Abstract
Background: The effect of long-chain polyunsaturated fatty acid (LCPUFA) intake on cognitive development is controversial. Most randomized trials have assessed cognition at 18 mo, although significant development of cognitive abilities (early executive function) emerge later.
Objective: The objective was to evaluate cognition beyond 18 mo and longitudinal cognitive change from 18 mo to 6 y in children who were fed variable amounts of docosahexaenoic acid (0.32%, 0.64%, and 0.96% of total fatty acids) and arachidonic acid (ARA; 0.64%) compared with children who were not fed LCPUFA as infants.
Design: Eighty-one children (19 placebo, 62 LCPUFA) who participated in a double-blind, randomized trial of LCPUFA supplementation as infants were re-enrolled at 18 mo and tested every 6 mo until 6 y on age-appropriate standardized and specific cognitive tests.
Results: LCPUFA supplementation did not influence performance on standardized tests of language and performance at 18 mo; however, significant positive effects were observed from 3 to 5 y on rule-learning and inhibition tasks, the Peabody Picture Vocabulary Test at 5 y, and the Weschler Primary Preschool Scales of Intelligence at 6 y. Effects of LCPUFAs were not found on tasks of spatial memory, simple inhibition, or advanced problem solving.
Conclusions: The data from this relatively small trial suggest that, although the effects of LCPUFAs may not always be evident on standardized developmental tasks at 18 mo, significant effects may emerge later on more specific or fine-grained tasks. The results imply that studies of nutrition and cognitive development should be powered to continue through early childhood. This parent trial was registered at clinicaltrials.gov as NCT00266825.
INTRODUCTION
The long-chain PUFAs (LCPUFAs)5 DHA (22:6n−3) and arachidonic acid (ARA, 20:4n−6) are concentrated in the central nervous system, and it has been suspected that variations in LCPUFA status could influence cognitive function. Because DHA concentrations in the central nervous system accumulate during late pregnancy through early childhood and are affected by diet in early development (1–3), the effects of LCPUFA status and dietary intake (particularly DHA) have been increasingly evaluated through the use of measures of early cognitive development (4–7).
The hypothesis that LCPUFAs might affect cognitive function has been supported by studies of nonhuman primate infants (8) and human infants (9–11). However, the results of LCPUFA studies using standardized outcomes in the second year are mixed. Some reports find positive effects of LCPUFAs on standardized measures of developmental status (12–15); however, systematic reviews and meta-analyses that rely mainly on performance on the Bayley Scales of Infant Development (BSID) at 18 mo have unanimously concluded that there is no benefit of dietary LCPUFA supplementation (16–22). A recent large trial of preterm infants showed no advantage of 1% DHA compared with 0.3% DHA on the BSID at 18 mo (23).
We have argued for a comprehensive and sensitive approach that assesses the developmental trajectories of specific measures of cognitive function to evaluate the effects of LCPUFA supplementation (24, 25). Here we report long-term follow-up of LCPUFA-supplemented infants from such a perspective. The data included are results of planned secondary outcomes at 1 of the 2 sites involved in the DHA Intake and Measurement of Neural Development study (NCT00753818 at www.clinicaltrials.gov). Positive effects of LCPUFA on infant visual acuity development are reported elsewhere in this journal (26). In addition, we reported positive effects of LCPUFAs on first-year attention in the full cohort studied at our site (10). The cognitive data presented here extends assessment from 18 mo to 6 y of age in children at our site, whose parents consented to longer follow-up. The data collected represents finer-grained laboratory-based measures of several discrete aspects of cognitive function obtained longitudinally. Standardized tests were administered at the end of the sampling period to include outcomes that are better known to scientists and practitioners from various disciplines.
SUBJECTS AND METHODS
Subjects
The trial enrolled healthy, full-term, formula-fed, singleton-birth infants (37–42 wk gestation; 2490–4200 g birth weight) born in the Kansas City metropolitan area between 3 September 2003 and 25 September 2005. Exclusion criteria included the receipt of human milk within 24 h of randomization—maternal and newborn health conditions known to interfere with normal growth and development (eg, intrauterine growth restriction) or with normal cognitive function (eg, congenital anomalies or established genetic diagnoses associated with intellectual disability), poor formula intake, or intolerance to cow milk infant formula. Infants were also excluded if born to mothers with physician-documented chronic illness (eg, HIV, renal or hepatic disease, type 1 or type 2 diabetes, alcoholism, or substance abuse).
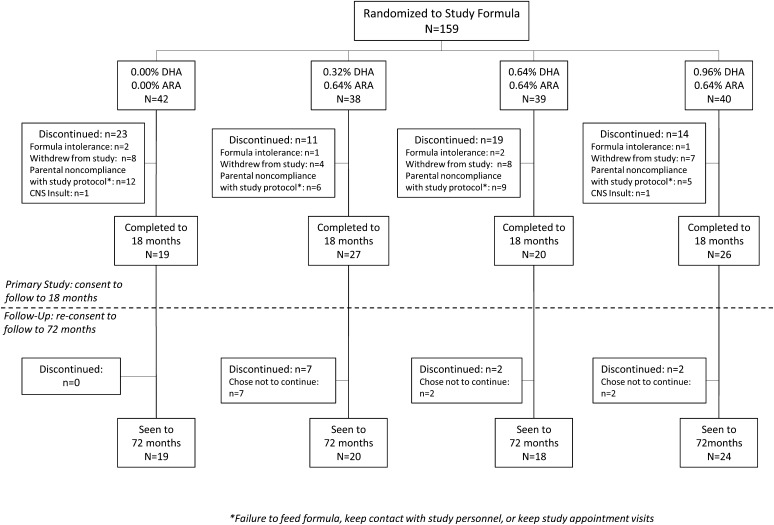
The sample was recruited from a population with a low socioeconomic status. The demographic characteristics of the sample enrolled in Kansas City (n = 159) and the sample that consented to the extended follow-up (n = 81) are shown in Table 1. The follow-up sample was very representative of the original sample. The only detectable difference between the original sample and the follow-up sample was the disproportionately smaller number of males who participated in follow-up: 47% males in the original and 37% males in the follow-up sample. Participant flow is shown in Figure 1. Informed consent was obtained from all participating families, and the study was approved by the Human Subjects Committee at the University of Kansas Medical Center.
TABLE 1.
