Abstract
Background: Two chemoprevention trials found that supplementation with β-carotene increased the risk of lung cancer and overall mortality. The biologic basis of these findings remains poorly understood.
Objective: The objective was to compare the on-study change in metabolomic profiles of men randomly assigned to receive or not receive β-carotene supplements in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study.
Design: The ATBC Study was a randomized, double-blind, placebo-controlled, primary cancer prevention trial; participants were Finnish male smokers assigned to 1 of 4 intervention groups: 1) α-tocopherol, 2) β-carotene, 3) both, or 4) placebo. Fifty participants with both baseline and follow-up fasting serum samples were randomly selected from each of these groups. Metabolomic profiling was conducted by mass spectrometry. The association between change in each metabolite over time and trial assignment (β-carotene or no β-carotene) was estimated by linear regression.
Results: We measured 489 metabolites, and 17 changed significantly (P < 0.05) in response to β-carotene supplementation. More of these 17 metabolites were of xenobiotic origin than would be expected by chance (9 of 60, or 15%; P = 0.00004). We also found a suggestive association with 1,5-anhydroglucitol—a marker of glycemic control (β = −0.379, P = 0.0071).
Conclusions: Male smokers supplemented with β-carotene developed metabolomic profiles consistent with the induction of cytochrome P450 enzymes, the primary metabolizers of xenobiotics in humans. These findings may shed light on the increased mortality associated with β-carotene supplementation in the ATBC Study and suggest the need to explore potential interactions between medication use and dietary supplements, particularly among smokers. This trial was registered at clinicaltrials.gov as NCT00342992.
INTRODUCTION
Despite laboratory and observational studies suggesting beneficial health effects of β-carotene supplementation on cancer and other diseases, 2 chemoprevention trials conducted in populations of smokers found the opposite (1, 2). The Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC)4 Study showed that male smokers supplemented with 20 mg β-carotene/d had a greater incidence of lung cancer (18%) and overall mortality (8%) than did men who did not receive β-carotene supplements (1). Similarly, the Beta-Carotene and Retinol Efficacy Trial reported that, among heavy smokers and asbestos-exposed workers, those supplemented with a combination of 30 mg β-carotene/d and 25,000 IU retinyl palmitate/d had a greater incidence of lung cancer (28%) and overall mortality (17%) than did women and men who received placebo (2). Subsequent research aimed at elucidating the biology underlying the adverse effects of β-carotene has suggested putative mechanisms for the increased lung cancer rates, including increased retinoic acid catabolism in the lungs via induction of cytochrome p450 (CYP) enzymes (3–5); however, the relevance to the increased overall mortality rate is poorly understood.
Metabolomic profiling of biologic samples is an analytic tool that allows for the measurement and identification of a broad array of low-molecular-weight biochemicals in a variety of matrices including blood, urine, or organ-specific tissue, among others (6). Other agnostic approaches, such as genome-wide association studies, have been successful in the identification of new biologic components and pathways of interest to human disease, including cancer. Similarly, examination of the human metabolome offers the potential to discover molecular species relevant to cancer etiology and early detection (7) and markers indicative of biological mechanisms for known nutrient effects. Thus, metabolomic profiling may shed light on how β-carotene supplementation affected overall mortality and cancer incidence in the aforementioned controlled trials (8).
To our knowledge, there are no published metabolomic studies of the biological response to supplementation with β-carotene. We therefore quantitatively assessed the pre- and postenrollment fasting serum metabolome of men randomly assigned to receive β-carotene in the ATBC Study and compared the changes that occurred with supplementation with those not supplemented with β-carotene.
