Abstract
Objective
To determine short-term outcomes of infants who had perinatal acidemia and were evaluated for hypothermia therapy but did not qualify based on a standardized neurologic examination.
Study design
Retrospective, single-site cohort study of inborn infants of ≥36 weeks gestation who had perinatal acidemia from October 2005-September 2008 and had a standardized neurologic examination performed by a certified neonatologist to assess eligibility for hypothermia therapy. An abnormal short-term nursery outcome was defined as death, seizures, brain magnetic resonance imaging consistent with hypoxic-ischemic encephalopathy, abnormal neurologic examination at discharge, gastrostomy tube feeding, or inability to nipple all feeds beyond the first week of age.
Results
One hundred forty-four (0.3%) of 46 887 newborns with perinatal acidemia had a neurologic examination performed that was either normal (n = 29) or consistent with mild encephalopathy (1 or 2 abnormal categories; n = 60). Of the latter infants classified as having mild encephalopathy, 12 (20%) experienced an abnormal short-term outcome (feeding difficulties, n = 8; abnormal neurologic examination at discharge, n = 7; abnormal brain magnetic resonance imaging, n = 6; seizures, n = 5; gastrostomy, n = 1; or death, n = 1).
Conclusions
Twenty percent of newborns with perinatal acidemia and a neurologic examination that revealed only mild encephalopathy had abnormal short-term outcomes that could be attributed to the encephalopathy. Adjunctive tools or biomarkers for optimal assessment of infants with fetal acidemia for hypothermia therapy are needed.
Hypoxic-ischemic encephalopathy (HIE) affects 2-5 infants per 1000 live births and remains an important cause of neonatal brain injury. Hypothermia therapy initiated within the first 6 hours of age reduced the risk of death or disability or increased the number of survivors without disability among infants with moderate or severe HIE.1-7 Newborns are screened for hypothermia therapy using biochemical criteria that show perinatal acidemia and subsequently selected for cooling based on a detailed neurologic examination that is consistent with moderate to severe HIE, with or without the use of an amplitude electroencephalogram.1-4,6,7
Infants diagnosed as having mild encephalopathy based on the neurologic examination are excluded from hypothermia therapy. In 1989, Robertson et al8 found that infants who did not progress beyond mild HIE in the first week of age had normal development on follow-up evaluation at 8 years of age, supporting that mild encephalopathy in the first week of life results in a normal long-term outcome. Subsequently, Van Handel et al9 reported that mild neonatal encephalopathy was associated with a negative effect on daily life behavioral functioning at 9-10 years of age. In addition, neonatal encephalopathy is a dynamic process in which the infant's neurologic status may worsen over the first 72 hours of age10,11 with progression from mild to moderate or even severe encephalopathy.10 Although the neurologic examination performed in the first 6 hours of age may classify newborns as having only mild or no encephalopathy, the current outcomes of these infants are not fully known. Therefore, the objective of this study was to determine the characteristics and short-term outcomes of a cohort of infants who had perinatal acidemia and were evaluated for hypothermia therapy in the first 6 hours of age but did not qualify based on a standardized neurologic examination that showed either mild or no encephalopathy.
Methods
This retrospective cohort study included all inborn infants of ≥36 weeks gestation and birth weight ≥1800 g who were admitted to the neonatal intensive care unit (NICU) at Parkland Memorial Hospital (PMH) with perinatal acidemia from October 2005-Sptember 2008 but did not receive hypothermia therapy because they were diagnosed with mild or no encephalopathy. Exclusion criteria included presence of congenital anomalies, imminent death, or transfer to an outside facility. These infants were identified from a prospective neonatal database and resuscitation registry that were described previously12 and included all admissions to the PMH NICU with perinatal acidosis. The study period corresponded to the time after publication of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) trial on whole body hypothermia7 when such therapy was performed routinely on newborns with moderate-to-severe HIE in the PMH NICU. The medical records of the infants and their mothers were reviewed and pertinent demographic, clinical, laboratory, and neuroimaging data were recorded. The study was approved with waiver of informed consent by the Institutional Review Board of the University of Texas Southwestern Medical Center.