Characteristics of the enrolled trial sample (n = 159), the follow-up sample (n = 81), and the 4 randomized groups1
| Formula group |
|||||||
| Enrolled (n =159) | Follow-up (n = 81) | 0.00% (n = 18) | 0.32% (n = 21) | 0.64% (n = 18) | 0.96% (n = 24) | P | |
| Maternal age (y) | 24.1 ± 5.12 | 23.9 ± 4.6 | 23.3 ± 4.5 | 24.5 ± 5.0 | 23.9 ± 3.7 | 23.8 ± 5.2 | NS |
| Maternal education (y) | 11.9 ± 1.7 | 12.0 ± 1.5 | 12.2 ± 1.8 | 12.0 ± 1.6 | 11.5 ± 1.3 | 12.1 ± 1.5 | NS |
| Income, by zip code (US$) | 35,960 ± 12,936 | 34,083 ± 10,804 | 34,847 ± 8767 | 34,989 ± 13,667 | 36,843 ± 12,363 | 30,905 ± 6749 | NS |
| No. of living children | 2.5 ± 1.3 | 2.5 ± 1.3 | 2.1 ± 1.2 | 2.7 ± 1.4 | 2.6 ± 1.4 | 2.4 ± 1.3 | NS |
| Smoking during pregnancy (packs/d) | 0.08 ± 0.19 | 0.11 ± 0.22 | 0.06 ± 0.12 | 0.13 ± 0.26 | 0.14 ± 0.26 | 0.10 ± 0.23 | NS |
| Parent PPVT3 | 89.5 ± 13.4 | 88.2 ± 12.0 | 86.2 ± 13.4 | 89.4 ± 8.8 | 94.0 ± 12.0 | 85.1 ± 12.4 | NS |
| Ethnicity (%) | NS | ||||||
| African American | 60.6 | 63.9 | 83.3 | 52.4 | 55.6 | 64.0 | |
| White | 38.3 | 34.9 | 16.7 | 42.9 | 44.4 | 36.0 | |
| Other | 1.1 | 1.2 | 0.0 | 4.8 | 0.0 | 0.0 | |
Analyses of continuous variables were tested by comparing initially enrolled subjects with subjects who agreed to the follow-up study (t test) and against the 4 randomized groups (ANOVA), except smoking (nonparametric). Categorical variables were analyzed by using chi-square tests.
Mean ± SD (all such values).
PPVT, Peabody Picture Vocabulary Test.
FIGURE 1.
Participant flow in the follow-up study. The primary study (26) followed children to 18 mo. The current study reports on measures taken from 18 to 72 mo of age. ARA, arachidonic acid; CNS, central nervous system.
Design and randomization
Infants were randomly assigned after delivery to infant formulas that varied only in LCPUFA content. Control infants were fed formula containing no DHA or ARA. The other 3 groups received formulas containing 0.32% of fatty acids from DHA (17 mg/100 kcal), 0.64% from DHA (34 mg/100 kcal), or 0.96% from DHA (51 mg/100 kcal); these 3 latter formulas also included 0.64% of fatty acids (34 mg/100 kcal) from ARA. Infants were fed the study formulas for the first 12 mo without limits. The study remained blinded to the parents and all personnel conducting nutritional and developmental assessments until the last child reached 6 y of age.
Sample size
Of 159 infants who were enrolled to be followed to 18 mo in the parent trial, 106 completed the study. Eighty-one of these families consented to follow-up to 6 y The attrition observed from birth to 6 y is in keeping with other large-scale long-term longitudinal studies that we have conducted in following children from infancy to the preschool years (27). Across the follow-up from 18 through 72 mo of age, the total sample size tested remained constant at 81, which was distributed among the 4 formula groups as follows: n = 18 for 0.00% DHA/0.00% ARA, n = 21 for 0.32% DHA/0.64% ARA, n = 18 for 0.64% DHA/0.64% ARA, and n = 24 for 0.96% DHA/0.64% ARA. At each age, the usability of data from each task administered was determined by those administering the tasks; as a result, the actual analysis for any age point may be less than these totals (actual sample sizes are shown in the figures, where appropriate); however, the use of mixed-model analyses (see the Statistics section below) made use of all available data in the evaluation of the effects of supplementation. The University of Kansas Medical Center Human Subjects Committee (HSC) approved both the original randomized trial (HSC no. 9198) and the follow up (HSC no. 10205).
Measures
The longitudinal schedule of measures is shown in Table 2. The tasks administered in the preschool period focused on executive function (working memory, inhibitory control, attentional flexibility, planning, and strategy on various complex tasks), verbal performance, and intelligence. The tasks were chosen to be developmentally sensitive and age appropriate. Maternal smoking history (packs/d; Table 1) did not correlate with any developmental outcome measures. Only 3 women in the entire sample reported alcohol use during pregnancy, and only 1 reported alcohol use more than once per week.
TABLE 2.
Schedule of longitudinal assessments
| Age at longitudinal visit |
||||||||
| Cognitive task/assessment instrument | 18 mo | 24 mo | 30 mo | 36 mo | 42 mo | 48 mo | 60 mo | 72 mo |
| Bayley Scales of Infant Development, 2nd edition | x | |||||||
| MacArthur-Bates Communicative Development Inventory | x | |||||||
| Delayed Response | x | x | x | |||||
| Bear-Dragon Go/No Go | x | x | x | |||||
| Stroop (red/yellow and day/night) | x | x | x | x | ||||
| Dimensional Change Card Sort task | x | x | x | x | ||||
| Tower of Hanoi | x | x | x | |||||
| Peabody Picture Vocabulary Test, 4th edition | x | |||||||
| Weschler Primary Preschool Test of Intelligence, 3rd edition | x | |||||||
Bayley Scales of Infant Development, version 2, at 18 mo
The Bayley Scales of Infant Development, version 2 (BSID-2) is a well-standardized and common assessment of infant developmental status (28). The BSID-2 is applicable from birth to 3 y of age and yields composite scores (normed at 100) for both a Mental Developmental Index (MDI) and a Psychomotor Development Index (PDI).
MacArthur-Bates Communicative Development Inventory at 18 mo
The MacArthur-Bates Communicative Development Inventory (MBCDI) is a measure of early communicative competence. It is a parent-report measure that has been validated (29) against actual child language diaries. The primary dependent measure for this study was the child's productive (expressive) vocabulary—a measure of the number of the words that the parent reports the child actually says or has said.
Delayed Response task at 24, 30, and 36 mo
The Delayed Response (DR) task (30) is an assessment of spatial memory. It was conducted by using a raised box (1.0 m × 0.5 m × 0.25 m) in which a toy was buried in various locations in a box of lentils, and the child was required to find it after a 3- or 10-s delay. With the child seated on their caregiver's lap at the center point of the box, a small toy was buried (at 24 mo, 0.33 m from the center point of the box; at 36 mo, 0.16 m from the center point) in full view of the child to the child's left or right (counterbalanced across children), and the child was allowed to find the toy after the prescribed delay. Three trials were conducted using a 3-s delay at one location, and then the position location was reversed for 2 more trials using a 10-s delay. The session was videorecorded, and the accuracy of the child's searches was coded (in inches) from the recording. Approximately 25% of the sessions were coded by a second observer, and the average interobserver reliability (across all ages) for distance between the actual location and the point of the child's search was r = 0.93, and any coded difference of ≥0.5 inches (1.27 cm) more was resolved among the coders.