SUBJECTS AND METHODS
Study population
The ATBC Study was a double-blind, placebo-controlled, primary cancer prevention trial designed to test the influence of supplementation with α-tocopherol or β-carotene on cancer incidence (9). Men who were between 50 and 69 y of age and who smoked ≥5 cigarettes/d were recruited between 1985 and 1988 in southwestern Finland. Trial participants (n = 29,133) were randomly assigned to 1 of 4 groups based on a 2 × 2 factorial design: 1) α-tocopherol (dl-α-tocopheryl acetate (50 mg/d), 2) β-carotene (20 mg/d), 3) both supplements, or 4) placebo. Supplementation continued for 5 to 8 y until the trial ended on 30 April 1993. Fasting blood samples were collected from all participants during their baseline visit. In addition, blood samples were collected annually from a random sample of participants. The blood samples were divided into aliquots and stored at −70°C. Written informed consent was obtained from all trial participants, and the ATBC Study was approved by institutional review boards at both the US National Cancer Institute and the Finnish National Public Health Institute. For the current analysis, 50 individuals were chosen from each of the 4 intervention groups for a total sample of 200 individuals. To be eligible for inclusion, participants were required to 1) have both a pre- and postrandomization fasting serum sample available with the follow-up sample collected ≥1 y after the baseline blood collection (mean: 3.3 y; range 1.0–6.9 y) and start of supplementation, and 2) have no diagnosis of cancer within 5 y after the follow-up blood collection.
Metabolomic analysis
Metabolomic profiling was conducted on baseline and follow-up serum samples at Metabolon Inc (Durham, NC) according to the protocol described below.
Sample accessioning
Each sample received was accessioned into a laboratory information management system (LIMS) and was assigned by the LIMS a unique identifier that was associated with the original source identifier only. This identifier was used to track all sample handling, tasks, results etc. The samples (and all derived aliquots) were tracked by LIMS. All portions of any sample were automatically assigned their own unique identifiers by LIMS when a new task was created; the relation of these samples was also tracked. All samples were maintained at −80°C until processed.
Sample preparation
Samples were prepared as described previously (10). Briefly, sample extraction was conducted by using aqueous methanol, delivered by an automated liquid handler to remove the protein fraction while allowing maximum recovery of small molecules. The resulting extract was divided into 4 fractions: 1 for analysis by ultraHPLC tandem mass spectroscopy (UPLC/MS/MS2) in the positive mode, 1 for UPLC/MS/MS2 (negative mode), 1 for gas chromatography–mass spectrometry (GC-MS), and 1 for backup. Samples were placed briefly on a TurboVap (Zymark) to remove the organic solvent. Each sample was then frozen and dried under vacuum. Samples were then prepared for the appropriate instrument, either UPLC/MS/MS2 or GC-MS.
UPLC/MS/MS2
The sample extracts destined for UPLC/MS/MS2 positive- and negative-mode analyses were reconstituted and analyzed as described previously (10) under Methods/UHPLC Analyses, with the exception that the data were collected by using a ThermoFisher Scientific Orbitrap Elite high-resolution/accurate mass mass spectrometer. Briefly, 2 of the dried extracts were reconstituted in acidic or basic LC-compatible solvents, respectively. The acidic reconstituted extract was analyzed under positive ion optimized MS conditions, and the basic extract was analyzed by using negative-ion MS optimized conditions in 2 independent injections by using separate dedicated columns. The Orbitrap Elite used a heated electrospray ionization source and orbitrap mass analyzer operated at 30,000 mass resolution, scanned 80–1000 m/z, and alternated between MS and data-dependent MS2 scans by using dynamic exclusion.
Gas chromatography–mass spectroscopy
The samples destined for GC-MS analysis were redried under vacuum desiccation for a minimum of 24 h before being derivatized under dried nitrogen with the use of bistrimethyl-silyl-triflouroacetamide. The GC column was 5% phenyl, and the temperature ramp was from 40° to 300°C in a 16-min period. Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer by using electron impact ionization. The instrument was tuned and calibrated for mass resolution and mass accuracy on a daily basis. The information output from the raw data files was automatically extracted as discussed below.
Quality assurance/quality control
For quality assurance/quality control (QC) purposes, additional samples were included with each day's analysis. These samples included extracts of a pool of well-characterized human plasma, extracts of a pool created from a small aliquot of the experimental samples, and process blanks. QC samples were spaced evenly among the injections, and all experimental samples were randomly distributed throughout the run. A selection of QC compounds was added to every sample for chromatographic alignment, including those under test. These compounds were carefully chosen so as not to interfere with the measurement of the endogenous compounds.