Umbilical cord arterial blood sampling was obtained routinely on all deliveries at PMH from double clamped sections of umbilical cord.13 Newborns with perinatal acidemia were identified by the same criteria used to screen for HIE in the NICHD NRN study of whole body hypothermia.7 Specifically, these clinical and biochemical screening criteria consisted of: (1) a pH ≤7.0 or a base deficit ≥16 mEq/L on umbilical cord blood or any postnatal blood sample within 1 hour of age; or (2) history of an acute perinatal event and either: (1) no blood gas available or (2) a pH from 7.01-7.15 or a base deficit from 10-15.9 mEq/L, along with a 10-minute Apgar score ≤5, or assisted ventilation initiated at birth and continued for at least 10 minutes.
If these criteria were met, then the newborn had a neurologic exam performed by a certified attending neonatologist to assess eligibility for whole body hypothermia therapy. Serial examinations were not performed. To become a certified examiner, the neonatologist must have performed a neurologic examination on 2 newborns with perinatal acidosis, and his/her examination had to be similar (<2 differences allowed) to one performed on the same baby and at a similar time by a previously certified examiner. Certification was obtained through participation in the NRN.
The neurologic examination was the same as the one used in the NICHD whole body hypothermia trial.7 Specifically, moderate or severe HIE, respectively, was classified based on: (1) level of consciousness (lethargy; stupor/coma); (2) spontaneous activity (decreased; no activity); (3) posture (distal flexion; complete extension; decerebrate); (4) tone (hypotonic; flaccid); (5) primitive reflexes (weak to absent suck or Moro); and (6) autonomic nervous system signs (pupils [constricted; deviation/dilated/nonreactive to light], heart rate [bradycardia; variable heart rate], and breathing patterns [periodic breathing; apnea]). Each category was scored as 1 (normal or mild), 2 (moderate), or 3 (severe). If ≥3 categories had a score of ≥2, the infant received hypothermia therapy. Otherwise, infants were not cooled and these constitute the study population. If the examiner was not definitive in scoring the infant in any particular category (eg, a score of “1-2”), the worst score of the two was assigned. Infants were grouped based on how many abnormal categories (0 abnormal, 1 abnormal, or 2 abnormal) they had on the early neurologic examination. We defined a priori infants with no abnormal categories as not having encephalopathy and those with 1 or 2 abnormal categories as having mild encephalopathy.
The medical records of infants who did not have whole body hypothermia due to the early neurologic examination being consistent with mild or no encephalopathy were reviewed for short-term outcomes. Abnormal short-term outcome was defined a priori as any one of the following: death, seizures, abnormal brain magnetic resonance imaging (MRI), abnormal neurologic examination at discharge as determined by the NICU provider, gastrostomy tube feeding, or inability to achieve full nipple feeds until after the first week of age and that required consultation by speech therapy.14 One experienced pediatric neuroradiologist (M.M.), who was blinded to the infant's clinical status, reviewed the MRI studies. MRI studies were performed as clinically indicated on a 3T MR unit at Children's Medical Center with software v. R2.6 (Philips Healthcare Systems, Andover, Massachusetts). The MRI protocol consisted of routine anatomic images and MR proton spectroscopy. Conventional images were reviewed for abnormalities consisting of a predominant basal ganglia/thalamus injury pattern, or a watershed pattern. The severity of injury in the different patterns was scored using a validated MRI Barkovich score.15
Statistical Analyses
Data analysis was performed using Sigma Plot (v. 11.0, Chicago, Illinois). Student t test (2-sided) and Fisher exact tests were used where appropriate. A χ2 test was performed on all categorical data. A P value of <.05 was considered to be statistically significant. Positive predictive value (PPV), negative predictive value (NPV), sensitivity, specificity, and likelihood ratios were assessed for each individual component of the neurologic examination as well as a combination of categories.