Bear-Dragon Go/No-Go Task at 36, 42, and 48 mo
The Bear/Dragon task (31) is a variant of the classic “Simon Says” game, in which children follow the commands of a “Bear” puppet but ignore the commands of a “Dragon” puppet. The commands are common actions that children know how to perform (eg, sticking out one's tongue, touching one's toes). After 5 practice trials with feedback, 10 test trials were administered (Bear/Dragon commands alternated). Responses were scored from 0 to 3 for each of 10 trials, based on both following the Bear commands and inhibiting after the Dragon commands. The highest total score possible was 30, and a proportion score out of this total was calculated. Calculation of scores was double-checked for all participants; for any questionable responses, raters referred back to the video of the session to confirm or correct scoring.
Stroop tasks at 36, 42, 48, and 60 mo
Children were required to follow a rule that ran counter to an automatic response. In the first (red/yellow) form of the task (32), the child was asked to point to an opposite color when experimenters said the name of common red/yellow pairings (eg, apple/banana, ketchup/mustard, and Elmo/Big Bird). Experimenters instructed the child to point to, for example, the yellow square when the experimenter said apple, and to the red square if the experimenter said banana. In the second (day/night) variant (33), the child was presented with a yellow moon with stars on a black background or a yellow sun on a white background. After the child confirmed that they understood the moon/night and sun/day association, the child was then asked to say “day” when the moon was presented and “night” when the sun was presented. In both tasks, children were given up to 8 practice trials with feedback and further instruction as needed, and then 16 test trials were administered (with lateral position of the 2 choices counterbalanced across trials). The primary dependent variable for both tasks was the number correct. Again, calculation of scores was double-checked, and raters referred back to the video of the session to correct scoring for any questionable responses.
Dimensional Change Card Sort at 36, 42, 48, and 60 mo
The Dimensional Change Card Sort (DCCS) task is based on classic discrimination learning and is administered according to a standard protocol (34); it provides data on rule learning and the ability to inhibit use of an old rule when a new one is introduced. Children were presented with cards that could be sorted on the basis of either shape or color; after sorting cards on one dimension (eg, shape: rabbits or cars), they were asked to sort cards on the other dimension (eg, to color: red or blue). Before each trial, the tester pointed to each box and stated which category was to be sorted into which box; then, after showing a card, the tester labeled the card to facilitate sorting. Six trials followed after 2 practice trials; the child was reminded of the rule before each trial. After 6 test trials, the rule was switched. Correct responses were recorded for both pre- and postswitch phases. Calculation of scores was double-checked for all participants, and raters referred back to the video of the session to correct scoring for any questionable responses.
Tower of Hanoi task at 48, 60, and 72 mo
The Tower of Hanoi is a traditional puzzle that is used as a neuropsychological and developmental assessment of rule-learning and maintenance, goal-directed behavior, planning, and error correction (35). Three different-sized disks are stacked in ascending order of size on 1 of 3 pegs. The goal for the child was to move the ordered stack to another peg, with the following constraints: 1) moving only one disk at a time, 2) moving only the topmost disk from any stack, and 3) following the rule that only smaller disks could be placed on top of larger ones; the optimal solution requires 7 steps. For testing with children, the task starts 2 steps from the final solution, and the child must demonstrate 2 consecutive solutions solving in the fewest steps possible to advance to the next level of the task. If the child could advance after 6 trials at a given level, that level was considered to be ceiling level and testing was stopped. In addition to the child's ceiling level, we calculated a processing efficiency score in which higher scores were given if optimal solutions were realized in early trials rather than in later trials. All score calculations were double-checked for errors. Because coding is a very objective task, only 10% of the sessions were coded a second time; in these cases, observers were found to be in 100% in agreement.
Peabody Picture Vocabulary Test, 3rd edition at 60 mo
The Peabody Picture Vocabulary Test, 3rd edition (PPVT-III) is a well-standardized and widely used measure of vocabulary for individuals from 2.5 to 90 y of age (36); it is used in some cases as an indicant of verbal intelligence quotient (IQ) or as a surrogate for overall IQ.
The Weschler Preschool Primary Intelligence Scale, 3rd edition at 72 mo
The Weschler Preschool Primary Intelligence Scale, 3rd edition (WPPSI-III) is among the most widely used and best-standardized tests of early intelligence available (37). At this age, there are 8 core subtests (Information, Vocabulary, Word Reasoning, Block Design, Matrix Reasoning, Picture Concepts, Symbol Search, and Coding), which are combined in various configurations to provide a Verbal IQ, a Performance IQ, a Full-Scale IQ score, and a Processing Speed Quotient.
Statistics
Characteristics of the population and data were analyzed by using SPSS/PASW (version 20; IBM Corporation). For the population characteristics evaluated in Table 1, we used ANOVA, except for smoking, for which a nonparametric analysis was conducted. For data on which repeated measures were available, we used mixed-model analyses; these analyses use all of the available data and avoid the list-wise deletion issues (and loss of power) that are common with repeated-measures ANOVAs. Covariances were left unstructured (a conservative setting), and the subjects were included as a random factor. The initial analyses were performed in the 4 groups of the study (control and 3 LCPUFA groups). When appropriate, we performed additional analyses that included 2 covariates that represented variability specific to the population we sampled: maternal PPVT (obtained during testing visits) and household income (derived from the zip code of the participant's listed residence). Income is a consistent correlate with race, which we confirmed in the current data set (η2 = 0.31, P < 0.001); however, we examined the influence of ethnicity and found that it did not affect the outcome of effects of the formula reported below. Significant effects from these analyses were followed up with Dunnett's tests, which contrasted each of the LCPUFA groups against the control group, assuming a positive effect of dietary LCPUFAs. In addition, secondary analyses were conducted in some cases in which the 3 LCPUFA groups were collapsed to form a single group, which was then tested against the control group; because it was hypothesized that groups fed LCPUFAs would perform better than the control group, these were evaluated with one-tailed tests. For assessments in which no repeated measures were taken (ie, the BSID-2, MBCDI productive vocabulary, PPVT-III, and WPPSI-III), simple ANOVAs were run to compare the 4 groups, and secondary comparisons between the 3 LCPUFA groups collapsed and the control group were conducted by using a t test. For all analyses we report the test statistic (F; variance of the group means divided by the within group variances). P ≤ 0.05 was considered to be significant.