Data extraction and compound identification
Raw data were extracted, peak-identified, and QC processed as described previously (10, 11). Briefly, compounds were identified by comparison of experimental data to the laboratory's authenticated standard library of purified standards or recurrent unknown entities. This library contains the retention time/index, m/z of the intact standard and its fragmentation spectral data (MS2 or MS/MS), and chromatographic data on all molecules present in the library. Biochemical identifications were based on 3 criteria: retention index within a narrow retention time/index window of the proposed identification, accurate mass match to the library +/− 0.005 amu, and the MS/MS forward and reverse scores between the experimental data and authentic standards. Although there may be similarities between these molecules based on one of these factors, the use of all 3 data points can be used to distinguish and differentiate biochemicals. More than 3000 commercially available purified standard compounds have been acquired and registered into LIMS for distribution to both the LC and GC platforms for determination of their analytic characteristics. A total of 516 compounds were identified in our samples; any compound with <5 nonmissing observations in either the baseline or follow-up sample was excluded, which left 489 metabolites for analysis. Metabolites were then grouped into 10 mutually exclusive chemical classes (amino acids, carbohydrates, cofactors and vitamins, energy metabolites, lipids, nonstandard amino acids, nucleotide metabolites, peptides, and xenobiotics) based on the available literature. For the analysis, amino acids were separated into the standard amino acids (ie, those that are precursors of proteins directly encoded by the genetic code) and nonstandard amino acids (ie, those not incorporated into proteins or encoded by the genetic code). Each batch of samples submitted for analysis contained blinded QC samples from this study's participants as well as from another cohort. The median and IQR of the intraclass correlation coefficient across the metabolites was 0.84 (0.52–0.96). These intraclass correlation coefficients were similar to those seen previously in samples analyzed by this laboratory (12).
Statistical analysis
To account for batch variability, metabolite concentrations were divided by their batch's median value. A total of 36 samples were included in each batch, and the baseline and follow-up blood samples from an individual were included in the same batch with one another. Normalized metabolite concentrations were then log transformed, and missing values were imputed to the minimum of nonmissing values. In our primary analysis, we modeled the association between change in log-metabolite concentrations and trial assignment (ie, β-carotene or no β-carotene) by linear regression. Analyses were conducted with stratification by number of cigarettes smoked per day, alcohol consumption, duration of supplementation with β-carotene, and trial α-tocopherol supplementation. The threshold for statistical significance for our main analysis was 0.0001022495 based on a Bonferroni correction for 489 tests to obtain a family-wise error rate of 0.05, or 0.00021 based on a permutation correction. For the latter, we calculated the minimum P value for each of 10,000 permuted data sets, where we permuted the group assignment, and found the 0.05 quantile to be 0.00021. For stratified analyses of our most significant metabolites from our main analysis, the threshold for statistical significance was 0.0029 based on a Bonferroni correction for 17 tests. We further examined whether a particular chemical class of metabolites was over- or underrepresented among our top hits. For this analysis, we categorized metabolites as below a P value of 0.05 (yes or no) and as belonging to a chemical class (yes or no) and then used a Fisher's exact test to determine whether the representation among the top hits was statistically significant. For this analysis, the threshold for statistical significance was 0.0056 based on a Bonferroni correction for 9 tests. All analyses were performed by using SAS version 9.1.3 (SAS Institute).
RESULTS
Prerandomization characteristics of the study population by the trial β-carotene assignment are shown in Table 1. As expected, because of the randomized nature of the trial, the distributions of factors were well balanced by supplementation arm, with the exception of fruit intake, which was slightly higher among men in the β-carotene arm (Table 1).
TABLE 1.