Results
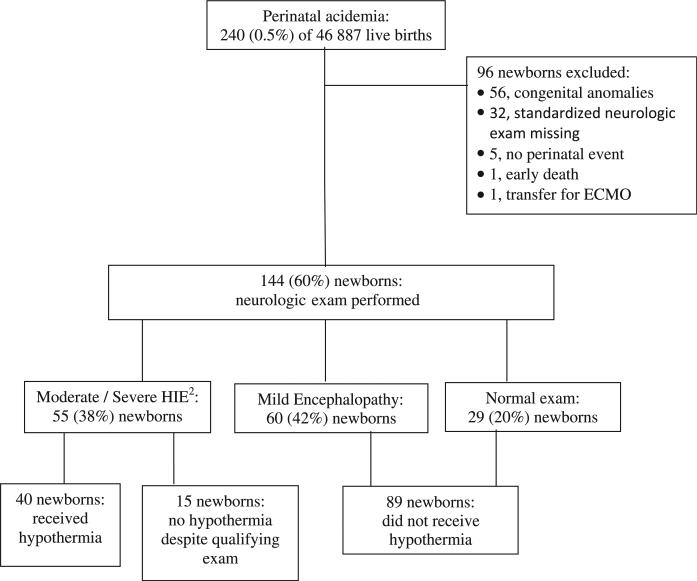
In this 3-year cohort, 240 (0.5%) of 46 887 live births had perinatal acidemia and were admitted to the NICU (Figure). Of these, 176 met criteria for a neurologic examination, and 144 (82%) had an examination by a certified examiner performed within 6 hours of birth to assess eligibility for hypothermia therapy. Twenty-nine (20%) newborns had a normal examination, 60 (42%) had a neurologic examination consistent with mild encephalopathy, and 48 (33%) had moderate HIE and 7 (5%) had severe HIE. Of the 144 newborns, 104 (72%) did not receive hypothermia therapy, and in 89 (86%) infants, this was due to an assessment of their having either no (n = 29) or mild (n = 60) encephalopathy. These 89 infants form the study population. There were 15 (14%) infants who did not receive hypothermia therapy despite having a neurologic examination consistent with moderate to severe HIE. Reasons for not implementing hypothermia therapy in these 15 infants included: sedation (fentanyl, n = 3), elevated serum magnesium concentration (n = 2), borderline examination (assessments of “1-2,” n = 8), or error in assessment of breathing patterns because it was not recognized that mechanical ventilation assigns the infant to “severe” in that category (n = 2).
Figure.
Inborn infants of ≥36 wk gestation and birth weight ≥1800 g who were admitted to the NICU at PMH with perinatal acidemia from October 2005 to September 2008. ECMO, extracorporeal membrane oxygenation.
Among the 89 infants with no or mild encephalopathy, the age that the neurologic examination was performed was not statistically different between those with no encephalopathy (mean, 4.1 hours; range, 3.0-5.4) vs those with mild encephalopathy (mean, 4.2 hours; range, 3.2-5.0; P = .99). Of these 89 infants, 29 (33%) had no abnormalities on neurologic examination, and 29 (33%) had 1 abnormal category and another 31 (35%) infants had 2 abnormalities. Among the 29 infants with only 1 abnormal category on neurologic examination, 3 (10%) had decreased activity, 19 (66%) had abnormal muscle tone, 5 (17%) had abnormal primitive reflexes, and 4 (14%) with abnormal autonomic nervous system findings. Of the 31 newborns with 2 abnormal categories, 4 (13%) had lethargy, 7 (23%) decreased activity, 2 (6%) abnormal posture, 25 (81%) abnormal tone, 17 (55%) abnormal primitive reflexes, and 7 (23%) abnormal autonomic system findings.
The baseline characteristics among the infants with no encephalopathy compared with those with 1 or 2 abnormal categories were similar except for significantly longer duration of rupture of membranes, lower Apgar scores at 1 and 5 minutes, and delivery room intubation among those with mild encephalopathy (Table I). In addition, infants with mild encephalopathy had a significantly higher base deficit on the first postnatal blood gas (P = .02), and the umbilical cord blood gas results did not differ.
