Abstract
Background: High sugar-sweetened beverage (SSB) consumption is associated with cardiometabolic disturbances in adults, but this relation is relatively unexplored in children and adolescents.
Objective: We tested the hypothesis that higher SSB intakes are associated with increases in cardiometabolic risk factors between 14 and 17 y of age.
Design: Data were provided by 1433 adolescent offspring from the Western Australian Pregnancy Cohort (Raine) Study. At 14 and 17 y of age, SSB intakes were estimated by using a food-frequency questionnaire; body mass index (BMI), waist circumference, blood pressure, fasting serum lipids, glucose, and insulin were measured, and overall cardiometabolic risk was estimated. Prospective associations between cardiovascular disease risk factors and SSB intake were examined with adjustment for age, pubertal stage, physical fitness, socioeconomic status, and major dietary patterns.
Results: The average SSB intake in consumers (89%) was 335 g/d or 1.3 servings/d. Girls who moved into the top tertile of SSB consumption (>1.3 servings/d) between 14 and 17 y of age had increases in BMI (3.8%; 95% CI: 1.8%, 5.7%), increased overweight and obesity risk (OR: 4.8, 95% CI: 2.1, 11.4), and greater overall cardiometabolic risk (OR: 3.2; 95% CI: 1.6, 6.2) (all P-trend ≤ 0.001). Girls and boys who moved into the top tertile of SSB intake showed increases in triglycerides (7.0–8.4%; P-trend ≤ 0.03), and boys showed reductions in HDL cholesterol (−3.1%; 95% CI: −6.2%, 0.1%; P-trend < 0.04) independent of BMI. Some associations were attenuated after adjustment for major dietary patterns.
Conclusion: Increased SSB intake may be an important predictor of cardiometabolic risk in young people, independent of weight status.
See corresponding editorial on page 261.
INTRODUCTION
Sugar-sweetened beverages (SSBs)4 have been linked with weight gain, type 2 diabetes, and increased cardiovascular disease (CVD) risk in adults (1, 2). SSBs, which include carbonated (soft) drinks and fruit drinks with added sugar, are purported to increase obesity risk primarily because they provide a liquid form of energy that has less impact on satiety than isoenergetic food (3, 4). SSBs may also have direct effects on cardiometabolic health, independent of weight gain. Experimental and observational studies have shown that high SSB consumption increases the dietary glycemic load, which can lead to insulin resistance, impaired β cell function, and inflammation (1). In addition, high intakes of fructose sweeteners in SSB have been linked to visceral adiposity, hepatic lipogenesis, and hypertension (1, 5)
Children and adolescents in the United States and Australia are high consumers of SSBs (6, 7). Much of the research on SSB consumption in young people has focused on obesity (8); however, there is increasing evidence of cardiometabolic disturbances in children and adolescents (9, 10). To our knowledge, only 2 cross-sectional studies have examined cardiometabolic factors in relation to SSBs in young people (11, 12). A better understanding of the relations between SSBs and cardiometabolic health in young people is required to develop public health nutrition policy for reducing risk of a range of noncommunicable diseases, not only obesity.
This study investigated prospective associations between SSB consumption and cardiometabolic risk factors in a cohort of adolescents for whom diet has been well characterized. It was hypothesized that increases in SSB consumption between 14 and 17 y of age would be positively associated with a greater odds of overweight or obesity and unfavorable changes in CVD risk factors, independent of body weight.
SUBJECTS AND METHODS
Study population
Data were provided by adolescents from the Western Australian Pregnancy Cohort (Raine) Study as detailed previously (13). In brief, 2900 pregnant women at 16–20 wk gestation were recruited through public and private antenatal clinics in Perth, Western Australia, between 1989 and 1991. Of these subjects, 2804 women (97%) had 2868 live births. These children and their families have been followed up at regular intervals since. This article used data from follow-ups at 14 y (2003–2006) and 17 y (2006–2009) of age. Sociodemographic details of the cohort have been published previously (14).
Ethics
All data collection for the Raine Study occurred in accordance with Australian National Health and Medical Research Council Guidelines for Ethical Conduct in Human Research and was approved by the ethics committees of King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, Perth Western Australia. Informed written consent was obtained from adolescents and their primary caregivers.
Dietary assessments
At both follow-ups, study participants completed a semiquantitative food-frequency questionnaire (FFQ) that assessed the usual dietary intake over the previous year (at 14 y of age, this was done with assistance from the parent). The FFQ has been described in detail previously (14) and was evaluated in this cohort for relative validity (15). Dietary misreporting at 14 and 17 y of age was assessed by using the ratio of reported energy intake relative to the estimated energy requirement (16). The Goldberg equation (17) was used to estimate cutoffs for the energy intake:estimated energy requirement to identify probable dietary underreporters (25% at 14 y and 37% at 17 y), plausible reporters (63% and 54%), and overreporters. The FFQ included questions on the frequency of consumption and usual portion size of all carbonated (soft) drinks, cordials or squash (fruit drink concentrate), and fruit juice drinks (with the exclusion of 100% juice). These items were combined to estimate the daily intake of SSBs (g/d), with the exclusion of artificially sweetened or diet beverages. The distribution of SSB intakes was highly skewed, and transformation of the data was not possible because of a large number of individuals with zero intakes. Therefore, SSB intakes were categorized by using tertile cutoffs and rounding these to practical serving sizes to create 3 approximately balanced levels of intake as follows: 0–0.5 servings/d (0–130 g/d; mean ± SD: 48 ± 39 g/d), >0.5–1.3 servings/d (130–329 g/d; mean ± SD: 223 ± 59 g/d), and >1.3 servings/d (331–2876 g/d; mean ± SD: 665 ± 351 g/d), with the assumption that one serving was equivalent to 1 cup [250 mL (8.45 oz) or 261 g].
