression models (13) to derive approximate maximum likelihood estimates for PR model regardless of the number of independent variables. The PR instead of odds ratio was reported because the former is often more interpretable (14).
Results
Among the valid samples of 2,398, 35% were men and 65% were women. Women were older than men in the sample (54.6 ±10.7 years vs. 53±10.9 years, p-value<0.0001). While there was no sex-related difference in abdominal aortic calcification scores (women: 313.2±640.9; men: 291.6±674.5; p-value=0.4405), women had lower CAC scores (108.0±368.1 vs. 187.6±591.0 for women and men, respectively; p-value<0.0001). Mean ABI were 1.14±0.004 for women and 1.19±0.005 for men (p>0.0001).
A large majority (84.6%) of participants in had ABI’s in the normal range (1.0–1.39); while the remainder of the sample fell into borderline (6.8%), low (4.6%) and high (4.0%) ABI categories. Table 1 represents mean (SD) and percent distribution of major characteristics stratified by the categories of ABI and the results of linearity test between ABI and ABI was significantly increased with increase of body mass index. Older participants were more likely to have lower ABI (p>0.0001). Mean Agatston scores for CAC and AAC were highest in the low ABI group, showing a significant inverse linear association with ABI. High-density lipoprotein cholesterol was higher in the low and borderline ABI groups than the normal and high ABI groups with a significant inverse trend. A higher proportion of current smokers and those who took cholesterol reduction medication (LPM) was observed among the low or borderline ABI group. While age, body mass index, and AAC were significantly associated with ABI among both genders, a significant association of ABI was found with HDL cholesterol and CAC only among females and with CRP, current smoking and LPM only among males. CRP was inversely associated with ABI among men.
Table 1.
Mean and proportion of major characteristics by ABI categories
| ABI categories | p-value for linear trend | ||||
|---|---|---|---|---|---|
| Low ABI(<0.9) [n=110] | Borderline ABI (0.90– 0.99) [n=164] | Normal ABI (1– 1.39) [n=2028] | High ABI (>1.4) [n=96] | ||
| Age (year) | 59.0 | 53.2 | 53.6 | 52.5 | <.0001 |
| Body Mass Index (kg/m2) | 29.3 | 30.4 | 30.8 | 34.3 | <.0001 |
| Systolic Blood Pressure (mmHg) | 126.7 | 126.2 | 125.3 | 124.0 | 0.5829 |
| Diastolic Blood Pressure (mmHg) | 79.9 | 79.0 | 79.9 | 79.1 | 0.1692 |
| C-reactive protein (mg/L) | 4.9 | 3.9 | 4.2 | 5.3 | 0.7401 |
| HDL cholesterol (mg/dL) | 55.02 | 55.78 | 51.72 | 47.95 | <.0001 |