Table I.
Characteristics of the 89 mothers and newborns who had perinatal acidemia but did not receive hypothermia therapy based on the neurologic exam that was either normal or consistent with mild encephalopathy
| No encephalopathy* | Mild encephalopathy* | P value | |
|---|---|---|---|
| No. of mothers | 29 | 60 | - |
| Age (y)† | 26 ± 7 | 25± 6 | .57 |
| Race/ethnicity | |||
| Hispanic | 23 (79%) | 47 (78%) | .87 |
| Black | 3 (11%) | 6 (10%) | 1.0 |
| White | 1 (4%) | 5 (8%) | .66 |
| Asian | 2 (7%) | 2 (3%) | .59 |
| Hypertension | 2 (7%) | 10 (16%) | .32 |
| Diabetes | 2 (7%) | 6 (10%) | 1.0 |
| Pre-eclampsia | 0 | 5 (8%) | .17 |
| Cesarean delivery | 23 (79%) | 33 (55%) | .05 |
| Emergency cesarean delivery | 9 (33%) | 15 (24%) | .53 |
| Prolapsed cord | 0 | 2 (3%) | 1.0 |
| Placental abruption | 1 (4%) | 7 (11%) | .27 |
| Maternal pyrexia (T ≥38°C) | 5 (18%) | 20 (33%) | .18 |
| Rupture of membranes (h)† | 5±6 | 9±9 | .004 |
| No. of infants | 29 | 60 | - |
| Birth weight (g)† | 3373 ± 566 | 3229 ± 569 | .27 |
| Gestational age (wk)† | 39 ± 1 | 40 ± 1 | .20 |
| Sex male | 18 (62%) | 34 (57%) | .80 |
| Apgar score 1 min‡ | 4 (3-7) | 3 (1-5) | .002 |
| Apgar score 5 min‡ | 8 (7-9) | 7 (5-8) | <.001 |
| Intubation (delivery room) | 2 (7%) | 27 (45%) | <.001 |
| Cardiopulmonary resuscitation (delivery room) | 0 | 2 (3%) | 1.00 |
| Blood gas, umbilical cord (n = 86) | |||
| pH‡ | 6.98 (6.95-7.04) | 7.02 (6.94-7.10) | .31 |
| Base deficit† | –17 ± 3 | –17±4 | .86 |
| Blood gas, first postnatal sample§ | |||
| pH‡ | 7.28 (7.23-7.35) | 7.26 (7.15-7.32) | .10 |
| Base deficit† | –9 ± 5 | –12±7 | .02 |
| Hypotension | 0 | 4 (6%) | .31 |
| Pulmonary artery hypertension | 0 | 6 (10%) | .17 |
| Early-onset sepsis | 1 (4%)¶ | 0 | .30 |
| Length of stay (d)‡ | 5 (4-8) | 8 (6-8) | .009 |
| Abnormal outcome at discharge | |||
| Seizures | 0 | 5 (8%) | .17 |
| Anticonvulsant medication | 0 | 2 (3%) | 1.0 |
| Abnormal neurologic exam (n = 84) | 1 | 7 | .43 |
| Feeding (nippling) difficulties | 1 (4%) | 8 (13%) | .26 |
| Days to full oral feeds (n = 86)‡ | 3 (2-4) | 4 (3-5) | <.001 |
| Gastrostomy tube feeding | 0 | 1 (2%) | 1.0 |
| Death | 0 | 1 (2%) | 1.0 |
| Brain MRI | |||
| Number performed | 1 (3%) | 9 (15%) | .14 |
| Age performed (d)† | 12 | 10±8 | .82 |
| Basal ganglia abnormalities | 0 | 3 | 1.0 |
| Watershed abnormalities | 0 | 2 | .44 |
| DWI abnormalities | 0 | 2 | 1.0 |
| Any abnormal finding | 0 | 6 | 1.0 |
| Normal | 1 | 3 | 1.0 |
| Any abnormal outcome∥ | 1 (4) | 12 (20) | .05 |
DWI, diffusion weighted image.
