Summary
Background
Formin proteins nucleate actin filaments de novo and stay associated with the growing barbed end. Whereas the formin-homology (FH) 2 domains mediate processive association, the FH1 domains—in concert with the actin-monomer-binding protein profilin—increase the rate of barbed-end elongation. The mechanism by which this effect is achieved is not well understood.
Results
We used total internal reflection fluorescence microscopy to measure the effect of profilin on the elongation of single actin filaments associated with FH1FH2 constructs (derived from the formin Bni1p from S. cerevisiae) with FH1 domains containing one to eight profilin-binding polyproline tracks. Over a large range of profilin concentrations (0.5–25 μM), the rate of barbed-end elongation increases with the number of polyproline tracks in the FH1 domain. The binding of profilin-actin to the FH1 domain is the rate-limiting step (up to rates of at least 88 s−1) in FH1-mediated transfer of actin subunits to the barbed end. Dissociation of formins from barbed ends growing in the presence of profilin is proportional to the elongation rate. Profilin profoundly inhibits nucleation by FH2 and FH1FH2 constructs, but profilin-actin bound to FH1 might contribute weakly to nucleation.
Conclusions
To achieve fast elongation, formin FH1 domains bind profilin-actin complexes and deliver them rapidly to the barbed end associated with the FH2 domain. Because subunit addition promotes dissociation of FH2 domains from growing barbed ends, FH2 domains must pass through a state that is prone to dissociation during each cycle of actin subunit addition coupled to formin translocation.
Introduction
Formin proteins are required for the assembly of various actin structures in eukaryotes, including polarized cables in budding yeast [1], contractile rings in fission yeast [2], interphase cables in fission yeast [3], and stress fibers [4] and filopodia [5] in animal cells. Along with Arp2/3 complex and Spire, formins are one of three families of well-characterized proteins that nucleate actin filaments. These proteins differ mechanistically in how they promote nucleation. Whereas the other proteins allow growth of free barbed ends following nucleation [6, 7], formins remain bound to the growing barbed end of a nucleated filament [8–11]—a phenomenon known as processive association.
The highly conserved formin-homology (FH) 2 domain of many formins suffices for nucleation [12–15] and processive association in vitro [9]. FH2 domains, typically ∼400–500 amino acids long [16, 17], form dimers that encircle the barbed end of an actin filament [18]. Barbed ends associated with many types of FH2-containing constructs elongate slower than free ends [9, 10, 19, 20]. To explain this behavior, some have proposed that ends associated with FH2 equilibrate between an open state, which allows addition of actin subunits, and a closed state, which prevents subunit addition [18].
The FH1 domains of most formins lie N-terminal to the FH2 domain and are predicted to be disordered except for multiple discrete stretches of contiguous proline residues [16]. These polyproline tracks are expected to form rigid type-II polyproline helices, which serve as binding sites for the actin-monomer-binding protein profilin [2, 21]. Profilin increases the rate of elongation of actin-filament barbed ends associated with formins containing both FH1 and FH2 domains (FH1FH2) [9–11, 19], often above the diffusion-limited rate of elongation of free barbed ends [22]. Given that the elongation rates of barbed ends associated with FH1FH2 domains from various formins are proportional to the concentration of profilin-actin, one model to explain their behavior is that multiple polyproline tracks in FH1 domains each bind complexes of profilin-actin and deliver them to the formin-associated barbed end [9, 23].
FH1 domains are found in most eukaryotic formins [16] but vary widely in their numbers of polyproline tracks. Profilin increases the elongation rate of barbed ends associated with FH1FH2-formins roughly in proportion to the number of FH1 polyproline tracks [9]. The significance of this relationship, however, has not been clear because different formins contain unique FH2 domains, which alone differ in their effects on filament elongation. To study the effect of the number of FH1 profilin-binding sites on formin-associated elongation, we measured elongation with formin constructs containing identical FH2 domains but FH1 domains that differ in their numbers of polyproline tracks. We combined measurements of single filaments associated with formins with the full time courses of polymerization of bulk samples to evaluate the effect of the FH1 domain on nucleation of ends from actin complexed with profilin.
Results
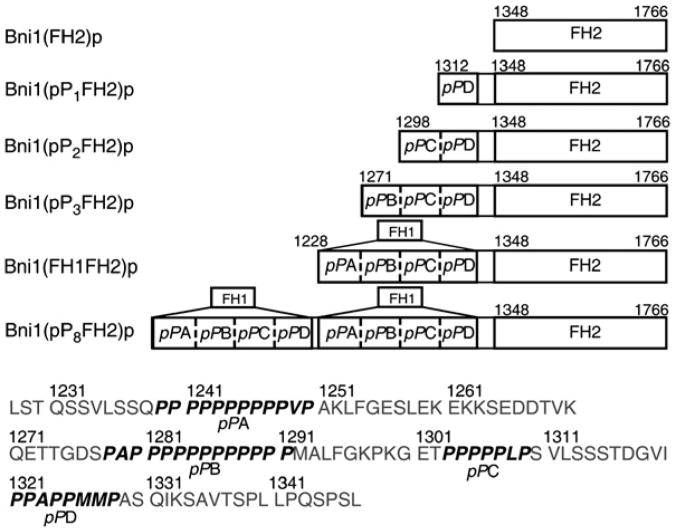
We made several FH1FH2 constructs with the Bni1p FH2 domain attached to variant FH1 domains. These FH1 domains were derived from Bni1p and contained between one and eight polyproline tracks (Figure 1). We named these constructs collectively Bni1(pPnFH2)p, in which n denotes the number of FH1 polyproline tracks . The FH1 domain of Bni1p has four polyproline tracks, three of which (A–C) fulfill the criterion for profilin binding—that is, they have at least six prolines out of seven consecutive residues [24, 25]. The profilin-binding capacity of the PPxPPxxP sequence of track D was less certain, but it is active, because ends associated with Bni1(pP1FH2)p in the presence of profilin elongate faster than those associated with the FH2 domain alone. We also note that both tracks A and B are long enough to bind two profilins [26, 27]. Bni1(FH1FH2)p, with four polyproline tracks, has been studied previously [9, 10, 12, 14, 28]. Bni1(pP8FH2)p contains two consecutive full-length FH1 domains.
Figure 1. Domain Maps of Bni1p FH1 Variants Used in this Study.
Residue numbers are shown to mark domain boundaries and polyproline tracks pPA, pPB, pPC, and pPD. The amino acid sequence of the Bni1p FH1 domain (residues 1228–1347) is given at the bottom.
We used time-lapse total internal reflection fluorescence microscopy (TIRFM) and actin, with the dye Oregon-green conjugated to Cys-374 (33% of the total actin), to directly observe the growth of individual actin filaments (Figure 2). We measured rates of elongation in association with Bni1(pPnFH2)p over a range of concentrations of S. cerevisiae profilin (Figure 3) and muscle actin monomers (Figure 4).
