Abstract
Background:
Due to the high incidence of deaths from breast cancer, high cost of treatment and limited resources, the need to formulate and implement effective programs in reducing the burden of disease is obvious. Care, control and creation of cancer information system having an infrastructure from collection of minimum data sets (MDS) are the top priorities of research in Iran’s Ministry of Health.
Methods:
This is an applied descriptive research with comparative approach implemented in 2010. MDS for breast cancer on selected countries were searched and reviewed and proposed model based on the country’s need was designed. Research data were implemented in 2 stages; assessment of MDS on selected countries and the validation of the proposed model through several meetings that has been carried out by the Undersecretary for Research and Technology and several oncologists and pathologists.
Results:
The MDS is composed of 11 parameters in the form of fields in closed structured arrangements with consideration to coding responses. These parameters include: hospital data, demography, referral, physical examination and investigation, diagnostic information, pathology, treatment, palliative care, completion of primary treatment, clinical trials and follow-up. This form is available for use in the cancer registry database.
Conclusion:
MDS provides an opportunity to strengthen communication between performed researches and research results for the improvement of programs, policies and strategies and provides positive effect on equality in the health system. Although the stages of creating the MDS for breast cancer has been successful, but many challenges has been met until its completion.
Keywords: Breast cancer, Data, Iran, Information, Cancer Registry
Introduction
Universally cancer is an important factor in the burden of disease. It is an important cause of morbidity and mortality(1). It is estimated that by 2025 the number of cancer patients will increase to 15 million in comparison to the 10 million in 2002 of which 60% are accounted to the developing countries(2). During the last decades the risk of breast cancer in industrialized and the developing countries have increased from 1–2% annually (3–4). Annually, about 8040 new cases of breast cancer are predicted in the country of which 7778 cases are Iranian women with ages between 45–55 years (5). In Iran, 6% of the burden of disease is from cancer cases and the 5th cause of cancer mortality in women which is ranked 3rd after gastric cancer and leukemia. These figures are the result of the research performed by the Cancer Research Center in Shahid Beheshti University of Medical Sciences (5). Therefore, meticulous attention to health status is very important and early diagnosis is the key step in improving prognosis (6–7).
In recent years various research consortiums with the purpose of facilitating multidisciplinary and multi institutional researches have been implemented and regardless of the consortia’s aim, this data collection needs a standard method for collecting, storing, transferring and reporting of data to other institutions involved (8). The Royal College of Pathologist have done a move to present a MDS for reporting cancer cases with the aim of creating an environment of comparing pathologic reports in a standard form (9). The National Cancer Association of Ireland (2011) has stated that MDS for all cancer cases must be collected at the national level, these data sets aside from guaranteeing comprehensive and accurate information on types of cancer also provide insights to similar developments in related areas such as preventive care (10). Standardization and uniformity of data elements allows the feasibility of comparing the collected data from different centers and through comparison of these data, validation from other countries and implementation of internal researches and statistical studies can be gained (11). Despite the fact that breast cancer is one important field in medical research but the presence of certain gaps has been documented which is highly important for patients and expert specialists (12).
Considering the high incidence of mortality rate from breast cancer, the high cost of treatment and the limited resources, it is necessary that the Ministry of Health and policy makers should put more emphasis on this issue. Therefore, the need to formulate and implement effective programs in reducing the burden of disease has become evident. Care, control and creation of breast cancer information system as a priority for research of the MOH in the country is highly suggested. The MDS is an important step towards improving services to cancer patients and this improvement will take place only through the collection and use of correct data (13).
The present study, with the aim of comparing the MDS for breast cancer in countries such as the United States of America, Australia, Britain, Canada and Ireland was done to ultimately equip the country with a national model.