No encephalopathy defined as a neurologic exam with no abnormal category. Mild encephalopathy defined as presence of 1 or 2 abnormal categories on neurologic exam.
Mean ± SD.
Median (range).
Two newborns had capillary blood gas only.
Group b Streptococcus.
Death, seizures, abnormal brain MRI, abnormal neurologic exam at discharge, gastrostomy tube feeding, or feeding (nippling) difficulties beyond the first week of age.
Of the 29 infants who had a normal neurologic examination, 1 had an abnormal short-term outcome of feeding difficulty and an abnormal neurologic examination at discharge (Table I). On brain MRI, this infant had an ectopic pituitary but no evidence of hypoxic-ischemic brain injury. Of the 60 infants classified as having mild encephalopathy on initial examination, 12 (20%) experienced an abnormal short-term outcome (Table I). Five infants classified as having mild encephalopathy developed seizures between 12 and 40 hours of age indicating progression to more severe encephalopathy. Four of these 5 infants had abnormal MRIs with areas of injury in the watershed regions (n = 2) and basal ganglia (n = 1), as well as abnormalities in diffusion weighted images (n = 1), all of which occur in association with HIE.15,16 The fifth infant did not have a brain MRI performed, but had intractable seizures starting at 15 hours of age and died 2 hours later from multisystem organ failure secondary to hypoxic-ischemic injury. This infant had a neurologic examination performed at 3 hours of age that demonstrated 3 abnormal categories consistent with moderate HIE, but on re-examination 1 hour later, there were only 2 abnormal categories and therefore he was not cooled. Of the 8 infants who experienced feeding difficulties, 1 had placement of a gastrostomy tube secondary to poor nippling. Seven infants had an abnormal neurologic examination at discharge (hypotonia, 4; hypertonia, 2; facial palsy, 1). The 12 infants with abnormal short-term outcomes were significantly more likely to have a lower Apgar score at 5 minutes, be intubated in the delivery room, and on their first postnatal blood gas, have a lower blood pH and higher base deficit (P < .05; Table II).
Table II.
Short-term hospital outcomes in 89 infants with perinatal acidemia and a neurologic exam that showed no or mild encephalopathy
| Short-term hospital outcomes* |
|||
|---|---|---|---|
| Normal | Abnormal | P value | |
| No. of infants | 76 | 12 | - |
| Birth weight (g)† | 3290 ± 555 | 3199 ± 659 | .60 |
| Gestational age (wk)‡ | 40 (39-40) | 40 (39-40) | .20 |
| Sex male | 46 (60%) | 7 (54%) | .65 |
| Cesarean delivery | 46 (60%) | 9 (69%) | .5 |
| Emergency cesarean delivery | 21 (28%) | 4 (31%) | 1.0 |
| Rupture of membranes (h)‡ | 5.5 (0.8-12) | 5.5 (0-10) | .80 |
| Apgar score 1 min‡ | 3 (2-6) | 2 (1-4) | .06 |
| Apgar score 5 min‡ | 7 (6-9) | 6 (4-7) | .02 |
| Intubation in delivery room | 20 (26%) | 9 (75%) | .002 |
| Blood gas, umbilical cord (n = 86) | |||
| pH‡ | 7.00 (6.95-7.07) | 6.98 (6.90-7.10) | .50 |
| Base deficit‡ | –17 (–16 → –19) | –21 (–12 → –23) | .18 |
| Blood gas, first postnatal sample§ | |||
| pH‡ | 7.27 (7.23-7.34) | 7.14 (7.09-7.26) | .01 |
| Base deficit‡ | –10 (–6 → –14) | –16 (–12 → –21) | .01 |
| Mild encephalopathy | 50 (66%) | 11 (92%) | .10 |
| Age at neurologic exam (h)‡ | 4.1 (3-5) | 3.7 (3-5) | .89 |
Death, seizures, abnormal brain MRI, abnormal neurologic exam at discharge, gastrostomy tube feeding, or feeding (nippling) difficulties beyond the first week of age.