RESULTS
BSID-2 and -I
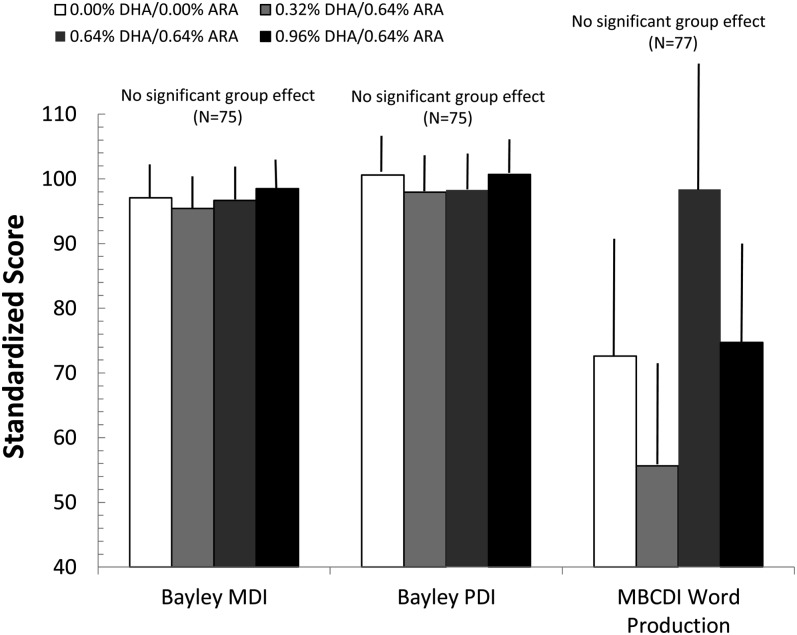
One-factor ANOVAs performed on the BSID-2 yielded no significant differences as a function of group: F(3,72) = 0.03 for the MDI (NS) and F(3,65) = 0.46 for the PDI (NS). These results were unchanged when maternal PPVT-III and household income were covaried in the analyses: F(3,67) = 0.40 for the MDI (NS), F(3,66) = 0.11 for the PDI (NS), and F(3,70) = 0.86 for the MBCDI (NS). The primary measure of interest from the MBCDI was total word production. This measure did not vary as a function of group: F(3, 82) = 0.68 (NS); because of the high variability of vocabulary at 18 mo, this variable was also subject to nonparametric analyses and parametric analyses (with covariates) of transformed values, with the same results. Means (±SEs) for these measures (corrected for covariates) are shown as a function of the 4 randomized formula groups in Figure 2. Furthermore, no significant effects were observed when the 3 LCPUFA groups were collapsed and tested against the control group, and the results were unchanged if maternal PPVT-III and household income were entered in covariates. The effect sizes (Cohen's d) observed for these comparisons were 0.004 and 0.187, respectively, for the MDI and PDI (with the latter actually favoring the control group) and d = 0.029 for MBCDI productive vocabulary.
FIGURE 2.
Mean (±SE) scores for the BSID-2 subscales and MBCDI productive vocabulary test (adjusted for covariates) at 18 mo as a function of group assignment. Neither ANOVA nor ANCOVA showed any significant differences as a function of formula on these measures. ARA, arachidonic acid; BSID, Bayley Scales of Infant Development; MBCDI, MacArthur-Bates Communicative Development Inventory; MDI, Mental Developmental Index; PDI, Psychomotor Development Index.
DR
A group (4 categories) × age (3 categories) × side (2 categories) × trial (3 categories) mixed-model analysis was conducted on performance data from the DR task. This analysis yielded no significant results, and no further results are shown or discussed.
Bear-Dragon
A group (4 categories) × age (3 categories) × trial type (2 categories; Bear compared with Dragon) mixed-model analysis yielded several significant findings, although none showed an effect of LCPUFA dose. Significant effects included a main effect of age [F(2, 53.955) = 33.667, P < 0.001], as children improved their performance across the 3 ages tested. In addition, a significant main effect of trial type was shown [F(1, 60.249) = 273.840, P < 0.001], as children overall performed significantly better on the Bear tasks (which required action) than on the Dragon tasks (which required inhibition). Both main effects were qualified by an age × trial type interaction [F(2, 54.635) = 21.372, P < 0.001], as children improved more dramatically in the Dragon trials with age than in the Bear trials. None of the other effects (all of which involved group) were statistically significant or nearly such.
DCCS
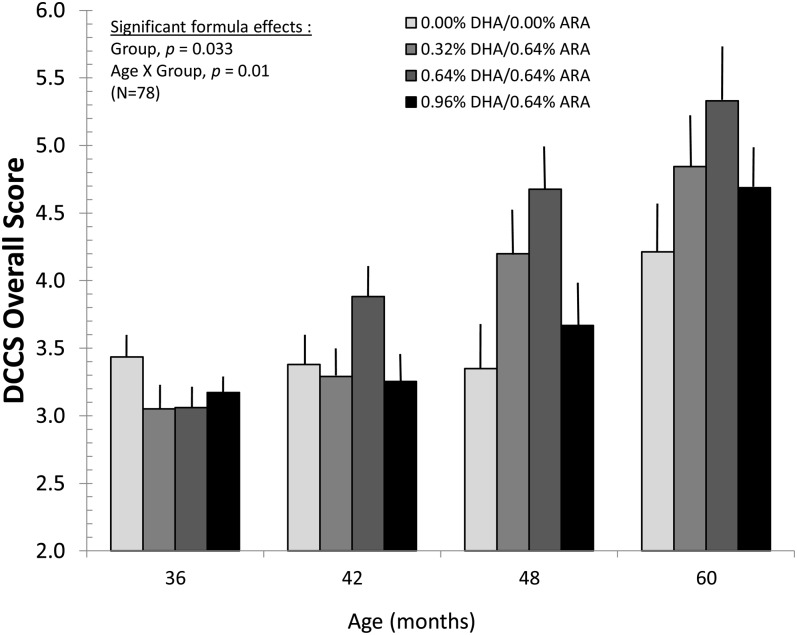
The earliest major effect of LCPUFA intake in the current study was observed on the DCCS; overall scores were analyzed by using an age (4 categories) × group (4 categories) × preswitch/postswitch (2 categories) mixed model. Main effects emerged for age [F(3, 67.336) = 33.832, P < 0.001], as older children showed improved scores, and for preswitch/postswitch mixed model [F(1, 72.390) = 228.374, P < 0.001], as children performed better on trials while learning the initial contingencies (preswitch) than on trials when the initial contingencies were changed (postswitch). These 2 main effects were qualified by a significant age × preswitch/postswitch interaction [F(3, 69.337) = 3.389, P = 0.023], as performance increased more dramatically across ages for postreversal trials than for prereversal trials. With respect to LCPUFA intake, a main effect also emerged for group [F(3, 72.117) = 3.062, P = 0.033], as the middle 2 DHA groups (0.32% DHA/0.64% ARA and 0.64% DHA/0.64% ARA) performed significantly better than the control group and the highest DHA group. This main effect was qualified by a significant group × age interaction [F(9, 67.458) = 2.673, P = 0.01]; means (±SEs) for this interaction are shown in Figure 3. As is evident from the figure, overall performance on the DCCS improved linearly from 36 to 48 mo for the 2 middle-dose groups; performance in the highest dose group increased from 42 to 48 mo of age. The control group showed no improvement on this task until 60 mo of age. These findings were unchanged by the addition of maternal PPVT and household income as covariates; the critical group × age interaction remained significant [F(9, 67.069) = 2.606, P = 0.012], as did the other significant terms described here.