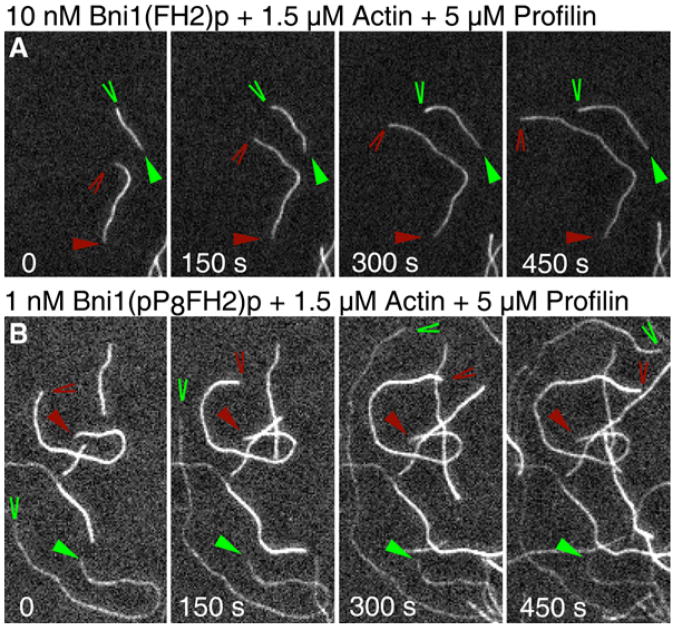
Figure 2. Time-Lapse TIRFM of Actin Polymerization in the Presence of Bni1(FH2)p or Bni1(pP8FH2)p with Profilin.
Conditions: 1.5 μM actin monomers (33% Oregon green) and 5 μM profilin in microscopy buffer (9.6 mM imidazole, pH 7.0, 48 mM KCl, 0.96 mM MgCl2, 0.96 mM EGTA, 96 mM DTT, 1.92 mM ATP, 50 mM CaCl2, 14.4 mM glucose, 19.2 mg/ml catalase, 96 mg/ml glucose oxidase, 0.48% methylcellulose [4000 cP at 2%], 0.19% BSA) with (A) 10 nM Bni1(FH2)p or (B) 1 nM Bni1(pP8FH2)p in PF buffer. The time series of images shows growth of free barbed ends (red wedges) and formin-associated barbed ends (green wedges) in the same field. Triangles mark pointed ends. Formin-associated barbed ends grew at an average rate of 32.6 subunits/s for Bni1(pP8FH2)p and 4.5 subunits/s for Bni1(FH2)p. Free barbed ends in the same viewing fields grew at 13.1 subunits/s for Bni1(pP8FH2)p and 11.7 subunits/s for Bni1(FH2)p.
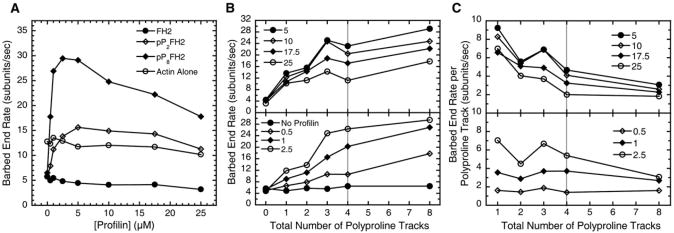
Figure 3. The Effect of the Number of the FH1 Polyproline Tracks on Barbed-End Elongation with Profilin.
Conditions: 1.5 μM actin (33% Oregon green) in microscopy buffer with varying concentrations of profilin. All data were collected with TIRFM. All Bni1(pPnFH2)p-associated rates are normalized to that of Bni1(FH2)p (equation 1).
(A) The dependence of the elongation rate of barbed ends on the concentration of profilin. Rates for ends associated with Bni1(pP2FH2)p and Bni1(pP8FH2)p are shown as representative plots for the Bni1p FH1 variants. The rates for free barbed ends and ends associated with Bni1(FH2)p were measured in the same viewing fields.
(B and C) Plots are split into upper and lower panels for clarity. Gray vertical lines indicate polyproline track number of Bni1(FH1FH2)p on the x axis. (B) The dependence of the rate of elongation of barbed ends associated with Bni1(pPnFH2)p constructs on the total number of polyproline tracks in the FH1 domain for a range of profilin concentrations (μM). (C) The barbed-end rate per polyproline track (equation 2) versus the total number of FH1 polyproline tracks (per formin subunit) at indicated micromolar concentrations of profilin.
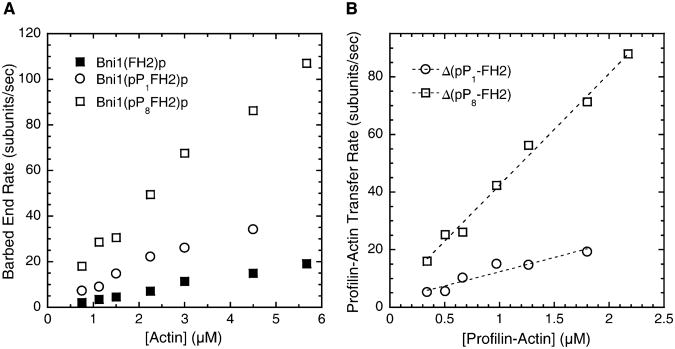
Figure 4. Dependence of the Elongation Rate of Barbed Ends Associated with Bni1(pP8FH2)p, Bni1(pP1FH2)p, or Bni1(FH2)p on the Concentration of Profilin-Actin.
Conditions: Varying concentrations of actin monomers (33%Oregon Green) with 5 μM profiling in microscopy buffer. All data were collected with TIRFM. The dependence of the rates of elongation with profilin on the bulk actin-monomer concentration.
Determination of the profilin-actin transfer rate constant for Bni1(pP1FH2)p and Bni1(pP8FH2)p. The differences in the rates of elongation mediated by Bni1(pPnFH2)p and Bni1(FH2)p (ΔpPn-FH2) versus the profilin-actin concentration (calculated from the profilin and actin concentrations and the Kd of their interaction [32]) are shown. The slopes yield apparent second-order association rate constants of 9.9 μM−1 s−1 for Bni1(pP1FH2)p (R2 = 0.91) and 39 μM−1 s−1 for Bni1(pP8FH2)p (R2 = 0.99).
Samples contained filaments with barbed ends associated with formins as well as free barbed ends; we used the free barbed ends as internal controls for the effects of formin association. To distinguish free ends from ends associated with a formin in a given viewing field, we observed differences in the elongation rates of filaments, the fluorescence intensities of the filaments, or both. Without profilin, free and formin-associated filaments were similarly fluorescent, but ends associated with a formin grewat ∼40%–50% the rate of free barbed ends (Figure 3A). In the presence of profilin, free barbed ends and those associated with Bni1(FH2)p had similar fluorescence intensities, but ends associated with FH2 alone grew substantially slower than free barbed ends (Figures 2A and 3A). By contrast, ends growing in association with the Bni1(pPnFH2)p FH1 variants were dimmer in the presence of profilin than free barbed ends (Figure 2B). This behavior can be understood by considering that FH1-bound profilin-actin is highly favored for addition to barbed ends over bulk-phase actin [9, 23] and that profilin has a higher affinity for unlabeled actin than for actin modified at Cys-374 [29]. At many concentrations of profilin, ends associated with Bni1-(pPnFH2)p constructs could be further distinguished from free barbed ends, because the formin-associated filaments grew substantially faster (Figures 2B and 3).