Participant characteristics by β-carotene assignment in the ATBC Study1
| No β-carotene(n = 100) | β-Carotene(n = 100) | P value | |
| Age (y) | 57.5 ± 4.82 | 57.9 ± 5.1 | 0.71 |
| Height (cm) | 175 ± 6.1 | 174 ± 6.4 | 0.78 |
| Weight (kg) | 78.8 ± 11.2 | 79.2 ± 12.1 | 0.69 |
| BMI (kg/m2) | 25.9 ± 3.1 | 26.1 ± 3.6 | 0.65 |
| Smoking (cigarettes/d) | 20.3 ± 10.4 | 19.7 ± 8.4 | 0.92 |
| Smoking duration (y) | 35.0 ± 9.4 | 35.9 ± 9.6 | 0.46 |
| Quit smoking at follow-up visit (%) | 26.0 | 25.0 | 0.97 |
| Physically active (%) | 26.0 | 22.0 | 0.51 |
| > Elementary school education (%) | 29.0 | 23.0 | 0.33 |
| Dietary intake | |||
| Total energy (kcal/d) | 2770 ± 710 | 2768 ± 676 | 0.96 |
| Fruit (g/d) | 196 ± 147 | 264 ± 208 | 0.03 |
| Vegetables (g/d) | 323 ± 140 | 302 ± 102 | 0.58 |
| Red meat (g/d) | 78.4 ± 31.5 | 72.4 ± 28.9 | 0.23 |
| Coffee (g/d) | 652 ± 331 | 650 ± 351 | 0.75 |
| Tea (g/d) | 59.3 ± 114 | 61.6 ± 112 | 0.82 |
| Alcohol, ethanol (g/d) | 15.2 ± 18.6 | 13.1 ± 13.9 | 0.86 |
| Serum concentrations | |||
| Total cholesterol (mmol/L) | |||
| Baseline | 6.4 ± 1.1 | 6.4 ± 1.0 | 0.77 |
| Follow-up | 6.1 ± 1.1 | 6.3 ± 1.1 | 0.42 |
| α-Tocopherol (mg/L) | |||
| Baseline | 12.3 ± 3.0 | 12.3 ± 3.0 | 0.81 |
| Follow-up | 15.6 ± 4.5 | 15.7 ± 4.8 | 0.84 |
| β-Carotene (μg/L) | |||
| Baseline | 270 ± 249 | 242 ± 135 | 1.0 |
| Follow-up | 268 ± 222 | 3633 ± 1448 | <0.0001 |
| Retinol (μg/L) | |||
| Baseline | 587 ± 118 | 574 ± 111 | 0.35 |
| Follow-up | 595 ± 129 | 616 ± 128 | 0.34 |
All variables are from the baseline questionnaire unless otherwise indicated. ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention.
Mean ± SD (all such values).
A total of 489 metabolites were measured in our analytic set. However, after correction for multiple comparisons, no compound changed significantly in fasting serum during supplementation with β-carotene as compared with no β-carotene supplementation. Results for the 17 metabolites that changed in response to β-carotene supplementation at the nominal statistical level of P < 0.05 are shown in Table 2; findings for all 489 metabolites appear elsewhere (see Supplemental Table 1 under “Supplemental data” in the online issue). Our results were very similar when we used change in serum β-carotene concentration as the exposure, rather than β-carotene trial assignment, both overall and among the β-carotene group (data not shown). No statistically significant interaction was found between any of the top 17 metabolites and any of the following factors: duration of supplementation with β-carotene, number of cigarettes smoked per day, alcohol consumption, or trial α-tocopherol supplementation.
TABLE 2.