Cardiometabolic risk factors
Study participants were requested to attend the study clinic for physical assessments at both follow-ups. Calibrated measurements of height and weight were made by using electronic chair scales and a stadiometer. Overweight and obesity were defined by using International Obesity Task Force cutoffs for BMI (18). Waist circumference (WC) was measured at the level of the umbilicus to the nearest 0.1 cm, and the average of 2 measurements was used. Blood pressure was measured by using an oscillometric spygmanometer (Dinamap) after subjects rested supine for 5 min. Diastolic blood pressure (DBP) and systolic blood pressure (SBP) were recorded every 2 min for 10 min; average values, with the exclusion of the first measurement, were used for analyses.
Blood samples were collected at both follow-ups in the participant's home the morning after an overnight fast. Assays to determine serum insulin, glucose, triglycerides, HDL cholesterol, and calculated LDL cholesterol were conducted at PathWest Laboratories at Royal Perth Hospital by using standardized methods detailed previously (19). To estimate insulin resistance, HOMA-IR was calculated as
 |
To avoid adult-derived cutoffs for the metabolic syndrome, a 2-step cluster analysis was previously conducted to identify children at greater metabolic risk (19, 20). In brief, separate cluster analyses were conducted for boys and girls and included BMI, SBP, serum triglycerides, and HOMA-IR. This method classified participants into 2 distinct groups or clusters as follows: a high–metabolic risk cluster or a low–metabolic risk cluster. At both follow-ups, membership in the high–metabolic risk cluster was associated with significantly greater BMI, WC, SBP, DBP, fasting insulin, HOMA-IR, glucose, triglycerides, and LDL cholesterol and lower fasting HDL cholesterol than for subjects classified in the low–metabolic risk cluster (20).
Other assessments
Pubertal development was estimated by using self-reported Tanner stage (pubic hair development) at both follow-ups. Physical fitness was measured by using the Physical Working Capacity 170 test on a bicycle ergometer at each clinic visit as a more-objective measure of physical activity that is highly correlated with self-reported physical activity in this cohort (21). Markers of socioeconomic status included maternal education, which was assessed by using a questionnaire during pregnancy and categorized as low (≤10 y of education) or high (>10 y of education), and family income, which was reported in the parent questionnaire at each follow-up and categorized into quartiles.
SSB intakes may be a marker for a poor-quality diet. We have previously described 2 major dietary patterns in this cohort, which were identified by applying a factor analysis to all food intakes estimated by using the FFQ (14), and their cross-sectional associations with cardiometabolic risk factors (22). At both 14 and 17 y of age, a Western dietary pattern was characterized by high intakes of fat, saturated fat, cholesterol, and refined sugars and correlated with SSB consumption (girls: r = 0.44, P < 0.001; boys: r = 0.39, P < 0.001). A healthy pattern was characterized by high intakes of fiber and micronutrients. Therefore, each participant's z score for both dietary patterns at each follow-up was examined as a potential confounder of relations between SSB intake and cardiometabolic risk factors.
Statistics
Cohort characteristics were first described according to tertiles of SSB intake at baseline (14 y of age). Longitudinal (mixed-effects) models were used to quantify prospective relations between tertiles of SSB intake and cardiometabolic outcomes between 14 and 17 y of age, including a random subject effect (23). Mixed logistic regression models included a dichotomous outcome [overweight or obese (yes or no) or being classified in the high–metabolic risk cluster (yes or no)] and tertile of SSB intake as the main predictor variable of interest, using all available measurements collected at 14 and 17 y of age. Mixed linear regression models included a continuous outcome measurement (BMI, WC, fasting HDL cholesterol, LDL cholesterol, glucose, HOMA-IR, insulin, SBP, or DBP) and the tertile of SSB intake as the main predictor variable of interest, using all available measurements. For both models, the regression coefficient for the tertile of SSB intake was interpreted as the change in outcome associated with each tertile increase in SSB intake (relative to staying in the lowest tertile) observed between 14 and 17 y of age. Because some continuous outcomes were not normally distributed, these were log transformed before analyses so that regression coefficients were interpreted as the percentage of change in the outcome associated with each tertile increase in SSB intake between 14 and 17 y of age. STATA software was used for all statistical analyses (24).
Boys and girls were analyzed separately. Models were first adjusted for age, dietary misreporting, pubertal development, and physical fitness as time-varying covariates and maternal education and family income as fixed covariates. Additional adjustment was made for BMI at 14 and 17 y of age, when appropriate, to test the strength of associations independently of overweight and obesity. A third model was additionally adjusted for Western and healthy dietary pattern z scores at both time points. Because total energy intake is thought to mediate the effect of SSBs as it is on the causal pathway (via weight gain) (1), it was tested in separate models. Tests for trend across tertiles of SSB intake were based on the P value for the SSB tertile when modeled as a continuous variable. Subjects who reported not fasting before venepuncture were excluded from analyses (n = 79 at 14 y of age; n = 33 at 17 y of age).
RESULTS
A total of 1860 adolescents participated in the 14-y follow up; of these adolescents, 1667 subjects (48% girls) completed dietary assessments and 1294 (48% girls) provided a blood sample. One thousand seven hundred fifty-four adolescents participated in the 17-y follow up; of those, 1009 subjects (54% girls) completed the dietary assessment, and 1236 subjects (48% girls) provided a blood sample.