FIGURE 3.
Mean (±SE) overall DCCS scores as a function of group assignment and age at assessment. Infants who received supplemented formula had accelerated developmental improvement on this task compared with the control group. Mixed-model analyses showed positive effects of diet on this task, which were most evident in the comparison of controls with the 0.32% DHA and 0.64% DHA groups at 48 and 60 mo. ARA, arachidonic acid; DCCS, Dimensional Change Card Sort.
The pattern of effects yielded by the 4-group analysis persisted when the 3 LCPUFA groups were collapsed and tested against the control group. The LCPUFA/control × age × preswitch/postswitch mixed model yielded significant effects for age [F(3, 69.759) = 15.174, P < 0.001], preswitch/postswitch [F(1, 77.037) = 179.279, P < 0.001], and for the age × LCPUFA/control interaction [F(3, 69.785) = 4.389, P = 0.007]. In this analysis, the terms were nearly significant for the LCPUFA/control main effect [F(1, 75.671) = 2.775, P = 0.10] and the age × preswitch/postswitch interaction [F(3, 73.019) = 2.232, P = 0.092]. The effect size for the LCPUFA/control comparison was d = 0.50, which favored the LCPUFA group. Again, this outcome was unchanged when maternal PPVT-III and household income were added as covariates; the age × LCPUFA/control interaction remained significant [F(3, 69.435) = 4.290, P = 0.008].
Stroop tasks
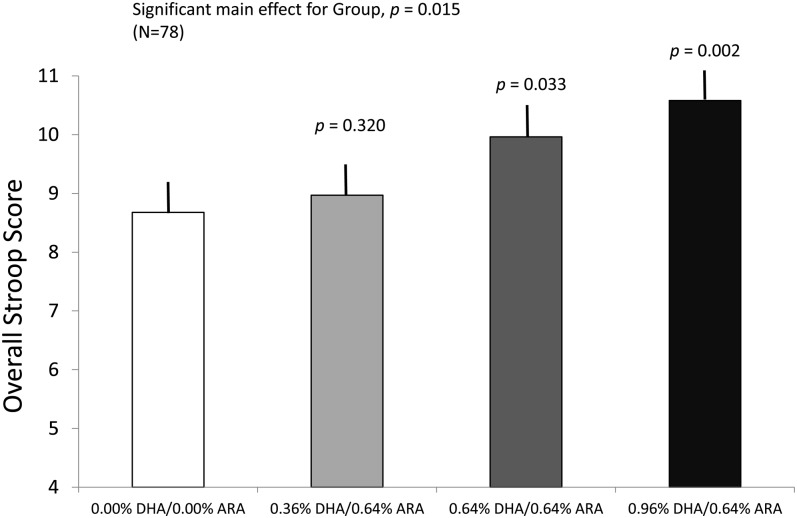
Along with group (4 categories) and age (4 categories), there were 2 variants of the Stroop task. Because performance scores were on the same scale for both tasks, we entered task (2 categories) as a factor in the mixed-model analysis. The analysis yielded significant main effects of group [F(3, 67.407) = 3.723, P = 0.015], as, overall, performance of the children assigned to the 2 higher LCPUFA doses (0.64% DHA/0.64% ARA and 0.96% DHA/0.64% ARA) was significantly greater than that of the control group (P = 0.03 and P = 0.002, respectively). The lowest LCPUFA dose (0.32% DHA/0.64% ARA) was intermediate and did not differ from either the control group or the 2 other dose groups. Along with the group effects, we also observed a significant main effect of age [F(3, 65.198) = 24.232, P < 0.001], as the children's performance improved from 36 to 60 mo of age. No other significant main effects or interactions emerged. Means (±SEs) from the main effect of the formula groups (collapsed across both age and task) are shown in Figure 4. This results were unchanged when maternal PPVT-III and household income were added as covariates, ie, the group main effect remained significant [F(3, 65.930) = 3.548, P = 0.019].
FIGURE 4.
Mean (±SE) overall Stroop task scores as a function of group assignment, collapsed across all ages of testing and across the 2 different Stroop variants. Omnibus statistical tests were carried out with mixed-model analyses; the P values represent differences between each long-chain PUFA group and the control group. Infants who received 0.64% DHA and 0.96% DHA performed better than did the control group; the 0.32% group was intermediate between these groups and the control group. ARA, arachidonic acid.
As with other measures for which significant formula effects were observed, this pattern of results persisted when the 3 LCPUFA groups were collapsed and tested against the control group. A mixed-model analysis of LCPUFA/control × age × Stroop task yielded additive main effects of the first 2 factors, but no other significant main effects or interactions emerged. The main effect of LCPUFA/control [F(1, 71.159) = 4.407, P = 0.039] was attributable to overall superior performance on the task by the LCPUFA groups; the effect size for this comparison was d = 0.74. The significant main effect of age [F(3, 68.990) = 15.081, P < 0.001] was attributable to linear improvement on the task across the ages tested. After control for maternal PPVT-III and household income, the main effect of LCPUFA/control remained significant [F(1, 69.824) = 5.133, P = 0.027].
Tower of Hanoi
From the Tower of Hanoi task, 2 measures were analyzed in the children's attempts to solve the problem: the maximum number of steps passed (a measure of how close they came to solving the problem) and a total processing efficiency score (a reflection of how quickly the child achieved the 2 optimal solutions needed to advance to the next step). For both measures, we conducted group (4 categories) × age (3 categories) mixed-model analyses, and, for both measures, we obtained significant effects of age [F(2, 54.556) = 105.208, P < 0.001 for maximum steps; F(2, 52.586) = 107.695, P < 0.001 for efficiency score], as children showed linear improvement in the measures from 48 to 72 mo of age. Neither the main effect of group nor the group × age interaction was significant. This was also true when the 3 LCPUFA groups were collapsed and tested against the control formula.