Metabolites that changed significantly (P < 0.05) in response to β-carotene supplementation in the ATBC Study1
| Metabolite | Chemical class | Number > LOD | Effect size2 | P value |
| Threonine | Amino acid | 200 | −0.381 | 0.0067 |
| 1,5-Anhydroglucitol | Carbohydrate | 200 | −0.379 | 0.0071 |
| Hippurate | Xenobiotics | 200 | 0.363 | 0.0099 |
| 3-(3-Hydroxyphenyl) propionate | Nonstandard amino acid | 158 | 0.357 | 0.0111 |
| 3-Hydroxyhippurate | Xenobiotics | 198 | 0.351 | 0.0126 |
| Dihydroferulic acid | Xenobiotics | 77 | 0.350 | 0.0131 |
| 2-Hydroxyacetaminophen sulfate | Xenobiotics | 24 | 0.334 | 0.0179 |
| 3,7-Dimethylurate | Xenobiotics | 158 | 0.318 | 0.0240 |
| 5-Acetylamino-6-amino-3-methyluracil | Xenobiotics | 196 | 0.318 | 0.0241 |
| β-Sitosterol | Lipid | 114 | −0.314 | 0.0262 |
| Xylose | Carbohydrate | 174 | −0.296 | 0.0358 |
| 1-Methylurate | Xenobiotics | 200 | 0.294 | 0.0372 |
| 2-Hydroxyisobutyrate | Nonstandard amino acid | 200 | 0.293 | 0.0378 |
| 10-Nonadecenoate (19:1n−9) | Lipid | 200 | 0.285 | 0.0434 |
| Catechol sulfate | Xenobiotics | 200 | 0.283 | 0.0451 |
| Cinnamoylglycine | Xenobiotics | 185 | 0.280 | 0.0475 |
| 7-Ketodeoxycholate | Lipid | 63 | 0.277 | 0.0498 |
ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention; LOD, limit of detection.
Effect size denotes the change in metabolite level (expressed in number of SDs on the log scale) for the β-carotene arm compared with the no β-carotene arm. The effect sizes and P values were estimated by linear regression.
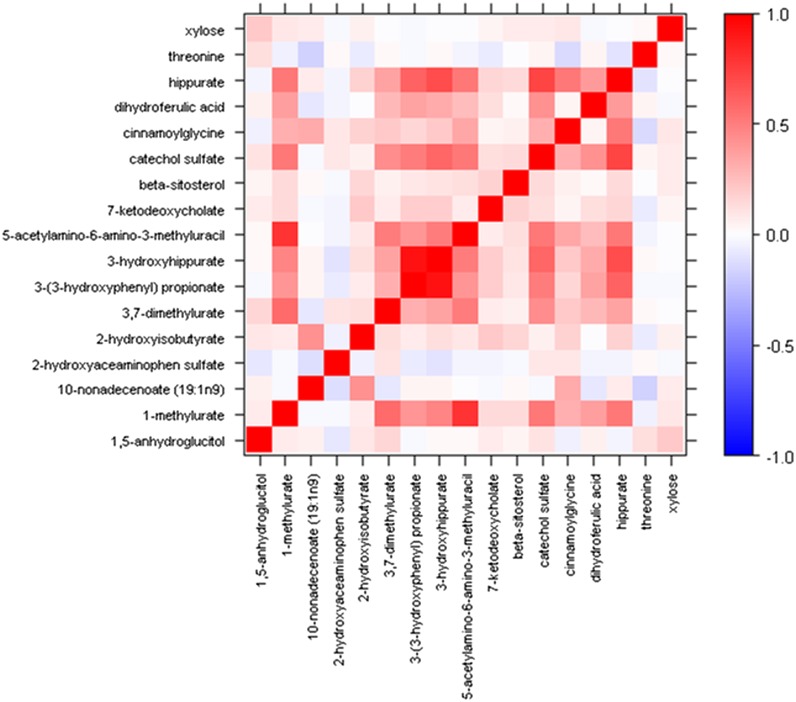
Examination of the chemical classes of the top 17 metabolites showed that a greater number than would be expected by chance alone were xenobiotics (9 of 60, 15%; P = 0.00004; Table 3). A modest intercorrelation was observed between these 17 metabolites (Figure 1), and a few were highly correlated: 3-(3-hydroxyphenyl) propionate and 3-hydroxyhippurate (r = 0.93) and 1-methylurate and 5-acetylamino-6-amino-3-methyluracil (r= 0.79). It is of interest that all 9 of the xenobiotic metabolites among the top 17 metabolites increased with β-carotene supplementation (Table 2). No other chemical class was over- or underrepresented in the most statistically significantly associated metabolites (Table 3).
TABLE 3.