Mean ± SD.
Median (range).
Two newborns had capillary blood gas only.
The predictive values of the various components of the neurologic examination for an abnormal short-term outcome were assessed among all 89 infants with either no or mild encephalopathy (Table III). Overall, the predictive value of any abnormal neurologic finding was poor, with PPV ranging from 0% to 54%. The highest PPV of 54% was achieved using the autonomic system findings and when 2 abnormal categories were combined with a base deficit ≥15 on a blood gas obtained within 1 hour after birth.
Table III.
Predictive indices of the categories of the standardized neurologic exam performed at <6 h of age with abnormal short-term outcomes at discharge from the NICU*
| PPV | NPV | Sensitivity | Specificity | Positive likelihood ratio | |
|---|---|---|---|---|---|
| Abnormal category of neurologic exam | |||||
| Level of consciousness | 25% | 86% | 8% | 96% | 1.9 |
| Activity | 22% | 86% | 15% | 91% | 1.7 |
| Posture | 0 | 85% | 0 | 97% | 0.03 |
| Suck or Moro | 23% | 88% | 38% | 78% | 1.7 |
| Muscle tone | 20% | 91% | 69% | 54% | 1.5 |
| Autonomic system | 54% | 91% | 46% | 93% | 7.0 |
| Two abnormal categories | 26% | 91% | 62% | 70% | 2.0 |
| Two abnormal categories and base deficit ≥15 | 54% | 92% | 54% | 92% | 6.8 |
Death, seizures, abnormal brain MRI, abnormal neurologic exam at discharge, gastrostomy tube feeding, or feeding (nippling) difficulties beyond the first week of age.
Discussion
Perinatal acidemia is the initial screening component in the sequence of events that culminates in the decision to use hypothermia. The majority of newborns with perinatal acidemia are not cooled because their neurologic examination does not have sufficient abnormalities to classify them as having moderate or severe HIE.17 The outcomes of these infants who are diagnosed with mild or no encephalopathy on an early neurologic examination are not fully known,18 and whether the current assessment of these infants with perinatal acidosis identifies only the more severely affected infants remains to be determined. We therefore undertook this study to describe the short-term outcomes of infants who were assessed as having no or mild encephalopathy.
We found that as many as 20% of newborns with mild encephalopathy will have abnormal short-term outcomes such as seizures, death from progressive asphyxial insult, abnormal brain MRI consistent with HIE, abnormal neurologic examination at discharge, gastrostomy tube feeding, or nippling difficulties in the NICU. These infants, compared with those with no encephalopathy on neurologic examination, were more likely to have a combination of low Apgar scores, need for intubation in the delivery room, and persistent acidosis on the first postnatal blood gas. Previous reports of infants with moderate to severe HIE have found similar risk factors among those with abnormal neurodevelopmental outcomes.19-22
Our results cast some doubts over the reliability of the early neurologic examination. Previously published data showed that examiners often face challenges in attempts to categorize the Sarnat examination shortly after birth.23 In our study, the predictive ability of each component of the early neurologic examination was poor, and no single component or even combination of 2 categories accurately predicted which infants would have abnormal short-term outcomes consistent with more severe encephalopathy. This is consistent with data from the randomized clinical trials on hypothermia therapy that required multiple abnormal components of the neurologic examination performed at less than 6 hours of age for eligibility for cooling. Future studies evaluating other scoring systems or classification trees are needed for optimal selection of infants for cooling and prediction of prognosis.22
The issue remains as to whether these infants had more than mild encephalopathy at birth but were not identified correctly or had progression to moderate or severe HIE during the nursery stay. The neurologic examination used by the NICHD NRN trial as well as in this study is a modified Sarnat examination performed in the first 6 hours of age. Sarnat and Sarnat performed neurologic examinations serially at 12-24 hourly intervals for the first 6 days of age.10 Moreover, studies that reported normal long-term outcomes of infants with mild encephalopathy used the highest stage of encephalopathy over the first 3 days of age.8,10 It is known that hypoxic-ischemic injury to the neonatal brain is a dynamic process that can progress to more severe encephalopathy over the first several days of age.10,11,14 In our cohort, 5 infants originally classified as having mild encephalopathy progressed to a more severe stage during the first 12 to 24 hours of age. It also is known that the neurologic examination can improve over the first 6 hours of age, but the optimal time to perform it remains unclear. It is hoped that results of the ongoing NICHD trial of late hypothermia initiated from 6 to 24 hours of age will help answer some of these questions.