Influence of the Number of FH1 Polyproline Tracks on Barbed-End Elongation with Profilin
Profilin stimulated the elongation of barbed ends associated with all of the Bni1(pPnFH2)p constructs, as observed previously for Bni1(FH1FH2)p and three other FH1FH2-formins [9–11, 19]. In all of our experiments with 1.5 μM actin, the rates of elongation of barbed ends associated with the Bni1(pPnFH2)p constructs peaked at 2.5 to 5 μM profilin and declined with higher profilin concentrations (Figure 3A). Profilin weakly inhibited elongation of barbed ends associated with the FH2 domain alone, with the rate decreasing slightly from 5.7 subunits/s without profilin to 3.2 subunits/s at 25 μM profilin. High concentrations of profilin also slightly inhibited elongation of free barbed ends.
The rates of elongation of filaments associated with Bni1(pPnFH2)p increased with the total number of FH1 polyproline tracks at all concentrations of profilin (Figure 3B), suggesting that the individual tracks of polyproline capture profilin-actin complexes from the bulk solution and transfer them onto the barbed end as proposed previously [9, 23]. In general, each additional track contributed less to the elongation rate as the total number of tracks increased (Figure 3C). This effect was more pronounced at higher profilin concentrations.
Profilin-Actin Binding to the FH1 Domain Limits Formin-Mediated Barbed-End Elongation
The elongation rates of barbed ends associated with Bni1(FH2)p, Bni1(pP1FH2)p, or Bni1(pP8FH2)p increased with the concentration of actin in the presence of 5 μM profilin (Figure 4A). FH1FH2-mediated polymerization in profilin-actin proceeds through multiple pathways, including addition of free actin and profilin-actin onto the formin-associated barbed ends in a manner independent of the FH1 domain [9, 23]. To correct for the contribution to elongation of pathways other than those related to FH1 and profilin-actin, we plotted the differences in elongation rates between filaments associated with the polyproline-containing constructs and FH2 alone (ΔpPn-FH2) versus the concentration of profilin-actin (Figure 4B). For both Bni1(pP1FH2)p and Bni1(pP8FH2)p, the contribution of FH1 to elongation, presumably through profilin-actin binding and transfer, was proportional to the profilin-actin concentration. For Bni1(pP8FH2)p, profilin-actin limited FH1-mediated elongation up to 88 subunits/s, showing that binding of profilin-actin to the FH1 domain limited transfer of profilin-actin by FH1 to the end of the filament. For both Bni1(pP1FH2)p and Bni1(pP8FH2)p, the slopes of these plots (Figure 4B) indicate how quickly FH1 transfers profilin-actin to the formin-associated barbed end. The single polyproline track pPD of Bni1(pP1FH2)p transfers profilin-actin to the FH2-associated barbed end with an apparent second-order rate constant of 9.9 μM−1 s−1. The seven additional FH1 polyproline tracks of Bni1(pP8FH2)p increase this transfer rate constant to 39 μ M−1 s−1.
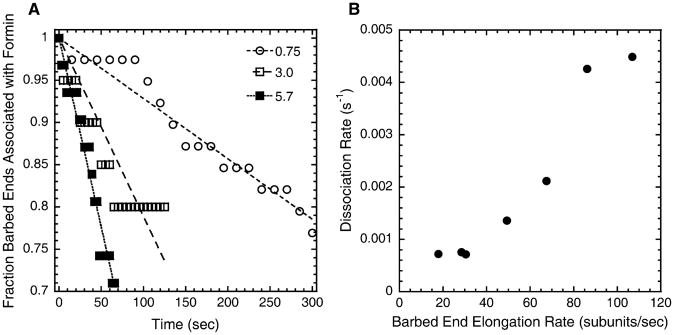
Dissociation of Formin Depends on the Elongation Rate
FH1FH2-formins are highly processive but have a finite probability of dissociating from growing barbed ends [9]. We measured the initial rate of dissociation of Bni1(pP8FH2)p from barbed ends growing in 5 μM profilin over a range of actin concentrations (Figure 5A) and found that the dissociation rate is roughly proportional to the elongation rate (Figure 5B), increasing from ∼0.001 s−1 When ends elongate at 18 subunits/s to ∼0.005 s−1 when ends elongate at >100 subunits/s. The probability of dissociation is therefore low—on the order of once per 20,000 subunit additions.
Figure 5. Dissociation of Bni1(pP8FH2)p from Barbed Ends.
Conditions as in Figure 4.
Time course of dissociation of Bni1(pP8FH2)p from growing barbed ends in 5 μM profilin at three actin concentrations (μM). Straight lines are fit to the first part of the time course of each reaction. R2 values for fits at the various actin concentrations were >0.70.
The dependence of the initial rate of dissociation of Bni1(pP8FH2)p from growing barbed ends (slopes from [A]) on the average barbed-end elongation rate of the filaments (from Figure 4A).
Effect of Profilin and the FH1 Domain on Nucleation of Actin Filaments by the FH2 Domain
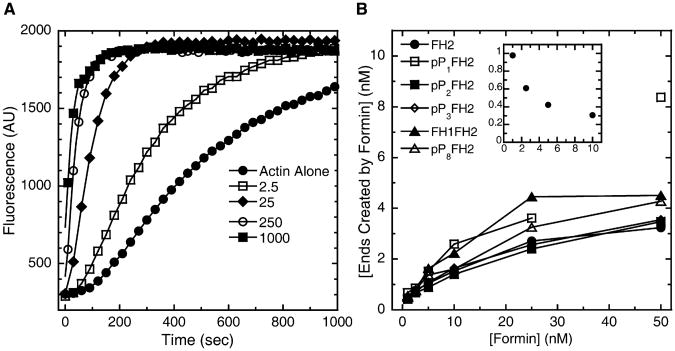
All of our Bni1p constructs stimulated spontaneous assembly of bulk samples of pyrene-labeled actin monomers (Figure 6A). As shown previously [12–14], the FH2 domain of Bni1p is sufficient for this activity.
Figure 6. The Bni1p FH1 Domain Does Not Influence Nucleation of Filaments of Free Actin by FH2.
Conditions: 4 μM actin monomers (20% pyrene) in polymerization buffer (10 mM imidazole, pH 7.0, 50 mM KCl, 1 mM EGTA, 1 mM MgCl2, supplemented with formin buffer).
(A) Representative time courses of spontaneous polymerization measured by fluorescence enhancement of pyrenyl-actin (λex = 362 nm; λem = 407 nm) in the presence of indicated nanomolar concentrations of Bni1(FH2)p.