| LDL Cholesterol (mg/dL) | 131.7 | 128.3 | 128.5 | 126.6 | 0.8573 |
| Coronary Artery Calcification (Agatston Score) | 476.1 | 132.1 | 120.1 | 83.8 | <.0001 |
| Abdominal Aortic Calcification (Agatston score) | 881.8 | 366.9 | 276.0 | 167.0 | <.0001 |
| Female (%) | 64.6 | 79.3 | 64.1 | 61.5 | 0.0549 |
| Diabetes (%) | 14.3 | 10.6 | 13.0 | 17.7 | 0.7332 |
| Hypertension (%) | 64.9 | 52.4 | 60.1 | 61.0 | 0.8933 |
| Current smoking (%) | 14.3 | 15.5 | 9.9 | 5.2 | 0.0035 |
| Cholesterol medication (LPM) (%) | 15.0 | 7.0 | 9.4 | 3.1 | 0.0203 |
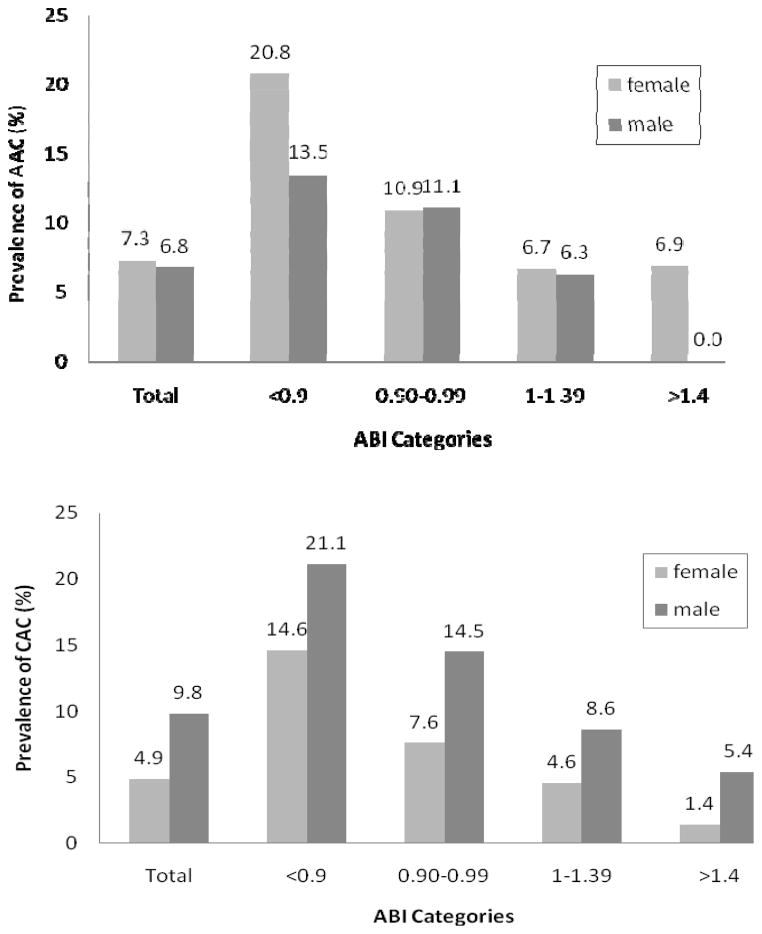
Figure 1 depicts the age-adjusted proportion of the AAC scores and the CAC scores greater than 75th percentile by ABI categories by gender. The risk of the significant AAC was higher among the lower ABI group regardless of gender (females: p < 0.0001, males: p=0.0265 for linear trend). The proportion of significant AAC scores was 20.8 % for females and 13.5% for males among the low ABI group, 10.9% for females and 11.1% males among the borderline ABI group, 6.7% for females and 6.3% males among the normal ABI, and 6.9% for females and 0% for males among the high ABI. The risk of significant AAC did not differ between females and males (7.3% versus 6.8%, p=0.6068). The proportion of the significant CAC scores was significantly higher in males than in females (9.8% versus 4.9%, p<0.0001). The risk of CAC was higher among the lower ABI group regardless of gender (females: p < 0.0001, males: p=0.0027 for linear trend).
Figure 1.
Age-adjusted prevalence of abdominal aortic calcification scores and coronary artery calcification scores greater than 75th percentile by ABI group.
Table 2 represents the results of the multivariable log-binomial regression applied to determine the relationship between AAC and ABI after adjusting major characteristic variables. In age-adjusted model, the prevalence of significant AAC scores was 1.97 times higher for participants having the low ABI (PR=1.97; 95% CI=1.50–2.59; p-value<0.0001) and 1.47 times higher for those having the borderline ABI (PR=1.47; 95% CI=1.04–2.09; p-value=0.0289) than those with the normal ABI. A non-significant trend was seen in the high ABI category for lower prevalence of AAC (PR=0.55; 95% CI=0.24–1.28; p-value=0.1662). Similar patterns persisted in the fully adjusted model. Sex-specific analyses revealed that a significantly higher prevalence in the low ABI and the borderline ABI groups was found only among women for both age- and fully adjusted models. Among men, the significantly higher prevalence was detected only in the low ABI for age-adjusted model.
Table 2.