Number of metabolites by chemical class and the association with β-carotene supplementation in the ATBC Study1
| Chemical class | No. of identified metabolites | No. of metabolites associated with β-carotene supplementation at P < 0.05 | P value2 |
| All | 489 | 17 | |
| Xenobiotics | 60 | 9 (15.0) | 0.00004 |
| Lipids | 181 | 3 (1.7) | 0.12 |
| Peptides | 70 | 0 (0) | 0.15 |
| Carbohydrates | 22 | 2 (9.1) | 0.17 |
| Amino acids3 | 20 | 1 (5.0) | 0.51 |
| Nonstandard amino acids3 | 88 | 2 (2.3) | 0.75 |
| Cofactors and vitamins | 21 | 0 (0) | 1.0 |
| Energy metabolites | 7 | 0 (0) | 1.0 |
| Nucleotide metabolites | 20 | 0 (0) | 1.0 |
Values in parentheses are percentages. ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention.
Calculated by using Fisher's exact test.
Amino acids are defined here as the 20 amino acids that are precursors to proteins and that are directly encoded by the universal genetic code. Nonstandard amino acids are those that are either not incorporated into proteins or not directly encoded by the universal genetic code.
FIGURE 1.
Heat map of the correlations between metabolites that changed significantly (P < 0.05) in response to β-carotene supplementation.
DISCUSSION
In this analysis of the metabolomic profile of fasting serum in a controlled trial population of male smokers, we identified 17 metabolites that appeared to change in men supplemented with β-carotene. To our knowledge, this was the first study to use global metabolomics analysis to characterize the biological effect of supplementation with β-carotene.
Several of the metabolites possibly altered in response to β-carotene supplementation provided potential biologic insights into the trial findings for β-carotene in the ATBC Study and Beta-Carotene and Retinol Efficacy Trial. One notable finding was the overrepresentation of xenobiotic metabolite signals among our most statistically significant associations with response to β-carotene, all of which showed increased concentrations during supplementation. CYP enzymes are primarily responsible for human xenobiotic metabolism, and previous experimental studies showed induction of several CYP isoforms in various tissues in response to supplementation with β-carotene, including CYP1A2 and CYP2E1 (3, 13). In fact, several of the top xenobiotic compounds identified in our analysis result from CYP1A2- or CYP2E1-mediated metabolism, including 3,7-dimethylurate and 1-methylurate. These are metabolites of theophylline, a drug used to treat asthma and chronic obstructive pulmonary disease and a metabolite of caffeine, and 5-acetylamino-6-amino-3-methyluracil—a caffeine metabolite. Both theophylline and caffeine are substrates for CYP1A2. Another top signal was 2-hydroxyacetaminophen sulfate, an oxidation product of acetaminophen after metabolism by CYP2E1 (with CYP1A2 serving as a secondary pathway) (14). One possible explanation for these findings is that men who received the β-carotene supplement began taking more theophylline or acetaminophen or increased their caffeine intake during follow-up relative to the men who did not receive β-carotene. However, both caffeine and theophylline were measured as part of the metabolomic profile, and neither changed with β-carotene supplementation (caffeine: P = 0.75; theophylline: P = 0.33; see Supplemental Table 1 under “Supplemental data” in the online issue). Several important medications used to treat cardiovascular diseases are also metabolized by CYP1A2, including propranolol, tizanidine, verapamil, and warfarin. Thus, our findings support the hypothesis that interactions between β-carotene supplementation and prescription (and nonprescription) medications may have led to adverse mortality outcomes in the ATBC Study. To our knowledge, this was the first study to provide evidence supporting CYP enzyme induction as a result of β-carotene supplementation in humans. Another dietary supplement known to interact with prescription medications is St John wort—an herbal preparation commonly used for depression and a potent inducer of CYP2C9 and CYP3A4 known to cause multiple clinically significant drug interactions (15). Our analysis was limited to those metabolites that were both present in the samples from our study population and detectable by the metabolomic platform. Importantly, of the cardiovascular medications known to be metabolized by CYP1A2, only propranolol and verapamil were detectable by the metabolomic platform, and only verapamil was present in our study sample (and only in 2 participants at baseline and in 5 participants at the second blood collection). Thus, a lack of direct evidence of increased cardiovascular medication metabolites in the β-carotene group should not be considered evidence against the drug interaction hypothesis. Future studies should directly examine the interaction between β-carotene supplementation and cardiovascular medications.