(B) Dependence of the concentration of ends nucleated (calculated from the elongation rate and the slope of the pyrenyl-fluorescence enhancement when half of the monomers were polymerized [equation 3]) on the concentrations of Bni1(FH2)p or Bni1(pPnFH2)p. The reported values of concentration of ends created by formin were obtained by subtraction of the value for the concentration of ends created through self-nucleation of actin monomers (typically ∼0.2 nM; calculated from time courses of assembly of actin alone) from the total concentration of ends. For Bni1(pP1FH2)p, the data point at 50 nM formin appears anomalous and is therefore not connected by a line to the rest of the data. Inset: Dependence of the concentration of ends created per formin dimer (calculated from the data in the main plot) on the formin concentration (nM). Data for Bni1(FH2)p are shown as a representative plot.
We measured nucleation by calculating the concentration of barbed ends present when half of the actin was polymerized. This calculation depended upon our knowing the rates of elongation (from microscopy) under all of these conditions. Without profilin, the presence of an FH1 domain with profilin-binding sites neither promoted nor inhibited the nucleation activity of the associated Bni1p FH2 domain over a range of formin concentrations (Figure 6B). Bni1(FH2)p (Figure 6B, inset) and our various Bni1(pPnFH2)p constructs (not shown) all produced a maximum of approximately one end per formin dimer. The efficiency of nucleation declined at higher formin concentrations, because the numerous growing ends rapidly depleted actin monomers before all of the FH2 dimers initiated new filaments.
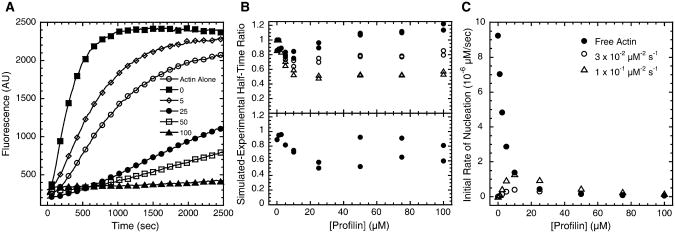
Profilin slowed polymer assembly from actin monomers in the presence of either Bni1(FH2)p (Figure 7A) or Bni1(FH1FH2)p (not shown). For Bni1(FH1FH2)p, we could not directly calculate the number of filaments formed from the time course of the fluorescence change, because profilin inhibits incorporation of labeled actin into the growing filaments associated with polyproline-containing formins (Figure 2B). The fluorescence of pyrenyl-actin, therefore, does not accurately reflect the total actin-polymer concentration. Instead, we used computational methods to simulate actin polymerization and estimate the nucleation activity of FH1FH2 in the presence of profilin. Our simulations took into account all of the interactions of profilin, actin, pyrenyl-actin, FH1 domains, FH2 domains, and filament ends, which together give rise to the observed signal from pyrenyl-actin in bulk polymerization assays (Figure S1 and Table S1, available online). We compared the polymerization half-times for experiments and simulations to judge the adequacy of hypothetical mechanisms and rate constants to account for observations. Perfect agreement between computational and solution data would yield a value of 1 at all profilin concentrations for the ratio of the simulated to experimental half-time.
Figure 7. The Influence of Profilin on Actin-Filament Nucleation by Bni1(FH2)p or Bni1(FH1FH2)p.
Conditions as in Figure 6, except pH = 7.5.
(A) Representative time courses of the polymerization of 4 μM actin monomers in the presence of 5 nM Bni1(FH2)p and indicated micromolar concentrations of profilin.
(B) Ratios of simulated to experimental half-times for the assembly of pyrenyl-actin (4 μM, 20% pyrene) with Bni1(FH1FH2)p (upper panel) or Bni1(FH2)p (lower panel) over a range of profilin concentrations. For each profilin concentration and formin construct tested as in (A), the half-time (the point in the assembly time course at which half the pyrenyl-actin had polymerized) of simulated assembly was divided by the half-time of the experimental measurements. For Bni1(FH1FH2)p, simulated to experimental half-time ratios are shown for simulated assembly with a mechanism having no reaction for profilin-actin nucleation (filled circles) or a reaction for nucleation of FH1-bound profilin-actin (reaction 14 [forward], Figure S1) with a rate constant of either 3 × 10−2 μM−2 s−1(open circles) or 1 × 10−1 μM−2 s−1 (open triangles). For each condition tested in simulated assembly, simulated to experimental half-time ratios are shown for two sets of experimental measurements.
(C)The dependence on profilin concentration of the initial rates of nucleation by FH1FH2 from free actin monomers (filled circles) or profilin-actin (open circles, open triangles) in the simulated polymerization reactions in (B). The reported values are the instantaneous rates at 15 s after the initiation of polymerization of reaction 5 (nucleation from free actin) or 14 (nucleation from profilin-actin) in Figure S1. This time point was chosen because in simulated polymerization it is near the outset of the polymerization reaction but far enough into the reaction to allow the individual pools of profilin, actin, and profilin-actin to equilibrate. For the plots of rates of nucleation from profilin-actin, the legend indicates the rate constant of the forward Reaction 14 tested in the simulations in (B). The initial rate of nucleation from free actin is identical for each simulated reaction with FH1FH2 in (B).
We used polymerization with Bni1(FH2)p and a range of profilin concentrations to establish a baseline for comparisons of simulated and experimental assembly reactions. Under these conditions, we expected this construct to nucleate ends only from free actin monomers [13]. The simulated time courses were somewhat faster than the experiments (Figure 7B, bottom panel), but the simulations closely matched the dependence of nucleation by formin on the profilin concentration (Figure S2). The divergences between the simulated and experimental time courses are probably due to uncertainties in the simulation parameters.
Simulations gave half-times similar to experiments for Bni1(FH1FH2)p, if one assumes that the formin nucleates ends exclusively from free actin monomers and not profilin-actin (Figure 7B, top panel). Simulated assembly agreed more closely with the experiment for Bni1(FH1FH2)p than for FH2 alone, especially at higher concentrations of profilin. Although participation of profilin-actin is not required to explain nucleation by Bni1(FH1FH2)p, we used simulations to test the hypothesis that the FH1 domain enables FH2 to use profilin-actin for nucleation [13]. We simulated assembly with a mechanism that allows the creation of an end from two profilin-actin complexes bound simultaneously to opposite FH1 domains on a single formin dimer (reaction 14 in Figure S1). Simulated assembly with this reaction in the mechanism was faster than that without this reaction, and the simulated half-times diverged more strongly from those observed in experiments (Figure 7B, top panel). The ratios of simulated to experimental half-times, however, were closer to those observed for the FH2 domain alone. This trend might represent the limit at which nucleation from profilin-actin can stimulate polymerization with FH1FH2.