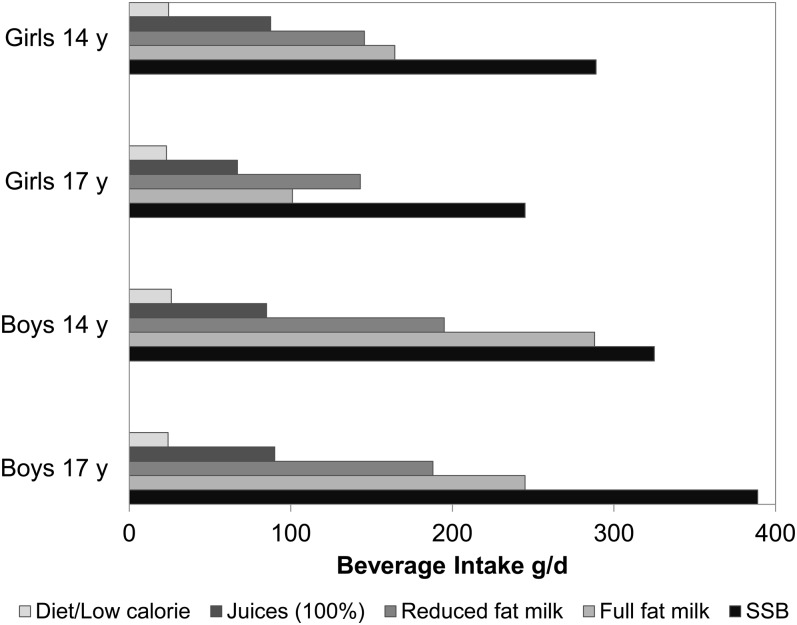
SSBs were the most consumed beverage type for girls and boys (Figure 1), and 89% of respondents were SSB consumers at each follow-up (not shown). SSBs provided 4–5% of total energy intakes of which ∼50% came from carbonated or soft drinks (data not shown). At baseline (14 y of age), the average BMI, WC, total energy intake, SBP, fasting triglycerides, and z score for the Western dietary pattern increased, whereas the average HDL cholesterol, glucose, and z score for the healthy dietary pattern decreased, with increasing intakes of SSBs (P-trend < 0.05) (Table 1). The prevalence of obesity, low maternal education, and low family income increased with higher SSB intakes (P < 0.05) (Table 2).
FIGURE 1.
Mean beverage intakes (g/d) in adolescents at 14 y of age (794 girls; 837 boys) and 17 y of age (541 girls; 468 boys) in the Western Australian Pregnancy (Raine) cohort. Of all beverages, SSBs were consumed in the largest amounts (boys and girls). The mean SSB consumption increased in boys and decreased in girls between 14 and 17 y of age. SSB, sugar-sweetened beverage.
TABLE 1.
Cardiometabolic and dietary characteristics by tertiles of SSB intake at 14 y of age in the Western Australian Pregnancy (Raine) cohort1
| Tertiles of SSB intake |
|||||||
| 1 |
2 |
3 |
|||||
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | P2 | |
| SSB intake (g/d) | 551 | 47.5 ± 37.1 | 534 | 223.8 ± 57.8 | 547 | 651.1 ± 321.2 | <0.001 |
| Age (y) | 551 | 14.0 ± 0.2 | 534 | 14.0 ± 0.2 | 547 | 14.0 ± 0.2 | 0.43 |
| BMI (kg/m2) | 481 | 21.0 ± 3.9 | 473 | 21.0 ± 4.0 | 475 | 21.7 ± 4.4 | <0.01 |
| Waist circumference (cm) | 474 | 74.2 ± 9.9 | 467 | 75.3 ± 11.2 | 473 | 76.8 ± 11.2 | <0.001 |
| Total energy intake (MJ) | 551 | 8.6 ± 2.8 | 534 | 9.7 ± 3.2 | 547 | 10.9 ± 3.4 | <0.001 |
| HDL cholesterol (mmol/L) | 414 | 1.43 ± 0.33 | 409 | 1.39 ± 0.31 | 409 | 1.37 ± 0.31 | 0.01 |
| LDL cholesterol (mmol/L) | 414 | 2.31 ± 0.61 | 409 | 2.33 ± 0.64 | 409 | 2.32 ± 0.65 | 0.89 |
| Glucose (mmol/L) | 414 | 4.85 ± 0.70 | 409 | 4.80 ± 0.41 | 409 | 4.76 ± 0.39 | 0.03 |
| Insulin (mU/L) | 413 | 12.7 ± 10.35 | 408 | 12.28 ± 9.20 | 409 | 12.09 ± 7.44 | 0.77 |
| HOMA-IR | 413 | 2.85 ± 2.88 | 408 | 2.69 ± 2.36 | 409 | 2.58 ± 1.65 | 0.52 |
| Triglycerides (mmol/L) | 414 | 0.96 ± 0.43 | 409 | 1.02 ± 0.68 | 409 | 1.05 ± 0.51 | <0.01 |
| Systolic blood pressure (mm Hg) | 482 | 112.3 ± 11.1 | 472 | 113 ± 10.8 | 474 | 113.8 ± 10.9 | 0.03 |
| Diastolic blood pressure (mm Hg) | 482 | 59.5 ± 7.0 | 472 | 59.2 ± 7.7 | 474 | 59.1 ± 7.5 | 0.07 |
| Physical fitness (watts) | 428 | 111 ± 30 | 412 | 110 ± 29 | 420 | 112 ± 30 | 0.56 |
| Healthy dietary pattern z score | 548 | 0.20 ± 0.91 | 529 | 0.04 ± 0.90 | 537 | −0.24 ± 0.83 | <0.001 |
| Western dietary pattern z score | 548 | −0.43 ± 0.67 | 529 | −0.03 ± 0.78 | 537 | 0.47 ± 0.90 | <0.001 |
SSB, sugar-sweetened beverage.
z test for linear trend (linear regression with the tertile of SSB intake modeled as a continuous variable).