Methods
This is an applied descriptive research with a comparative approach, implemented in 2010. Research data were implemented into 2 stages:
In the stage of assessment of the MDS for breast cancer, countries like United States, Australia, Britain, Ireland and Canada were chosen due their outstanding performances in health affairs, researches in the field of cancer and in designing the MDS for breast cancer. Eight information sources namely breast cancer specific data items for clinical cancer registration, Australian Government Department of Health and Ageing (14), Framework for Specialist MDS Development for Specific Cancers in Clinical Cancer Registry (15), SEER Program Coding and Staging Manual National Cancer Institute (16), Breast cancer surveillance consortium, Data dictionary (17), A Proposed Core National Cancer Dataset: National Cancer Registry, National Cancer Registry Ireland (18), Reporting Template for Breast Cancer Resection Reports, Faculty of Pathology Royal college of physicians (19), North Trent Breast Cancer Group, Guidelines for Treatment & Referral Breast cancer version 12 (20), Canadian Cancer Registry System Guide – 2009 Edition (21) were also assessed and reviewed in designing this minimum data set.
Data were collected through review of literature of electronic and printed scientific resources and also by asking the opinions of the selected experts and specialists within the country. Data of the selected countries were matched in accordance to the course of treatment designed for breast cancer patients from admission to discharge. Then the proposed model was determined based on common aspects and differentiation.
In the second stage which was the stage of validation, the proposed model was reviewed and evaluated throughout several meetings that have been carried out. The different elements of the MDS were discussed and opinions were exchanged on the oncologists and pathologists’ ongoing meetings.
For a period of one and a half months, 6 consecutive meetings that amounted to 135 person hours has been held by the Committee on Strategic Planning Management System attended by15 oncologists, six radiologists and 9 personnel from the Undersecretary for Research and Technology, Ministry of Health and Medical Education. In each session, one of the Information Management Specialist is responsible for coordinating the contents to be undertaken in the meeting, 2 persons from the Undersecretary for Research and Technology, Ministry of Health and Medical Education acts as note takers and one expert from the Undersecretary for Research and Technology has acted as coordinator of the meetings. All members of the meeting are also members of the Strategic Committee of Breast Cancer Management System of which their memberships were officially decreed by the Minister of Health. At the beginning of each session, the proposed model was introduced and each part was presented for discussion. Discussions and debate regarding each data element continues until consensus from the experts is reached then the minimum data set necessary in the proposed model is recorded and put in the desired place based on their relevance and priority. Collections of all items in the MDS were taken into consideration, meaning those items that are common and those which are not, were all included in the first draft. All specialists present in the meeting, have focused their attention to the following questions:
Are those necessary items in the initial draft be eliminated or changed?
Are the items to be included in the initial draft possess high importance in terms of decision making?
To which group in the classification should each item in the data set be most appropriate?
Based on the collected suggestions, opinions and recommendations from specialists, the MDS was organized and contains all the data that would fill up the important categories of the patient’s pathway necessary for the management of breast cancer.
Again this MDS was deliberated in terms of the following:
Whether each data of the MDS play an important role in evaluating clinical performances and in decision making.
The extent of accessibility of the data to most patients, this is an important item since if these data set are not accessible by most patients so, filling up the data is impossible and the data set will be useless.
For each data, clear and explicit definition has been set in order to avoid ambiguity upon completing the data set.
Data items were particularly focused on the consequences, outcomes and the direction of care, particularly in the pathology section.
The MDS in the cancer registry network was structured in a hierarchical order and can register an unlimited number of procedures. Finally, the proposed model based on the needs of the country was acculturated. This data set is composed of 11 parameters in the form of fields in closed structured arrangements with consideration to coding responses. This form is available for use in the cancer registry database.