For the simulated polymerization reactions in Figure 7B allowing nucleation from profilin-actin, we compared the initial rates of nucleation from free actin and profilin-actin to understand the contributions of both processes in mixtures of profilin and actin. Low concentrations of profilin strongly inhibit nucleation by free actin. The rate of nucleation from FH1-bound profilin-actin increases up to 10 μM total profilin. The rate decreases with higher profilin concentrations, because free profilin competes with profilin-actin for FH1 binding sites (Figure 7C). Profilin-actin contributes most strongly to nucleation at 10 μM total profilin, when profilin-actin is ∼1.7 times more abundant than free actin. For the maximum strength of simulated profilin-actin nucleation tested in Figure 7B, free actin and profilin-actin contribute to nucleation at roughly the same initial rate (∼1.3 × 10−6 μM/s) at 10 μM profilin. One hundred micromolar total profilin strongly inhibits nucleation through both pathways (initial rates on the order of 10−7 μM/s), because most of the actin is bound to profilin and free profilin blocks profilin-actin binding to FH1.
Discussion
The FH1 Domain Rapidly Transfers Profilin-Actin to the Barbed End
Our observations of Bni1p support the hypothesis that individual tracks of polyproline first bind and then transfer profilin-actin to the barbed end of filaments [9, 23]. From a structural perspective, FH1 domains, typified by flexible stretches of polypeptide interspersed with profilin-binding sites, seem ideally suited to this task. Although multiple possible pathways are available for actin to add onto a barbed end associated with FH1FH2 in the presence of profilin, Vavylonis et al. [23] calculated that with sufficient profilin, the dominant pathway involves binding of bulk-phase profilin-actin to the FH1 domain, followed by movement of FH1-bound profilin-actin through space to assemble the so-called ring complex with the barbed end, and finally dissociation of FH1 and profilin from the newly incorporated actin subunit to disassemble the ring complex and complete the elongation cycle [23]. Direct delivery of profilin-actin by FH1 to the barbed end is essential to account for the ability of profilin to increase the rate of elongation of barbed ends associated with FH1FH2. Mere elevation of the local concentration of profilin-actin by the FH1 domain followed by release into solution near the barbed end cannot explain how much profilin increases the elongation rates of barbed ends associated with FH1FH2 [23].
In a cell with a large pool of profilin-actin, an FH1 domain enables a formin to elongate filament ends rapidly. In vitro with sufficient profilin-actin, filaments associated with Bni1p can elongate at >100 subunits/s (Figure 4A), similar to rates observed with Bni1p-associated filaments emanating from the bud tip in S. cerevisiae [30, 31]. On the basis of our data for Bni1(pP8FH2)p-mediated elongation in profilin-actin (Figure 4), the dissociation equilibrium constant for profilin-actin [32], and the in vivo Bni1p-associated elongation rate of 110–180 subunits/s [30], we estimate that the cellular profilin-actin concentration near Bni1p at the bud tip is roughly 3–4 μM. This calculation assumes that, in vivo, Bni1p elongates barbed ends primarily through FH1-mediated profilin-actin transfer [23] and that rates measured with Bni1(pP8FH2)p are similar to that of wild-type Bni1p with four polyproline tracks (Figure 3).
Factors Governing the Rate of Profilin-Actin Transfer by FH1 Polyproline Tracks
Successive addition of up to 16 polyproline tracks to the Bni1p FH2 dimer [i.e., Bni1(pP8FH2)p] increased the rate of elongation of barbed ends in a “dose-dependent” manner up to ∼30 s−1 with a limiting concentration of profilin-actin (Figure 3). Therefore, many individual FH1 profilin-binding sites contribute to elongation, suggesting that each polyproline track captures profilin-actin complexes independently from the bulk solution and forms FH1-profilin-actin ring complexes with the barbed end [23].
The profilin-actin concentration limits the rate of elongation of barbed ends associated with FH2 constructs both with and without FH1 domains (Figure 4). For FH1FH2 constructs, subunit transfer by FH1 is limited by binding of bulk-phase profilin-actin to the FH1 domain up to transfer rates of ≥88 subunits/s, showing that the steps in the profilin-actin transfer pathway that follow association of profilin-actin with FH1—namely, assembly and disassembly of the ring complex—proceed at rates >88 s−1 (Figure 4B).
The rate of formin-mediated elongation increases with either the number of FH1-profilin-binding sites (Figure 3) or the bulk profilin-actin concentration (Figure 4), indicating that the density of FH1-bound profilin-actin molecules near the barbed end modulates the formin-associated elongation rate. However, the contributions of individual polyproline tracks to elongation diminish as their total numbers in the FH1 domain increase, an effect that becomes apparent at elongation rates of <30 subunits/s when the profilin-actin concentration is fixed (Figure 3). Similarly, barbed ends associated with mDia1(FH1FH2) containing either 6 or 14 polyproline tracks elongate at the same rate [9]. (The criteria employed in the previous study indicated that the mDia1 constructs had 5 and 11 polyproline tracks.) This trend suggests that FH1-bound profilin-actin at polyproline tracks that are distant from the barbed end assemble ring complexes less efficiently than those that are closer to the barbed end. Indeed, Vavylonis et al. [23] predicted that assembly of a ring complex with a given polyproline track decreases with that track's distance from the barbed end because the frequency of end-to-end collision of a flexible peptide like FH1 decreases with chain length.The diminished contributions to elongation of polyproline tracks at the N terminus of the FH1 domain are even more pronounced if polyproline tracks pPA and pPB bind two profilin-actin complexes each (Figure S3), as is expected from their high proline content [26, 27].
The single polyproline track pPD of Bni1(pP1FH2)p transfers profilin-actin to the formin-associated barbed end at a rate directly proportional to the profilin-actin concentration (Figure 4B). High efficiency of transfer would explain the ability of FH1 to channel profilin-actin onto the barbed end. If one assumes that profilin-actin binds FH1 domains with the same association rate constant (200 μM−1 s−1) as profilin binds polyproline [24], the apparent second-order profilin-actin transfer-rate constant of ∼10 μM−1 s−1 suggests that ∼5% of profilin-actin-binding events to pPD results in profilin-actin transfer by FH1 to increase the rate of subunit addition. However, the association rate constant for profilin-actin binding to the FH1 pPD track on a barbed-end-bound formin is likely to be less than the value of 200 μM−1 S− measured for free profilin to polyproline oligomers, because of the larger sizes and restricted mobility of the binding partners. The efficiency of profilin-actin transfer events relative to FH1 profilin-actin binding events is therefore probably higher than 5% for this polyproline track.
Inhibition of Formin-Mediated Barbed-End Elongation by Free Profilin
Similar to previous findings with FH1FH2 constructs from different formins [9], we observed the maximum rate of barbed-end elongation for the Bni1p constructs containing full or partial FH1 domains at a concentration of profilin (2.5 to 5 μM) somewhat higher than the concentration of actin (Figure 3A). This behavior supports the hypothesis that free profilin competes with profilin-actin for binding to FH1 polyproline tracks and thereby inhibits profilin-actin transfer onto the barbed end [9, 23].