TABLE 2.
Weight status, high–cardiometabolic risk cluster, and socioeconomic markers according to tertiles of SSB intake at 14 y of age in the Western Australian Pregnancy (Raine) cohort1
| Tertiles of SSB intake |
||||
| 1 | 2 | 3 | P2 | |
| M [n (%)] | 266 (31.8) | 280 (33.4) | 291 (34.8) | 0.21 |
| F [n (%)] | 285 (35.9) | 253 (31.9) | 256 (32.2) | — |
| Overweight or obese [n (%)] | 117 (32.0) | 111 (30.3) | 138 (37.7) | 0.10 |
| Overweight [n (%)] | 92 (35.1) | 77 (29.4) | 93 (35.5) | 0.36 |
| Obese [n (%)] | 25 (24.0) | 34 (32.7) | 45 (43.3) | 0.04 |
| High–metabolic risk cluster [n (%)]3 | 112 (31.6) | 112 (31.6) | 130 (36.7) | 0.25 |
| Low maternal education [n (%)] | 162 (28.0) | 171 (29.6) | 245 (42.4) | <0.001 |
| Family income (increasing quartiles) [n (%)] | ||||
| 1 | 127 (27.3) | 139 (29.9) | 199 (42.8) | <0.001 |
| 2 | 101 (30.8) | 108 (32.9) | 119 (36.3) | — |
| 3 | 179 (35.2) | 172 (33.9) | 157 (30.9) | — |
| 4 | 129 (44.2) | 104 (35.6) | 59 (20.2) | — |
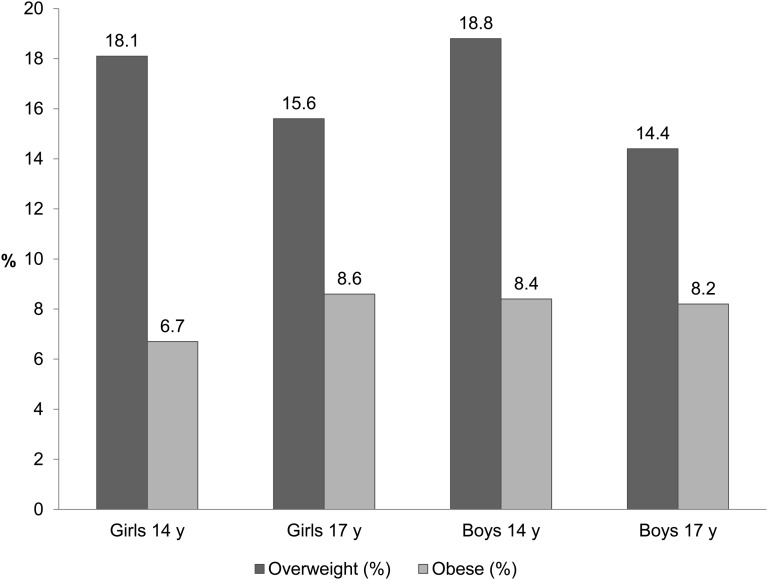
Between 14 and 17 y of age, obesity prevalence in girls increased (6.7–8.6%), whereas overweight decreased (18–15.6%) (Figure 2). Overweight prevalence decreased in boys (18.8–14.4%), but obesity did not alter. These results were not explained by overweight or obese boys being less likely to complete the 17y follow up; similar changes in prevalence were observed in participants who completed both follow-ups (not shown).
FIGURE 2.
Overweight and obesity prevalence at 14 and 17 y of age in the Western Australian Pregnancy (Raine) cohort. Proportions of overweight and obese adolescents in the Raine Study were based on International Obesity Task Force definitions (18) applied to BMI measured at 14 y of age (780 girls; 825 boys) and 17 y of age (620 girls; 631 boys). Between these ages, the prevalence of obesity increased, and overweight decreased in girls. In boys, the prevalence of obesity remained the same, and overweight decreased.
Results of the multivariate prospective analyses are shown in Tables 3 and 4. Girls who moved into the highest tertile of SSB consumption (>1.3 servings/d) between 14 and 17 y of age had a 4.8-times greater odds of overweight or obesity (95% CI: 2.1, 11.4; P-trend < 0.0001) than did girls who remained in the lowest SSB tertile (Table 3). The OR remained high after adjustment for the Western and healthy dietary patterns (OR: 3.8, 95% CI: 1.5, 9.3; P-trend = 0.005). Girls who moved into the highest tertile of SSB consumption between 14 and 17 y had a 3 times greater odds (OR: 3.2; 95% CI: 1.6, 6.2; P-trend = 0.001) of being classified at a greater overall metabolic risk at 17 y than did girls who remained in the lowest SSB tertile (Table 3). This relation persisted after adjustment for major dietary patterns (OR: 2.7; 95% CI: 1.3, 5.6; P-trend = 0.008). These associations were not observed in boys.
TABLE 3.