Results
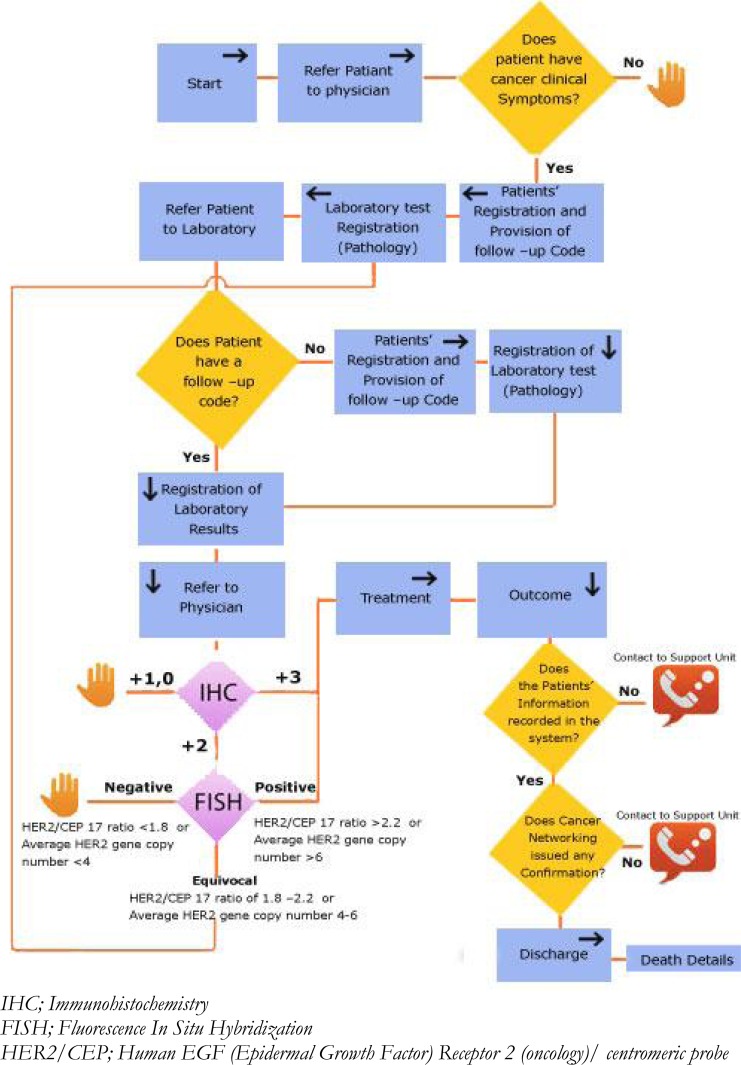
Figure1 (22), is a diagram showing patient cancer care pathway in Iran. This pathway allows the identification of the processes and outcomes that might develop during the patient’s treatment processes. This chart clearly defines the person to be approached and their corresponding responsibilities but this does not provide a complete information of all patients affected with cancer, therefore, cancer certificates and cancer registry contribute to the completeness of the information. Considering that this MDS is used for all breast cancer patients, all stages that a cancer patient must undergo are included. The health care provider concerned is the one responsible to gather all information regarding his patients. Each part of this data set must be completed by the responsible health care provider;(i.e.) surgeon involved must be the one to complete the necessary information regarding surgery and other related procedures, the pathologist concerned for completing the necessary information regarding chemotherapy and etc.
Fig. 1:
Patient’s care pathway in IRAN
In the analysis stage, the fact that at the present time there is no standard and uniform MDS available in the country, this data set was based on the tables on comparative evaluation of the MDS for breast cancer and the model that was approved by the experts in the format consisting of 11 sections in the form of fields in closed structured arrangements with consideration to coding responses which is presented in the following manner:
The first section includes hospital data that include: name of the hospital and hospital details. This section must be completed by the receptionist or head of the hospital’s data bank.
The second section is on demography and this entails the entire patient’s personal information like; name, surname, address, postcode, date of birth, gender, hospital record number, insurance serial number and the national code number respectively.
Third section includes the patient’s referral; the aim of this data category is to monitor the referral pathway the patient has passed through, the time from initial referral to all subsequent significant events.
