Abstract
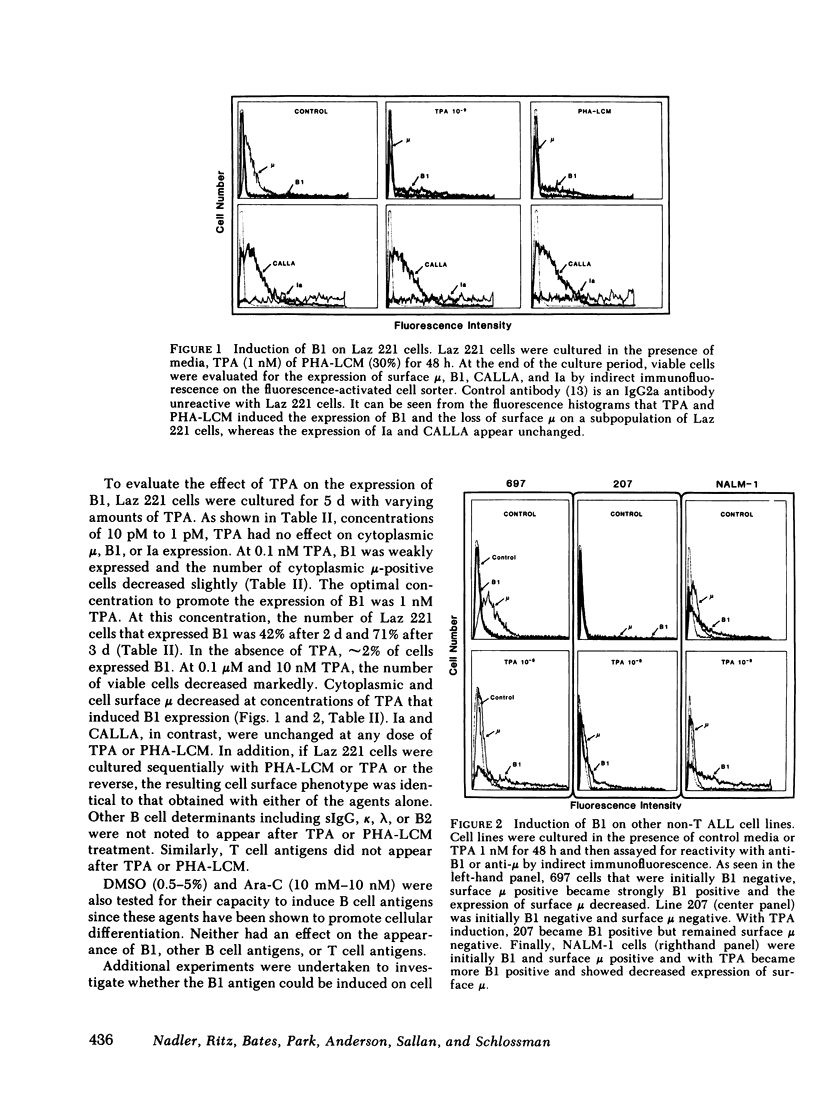
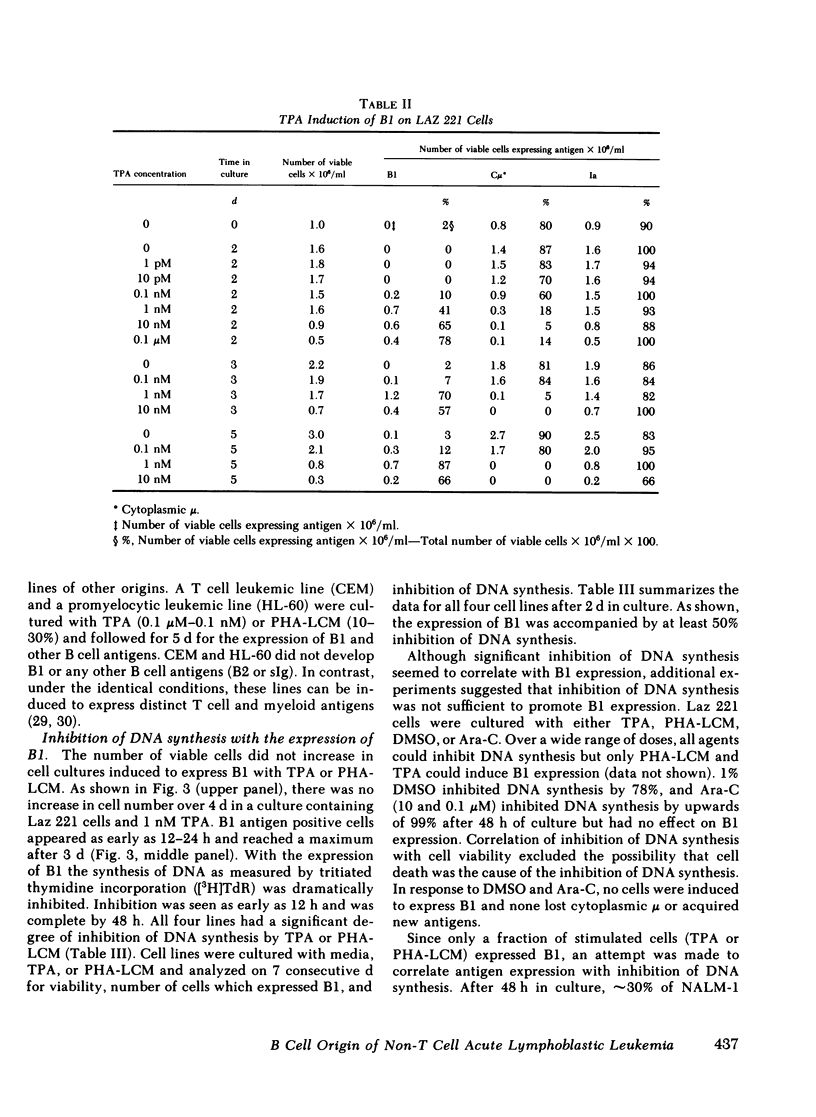
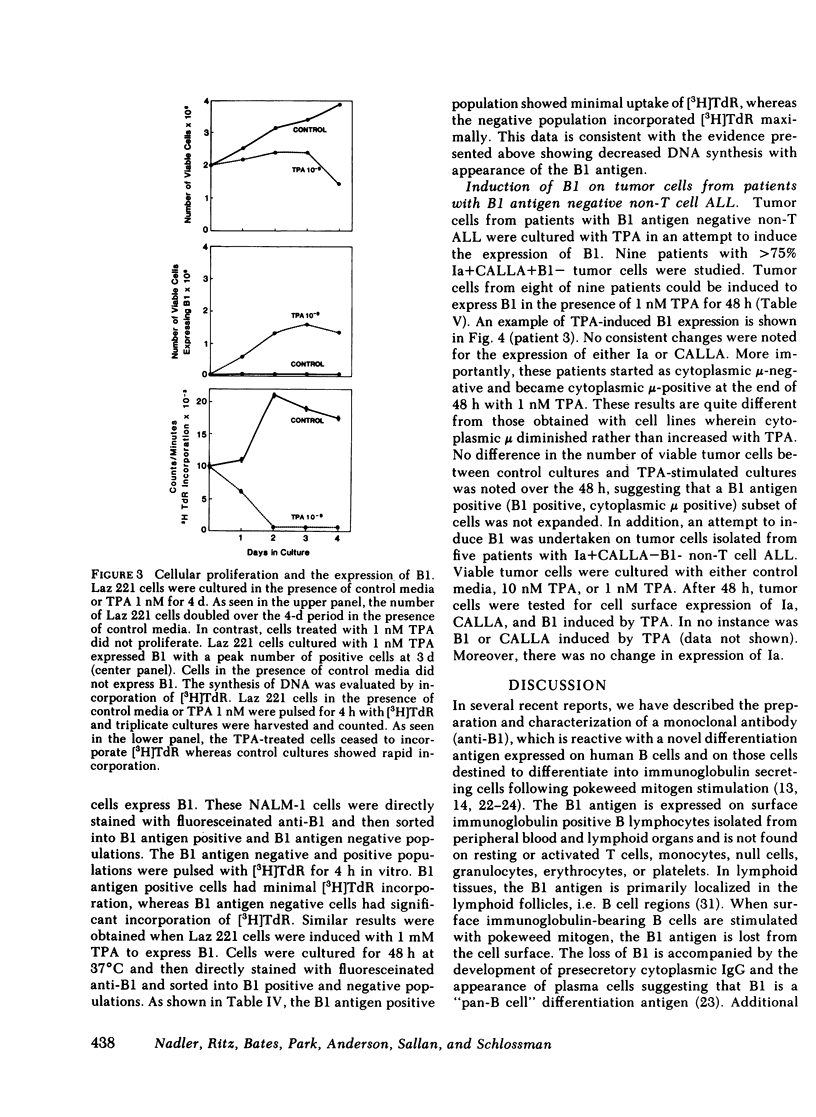
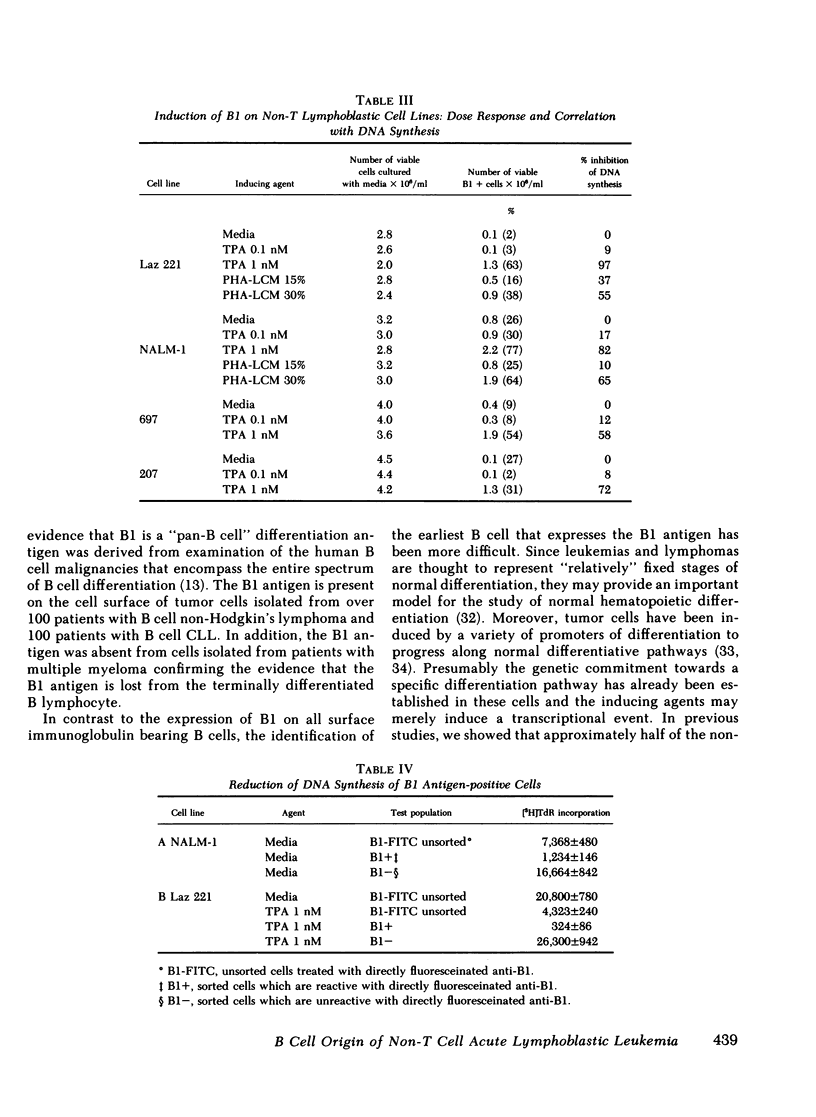
Leukemic cells from 70% of patients with Ia+CALLA+ non-T cell acute lymphoblastic leukemia (ALL) express an antigen (B1) found on all normal B lymphocytes. In this study, ALL cells that do not express the B1 antigen were studied in an attempt to further elucidate the cellular lineage of these tumors. Non-T cell ALL lines and tumor cells isolated from patients with non-T cell ALL that are Ia + CALLA + B1- were studied in vitro with a variety of agents known to promote cellular differentiation. Phorbol diester (TPA) or phytohemagglutinin conditioned leukocyte culture media were capable of inducing the expression of B1 on all four non-T cell ALL lines tested. In contrast, B1 could not be induced under the identical conditions on a promyelocytic leukemia line or a T cell lymphoblastic leukemia line. With the induction of B1 on non-T cell ALL lines, cytoplasmic mu-heavy chain (c mu) became undetectable, whereas the expression of CALLA and Ia were unchanged. The expression of B1 was accompanied by a decrease of cellular proliferation and DNA synthesis, but not significant morphologic changes were noted. In addition, no other B or T cell antigens were detected. The cellular origin of non-T cell ALL was further investigated using tumor cells isolated from leukemic patients. Tumor cells from eight patients with Ia + CALLA + B1-c mu- ALL could be induced in vitro with TPA to express both B1 and c mu. In contrast, cells from five