Results of multivariable log-binomial analysis to determine the association of ABI with abdominal aortic calcification (AAC)
| ABI Categories | Age-adjusted model | Fully adjusted model† | ||||||
|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | p-value | PR | 95% CI | p-value | |||
| All | ||||||||
| <0.9 | 1.97 | 1.50 | 2.59 | <0.0001 | 1.70 | 1.26 | 2.28 | 0.0004 |
| 0.90–0.99 | 1.47 | 1.04 | 2.09 | 0.0289 | 1.57 | 1.20 | 2.03 | 0.0008 |
| 1–1.39 | 1.00 | 1.00 | ||||||
| >1.4 | 0.55 | 0.24 | 1.28 | 0.1662 | 0.59 | 0.24 | 1.44 | 0.2431 |
| Females | ||||||||
| <0.9 | 2.13 | 1.57 | 2.89 | <0.0001 | 2.08 | 1.53 | 2.84 | <0.0001 |
| 0.90–0.99 | 1.48 | 1.01 | 2.17 | 0.0459 | 1.43 | 1.04 | 1.97 | 0.0298 |
| 1–1.39 | 1.00 | 1.00 | ||||||
| >1.4 | 0.98 | 0.45 | 2.16 | 0.9672 | 0.88 | 0.37 | 2.10 | 0.7755 |
| Males | ||||||||
| <0.9 | 1.72 | 1.03 | 2.87 | 0.0375 | 1.43 | 0.77 | 2.64 | 0.2519 |
| 0.90–0.99 | 1.38 | 0.63 | 3.00 | 0.4210 | 1.27 | 0.55 | 2.91 | 0.5731 |
| 1–1.39 | 1.00 | 1.00 | ||||||
| >1.4 | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
Adjusted for Age, Body Mass Index, Systolic Blood Pressure, Diastolic Blood Pressure, C-reactive protein, HDL cholesterol, LDL Cholesterol, gender, Diabetes, Hypertension, Current smoking, Cholesterol medication.
The results of the multivariable log-binomial regression to determine the relationship between coronary artery calcification and ankle-brachial index are provided in Table 3. The age-adjusted prevalence of the significant coronary artery calcification scores was significantly higher for participants having low ABI (PR=2.2; 95% CI=1.62–2.97; p-value<0.0001). A non-significant increase was noted for borderline ABI (PR=1.36; 95% CI=0.90–2.04; p-value=0.1456) and high ABI showed a non-significant decrease in calcification prevalence (PR=0.59; 95% CI=0.25–1.40; p-value=0.2344), compared with the normal ABI group. This pattern was consistent regardless of subgroups of gender models adjusted for age only; however, in the fully adjusted model, the relationship persisted among women, but was attenuated among men.
Table 3.
Results of multivariable log-binomial regression analysis to determine the association of ABI with coronary artery calcification (CAC)
| ABI categories | Age-adjusted model | Fully adjusted model† | ||||||
|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | p-value | PR | 95% CI | p-value | |||
| All | ||||||||
| <0.9 | 2.20 | 1.62 | 2.97 | <0.0001 | 1.55 | 1.12 | 2.14 | 0.0081 |
| 0.90–0.99 | 1.36 | 0.90 | 2.04 | 0.1456 | 1.60 | 1.05 | 2.46 | 0.0302 |
| 1–1.39 | 1.00 | 1.00 | ||||||
| >1.4 | 0.59 | 0.25 | 1.40 | 0.2344 | 0.53 | 0.19 | 1.50 | 0.2338 |
| Female | ||||||||
| <0.9 | 2.28 | 1.50 | 3.47 | 0.0001 | 2.56 | 1.63 | 4.03 | 0.0001 |
| 0.90–0.99 | 1.51 | 0.91 | 2.49 | 0.1082 | 1.99 | 1.19 | 3.32 | 0.0088 |
| 1–1.39 | 1.00 | 1.00 | ||||||
| >1.4 | 0.41 | 0.09 | 1.92 | 0.2586 | 0.45 | 0.09 | 2.17 | 0.3177 |
| Male | ||||||||
| <0.9 | 2.02 | 1.31 | 3.11 | 0.0015 | 1.59 | 0.95 | 2.66 | 0.0772 |
| 0.90–0.99 | 1.35 | 0.66 | 2.74 | 0.4111 | 1.27 | 0.58 | 2.78 | 0.5506 |
| 1–1.39 | 1.00 | 1.00 | ||||||
| >1.4 | 0.68 | 0.23 | 1.98 | 0.4783 | 0.73 | 0.19 | 2.71 | 0.6349 |
Adjusted for Age, Body Mass Index, Systolic Blood Pressure, Diastolic Blood Pressure, C-reactive protein, HDL cholesterol, LDL Cholesterol, gender, Diabetes, Hypertension, Current smoking, Cholesterol medication.