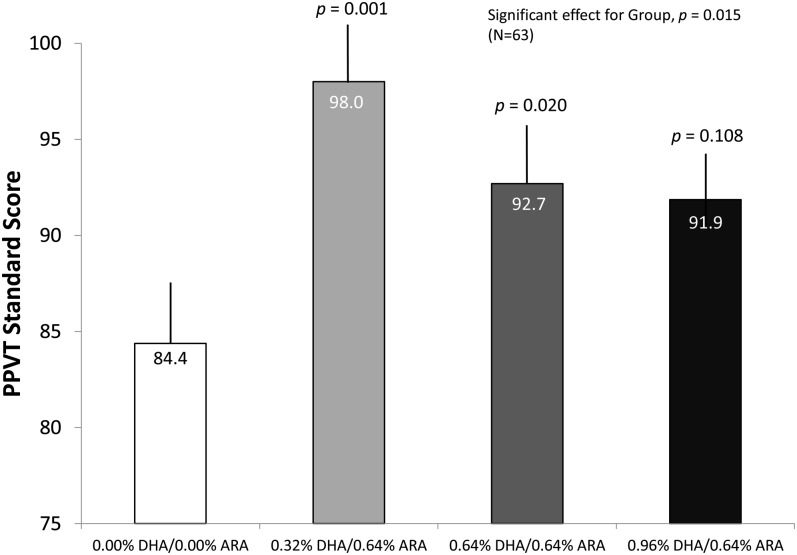
PPVT-III
Standard scores on the PPVT were subjected to a 1-factor group (4 categories) ANOVA. This analysis yielded a significant effect [F(3, 59) = 4.12, P = 0.01]. Follow-up analyses indicated that children assigned to the 0.32% DHA/0.64% ARA and 0.64% DHA/0.64% ARA groups scored significantly higher than did the control group (P = 0.001 and 0.020, respectively). The scores of the highest DHA group (0.96% DHA/0.64% ARA) fell intermediate between those of the lower dose groups and the control group, being statistically distinguishable from neither. The PPVT-III scores from this sample (particularly for the control group) are quite low, relative to the standard average score of 100; readers are reminded that this sample was drawn from a population with a very low socioeconomic status, where the average maternal PPVT-III score (Table 1) was also <90. When we covaried maternal PPVT-III score and household income in the ANOVA for the child's PPVT-III, the main effect of group persisted [F(3, 57) = 3.79, P = 0.015]. Means (±SEs) from this analysis (corrected for covariates) are shown in Figure 5.
FIGURE 5.
Mean (±SE) PPVT-III standard scores, adjusted for covariates, at age 5 y as a function of group assignment. Both one-factor ANOVA and ANCOVA analyses showed that infants who received the long-chain PUFA formula performed better on the PPVT-III. The effects were most evident in the 0.32% DHA and 0.64% DHA groups; the P values represent statistical test results against control group values; the numbers within the bars are means. ARA, arachidonic acid; PPVT-III, Peabody Picture Vocabulary Test, 3rd edition.
When the 3 supplemented groups were collapsed and tested against the control group, the positive effect persisted [t(61) = 2.80, P = 0.003], with an effect size of d = 0.85. When the maternal PPVT-III score and household income were covaried in the analysis of the PPVT-III, the results for LCPUFA supplementation were unchanged [F(1, 59) = 8.24, P = 0.006].
WPPSI-III
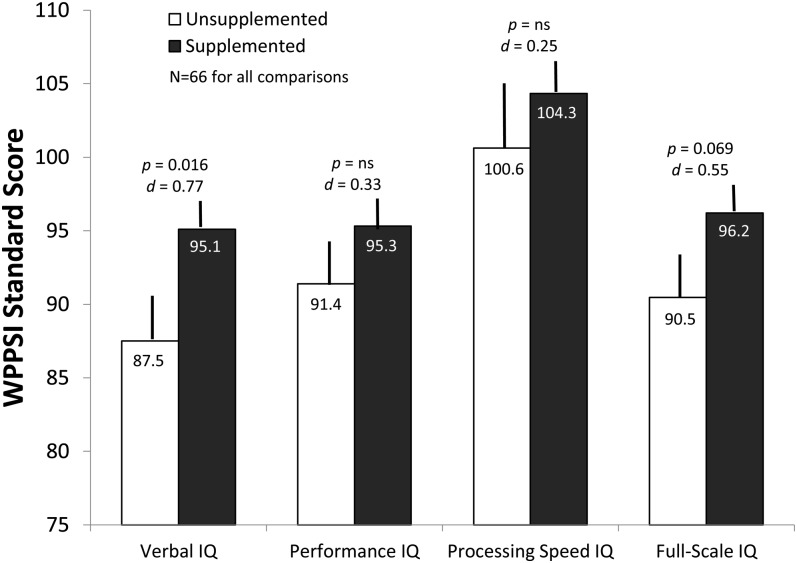
Measures derived from the WPPSI-III were also subjected to 1-factor group (4 categories) ANOVAs. The effects were not significant for the WPPSI-III Full Scale IQ [F(3, 62) = 1.53, NS], Performance IQ [F(3, 62) = 1.12, NS], and Processing Speed [F(3, 62) = 0.51, NS]. The analysis for the Verbal IQ subscale was nearly statistically significant; however [F(3, 62) = 2.31, P = 0. 085]; when maternal PPVT-III and household income is covaried in this analysis, the result is the same [F(3, 60) = 2.413, P = 0.075].
However, when the 3 LCPUFA groups were collapsed and tested against the control group (1-tailed tests, assuming that supplementation would not have a negative effect), the positive effect of both Verbal IQ [t(64) = 2.57, P = 0.006] and Full Scale IQ was statistically significant [t(64) = 1.92, P = 0.029]. The effect sizes (Cohen's d) for these 2 tests were 0.75 and 0.54, respectively. Again, when maternal PPVT-III scores and household income were covaried in these analysis, the result for Verbal IQ persisted [F(1, 62) = 6.67, P = 0.012], although the result for the Full-Scale IQ dropped below conventional significance levels [F(1, 62) = 3.43, P = 0.069]. Means (±SEs) and effect sizes for these analyses (again, corrected for covariates) are presented in Figure 6.
FIGURE 6.
Mean (±SE) WPPSI-III standard scores, adjusted for covariates, at age 6 y as a function of assignment to LCPUFA or control formula. Both ANOVA and ANCOVA analyses showed that infants who received the LCPUFA formula performed significantly better on the Verbal IQ subscale, and the differences were nearly significant for Full-Scale IQ. The numbers within the bars are means. The P values represent the results of ANCOVAs that compared values from the 3 LCPUFA groups (collapsed) with the control group; the d values represent the effect size for each comparison and were adjusted for covariates. IQ, intelligence quotient; LCPUFA, long-chain PUFA; WPPSI-III, Weschler Primary Preschool Scale of Intelligence, 3rd edition.
DISCUSSION
The cohort of children studied were enrolled at birth to study the efficacy of a dietary DHA intake varying from 0.32% to 0.96% of total fatty acids on development through 18 mo of age. Although no significant differences in performance at later ages was found between the 3 supplemented groups, positive effects on several measures were seen with the 2 lower DHA doses (ie, for DHA at 0.32% and 0.64% of fatty acids). One exception to this was performance on the Stroop tasks, for which only the groups provided 0.64% and 0.96% DHA performed significantly better than the control group. Earlier studies have found benefits on measures of cognitive development associated with an intake of 0.32% DHA (9, 14). With the exception of the Stroop tasks, performance of children in the highest DHA dose group (0.96%) was attenuated and intermediate relative to that of the control group and the 2 lower dose groups. This pattern was also observed in a previous report published on this sample (10), which raises the possibility of an upper limit to the benefits of feeding DHA.