patients with Ia + CALLA-B1-c mu- non-T cell ALL could not be induced with TPA to express CALLA, B1 or c mu. These studies suggest that the non-T cell ALL are heterogeneous and represent a spectrum of early B cell differentiation including the pre- pre-B cell (Ia + CALLA + B1-c mu-), the intermediate pre-B cell (Ia + CALLA +B1 + c mu-), and finally the "true" pre-B cell (Ia + CALLA + B1 + c mu+). The cellular origin of the remaining Ia + CALLA-B1-c mu- form of non-T cell ALL (20%) is still unknown.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson C. S., Kersey J. H., LeBien T. W. A monoclonal antibody (BA-1) reactive with cells of human B lymphocyte lineage. J Immunol. 1981 Jan;126(1):83–88. [PubMed] [Google Scholar]
- Aye M. T., Niho Y., Till J. E., McCulloch E. A. Studies of leukemic cell populations in culture. Blood. 1974 Aug;44(2):205–219. [PubMed] [Google Scholar]
- Baserga R. The cell cycle. N Engl J Med. 1981 Feb 19;304(8):453–459. doi: 10.1056/NEJM198102193040803. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Nadler L. M., Stashenko P., McCluskey R. T., Schlossman S. F. Stages of B cell differentiation in human lymphoid tissue. J Exp Med. 1981 Sep 1;154(3):737–749. doi: 10.1084/jem.154.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouet J. C., Preud'homme J. L., Penit C., Valensi F., Rouget P., Seligmann M. Acute lymphoblastic leukemia with pre-B-cell characteristics. Blood. 1979 Jul;54(1):269–273. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fu S. M., Winchester R. J., Kunkel H. G. The occurrence of the HL-B alloantigens on the cells of unclassified acute lymphoblastic leukemias. J Exp Med. 1975 Nov 1;142(5):1334–1338. doi: 10.1084/jem.142.