Discussion
In this sample from the JHS who at baseline were free of clinical coronary heart disease, we were able to discern an inverse relationship between clinically relevant categories of ABI and the presence of central vascular calcification measured 5.5 years later. Similar findings have been noted by others (15,16,17). However the present study complements and extends the literature by simultaneously studying both coronary and aortic calcification in the largest group of African Americans ever examined by MDCT in a population-based study. Our principal findings are that 1) ABI was significantly associated with AAC and CAC; the lower ABI, the higher prevalence of significant AAC and CAC; 2) peripheral vascular disease characterized by high (>1.4) ABI was not associated with atherosclerotic calcification; and 3) the significant inverse relationship between ABI and prevalence of AAC appears to be pronounced in women, but did not reach statistical significance in men in the JHS cohort.
Other studies have made significant observations on peripheral disease and subclinical atherosclerosis as detected by vascular calcification. The Rancho Bernardo Study which consisted of nearly all Caucasian and middle to upper class participants (17), The Rotterdam Coronary Calcification study which features a Dutch cohort (16), and the Multi-Ethnic Study of Atherosclerosis all found that lower ABI’s were associated with significantly higher prevalence of subclinical atherosclerosis in men. Only MESA had a significant proportion of African Americans available for analysis (28 percent (n= 1,187)). Notably in MESA, the relationship of ABI and CAC was most profound in the African American subsample (15).
There have been several studies that have looked at the relationship of ABI and CAC, and until recently, few have reported data on the relationship of ABI to aortic calcification (AAC) (18). Our results for the ABI/AAC relationship parallel those seen with ABI and CAC; i.e., as the ABI decreases, to the clinical diagnoses of PAD, abdominal aortic calcification increases. While the results of other studies anticipate this finding, they have been methodologically distinct, employing either standard radiography (19) or postmortem examination of subjects who had reported symptoms of clinical PAD (20). However, recent results from MESA utilizing EBCT to measure AAC noted a similar inverse relationship with increasing calcification and ABI <0.9. MDCT allows for a broader range of detection and a more highly quantitative assessment of aortic calcium than the more classical approaches.
The chief limitation of this study is that it is essentially cross-sectional in design, though clear associations between ABI and calcification of major vessels detectable by modern techniques is demonstrated, direction of causality cannot be inferred. Also there is a 5.5 year delay between the determination of ABI at JHS Exam 1 and CT evaluation during Exam 2. Further, the relatively low number of men in the study reduced the statistical power of the analysis to detect meaningful associations between ABI and calcification in men. Therefore the lack of a clear inverse relationship in men noted above must be regarded with caution.
ABI is non-invasive diagnostic tool which can predict the likelihood of CT-detectable major vessel calcification. Since such calcification is a manifestation of subclinical disease which has been consistently shown to be associated with high risk for coronary events and stroke, ABI may have a special role in risk stratification and management for a highly prevalent and frequently fatal disease. Direct ascertainment of calcium scores with use of MDCT is known to be clinically useful, but is costly and exposes individuals to the equivalent of ~26 standard chest x-rays per examination, making large scale use of this modality clinically and economically prohibitive. ABI is easy to perform, low-cost, nearly universally available and easily adapted to primary care settings. Since CVD and stroke are most prevalent in the regions and among the participants who have the greatest challenges with access to appropriate or advanced care, ABI may be a particularly important diagnostic modality in the management of CVD risk.
Conclusion
ABI was significantly and inversely associated with AAC and CAC. High ABI values, also indicative of abnormal circulation, were not, however, associated with increased calcification. Lastly, the inverse relationship with AAC and ABI appears to be stronger amongst women. ABI can provide clinicians with a simple, inexpensive additional tool to assess vascular health and cardiovascular risk without exposing the patient to ionizing radiation. While the ABI can provide for an immediate indication of the degree of vascular disease, it would be imperative to study this modality longitudinally to understand the full prognostic significance.
Acknowledgments
The authors thank the staff and participants in the Jackson Heart Study for important contributions. The JHS was supported by the NIH: National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities (NIMHD) [Contracts N01-HC-95170, N01-HC-95171, and N01-HC-95172]. This study was partially supported by NIMHD grant number U54MD008149, to Dr Jae Eun Lee.