Although positive effects of the LCPUFA manipulation were observed on some of the measures taken, not all of the measures included in the follow-up were affected by dietary LCPUFA intake. Even early in life, cognitive function is not a general or monolithic construct, and, when outcomes reflect independent or dissociable functions, LCPUFA may well affect some measures of cognitive/intellectual function, but may leave others unaffected. In this sample, the effects of LCPUFA intake are seen most clearly in early measures of attention (10), preschool measures of rule learning and implementation (Stroop and DCCS), and later measures of verbal ability (PPVT and WPPSI). LCPUFA was not observed to affect measures of spatial memory (eg, the DR task), simple inhibition (Bear-Dragon), or advanced problem solving (eg, the Tower of Hanoi). Whereas the sample size of this trial may preclude this study from being regarded as a definitive demonstration of the specificity of the effects of LCPUFAs, these findings may contribute to the development of more sophisticated hypotheses about the effects of LCPUFA on the development of cognition.
LCPUFA provision in this sample was observed earlier to affect visual development (26) and attentional development across the first year (10). We report here the positive effects of several important measures of executive function during early childhood and on various measures of vocabulary and IQ at the threshold of school age. Notably, we found no effect of LCPUFAs on the BSID (a well-standardized indicator of fundamental developmental status) at 18 mo of age. The lack of an observed effect at 18 mo could have been a result of the timing of the assessment, or it is also possible that the BSID was not consistently sensitive to the effects of LCPUFA supplementation. That said, 2 individual studies have found positive effects of LCPUFAs on the BSID in term and preterm infants (14, 38), and the parallel DHA Intake and Measurement of Neural Development cohort from Dallas also showed positive effects on the BSID when the 3 LCPUFA groups were combined and compared against the control group (15). The consideration of this issue is important, given that the BSID is a featured outcome measure in many recent meta-analyses (16–19, 22), which conclude that LCPUFA supplementation conveys no benefit to infant cognitive development. The BSID yields a composite score derived from infants’ attainment of normative developmental milestones and may not always provide the detailed assays of specific cognitive mechanisms that are available from more sophisticated laboratory tasks, such as those included in this longitudinal schedule (39). The current data, in which effects of LCPUFA are not seen on the BSID but are seen on other measures both before (10, 26) and after the BSID, raised the question of whether the BSID should be used as the primary measure for evaluating the effects of LCPUFA on cognitive development. Consistent with this point is the fact that one other randomized trial of LCPUFA supplementation of term infants found positive effects of LCPUFA on a measure of cognitive function at 5 y of age (40), despite the fact that assessment on the BSID MDI at 30 mo did not show benefits.
Several important limitations of the current trial should be considered when evaluating the ultimate generalizability of these findings. First, the follow-up to this trial was conducted with a total of 81 full-term infant subjects (∼20 per group), with final analyses based on even fewer children because of the nature of longitudinal measurements in very young children (eg, missed appointments or failure to complete tasks because of refusal or behavioral state). The re-enrolled children appear similar to those in the original study, except that parents of male infants were somewhat less likely to re-enroll; thus, group differences might have been influenced by parents’ self-selection to continue follow-up. Whereas we found statistically significant results, the results from smaller trials, such as this one, may be less reliable, because outcomes are subject to type I (random) error, and the inclusion of numerous outcome variables may have also compounded this issue. That said, larger trials will necessarily sacrifice the granularity of measurement that this trial has afforded to the field. Another limitation was that the trial was conducted in an inner-city largely African American population with a low socioeconomic status; thus, it remains unclear whether the kinds of cognitive gains observed in this population are generalizable to other populations. A third limitation was that there are many factors known to negatively influence cognitive ability. Whereas some of these factors were exclusion criteria in the parent trial (eg, low birth weight/gestation, parental alcohol and drug use, and poor general health of the mother and newborn), we did not have direct measures on the child's home or community experience that may have influenced the outcomes observed here; however, we did measure maternal vocabulary and household income and covaried them in the analyses of our standardized outcomes.
In summary, the data from this trial suggest benefits on several measures of cognitive development into early childhood after LCPUFA provision for the first 12 mo of life. Specifically, LCPUFA supplementation in infancy was associated with improved performance on several assessments of executive function and on verbal measures derived from standardized tests at 5 y (PPVT-III) and 6 y (WPPSI-III). We believe that these outcomes are worth evaluating in future trials of LCPUFA supplementation. We also believe that studies to improve nutrition with the hypothesis of enhancing cognitive function should be powered to include similar assessments into childhood. and cognition in general, because our data suggest that improving LCPUFA status during an early and finite period may have lasting effects on cognitive function through childhood.
Acknowledgments
The authors’ responsibilities were as follows—SEC and JC: were involved in the design of the initial clinical trial; JC, SEC, and CLC: designed the follow-up; DJS: coordinated the collection of the follow-up measures and ensured data integrity; EHK, DJS, and JMT: coordinated participant scheduling; KMG and CB: assisted with the data collection; and JC: analyzed the data and wrote the manuscript. JC and SEC are occasional consultants to Mead Johnson Nutrition but have no financial interest or holdings in the company. The remaining authors declare no conflicts or financial interests or holdings.
Footnotes
Abbreviations used: ARA, arachidonic acid; BSID, Bayley Scales of Infant Development; DCCS, Dimensional Change Card Sort Test; DR, Delayed Response; HSC, Human Subjects Committee; IQ, intelligence quotient; LCPUFA, long-chain PUFA; MBCDI, MacArthur-Bates Communicative Development Inventory; MDI, Mental Developmental Index; PDI, Psychomotor Development Index; PPVT-III, Peabody Picture Vocabulary Test, 3rd edition; WPPSI-III, Weschler Primary Preschool Scale of Intelligence, 3rd edition.