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Brown G., Rapson N. T., Lister T. A. Antisera to acute lymphoblastic leukemia cells. Clin Immunol Immunopathol. 1975 May;4(1):67–84. doi: 10.1016/0090-1229(75)90041-0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Verbi W., Kemshead J., Kennett R. A monoclonal antibody identifying a cell surface antigen shared by common acute lymphoblastic leukemias and B lineage cells. Blood. 1980 Dec;56(6):1141–1144. [PubMed] [Google Scholar]
- Greaves M., Verbi W., Vogler L., Cooper M., Ellis R., Ganeshaguru K., Hoffbrand V., Janossy G., Bollum F. J. Antigenic and enzymatic phenotypes of the pre-B subclass of acute lymphoblastic leukaemia. Leuk Res. 1979;3(6):353–362. doi: 10.1016/0145-2126(79)90032-8. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Bar-Eli M., Territo M. Phorbol diester-induced macrophage differentiation of leukemic blasts from patients with human myelogenous leukemia. J Clin Invest. 1980 Nov;66(5):1101–1108. doi: 10.1172/JCI109939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus H., Barell E. F., Krishan A., Livingston D. M., Harris K., Schlossman S. F., Chess L. Characterization of a unique cell line (LAZ 221) from human acute lymphocytic ("null" cell) leukemia. Cancer Res. 1978 May;38(5):1362–1367. [PubMed] [Google Scholar]