Odds of overweight or obesity and high–metabolic risk cluster associated with each tertile increase in SSB intake between 14 and 17 y of age1
| Model 1 |
Model 2 |
|||
| SSB tertiles | OR (95% CI) | P2 | OR (95% CI) | P2 |
| Overweight or obese | ||||
| Girls (n = 624) | ||||
| 1 | 1.0 | 1.0 | — | |
| 2 | 1.3 (0.6, 2.8) | 0.54 | 1.1 (0.5, 2.5) | 0.75 |
| 3 | 4.8 (2.1, 11.4) | <0.001 | 3.8 (1.5, 9.3) | 0.004 |
| P-trend | — | <0.001 | — | 0.005 |
| Boys (n = 680) | ||||
| 1 | 1.0 | 1.0 | — | |
| 2 | 1.5 (0.7, 3.3) | 0.28 | 1.5 (0.6, 3.3) | 0.37 |
| 3 | 1.2 (0.6, 2.7) | 0.60 | 0.8 (0.3, 2.1) | 0.76 |
| P-trend | — | 0.65 | — | 0.72 |
| High–metabolic risk cluster3 | ||||
| Girls (n = 537) | ||||
| 1 | 1.0 | 1.0 | — | |
| 2 | 1.3 (0.7, 2.4) | 0.42 | 1.2 (0.6, 2.3) | 0.52 |
| 3 | 3.2 (1.6, 6.2) | 0.001 | 2.7 (1.3, 5.6) | 0.007 |
| P-trend | — | 0.001 | — | 0.008 |
| Boys (n = 587) | ||||
| 1 | 1.0 | — | 1.0 | — |
| 2 | 1.2 (0.6, 2.6) | 0.59 | 1.2 (0.5, 2.5) | 0.73 |
| 3 | 1.3 (0.6, 2.8) | 0.46 | 1.0 (0.4, 2.4) | 0.95 |
| P-trend | — | 0.46 | — | 0.96 |
Model 1 (mixed logistic regression model) was adjusted for age, pubertal stage, physical fitness, dietary misreporting, maternal education, and family income. Model 2 was adjusted as for model 1 and for healthy and Western dietary pattern scores. SSB intake was determined according to population tertiles as follows: tertile 1: 0–0.5 serving/d (0–130 g/d); tertile 2: >0.5–1.3 servings/d (130–329 g/d); and tertile 3: >1.3 servings/d (331–2876 g/d), with the assumption that one serving is equivalent to 1 cup (250 mL or 8.45 oz) or 261 g. ORs (95% CIs) were associated with movement into the SSB tertile between 14 and 17 y of age relative to staying in the lowest tertile. SSB, sugar-sweetened beverage.
z test [null hypothesis (Ho): OR = 1].
TABLE 4.
Changes in cardiovascular risk factors associated with each tertile increase in SSB intake between 14 and 17 y of age1
| Model 1 |
Model 2 |
Model 3 |
||||
| SSB tertiles | Percentage of change (95% CI) | P2 | Percentage of change (95% CI) | P2 | Percentage of change (95% CI) | P2 |
| BMI | ||||||
| Girls (n = 660) | ||||||
| 1 | 0 | — | — | 0 | ||
| 2 | 0.5 (−1.2, 2.2) | 0.59 | — | — | 0.4 (−1.3, 2.1) | 0.64 |
| 3 | 3.8 (1.8, 5.7) | <0.001 | — | — | 3.6 (1.5, 5.8) | 0.001 |
| P-trend | — | <0.001 | — | — | — | 0.002 |
| Boys (n = 706) | ||||||
| 1 | 0 | — | — | 0 | ||
| 2 | 0.6 (−1.3, 2.5) | 0.54 | — | — | 0.3 (−1.6, 2.3) | 0.75 |
| 3 | 1.5 (−0.5, 3.5) | 0.14 | — | — | 0.8 (−1.3, 2.9) | 0.46 |
| P-trend | — | 0.14 | — | — | — | 0.46 |
| WC | ||||||
| Girls (n = 656) | ||||||
| 1 | 0 | — | 0 | — | 0 | — |
| 2 | 1.2 (−0.3, 2.7) | 0.11 | 0.9 (0.02, 1.8) | 0.04 | 0.8 (−0.1, 1.7) | 0.08 |
| 3 | 4.2 (2.5, 5.9) | <0.001 | 1.2 (0.2, 2.2) | 0.014 | 0.9 (−0.2, 2.0) | 0.09 |
| P-trend | — | <0.001 | — | 0.011 | — | 0.07 |
| Boys (n = 704) | ||||||
| 1 | 0 | — | 0 | — | 0 | — |
| 2 | 2.1 (0.5, 3.6) | 0.009 | 1.3 (0.4, 2.3) | 0.006 | 1.3 (0.3, 2.2) | 0.011 |
| 3 | 2.3 (0.7, 4.0) | 0.005 | 1.2 (0.3, 2.2) | 0.013 | 1.4 (0.2, 2.3) | 0.019 |
| P-trend | — | 0.007 | — | 0.019 | — | 0.025 |
| Triglycerides | ||||||
| Girls (n = 537) | ||||||
| 1 | 0 | — | 0 | 0 | ||
| 2 | 4.2 (−1.8, 10.3) | 0.172 | 4.2 (−1.7, 10.2) | 0.17 | 3.8 (−2.4, 9.9) | 0.23 |
| 3 | 10.8 (4.2, 17.3) | 0.001 | 7.0 (0.4, 13.5) | 0.037 | 6.2 (−1.2, 13.7) | 0.10 |
| P-trend | — | 0.001 | — | 0.033 | — | 0.09 |
| Boys (n = 587) | ||||||
| 1 | 0 | — | 0 | 0 | — | |
| 2 | 0 (−7.0, 7.0) | 0.99 | −2.2 (−9.0, 4.6) | 0.52 | −3.5 (−10.5, 3.5) | 0.33 |
| 3 | 10.4 (3.4, 17.5) | 0.004 | 8.4 (1.6, 15.3) | 0.016 | 6.7 (−0.8, 14.1) | 0.08 |
| P-trend | — | 0.003 | — | 0.011 | — | 0.06 |
| HDL cholesterol | ||||||
| Girls (n = 537) | ||||||
| 1 | 0 | — | 0 | — | 0 | — |
| 2 | −1.2 (−4.6, 2.2) | 0.49 | −1.2 (−4.5, 2.1) | 0.46 | −1.4 (−4.8, 1.9) | 0.40 |