REFERENCES
- 1.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty-acid composition of brain, retina, and erythrocytes in breast-fed and formula-fed infants. Am J Clin Nutr 1994;60:189–94. [DOI] [PubMed] [Google Scholar]
- 2.Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids 1996;31:859–65. [DOI] [PubMed] [Google Scholar]
- 3.Yavin E, Glozman S, Green P. Docosahexaenoic acid accumulation in the prenatal brain—prooxidant and antioxidant features. J Mol Neurosci 2001;16:229–35. [DOI] [PubMed] [Google Scholar]
- 4.Cheatham CL, Colombo J, Carlson SE. n−3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. Am J Clin Nutr 2006;83:1458S–66S. [DOI] [PubMed] [Google Scholar]
- 5.Willatts P, Forsyth JS. The role of long-chain polyunsaturated fatty acids in infant cognitive development. Prostaglandins Leukot Essent Fatty Acids 2000;63:95–100. [DOI] [PubMed] [Google Scholar]
- 6.Luzon-Toro B, Schaefer S, Wirths O, Jimenez-Lopez J, Boza-Puerta J, Geerlings A, Falkai P, Bayer TA. Dietary fatty acids and brain mechanism of action throughout the lifespan. Neurol Psychiatry Brain Res 2004;11:149–60. [Google Scholar]
- 7.Wainwright P. Effect of nutritional manipulation on brain function: implications for future research. Am J Clin Nutr 2006;83:919–21. [DOI] [PubMed] [Google Scholar]
- 8.Reisbick S, Neuringer M, Gohl E, Wald R, Anderson GJ. Visual attention in infant monkeys: effects of dietary fatty acids and age. Dev Psychol 1997;33:387–95. [DOI] [PubMed] [Google Scholar]
- 9.Drover J, Hoffman DR, Castañeda YS, Morale SE, Birch EE. Three randomized controlled trials of early long-chain polyunsaturated fatty acid supplementation on means-end problem solving in nine month-olds. Child Dev 2009;80:1376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res 2011;70:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werkman SH, Carlson SE. A randomized trial of visual attention in preterm infants fed docosahexaenoic acid until nine months. Lipids 1996;31:91–7. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs EB, Ross S, Kennedy K, Weaver LT, Lucas A, Fewtrell MS. 10-Year cognition in preterms after random assignment to fatty acid supplementation in infancy. Pediatrics 2011;128:e890–8. [DOI] [PubMed] [Google Scholar]
- 13.Birch EE, Garfield S, Castaneda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev 2007;83:279–84. [DOI] [PubMed] [Google Scholar]
- 14.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 2000;42:174–81. [DOI] [PubMed] [Google Scholar]
- 15.Drover JR, Hoffman DR, Castaneda YS, Morale SE, Garfield S, Wheaton DH, Birch EE. Cognitive function in 18-month-old term infants of the DIAMOND study: a randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Hum Dev 2011;87:223–30. [DOI] [PubMed] [Google Scholar]
- 16.Beyerlein A, Hadders-Algra M, Kennedy K, Fewtrell M, Singhal A, Rosenfeld E, Lucas A, Bouwstra H, Koletzko B, VonKries R. Infant formula supplementation with long-chain polyunsaturated fatty acids has no effect on Bayley developmental scores at 18 months of age—IPD meta-analysis of four large clinical trials. J Pediatr Gastroenterol Nutr 2010;50:79–84. [DOI] [PubMed] [Google Scholar]
- 17.Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: a systematic review of randomized controlled trials. Am J Clin Nutr 2008;87:912–20. [DOI] [PubMed] [Google Scholar]
- 18.Simmer K, Patole SK, Rao SC. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev 2008;CD000376. [DOI] [PubMed] [Google Scholar]
- 19.Hadders-Algra M, Bouwstra H, van Goor SA, Dijck-Brouwer DA, Muskiet FA. Prenatal and early postnatal fatty acid status and neurodevelopmental outcome. J Perinat Med 2007;35(suppl 1):S28–34. [DOI] [PubMed] [Google Scholar]
- 20.Simmer K, Patole SK, Rao SC. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev 2011;Dec 7:CD000376. [DOI] [PubMed] [Google Scholar]
- 21.Schulzke SM, Patole SK, Simmer K. Long-chain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev 2011;Feb 16:CD000375. [DOI] [PubMed] [Google Scholar]
- 22.Qawasmi A, Landeros-Weisenberger A, Leckman JF, Bloch MH. Meta-analysis of long-chain polyunsaturated fatty acid supplementation of formula and infant cognition. Pediatrics 2012;129:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, Simmer K, Colditz PB, Morris S, Smithers LG, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA 2009;301:175–82. [DOI] [PubMed] [Google Scholar]
- 24.Colombo J. Recent advances in infant cognition: implications for long-chain polyunsaturated fatty acid supplementation studies. Lipids 2001;36:919–26. [DOI] [PubMed] [Google Scholar]
- 25.Wainwright PE, Colombo J. Nutrition and the development of cognitive functions: interpretation of behavioral studies in animals and human infants. Am J Clin Nutr 2006;84:961–70. [DOI] [PubMed] [Google Scholar]
- 26.Birch EE, Carlson SE, Hoffman DR, Fitzgerald-Gustafson KM, Fu VL, Drover JR, Castaneda YS, Minns L, Wheaton DK, Mundy D, et al. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr 2010;91:848–59. [DOI] [PubMed] [Google Scholar]
- 27.Colombo J, Shaddy DJ, Richman WA, Maikranz JM, Blaga OM. The developmental course of habituation in infancy and preschool outcome. Infancy 2004;5:1–38. [Google Scholar]
- 28.Bayley N. 2nd ed. San Antonio, TX: The Psychological Corporation, 1970. [Google Scholar]
- 29.Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. Monogr Soc Res Child Dev 1994;59:1–173. [PubMed] [Google Scholar]
- 30.Schutte AR, Spencer JP, Schoner G. Testing the dynamic field theory: working memory for locations becomes more spatially precise over development. Child Dev 2003;74:1393–417. [DOI] [PubMed] [Google Scholar]
- 31.Carlson SM, Moses LJ, Claxton LJ. Individual differences in executive functioning and theory of mind: an investigation of inhibitory control and planning ability. J Exp Child Psychol 2004;87:299–319. [DOI] [PubMed] [Google Scholar]
- 32.Carlson SM, Moses LJ. Individual differences in inhibitory control and children's theory of mind. Child Dev 2001;72:1032–53. [DOI] [PubMed] [Google Scholar]
- 33.Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: performance of children 3.5-7 years old on a Stroop-like day-night test. Cognition 1994;53:129–53. [DOI] [PubMed] [Google Scholar]
- 34.Zelazo PD. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nat Protoc 2006;1:297–301. [DOI] [PubMed] [Google Scholar]
- 35.Welsh MC, Pennington BF, Groisser DB. A normative developmental study of executive function: a window on prefrontal function in children. Dev Neuropsychol 1991;7:131–49. [Google Scholar]
- 36.Dunn LM, Dunn DN. 4th ed. San Antonio, TX: Pearson/PsyCorp, 2007. [Google Scholar]
- 37.Weschler D. Upper Saddle River, NJ: Pearson, 2002. [Google Scholar]
- 38.Clandinin MT, Van Aerde JE, Merkel KL, Harris CL, Springer MA, Hansen JW, Diersen-Schade DA. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr 2005;146:461–8. [DOI] [PubMed] [Google Scholar]
- 39.Colombo J. Infant cognition: predicting later intellectual functioning. Newbury Park, CA: Sage Publications Inc, 1993.
- 40.Jensen CL, Voigt RG, Llorente AM, Peters SU, Prager TC, Zou YLL, Rozelle JC, Turcich MR, Fraley JK, Anderson RE, et al. Effects of early maternal docosahexaenoic acid intake on neuropsychological status and visual acuity at five years of age of breast-fed term infants. J Pediatr 2010;157:900–5. [DOI] [PubMed] [Google Scholar]