- Metzgar R. S., Borowitz M. J., Jones N. H., Dowell B. L. Distribution of common acute lymphoblastic leukemia antigen in nonhematopoietic tissues. J Exp Med. 1981 Oct 1;154(4):1249–1254. doi: 10.1084/jem.154.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Tsubota T., Greaves M. F., Walters T. R. A non-T, non-B human leukemia cell line (NALM-1): establishment of the cell line and presence of leukemia-associated antigens. J Natl Cancer Inst. 1977 Jul;59(1):83–87. doi: 10.1093/jnci/59.1.83. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Ritz J., Hardy R., Pesando J. M., Schlossman S. F., Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981 Jan;67(1):134–140. doi: 10.1172/JCI110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Schlossman S. F. A monoclonal antibody defining a lymphoma-associated antigen in man. J Immunol. 1980 Aug;125(2):570–577. [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., van Agthoven A., Terhorst C., Schlossman S. F. Characterization of a human B cell-specific antigen (B2) distinct from B1. J Immunol. 1981 May;126(5):1941–1947. [PubMed] [Google Scholar]
- Nagasawa K., Mak T. W. Phorbol esters induce differentiation in human malignant T lymphoblasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2964–2968. doi: 10.1073/pnas.77.5.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesando J. M., Ritz J., Lazarus H., Costello S. B., Sallan S., Schlossman S. F. Leukemia-associated antigens in ALL. Blood. 1979 Dec;54(6):1240–1248. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Ritz J., Nadler L. M., Bhan A. K., Notis-McConarty J., Pesando J. M., Schlossman S. F. Expression of common acute lymphoblastic leukemia antigen (CALLA) by lymphomas of B-cell and T-cell lineage. Blood. 1981 Sep;58(3):648–652. [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Seligmann M. B-cell neoplasia in man. Lancet. 