We also found a suggestive association with 1,5-anhydroglucitol, a marker of glycemic control. This metabolite decreased with β-carotene supplementation, which suggests that those who received the β-carotene supplement experienced poorer glycemic control over time than did those who did not receive β-carotene. This finding is biologically plausible: animals exposed to tobacco smoke and supplemented with β-carotene have lower concentrations of retinoic acid and lower retinoic acid receptor-β expression (5), and a role for retinoids and retinoic acid receptor-β in the etiology and possibly even the treatment of type 2 diabetes has been described (16). Poorer glycemic control in the β-carotene arm could have implications for the finding of overall increased mortality among those receiving the β-carotene supplement in the ATBC Study because individuals with type 2 diabetes experience earlier mortality, particularly from cardiovascular disease (17), which was the most common cause of death among the ATBC Study participants (1). It should be noted, however, that other markers of glycemic control, such as glucose itself, were not altered by β-carotene supplementation (glucose: P = 0.57; see Supplementary Table 1 under “Supplemental data” in the online issue). However, 1,5-anhydroglucitol has been suggested as a better marker of glycemic fluctuations than fasting glucose concentrations, and these fluctuations are more strongly associated with cardiovascular disease mortality (18). Alternatively, this finding may be due to chance.
Our study had many important strengths, perhaps foremost of which was the randomized, double-blind, placebo-controlled design of the parent trial and our measurement of metabolomic profiles both before and after supplementation. In addition, the metabolomic platform we used permitted identification of a large number of metabolites (>500) and a more comprehensive characterization of the biological response to β-carotene supplementation. We did not discover individual metabolites that were statistically significantly altered by β-carotene supplementation after correction for multiple comparisons; therefore, such findings (eg, for 1,5-anhydroglucitol) may have been due to chance. At the same time, it should be noted that the representation of multiple xenobiotic metabolites among our top hits was statistically significantly greater than expected by chance alone, even after adjustment for 9 comparisons at a family-wise error rate of 0.05. Our study population was limited to male smokers of white ancestry, and the trial tested only one β-carotene dosage, which was both quite high and very bioavailable compared with food sources of β-carotene; these factors may have limited the generalizability of our findings. It is important to note that cigarette smoke is also a known inducer of CYP1A2 and CYP2E1, and alcohol intake is a known inducer of CYP2E1; experimental data show ferret lung CYP1A2 induction in response to both β-carotene and cigarette smoke, with the strongest change resulting from exposure to both (3), and the original trial finding in ATBC of an increased risk of lung cancer for those supplemented with β-carotene was stronger among men who drank more (19). We examined the association between our top metabolites and β-carotene supplementation stratified by these factors. However, we found no statistically significant interactions with either cigarettes smoked per day or alcohol intake, particularly the associations between the metabolites known to result from CYP1A2- or CYP2E1-mediated metabolism discussed above, and β-carotene supplementation did not differ by either smoking (<20 compared with ≥20 cigarettes/d: 3,7-dimethylurate β<20 = 0.29, β≥20 = 0.26, P = 0.88; 1-methylurate β<20 = 0.31, β≥20 = 0.23, P = 0.73; 2-hydroxyacetaminophen sulfate β<20 = 0.41, β≥20 = 0.40, P = 0.98) or alcohol intake (<median compared with ≥median intake: 3,7-dimethylurate βlow = 0.43, βhigh = 0.22, P = 0.46; 1-methylurate βlow = 0.25, βhigh = 0.36, P = 0.70; 2-hydroxyacetaminophen sulfate βlow = 0.41, βhigh = 0.24, P = 0.57). Despite the lack of interaction in our study, however, there remains the possibility that the associations we observed may differ for nonsmokers, by alcohol consumption, or within those supplemented with a different dose of β-carotene.