Major limitations of this study include its retrospective design with data collection on short-term outcomes and neurologic examinations based on review of past medical records. Documentation bias also may have played a role in that 13% of infants with perinatal acidemia had missing standardized neurologic examinations. Although it was known that these infants did not receive hypothermia, it was not possible to assess whether the neurologic examination, normally documented in a paper document, was lost or not done, and thus these infants were excluded from analysis. Another limitation is that inter-rater reliability testing between examiners was not known beyond the initial certification examination. However, the majority of neurologic examinations (73%) during the study period were performed by only 2 neonatologists. Also, serial neurologic examinations were not done routinely to detect late progression of encephalopathy, the discharge neurologic examination was not standardized, and there was lack of information on neurodevelopmental outcomes beyond nursery discharge. Finally, only 11% of study infants had a brain MRI performed, and it may be that the 20% of abnormal outcomes among infants with mild encephalopathy may represent an underestimation of the true incidence. Such missing information is vital to refine strategies for selection of infants for neuroprotective therapies.18
There is a need to accurately identify infants with encephalopathy in a timely fashion. Moderate encephalopathy is the most challenging stage of encephalopathy to detect clinically, yet it is the stage with the most potential for benefit.11,24,25 The results of this study suggest that the early neurologic examination may not detect all newborns who could potentially benefit from hypothermia treatment. It is possible that using the amplitude electroencephalogram as an additional diagnostic criterion for detection of more severe HIE among infants with ≤2 abnormal categories on neurologic examination may capture some of these infants.23,26-28 Continued investigation for early biomarkers to assess neonatal encephalopathy is warranted.
In conclusion, as many as 20% of newborns with perinatal acidemia and a neurologic examination consistent with only mild encephalopathy had abnormal short-term neurologic outcomes during the NICU stay. The identification of all infants who may potentially benefit from neuroprotective therapies such as hypothermia remains a challenge.
Acknowledgments
The authors thank Ms Karen Kirby for secretarial assistance.
L.C. is supported by the National Center for Research Resources, National Institutes of Health (KL2RR024983 and UL1 RR024982, North and Central Texas Clinical and Translational Science Initiative).
Glossary
- HIE
Hypoxic-ischemic encephalopathy
- MRI
Magnetic resonance imaging
- NICHD
National Institute of Child Health and Human Development
- NICU
Neonatal intensive care unit
- NPV
Negative predictive value
- NRN
Neonatal Research Network
- PMH
Parkland Memorial Hospital
- PPV
Positive predictive value
Footnotes
Presented in part at the Pediatric Academic Societies Meeting, May 1-4, 2010, Vancouver, British Columbia, Canada.
The authors declare no conflicts of interest.