1974 Nov 23;2(7891):1230–1233. doi: 10.1016/s0140-6736(74)90748-x. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Chess L., Humphreys R. E., Strominger J. L. Distribution of Ia-like molecules on the surface of normal and leukemic human cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1288–1292. doi: 10.1073/pnas.73.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3848–3852. doi: 10.1073/pnas.78.6.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Griffin J. D., Ritz J., Nadler L. M., Abrams T., Schlossman S. F. Expression of normal monocyte-macrophage differentiation antigens on HL60 promyelocytes undergoing differentiation induced by leukocyte-conditioned medium or phorbol diester. Leuk Res. 1981;5(6):491–495. doi: 10.1016/0145-2126(81)90119-3. [DOI] [PubMed] [Google Scholar]
- Tötterman T. H., Nilsson K., Sundström C. Phorbol ester-induced differentiation of chronic lymphocytic leukaemia cells. Nature. 1980 Nov 13;288(5787):176–178. doi: 10.1038/288176a0. [DOI] [PubMed] [Google Scholar]
- Vogler L. B., Crist W. M., Bockman D. E., Pearl E. R., Lawton A. R., Cooper M. D. Pre-B-cell leukemia. A new phenotype of childhood lymphoblastic leukemia. N Engl J Med. 1978 Apr 20;298(16):872–878. doi: 10.1056/NEJM197804202981603. [DOI] [PubMed] [Google Scholar]
- Vogler L. B., Preud'homme J. L., Seligmann M., Gathings W. E., Crist W. M., Cooper M. D., Bollum F. J. Diversity of immunoglobulin expression in leukaemic cells resembling B-lymphocyte precursors. Nature. 1981 Mar 26;290(5804):339–341. doi: 10.1038/290339a0. [DOI] [PubMed] [Google Scholar]