Dissociation of Formins from Growing Barbed Ends and Translocation of Formin
Translocation of an FH2 dimer from the penultimate to the terminal subunit of a growing barbed end is the only step in formin-mediated elongation that is necessarily dependent on subunit addition. To stay processively associated with an elongating barbed end, a formin must translocate in locked step with subunit addition. The direct dependence of the dissociation rate of Bni1(pP8FH2)p from barbed ends on the rate of subunit addition (Figure 5) and a similar trend shown in previous studies of different formins [9] suggest that a formin has a much higher probability of dissociating from a filament end during some state related to translocation than during any other step in the elongation process. Indeed, translocation is a natural candidate for a process related to dissociation because a formin must partially dissociate from the filament to shift its position along the growing barbed end. Moreover, because subunit addition induces dissociation, actin-monomer association with the barbed end must precede translocation.
To understand what state of formin associated with the barbed end might be related to dissociation from growing ends, we consider our results in relation to the structure of the FH2 domain of Bni1p that is associated with actin and to current models of processive association of formins with the barbed end. In the dominant conformation of actin filaments, the subunits are rotated 167° relative to their neighbors along the short-pitch helix [33, 34]. In contrast, association with FH2 in crystals induces a filament-like polymer of actin with subunits related to each other by a 180° rotation, a conformation that is not expected to allow subunit addition [18]. On the basis of this observation, Otomo et al. [18] proposed that association of a FH2 dimer with the barbed end induces the 180° conformation of the three terminal subunits. In this state the formin is bound at all of its points of contact to actin through the so-called knob and post sites, and the barbed end is blocked for subunit addition [18]. Many researchers have proposed that in order to enable subunit addition and to simultaneously maintain contact with the barbed end, the FH2 dimer releases a fraction of its contacts to partially dissociate from the filament and translocate one of its subunits toward the barbed end. This process allows the filament to relax to the 167° conformation that is competent for elongation [18, 35, 36]. Although this view might account for processive association of formins, models in which formins partially dissociate to translocate before subunit addition are inconsistent with our finding that subunit addition precedes translocation.
Model for a Mechanical Cycle of Formin Translocation Coupled to Barbed-End Subunit Addition
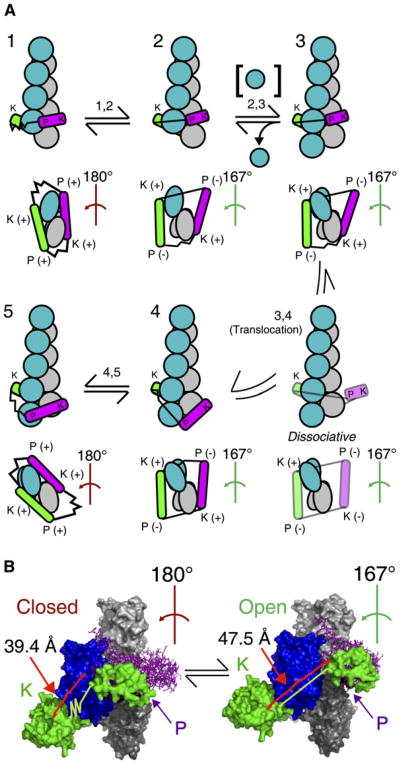
To explain our finding that the rate of formin dissociation from the barbed end increases with the elongation rate, we propose a model for the mechanical cycle of formin-mediated barbed-end elongation that differs from previous models in the feature that the FH2 dimer advances in the barbed-end direction, or translocates, only after subunit addition (Figure 8 and Figure S4). Other models suggest that the open state of formin associated with the barbed end, during which subunit addition is allowed, is gated by translocation of formin along the end and the resultant relaxation of the filament to the 167° conformation [18, 35, 36]. We propose that only relaxation of the filament without formin translocation is required for subunit addition. Our model thereby offers a novel structural interpretation of the open state of formin on the barbed end that is distinct from the translocation state.
Figure 8. Model for Translocation of a Formin FH2 Domain Coupled to Actin Subunit Addition to the Barbed End.
(A) The mechanical cycle of subunit addition coupled to translocation of formin is schematically illustrated from the upper left in clockwise order as a series of states 1-5. (Figure S4 shows this cycle with space-filling images derived from PDB files.) To proceed from state X to state Y, formin must pass through step X,Y indicated above the reaction arrows. For each state, the upper image displays the filament at a view normal to the filament axis, with the barbed end pointing down. The actin filament is shown with gray subunits along one long-pitch strand and blue subunits in the other strand. The lower images are views of the barbed end. The helical twist along the short-pitch helix of the three terminal barbed-end subunits is indicated for each state and is colored either red to indicate a conformation that prevents subunit addition (closed state) or green to indicate one that permits addition (open state). (Note that the 180° helical twist in closed states 1 and 5 does not propagate into the filament past the third subunit from the barbed end.) One subunit of the FH2 dimer is shown in green, the other in magenta. Each FH2 subunit has two sites to interact with actin: the knob (K) and the post (P). These sites are indicated in each image, and their states of association with the filament are indicated as (+) for bound and (‒) for unbound in barbed-end-on views. The flexible linkers of each FH2 subunit (Bni1p residues 1401-1417) are depicted as either stretched or relaxed springs (see [B]). The FH2 subunits of the transient translocation intermediate between states 3 and 4 are partially transparent.
(B) Space-filling models of the proposed closed state with a 180° filament conformation and the open state with a 167° filament conformation. The yellow lines show the distances that the FH2 flexible linker must span between the leading subunit's knob and the trailing subunit's post (from the carboxyl-terminus of residue 1400 to the amino-terminus of 1418 of the leading subunit) in the two states. Illustration of the linkers as stretched or relaxed springs emphasizes the differences in the extension of the linkers in the two states. The 180° filament conformation with Bni1(FH2)p is from PDB file 1Y64. The 167° conformation was modeled by overlaying the Bni1(FH2)p-actin structure with the atomic model of the actin filament (from K.C. Holmes, ftp://149.217.48.3/pub/holmes/pdb/actin_helix_93.pdb); the FH2 subunits were rotated about the filament axis while the knob-site attachments of the FH2 subunits to the actin subunits were maintained as in the structure [18]. Images were rendered with PYMOL (Delano Scientific, Palo Alto, California).