In this metabolomics analysis of participants from a large, randomized trial of male smokers, we found that the metabolomic profiles of response to β-carotene supplementation point to induction of CYP1A2 and CYP2E1 and, possibly, to dysregulated glycemic control. These findings may shed light on the increased mortality resulting from β-carotene supplementation in the ATBC Study and call attention to the need for greater understanding of possible interactions between dietary supplements and other pharmacologic agents.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—JV and DA: designed the research; AME and EDK: conducted the research; EDK: provided the essential materials; AMM and JNS: analyzed the data or performed the statistical analysis; AMM, JNS, SCM, SJW, JV, and DA: wrote the manuscript; and AMM: had responsibility for the final content. AME and EDK are employed by Metabolon Inc. AMM, JNS, SCM, SJW, JV, and DA had no conflicts of interest to disclose.
Footnotes
Abbreviations used: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention; CYP, cytochrome P450; GC, gas chromatography; LIMS, laboratory information management system; MS, mass spectroscopy; QC, quality control; UPLC/MS/MS2, ultraHPLC tandem mass spectroscopy.
REFERENCES
- 1.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35. [DOI] [PubMed] [Google Scholar]
- 2.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150–5. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Russell RM, Wang XD. Exposing ferrets to cigarette smoke and a pharmacological dose of beta-carotene supplementation enhance in vitro retinoic acid catabolism in lungs via induction of cytochrome P450 enzymes. J Nutr 2003;133:173–9. [DOI] [PubMed] [Google Scholar]
- 4.Wright ME, Groshong SD, Husgafvel-Pursiainen K, Genova E, Lucia MS, Wolff H, Virtamo J, Albanes D. Effects of beta-carotene supplementation on molecular markers of lung carcinogenesis in male smokers. Cancer Prev Res (Phila) 2010;3:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst 1999;91:60–6. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature 2008;455:1054–6. [DOI] [PubMed] [Google Scholar]
- 7.O'Connell TM. Recent advances in metabolomics in oncology. Bioanalysis 2012;4:431–51. [DOI] [PubMed] [Google Scholar]
- 8.Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, van Ommen B, Pujos-Guillot E, Verheij E, Wishart D, Wopereis S. Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics 2009;5:435–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The ATBC Cancer Prevention Study Group. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. Ann Epidemiol 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81:6656–67. [DOI] [PubMed] [Google Scholar]
- 11.Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminformatics 2010;2(1):9. [DOI] [PMC free article] [PubMed]
- 12.Sampson JN, Boca SM, Shu XO, Stolzenberg-Solomon RZ, Matthews CE, Hsing AW, Tan YT, Ji B, Chow W, Cai Q, et al. Metabolomics in epidemiology: sources of variability in metabolite measurements and implications. Cancer Epidemiol Biomarkers Prev 2013;22:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolini M, Antelli A, Pozzetti L, Spetlova D, Perocco P, Valgimigli L, Pedulli GF, Cantelli-Forti G. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis 2001;22:1483–95. [DOI] [PubMed] [Google Scholar]
- 14.A pharmacologic overview of Tylenol® (acetaminophen). Available from http://www.tylenolprofessional.com/pharmacology.html (cited 14 January 2013).
- 15.Di YM, Li CG, Xue CC, Zhou SF. Clinical drugs that interact with St. John's wort and implication in drug development. Curr Pharm Des 2008;14:1723–42. [DOI] [PubMed] [Google Scholar]
- 16.Villarroya F, Iglesias R, Giralt M. Retinoids and retinoid receptors in the control of energy balance: novel pharmacological strategies in obesity and diabetes. Curr Med Chem 2004;11:795–805. [DOI] [PubMed] [Google Scholar]
- 17.Dailey G. Overall mortality in diabetes mellitus: where do we stand today? Diabetes Technol Ther 2011;13(suppl 1):S65–74. [DOI] [PubMed] [Google Scholar]
- 18.Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn 2008;8:9–19. [DOI] [PubMed] [Google Scholar]
- 19.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 1996;88:1560–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.