The fourth section is about the data on patient’s investigation and physical examination which includes indicators that can be used to assess cancer. This indicator is intended to estimate the preciseness and accuracy of the cancer stages. Data in this section includes: breast exam, mammogram and ultrasound findings, FNA findings, core biopsy findings and nipple discharge cytology findings
The fifth section is about data related to diagnosis. This data category is intended to capture the date of diagnosis as required for the calculation of incidence data. The date of diagnosis is the month, day and year the tumor was first diagnosed by a medical practitioner, whether clinically or microscopically confirmed. Other data in this category includes; age at diagnosis, primary site, laterality, and performance status respectively.
The sixth section refers to the data on pathologic findings with an inclusion of their sub-categories together with the TNM classification (the TNM Nottingham standards was adopted). This section is to be filled-up and completed by a pathologist.
Section seven includes complete data regarding the patient’s treatment process and must be completed by an oncologist.
Section eight includes data on palliative care. Dates of contact between the patient and personnel/facilities providing palliative care services are collected in this category.
Section nine includes data regarding the patient’s treatment such as completion of primary treatment, the complications and toxicities that resulted from the treatment processes.
Section ten includes data on clinical trials.
Section eleven is on follow-up care.
On the patient’s identification, national code, hospital and laboratory codes based on standard codes defined by the MOH should be registered as well as the physician’s national licensure.
Discussion
Information in the health systems as a mediator to an effective care and treatment is a necessity and must be distributed properly between the users. The World Health Organization has stated that accurate, timely and accessible health care data plays a fundamental role in the program planning for the development and support of health services (23). In other words, health care providers in order to perform their professional activities and expertise demands accurate and timely information (24).
MDS provides an opportunity to strengthen communication between performed researches and exploit the results of researches to improve programs, policies and strategies of the country. Accurate and timely data is a potential in providing policymakers important principles in the health care industry (25).
In order to have an effective and successful decision making and an effective management implementation as well, the need for an efficient information system is felt for the reason that all information has a decisive role in the operation process (25).
The proposed MDS would led to the development of the health system performance and would have a positive effect on the four domains of the health system that include; sustainability, efficiency, effectiveness and accessibility (27).
In most studies that has been undertaken, in the development process of the minimum data set, usually, specialist in different related fields participated as a team and based on the opinions of the majority, past experiences and previous standards, a draft has been prepared (25).
In a study conducted in 2005 which was participated by pathologists, urologists, cancer registrars, data information managers and cancer researchers from 5 medical centers and National Institute for Specialized Cancer Programs, similar works and previous available standards have been utilized (28). In our study, the presence of oncologist and pathologist who are actively involved in researches regarding breast cancer were also utilized which is consistent with the above study but due to the fact that there was no previous standard breast cancer data set in Iran, therefore the comparative study was used in order to reached a preliminary model.
In addition, in this present study, aside from giving consideration to similar works done in various countries, the initial draft was designed and examined through the various meetings that were held by experts and specialists of the different fields. Finally, based on the opinions of the consensus, the MDS for breast cancer was finalized.
In another study, combine efforts were used to create a common minimum data set, in this study information regarding demography, pathology, treatment and clinical outcomes has been taken into consideration. After the approval of the first draft, this MDS was introduced into the information system and was submitted to the researchers for experimentation. This data set was composed of 145 elements and arranged into 5 major classifications such as; demography, clinical history, pathologic sampling and surgery, tissue block sampling, clinical results like; treatment, recurrence and vital status were classified. These data elements have been successfully able to respond to the needs of more than 60 end users and these data was used for 10 years to follow up patients. In our present study, based on the disease process, information was formatted into 11 sections in the form of fields in closed structured arrangements with consideration to coding responses. This data set includes 80 elements divided into 11 categories such as; hospital data, demography, referral, physical examination and investigation, diagnostic information, pathology, treatment, palliative care, completion of primary treatment, clinical trials and follow-up. This data set have been designed and made available in the data bank of the breast cancer website (29). In another study conducted in Italy in 2008, an MDS for stroke was developed based on prognostic factors and results of evaluation. The protocol was implemented as pilot then necessary changes and reforms were carried out (30). However, in our study, data set was obtained based on comparative study and based on consensus, but was not implemented as a pilot and therefore is not consistent with our study.