Figure 8A depicts the various states of association of a formin dimer with an actin filament during the process of translocation coupled to subunit addition. This schematic illustration is based on a space-filling model that takes into account the real dimensions of the formin and actin molecules (Figure S4). Our model provides a plausible explanation, based only on the flexibilities of formin dimer and actin filament, for the energetics of formin translocation. State 1 is the closed state that prevents subunit addition and is identical to the blocked state proposed in [18]. The knob and post sites of both formin subunits are bound to the three terminal actin subunits of the barbed end to induce a 180° helical twist of these subunits. We assume that state 1 is in rapid equilibrium with state 2, which we propose to be the open state. In state 2, the helical twist of the three terminal subunits is relaxed to 167°, so the flexible linker between the knob of the leading subunit and the post of the trailing subunit must stretch from 39.4 to 47.5 A (Figure 8B). We assume that this extension increases the energy of the formin bound to the end. (The opposite linker is slightly compressed in state 2.) If we assume that the globular regions of each FH2 polypeptide are not distorted during any steps in the cycle, it follows that the post sites of both FH2 subunits must be dissociated from actin in state 2 [18]. Addition of an actin monomer to the barbed end in the open state (step 2,3) results in a conformation with the FH2 subunits bound to actin through their knobs at the third and fourth subunits from the barbed end (state 3). In this state, the actin subunits at the tip are presumably related to the next subunit along the short-pitch helix by the standard 167° helical rotation. We assume that the actin subunits at the very tip of the filament are more torsionally flexible than internal subunits, in which helical twists of 167° or less are strongly favored [37]. Internal subunits bound to formin will therefore resist the relaxation of the stretched FH2 linker. We propose that the formin barbed-end complex relieves this high-energy transition state by translocating the trailing FH2 subunit to the new terminal barbed-end subunit (step 3,4), where flexibility of the actin subunits allows the formin to assume its lower-energy conformation. A tendency for formin to favor the compliant tip position of the filament provides a rationale for processive barbed-end association and the apparent absence of translocation toward the pointed end. Given that Bni1p stays associated with barbed ends growing at rates >100 subunits/s (Figure 4A), the transition state 3 preceding translocation must be very short lived. To complete the cycle of subunit addition, the knob site of the translocated formin subunit attaches to the newly added actin subunit to reestablish the open state 4; both post sites can then bind the filament to convert the complex to the closed state 5. States 4 and 5 are equivalent to states 2 and 1, but the filament is longer by one subunit and the orientations of the two FH2 subunits in relation to the barbed end are reversed.
In our structure-based model, association of the FH2 dimer with actin is weakest during translocation, when only one strong contact binds the FH2 dimer to the barbed end. This view of a loosely bound translocation intermediate in step 3,4 (Figure 8) is consistent with our observation (Figure 5) that subunit addition followed by translocation occasionally stimulates the complete dissociation of formin from growing barbed ends. The mechanism proposed in Figure 8 also suggests that a formin might get left behind in the interior of a growing filament and be unable to influence elongation if an actin subunit were to bind to the barbed end in state 3 before the formin translocates onto the end of the filament.
Our model predicts that FH2 dimers track with the long-pitch actin helix with each subunit addition. However, when bound simultaneously to a formin and an enzymatically inactive myosin, both attached to a glass slide, an elongating filament does not supercoil between the two attachment points. This observation raised the possibility that the translocating formin might “slip” about the filament axis [10,38]. The weakly bound translocation intermediate seems like the state that is most likely to allow the formin to slip under stress. However, neither the available structures nor observations of single filaments explain how the FH2 dimer might reorient when subjected to torque.
Our model for formin-mediated elongation provides a structural rationale for the variable ability of different formins to reduce barbed-end growth without profilin. Our explanation draws on the hypothesis that the occupancy of the open state increases with the length of the flexible linker connecting the knob and post sites of FH2 dimers [9]. The principle guiding our model for formin translocation coupled to subunit addition is the competition between the 167° conformation of the formin-barbed-end complex in the open state and the 180° conformation in the closed state. Whereas the actin filament favors the 167° conformation, the formin dimer favors the 180° conformation because of the energetic cost to the formin of stretching one of its FH2 linkers to at least 47.5 Å to accommodate the 167° conformation. Therefore, formins with longer linkers permit the open state with the 167° barbed-end conformation to be occupied a larger fraction of the time than do those with shorter linkers. Table S2 shows the relationship between elongation in association with various well-characterized formins and their flexible linker lengths. This table also provides information on the inferred values of the differences in energy for the open and closed states for these formins, as well as the forces required to stretch their FH2 flexible linkers.
Romero et al. [11, 39] proposed that ATP hydrolysis and release of the γ-phosphate from the terminal barbed-end actin subunit are required for processive translocation toward the barbed end. Our mechanical model uses the binding energy of each new actin subunit addition, rather than a chemical reaction, to explain translocation and might account for the ability of FH2 dimers to remain associated with barbed ends growing in ADP-actin [9].
The FH1 Domain Does Not Strongly Promote Nucleation with Profilin
The Bni1p FH2 domain nucleates barbed ends from free actin monomers, independent of attached FH1 profilin-binding sites as seen previously for mDia1 [40]. At low concentrations, all of our FH1-containing Bni1p constructs nucleated ends very efficiently with a yield of approximately one barbed end per formin dimer (Figure 6B, inset).
Similar to previous observations with mDia1 [40], profilin strongly inhibits assembly stimulated by either FH2 or FH1FH2 constructs of Bni1p. Indeed, profilin-actin does not contribute to nucleation by Bni1(FH2)p and is not required to account for nucleation by Bni1(FH1FH2)p (Figure 7). On the other hand, our data do not rule out a small contribution of profilin-actin to nucleation by Bni1(FH1FH2)p as proposed by Pring et al. [13].
Although profilin strongly inhibits nucleation, cells are likely to contain much more profilin-actin than free actin, so one must consider the possibility that FH1-mediated nucleation of ends is physiologically significant. Comparison between our observations in solution and those in simulations suggests that at high profilin concentrations, an FH1-mediated profilin-actin nucleation reaction is strongly inhibited and unlikely to proceed much faster than nucleation from the comparatively smaller pool of free actin (Figure 7C). Profilin-actin is therefore probably not the sole or perhaps even major source of new ends created by formin in the cell. Indeed, in live animal cells, a fragment containing only the FH2 domain of mDia1 is sufficient for apparent association with elongating filaments [8], suggesting that FH1 is not required for formin to utilize the cellular pool of actin.
On the other hand, full-length Bni1p lacking only the FH1 domain could not rescue the budding-related morphological defects of S. cerevisiae bni1-Δ cells [41], perturb the existing actin cytoskeleton in wild-type cells [42], or undergo actin-polymerization-dependent dissociation from the cell cortex [30]. If these phenotypes do not arise from a nucleation defect, perhaps the ability of FH1 to promote elongation with profilin is critical in cells. Another possibility is that Bni1p function depends on the binding of a factor other than profilin to the FH1 domain. Though no other FH1-binding partners for Bni1p have been identified, Src tyrosine kinase binds to the FH1 domain of the formin mDia2 from mouse [43].
Experimental Procedures
The Supplemental Data contain detailed descriptions of plasmid constructions, protein purifications and preparations, biochemical assays, and data analyses. We purified recombinant His6-tagged formin constructs and recombinant profilin essentially as described [19, 32]. Actin was purified from chicken skeletal muscle [44].We acquired time-lapse movies of individual fluorescent actin filaments growing in various polymerizing conditions by imaging with TIRFM [19, 45]. We measured the polymerization of pyrenyl-actin in bulk solution with fluorescence.