References
- 1.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [Epub 2010/09/22] [DOI] [PubMed] [Google Scholar]
- 2.Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomized trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 5.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurologic outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Robertson CM, Finer NN, Grace MG. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114:753–60. doi: 10.1016/s0022-3476(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 9.van Handel M, Swaab H, de Vries LS, Jongmans MJ. Behavioral outcome in children with a history of neonatal encephalopathy following perinatal asphyxia. J Pediatr Psychol. 2010;35:286–95. doi: 10.1093/jpepsy/jsp049. [DOI] [PubMed] [Google Scholar]
- 10.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, Molteno CD, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86:757–61. doi: 10.1111/j.1651-2227.1997.tb08581.x. [DOI] [PubMed] [Google Scholar]
- 12.Perlman JM, Risser R. Cardiopulmonary resuscitation in the delivery room. Associated clinical events. Arch Pediatr Adolesc Med. 1995;149:20–5. doi: 10.1001/archpedi.1995.02170130022005. [DOI] [PubMed] [Google Scholar]
- 13.Goldaber KG, Gilstrap LC III, Leveno KJ, Dax JS, McIntire DD. Pathologic fetal acidemia. Obstet Gynecol. 1991;78:1103–7. [PubMed] [Google Scholar]
- 14.Murray DM, Bala P, O'Connor CM, Ryan CA, Connolly S, Boylan GB. The predictive value of early neurologic examination in neonatal hypoxic-ischaemic encephalopathy and neurodevelopmental outcome at 24 months. Dev Med Child Neurol. 2010;52:e55–9. doi: 10.1111/j.1469-8749.2009.03550.x. [Epub 2010/01/01] [DOI] [PubMed] [Google Scholar]
- 15.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SP, Newton N, Ferriero DM, Partridge JC, Glidden DV, Barnwell A, et al. Predictors of 30-month outcome after perinatal depression: role of proton MRS and socioeconomic factors. Pediatr Res. 2002;52:71–7. doi: 10.1203/00006450-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 17.King TA, Jackson GL, Josey AS, Vedro DA, Hawkins H, Burton KM, et al. The effect of profound umbilical artery acidemia in term neonates admitted to a newborn nursery. J Pediatr. 1998;132:624–9. doi: 10.1016/s0022-3476(98)70350-6. [DOI] [PubMed] [Google Scholar]
- 18.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–4. doi: 10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- 19.Shah S, Tracy M, Smyth J. Postnatal lactate as an early predictor of short-term outcome after intrapartum asphyxia. J Perinatol. 2004;24:16–20. doi: 10.1038/sj.jp.7211023. [DOI] [PubMed] [Google Scholar]
- 20.Takenouchi T, Cuaycong M, Ross G, Engel M, Perlman JM. Chain of brain preservation—a concept to facilitate early identification and initiation of hypothermia to infants at high risk for brain injury. Resuscitation. 2010;81:1637–41. doi: 10.1016/j.resuscitation.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Toh VC. Early predictors of adverse outcome in term infants with post-asphyxial hypoxic ischaemic encephalopathy. Acta Paediatr. 2000;89:343–7. [PubMed] [Google Scholar]
- 22.Ambalavanan N, Carlo WA, Shankaran S, Bann CM, Emrich SL, Higgins RD, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–93. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 23.Shalak LF, Laptook AR, Velaphi SC, Perlman JM. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003;111:351–7. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
- 24.van de Riet JE, Vandenbussche FP, Le Cessie S, Keirse MJ. Newborn assessment and long-term adverse outcome: a systematic review. Am J Obstet Gynecol. 1999;180:1024–9. doi: 10.1016/s0002-9378(99)70676-9. [DOI] [PubMed] [Google Scholar]
- 25.Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL. Hypoxic-ischemic encephalopathy in term neonates: perinatal factors and outcome. J Pediatr. 1981;98:112–7. doi: 10.1016/s0022-3476(81)80555-0. [DOI] [PubMed] [Google Scholar]
- 26.Shankaran S, Pappas A, McDonald SA, Laptook AR, Bara R, Ehrenkranz RA, et al. Predictive value of an early amplitude integrated electroencephalogram and neurologic examination. Pediatrics. 2011;128:e112–20. doi: 10.1542/peds.2010-2036. [Epub 2011/06/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoresen M. Hypothermia after perinatal asphyxia: selection for treatment and cooling protocol. J Pediatr. 2011;158(2 Suppl):e45–9. doi: 10.1016/j.jpeds.2010.11.013. [Epub 2011/02/02] [DOI] [PubMed] [Google Scholar]
- 28.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–9. doi: 10.1542/peds.2009-2938. [Epub 2010/06/23] [DOI] [PubMed] [Google Scholar]