| 3 | −5.1 (−8.9, −1.4) | 0.007 | −2.2 (−5.9, 1.5) | 0.24 | −3.1 (−7.2, 1.0) | 0.14 |
| P-trend | — | 0.010 | — | 0.23 | — | 0.14 |
| Boys (n = 587) | ||||||
| 1 | 0 | — | 0 | — | 0 | — |
| 2 | 0.4 (−2.8, 3.6) | 0.79 | 1.2 (−1.9, 4.2) | 0.45 | 1.9 (−1.2, 5.1) | 0.23 |
| 3 | −3.8 (−7.1, −0.5) | 0.024 | −3.1 (−6.2, 0.1) | 0.05 | −2.3 (−5.6, 1.1) | 0.19 |
| P-trend | — | 0.017 | — | 0.038 | — | 0.14 |
| Systolic blood pressure | ||||||
| Girls (n = 660) | ||||||
| 1 | 0 | — | 0 | — | 0 | — |
| 2 | 0.2 (−1.1, 1.5) | 0.76 | 0.2 (−1.1, 1.5) | 0.79 | 0.1 (−1.2, 1.4) | 0.85 |
| 3 | 1.7 (0.3, 3.1) | 0.02 | 0.9 (−0.5, 2.3) | 0.22 | 0.8 (−0.8, 2.4) | 0.33 |
| P-trend | — | 0.02 | — | 0.24 | — | 0.36 |
| Boys (n = 706) | ||||||
| 1 | 0 | — | 0 | — | 0 | — |
| 2 | 0.5 (−0.9, 1.8) | 0.51 | 0.1 (−1.2, 1.5) | 0.84 | 0.3 (−1.0, 1.7) | 0.62 |
| 3 | 0.7 (−0.7, 2.1) | 0.34 | 0.3 (−1.1, 1.6) | 0.69 | 0.8 (−0.7, 2.2) | 0.29 |
| P-trend | — | 0.34 | — | 0.69 | — | 0.29 |
Model 1 was a mixed linear regression model adjusted for age, pubertal stage, physical fitness, dietary misreporting, maternal education, and family income. Model 2 was adjusted as for model 1 and for BMI. Model 3 was adjusted as for model 2 and for healthy and Western dietary pattern scores. SSB intake was determined according to population tertiles as follows: tertile 1: 0–0.5 serving/d (0–130 g/d); tertile 2: >0.5–1.3 servings/d (130–329 g/d); and tertile 3: >1.3 servings/d (331–2876 g/d), with the assumption that one serving is equivalent to 1 cup (250 mL or 8.45 oz) or 261 g. The percentage change (95% CI) in the outcome was associated with movement into the SSB tertile between 14 and 17 y of age relative to staying in the lowest tertile. SSB, sugar-sweetened beverage; WC, waist circumference.
z test [null hypothesis (Ho): Δ = 0].
Compared with staying in the lowest SSB tertile, moving into the highest tertile of SSB intake between 14 and 17 y of age was associated with average increases in BMI of 3.8% (95% CI: 1.8%, 5.7%; P-trend < 0.001) in girls, and this association remained after adjustment for dietary patterns (Table 4). Moving into the highest tertile of SSB consumption was associated with increases in WC that ranged from 2.3% (95% CI: 0.7%, 4.0%) to 4.2% (95% CI: 2.5%, 5.9%) in boys and girls, respectively. These relations were attenuated but remained significant after adjustment for BMI (girls and boys) and dietary pattern scores (boys only) (Table 4).
There was an increasing trend in fasting triglycerides with higher SSB intakes in boys and girls (P-trend ≤ 0.003). Moving into the highest tertile of SSB intake was associated with increases in triglycerides of 10.8% (95% CI: 4.2%, 17.3%; P-trend = 0.001) in girls and 10.4% (95% CI: 3.4%, 17.5%; P-trend = 0.003) in boys (Table 4). These associations were attenuated but remained significant after adjustment for BMI. After adjustment for dietary patterns, the associations were no longer significant (P-trend = 0.09 for girls; P = 0.06 for boys).
HDL cholesterol concentrations decreased with greater SSB intakes in boys and girls (P-trend ≤ 0.02). Compared with staying in the lowest tertile of SSB intake, moving into the highest tertile of SSB intake between 14 and 17 y of age was associated with an average reduction in HDL cholesterol of 5.1% (95% CI: 1.4%, 8.9%) in girls and 3.8% (95% CI: 0.5%, 7.1%) in boys (Table 4). This association persisted in boys after adjustment for BMI (P-trend = 0.038) but was no longer significant after adjustment for dietary patterns (P-trend = 0.14).
Small increases in SBP were suggested with increasing SSB intakes in girls (P-trend = 0.02) but not in boys (Table 4). Girls who moved into the highest tertile of SSB consumption showed average increases in SBP of 1.7% (P = 0.02) or ∼1.9 mm Hg (95% CI: 0.3, 3.5 mm Hg). This association was no longer significant after adjustment for BMI and major dietary patterns.