Supplementary Material
Acknowledgments
This work was supported by a National Institues of Health (NIH) research grant GM026338 to T.D.P. and a National Sciences Foundation Predoctoral Fellowship to A.P. We thank David Kovar and Dimitrios Vavylonis for extensive guidance and numerous discussions, Jeff Kuhn for help with image analyses of actin filaments observed with TIRFM, Brad Nolen for help with Figure 8B and Figure S4, and Janice Wong for help with some of the experiments.
Footnotes
Supplemental Data: Experimental procedures, as well as four figures and two tables, are available at http://www.current-biology.com/cgi/content/full/18/1/9/DC1/.
References
- 1.Sagot I, Klee SK, Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat Cell Biol. 2002;4:42–50. doi: 10.1038/ncb719. [DOI] [PubMed] [Google Scholar]
- 2.Chang F, Drubin D, Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feierbach B, Chang F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr Biol. 2001;11:1656–1665. doi: 10.1016/s0960-9822(01)00525-5. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15:129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Quinlan ME, Heuser JE, Kerkhoff E, Mullins RD. Drosophila Spire is an actin nucleation factor. Nature. 2005;433:382–388. doi: 10.1038/nature03241. [DOI] [PubMed] [Google Scholar]
- 7.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashida C, Miyoshi T, Fujita A, Oceguera-Yanez F, Monypenny J, Andou Y, Narumiya S, Watanabe N. Actin polymerization-driven molecular movement of mDia1 in living cells. Science. 2004;303:2007–2010. doi: 10.1126/science.1093923. [DOI] [PubMed] [Google Scholar]
- 9.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Kovar DR, Pollard TD. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc Natl Acad Sci USA. 2004;101:14725–14730. doi: 10.1073/pnas.0405902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119:419–429. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: Nucleation and barbed-end association. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 13.Pring M, Evangelista M, Boone C, Yang C, Zigmond SH. Mechanism of formin-induced nucleation of actin filaments. Biochemistry. 2003;42:486–496. doi: 10.1021/bi026520j. [DOI] [PubMed] [Google Scholar]
- 14.Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell. 2004;15:896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelot A, Guerin C, Huang S, Ingouff M, Richard S, Rodiuc N, Staiger CJ, Blanchoin L. The formin homology 1 domain modulates the actin nucleation and bundling activity of Arabidopsis FORMIN1. Plant Cell. 2005;17:2296–2313. doi: 10.1105/tpc.105.030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16:1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–723. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- 18.Otomo T, Tomchick DR, Otomo C, Panchal SC, Machius M, Rosen MK. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005;433:488–494. doi: 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- 19.Kovar DR, Kuhn JR, Tichy AL, Pollard TD. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J Cell Biol. 2003;161:875–887. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris ES, Li F, Higgs HN. The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J Biol Chem. 2004;279:20076–20087. doi: 10.1074/jbc.M312718200. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drenckhahn D, Pollard TD. Elongation of actin filaments is a diffusion-limited reaction at the barbed end and is accelerated by inert macromolecules. J Biol Chem. 1986;261:12754–12758. [PubMed] [Google Scholar]
- 23.Vavylonis D, Kovar DR, O'Shaughnessy B, Pollard TD. Model of formin-associated actin filament elongation. Mol Cell. 2006;21:455–466. doi: 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perelroizen I, Marchand JB, Blanchoin L, Didry D, Carlier MF. Interaction of profilin with G-actin and poly(L-proline) Biochemistry. 1994;33:8472–8478. doi: 10.1021/bi00194a011. [DOI] [PubMed] [Google Scholar]
- 25.Petrella EC, Machesky LM, Kaiser DA, Pollard TD. Structural requirements and thermodynamics of the interaction of proline peptides with profilin. Biochemistry. 1996;35:16535–16543. doi: 10.1021/bi961498d. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney NM, Janmey PA, Almo SC. Structure of the profilin-poly-L-proline complex involved in morphogenesis and cytoskeletal regulation. Nat Struct Biol. 1997;4:953–960. doi: 10.1038/nsb1197-953. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney NM, Rozwarski DA, Fedorov E, Fedorov AA, Almo SC. Profilin binds proline-rich ligands in two distinct amide backbone orientations. Nat Struct Biol. 1999;6:666–671. doi: 10.1038/10722. [DOI] [PubMed] [Google Scholar]
- 28.Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat Cell Biol. 2002;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- 29.Vinson VK, De La Cruz EM, Higgs HN, Pollard TD. Interactions of Acanthamoeba profilin with actin and nucleotides bound to actin. Biochemistry. 1998;37:10871–10880. doi: 10.1021/bi980093l. [DOI] [PubMed] [Google Scholar]
- 30.Buttery SM, Yoshida S, Pellman D. Yeast formins Bni1 and Bnr1 utilize different modes of cortical interaction during the assembly of actin cables. Mol Biol Cell. 2007;18:1826–1838. doi: 10.1091/mbc.E06-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehrenbacher KL, Yang HC, Gay AC, Huckaba TM, Pon LA. Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr Biol. 2004;14:1996–2004. doi: 10.1016/j.cub.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Eads JC, Mahoney NM, Vorobiev S, Bresnick AR, Wen KK, Rubenstein PA, Haarer BK, Almo SC. Structure determination and characterization of Saccharomyces cerevisiae profilin. Biochemistry. 1998;37:11171–11181. doi: 10.1021/bi9720033. [DOI] [PubMed] [Google Scholar]
- 33.Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 34.Huxley HE. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J Mol Biol. 1963;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- 35.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 36.Shemesh T, Kozlov MM. Actin polymerization upon processive capping by formin: A model for slowing and acceleration. Biophys J. 2007;92:1512–1521. doi: 10.1529/biophysj.106.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galkin VE, Orlova A, Lukoyanova N, Wriggers W, Egelman EH. Actin depolymerizing factor stabilizes an existing state of F-actin and can change the tilt of F-actin subunits. J Cell Biol. 2001;153:75–86. doi: 10.1083/jcb.153.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shemesh T, Otomo T, Rosen MK, Bershadsky AD, Kozlov MM. A novel mechanism of actin filament processive capping by formin: Solution of the rotation paradox. J Cell Biol. 2005;170:889–893. doi: 10.1083/jcb.200504156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero S, Didry D, Larquet E, Boisset N, Pantaloni D, Carlier MF. How ATP hydrolysis controls filament assembly from profilin-actin: Implication for formin processivity. J Biol Chem. 2007;282:8435–8445. doi: 10.1074/jbc.M609886200. [DOI] [PubMed] [Google Scholar]
- 40.Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–1340. doi: 10.1016/s0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki-Kuroda K, Yamamoto Y, Nohara H, Kinoshita M, Fujiwara T, Irie K, Takai Y. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:827–839. doi: 10.1128/MCB.21.3.827-839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evangelista M, Pruyne D, Amberg DC, Boone C, Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat Cell Biol. 2002;4:32–41. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- 43.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 44.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 45.Amann KJ, Pollard TD. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proc Natl Acad Sci USA. 2001;98:15009–15013. doi: 10.1073/pnas.211556398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.