References
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics. Compressed Mortality File 1999–2005. [Jul 6, 2009 1:26:58 PM];CDC WONDER On-line Database, compiled from Compressed Mortality File 1999–2005 Series 20 No. 2K. 2008 Accessed at http://wonder.cdc.gov/cmf-icd10.html.
- 2.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004 Feb 17;109(6):733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 3.Welten GM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, Bax JJ, van Sambeek MR, Poldermans D. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008 Apr 22;51(16):1588–96. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 4.Menke A, Muntner P, Wildman RP, Dreisbach AW, Raggi P. Relation of borderline peripheral arterial disease to cardiovascular disease risk. Am J Cardiol. 2006 Nov 1;98(9):1226–30. doi: 10.1016/j.amjcard.2006.05.056. Epub 2006 Sep 14. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992 Feb 6;326(6):381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 6.Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, Folsom AR, Rosamond WD. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study, 1987–2001. BMC Cardiovasc Disord. 2007 Jan 16;7:3. doi: 10.1186/1471-2261-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups: N Engl J Med. 2008 Mar 27;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal Aortic Calcific Deposits Are an Important Predictor of Vascular Morbidity and Mortality. Circulation. 2001 Mar 20;103(11):1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 9.Levitzky YS, Cupples LA, Murabito JM, Kannel WB, Kiel DP, Wilson PW, Wolf PA, O’Donnell CJ. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008 Feb 1;101(3):326–31. doi: 10.1016/j.amjcard.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African American: design and methods of the JHS. Ethn Dis. 2005 Autumn;15(4 Suppl 6):S6-4–17. [PubMed] [Google Scholar]
- 11.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting African-American research participation in the Jackson Heart Study:methods, response rates, and sample description. Ethn Dis. 2005 Autumn;15(4 Suppl 6):S6-18–29. [PubMed] [Google Scholar]
- 12.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi- Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 13.Deddens J, Petersen M, Lei X. SUGI-28: Seattle SAS Users Group International Proceedings. Seattle: Seattle SAS Users Group; 2003. Estimation of prevalence ratios when PROC GENMOD does not converge; pp. 1–6. paper 270–28. [Google Scholar]
- 14.Axelson O, Fredriksson M, Ekberg K. Use of the prevalence ratio v the prevalence odds ratio in view of confounding in cross sectional studies. Occup Environ Med. 1995;52:494. doi: 10.1136/oem.52.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005 Jul 1;162(1):33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 16.Oei HH, Vliegenthart R, Hak AE, Iglesias del Sol A, Hofman A, Oudkerk M, Witteman JC. The Association Between Coronary Calcification Assessed by Electron Beam Computed Tomography and Measures of Extracoronary Atherosclerosis The Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002 Jun 5;39(11):1745–51. doi: 10.1016/s0735-1097(02)01853-3. [DOI] [PubMed] [Google Scholar]
- 17.Allison MA, Laughlin GA, Barrett-Connor E, Langer R. Association between the ankle-brachial index and future coronary calcium (The Rancho Bernardo Study) Am J Cardiol. 2006 Jan 15;97(2):181–6. doi: 10.1016/j.amjcard.2005.08.019. Epub 2005 Nov 18. [DOI] [PubMed] [Google Scholar]
- 18.Wong ND, Lopez VA, Allison M, Detrano RC, Blumenthal RS, Folsom AR, Ouyang P, Criqui MH. Abdominal aortic calcium and multi-site atherosclerosis: The Multiethnic Study of Atherosclerosis. Atherosclerosis. Atherosclerosis. 2011 Feb;214(2):436–41. doi: 10.1016/j.atherosclerosis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niskanen LK, Suhonen M, Siitonen O, Lehtinen JM, Uusitupa MI. Aortic and lower limb artery calcification in type 2 (non-insulin-dependent) diabetic patients and non-diabetic control subjects. A five-year follow-up study. Atherosclerosis. 1990 Sep;84(1):61–71. doi: 10.1016/0021-9150(90)90009-8. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JR, Adams JH. Aortic size and aortic calcification. A necropsy study. Atherosclerosis. 1977 Aug;27(4):437–46. doi: 10.1016/0021-9150(77)90162-9. [DOI] [PubMed] [Google Scholar]