These associations were unchanged after additional adjustment for total energy intake (not shown). No significant associations were observed between SSB intake and fasting LDL cholesterol, HOMA-IR, glucose, insulin, or DBP (see Supplementary Table 1 under “Supplemental data” in the online issue).
DISCUSSION
These findings support experimental and observational studies on SSB and cardiometabolic health in adults, which have recently been reviewed in detail by other authors (1, 5). We observed that greater SSB intakes during adolescence were prospectively associated with greater overweight and obesity risk and higher overall cardiometabolic risk in girls and, specifically, unfavorable changes in WC, triglycerides, and HDL cholesterol, independent of BMI (for boys and girls). The follow-up period in this cohort of adolescents was relatively short (2 y), and these changes may accumulate over time.
The BMI-independent associations with triglycerides and HDL cholesterol indicated that high SSB intakes may affect lipid profiles via pathways that do not include excess weight. However, these relations were attenuated after adjustment for major dietary patterns, which suggested that SSB intakes may be an indicator of a poor diet associated with an unfavorable lipid profile. Nevertheless, large adult observational studies have linked SSB intakes with increased triglycerides and lower HDL-cholesterol concentrations (25, 26). In the current study, the Western dietary pattern was positively correlated with SSB intakes, and the inclusion of dietary pattern scores in some of our models may have led to overadjustment and attenuation of observed associations with SSB.
The relation between SSB intake, overweight, and obesity in children and adolescents has been fairly well accepted (27) and associations with obesity seen in this study corresponded with those in several longitudinal studies (8) and recent randomized trials (28, 29). However, to our knowledge, only 2 other observational studies have examined SSB intake and other cardiometabolic risk factors in young people. Both studies used cross-sectional US NHANES data that spanned 1999–2004, in which SSB consumption (soft drinks and fruit drinks, excluding pure fruit juices and diet beverages) was estimated by using one 24-h dietary recall. With the use of data from 12- to 19-y-olds (n = 6967), Bremer et al (11) reported that, in girls, each additional serving of SSBs (250 g) was associated with higher HOMA-IR (7%), triglycerides (2.25 mg/dL), SBP (0.38 mm Hg), WC (0.75 cm), and BMI percentile (0.84), and lower HDL cholesterol (0.73 mg/dL). In boys, SSB intake was associated with lower HDL cholesterol (−0.35 mg/dL) and greater WC (0.29 cm) and BMI percentile (0.78) per SSB serving. These associations were adjusted for age, sex, race, menarche, and energy intake but not BMI. Nguyen et al (12) examined SSB intake, uric acid concentrations, and blood pressure in 12–18-y-olds (n = 4938). Adolescents in the highest category of SSB consumption (>36 oz/d or 1.02 L/d) had higher mean SBP (2 mm Hg; 95% CI: 1–2 mm Hg) than that of adolescents who consumed no SSBs after adjustment for age, race, sex, total energy, and BMI z score. This association was similar in magnitude to that shown in the current study (in girls), although at a much higher amount of SSB intake. It is possible that the findings from these NHANES analyses were attenuated because of adjustment for total energy intake (1) and BMI.
SSBs have been hypothesized to influence cardiometabolic risk via their effect on the glycemic load and glucose metabolism (1). However, we did not observe any significant associations between SSB intakes, insulin, or insulin resistance in this adolescent cohort. This result may have been because of typical postpubertal declines in insulin and insulin resistance (30), which were observed in this cohort between 14 and 17 y of age (not shown).
Intakes of SSBs in the current study were high but were slightly lower than those reported by 14–16-y-olds in the 2007 Australian National Children's Nutrition and Physical Activity Survey (mean: 650 ± 465 g/d using a single 24-h dietary recall) (6). The proportion of energy from SSBs in the Raine cohort was approximately one-half of that reported by 14–18-y-olds in the NHANES 2005–2006 (7). Despite slightly lower SSB intakes in the Raine Study, we detected clinically important associations with cardiometabolic risk factors, which have implications for populations consuming higher intakes of SSB.
Associations between SSB intake, family income, and maternal education in this cohort highlighted the potential for parents to influence adolescent SSB intakes, possibly as the main purchasers of food and beverages consumed at home. In the Australian National Children's Nutrition and Physical Activity Survey, SSB intake was also strongly correlated with lower socioeconomic status, and 55% of all SSBs were consumed at home (6).
Some associations between SSB and cardiometabolic risk factors in this study were confined to girls. This result was likely due to sexual dimorphism in body composition or decreases in overweight prevalence in boys during the follow-up period. We have previously observed more-robust associations between diet and cardiometabolic risk factors in girls in this cohort (22).
A limitation of this study was the reduced number of participants who completed the FFQ at the second follow-up. At 17 y of age, study adolescents were asked to complete the FFQ rather than their parent or guardian (when study adolescents were 14 y of age), which may partly explain the reduced response and may have contributed to more dietary underreporting. The slightly lower response may have also led to a selective sample at 17 y that was biased toward cohort members who were more health conscious, which would make the findings less generalizable. However, if true, this bias would raise the possibility that our observations may have been underestimates of true associations between SSB and cardiometabolic risk factors in this cohort. Furthermore, although we attempted to control for major confounders in this study, residual confounding could not be ruled out.
This study benefited from the prospective design and use of longitudinal models that used all available data while accounting for within-person correlations in repeated measurements. The FFQ was designed to estimate typical food and beverage consumption over the previous year, thereby capturing infrequently consumed items and seasonal variations, which is not possible by using a single 24-h dietary recall. However, like all dietary assessment methods, FFQs have their inaccuracies. Dietary underreporting is common in adolescents regardless of the dietary assessment method used, and we attempted to control for this by using a standardized method. Unlike earlier studies (11, 12), we attempted to adjust for pubertal development. In addition, we examined a composite measure of overall cardiometabolic risk on the basis of the clustering of several CVD risk factors, which may be more informative than examining individual CVD risk factors.
In conclusion, this study is one of few studies to examine prospective associations between SSB intake and cardiometabolic risk factors in adolescents while considering BMI and major dietary patterns. The results provide evidence that moderate SSB consumption (>1.3 cup/d) during adolescence may have adverse consequences for cardiometabolic health, some of which may be independent of weight status. The results suggest that SSB consumption should be limited in young people to reduce future cardiometabolic risk.
Supplementary Material
Acknowledgments
We are extremely grateful to all families who took part in this study and the entire Raine Study team, including data collectors, cohort managers, data managers, clerical staff, research scientists, and volunteers. We acknowledge the Commonwealth Scientific and Industrial Research Organisation for the use of its FFQ.
The authors’ responsibilities were as follows—GLA, LJB, and WHO: designed the research; GLA: analyzed data and wrote the manuscript; RCH (metabolic cluster analysis), TAM (biochemistry), and WHO (dietary assessment) conducted research; GLA and SAJ: had primary responsibility for the final content of the manuscript; and all authors: contributed to data interpretation and manuscript preparation and approved the final version of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DBP, diastolic blood pressure; FFQ, food-frequency questionnaire; SBP, systolic blood pressure; SSB, sugar-sweetened beverage; WC, waist circumference.
REFERENCES
- 1.Malik VS, Popkin BM, Bray GA, Despres J-P, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik VS, Popkin BM, Bray GA, Despres J-P, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan A, Hu FB. Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care 2011;14:385–90. [DOI] [PubMed] [Google Scholar]
- 4.Cassady BA, Considine RV, Mattes RD. Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr 2012;95:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics 2012;129:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifton PA, Chan L, Moss CL, Miller MD, Cobiac L. Beverage intake and obesity in Australian children. Nutr Metab (Lond) 2011;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc 2010;110:1477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavey R-EW. How sweet it is: sugar-sweetened beverage consumption, obesity, and cardiovascular risk in childhood. J Am Diet Assoc 2010;110:1456–60. [DOI] [PubMed] [Google Scholar]
- 9.Bremer AA, Lustig RH. Effects of sugar-sweetened beverages on children. Pediatr Ann 2012;41:26–30. [DOI] [PubMed] [Google Scholar]
- 10.Daniels SR. Lipid concentrations in children and adolescents: it is not all about obesity. Am J Clin Nutr 2011;94:699–700. [DOI] [PubMed] [Google Scholar]
- 11.Bremer AA, Auinger P, Byrd RS. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: findings from the 1999-2004 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med 2009;163:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen S, Choi HK, Lustig RH, Hsu C-Y. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr 2009;154:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newnham JP, Evans SF, Michael CA, Stanley JF, Landau LI. Effects of frequent ultrasound during pregnancy - a randomised controlled trial. Lancet 1993;342:887–91. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosini GL, Oddy WH, Robinson M, O'Sullivan TA, Hands BP, de Klerk NH, Silburn S, Zubrick SR, Kendall GE, Stanley FJ, et al. Adolescent dietary patterns are associated with lifestyle and family psycho-social factors. Public Health Nutr 2009;12:1807–15. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosini GL, de Klerk NH, O'Sullivan TA, Beilin LJ, Oddy WH. The reliability of a food frequency questionnaire for use among adolescents. Eur J Clin Nutr 2009;63:1251–9. [DOI] [PubMed] [Google Scholar]
- 16.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39(suppl 1):5–41. [PubMed] [Google Scholar]
- 17.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991;45:569–81. [PubMed] [Google Scholar]
- 18.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–3. [DOI] [PMC free article] [PubMed]
- 19.Huang R-C, Mori TA, Burke V, Newnham J, Stanley FJ, Landau LI, Kendall GE, Oddy WH, Beilin LJ. Synergy between adiposity, insulin resistance, metabolic risk factors and inflammation in adolescents. Diabetes Care 2009;32:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang RC, Mori TA, Burrows S, Le Ha C, Oddy WH, Herbison C, Hands B, Beilin LJ. Sex dimorphism in the relation between early adiposity and cardiometabolic risk in adolescents. J Clin Endocrinol Metab 2012;97:E1014–22. [DOI] [PubMed] [Google Scholar]
- 21.Hands B, Larkin D, Parker H, Straker L, Perry M. The relationship among physical activity, motor competence and health-related fitness in 14-year-old adolescents. Scand J Med Sci Sports 2009;19:655–63. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosini GL, Huang RC, Mori TA, Hands BP, O'Sullivan TA, de Klerk NH, Beilin LJ, Oddy WH. Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr Metab Cardiovasc Dis 2010;20:274–83. [DOI] [PubMed] [Google Scholar]
- 23.Twisk JWR. Applied longitudinal data analysis for epidemiology. A practical guide. Cambridge, United Kingdom: Cambridge University Press, 2003. [Google Scholar]
- 24.Stata Corp. Stata/SE 12.1 for Windows. College Station, TX: StataCorp LP, 2012. [Google Scholar]
- 25.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8. [DOI] [PubMed] [Google Scholar]
- 26.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs NF, Jacobson MS; American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics 2003;112:424–30. [DOI] [PubMed] [Google Scholar]
- 28.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397–406. [DOI] [PubMed] [Google Scholar]
- 29.Ebbeling CB, Feldman HA, Chomitz VR, Ellenbogen SJ, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.