Results of another study conducted in Scotland have showed that the MDS of the Scottish Cancer Therapy Network could be a useful tool for any future prospective studies regarding breast cancer. The MDS is a robust mechanism for collecting meaningful and comparable data at the regional and national level (31).
Although the stages of creating the MDS for breast cancer has proven a success but its process of completion has been faced with several challenges. Holding face to face meetings does not always provide enough opportunities for the administrators to clearly explain their intensions and the future use of this data set, the resolving of the different views and opinions, discussions and dialogues must be completely implemented.
Based on experience and lessons learned in developing the minimum set of data for breast cancer, the following strategies are recommended for the research team that, in the near future efforts should be made to create and develop other MDS in various fields. In order to prevent possible duplication of works, a time frame of at least 6 months be allowed for creating and approving an MDS for a certain disease. Creation of a teamwork composed of specialists in epidemiology, biostatistics, health information managers, and other specialist depending of the type of the MDS to be developed in order that different dimensions will be considered. Based from the consensus and for the purpose of retrieving fast results, the researchers opted not to use the Delphi technique in this study. If the face to face meeting is to be employed, a whole day must be devoted for the meeting in order to have appropriate discussion and yield better results. Also, in finalizing the data set, technologic tools such as e-mails and forums were also utilized. Preliminary studies and preparation of the preliminary model before the start of a meeting have helped much in greatly reducing contradictions and incompatibility.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
The authors would like to express their thanks to Dr. Farzami Dr. Merzaie and Mr. Ghooshoni for their cooperation and support in the completion of this project. The authors declare that there is no conflict of interest.
References
- 1.Keshavarzi F, Noughani AE, Ayoubian MH, et al. Sequence Variants of BRCA1 and BRCA2 Genes in Four Iranian Families with Breast and Ovarian Cancer. Iranian J Publ Health. 2011;40(2):57–66. [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Regional Office for the Eastern Mediterranean Guidelines for management of breast cancer. EMRO Technical Publications Series31 2006 [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 4.Mamoon N, et al. Breast carcinoma over three decades in northern Pakistan — are we getting anywhere. JPMA. 2009;59:12. [PubMed] [Google Scholar]
- 5.Cancer Research Center . The Do’s and Don’ts, Recommendations for the Islamic Republic of Iran. Shahid Beheshti University; Tehran: 2009. pp. 1–20. [Google Scholar]
- 6.Fletcher CP, Hirdes PJ. Assessing the Health and Functional Status of Older Women with Breast Cancer Using the Minimum Data Set-Home Care (MDS-HC) Canadian Journal of Public Health. 2001;92:6. doi: 10.1007/BF03404540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, CaiGang L, Jun Z, Yang L, et al. Screen for specific proteins in the serum of patients with breast cancer using the technique of SELDI-TOF-MS. Journal of Chinese Clinical Medicine. 2006;4:6. [Google Scholar]
- Winget MD, et al. Development of common data elements: the experience of and recommendations from the early detection research network. International Journal of Medical Informatics. 2003;70:41–48. doi: 10.1016/s1386-5056(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 9.King PM, Blazeby JM, Gupta J, et al. Upper gastrointestinal cancer pathology reporting: a regional audit to compare standards with minimum datasets. J Clin Pathol. 2004;57:702–705. doi: 10.1136/jcp.2003.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDevitt J, Comber HA. Proposed CORE national Cancer Dataset: National Cancer Registry. National Cancer Registry Version 1.0; Ireland: 2011. Feb 09, 2013. [Google Scholar]
- 11.Latour KM, Maki SE. Health Information Management Concepts, Principles, and Practice. 2nd ed. AHIMA Press; Chicago: 2006. [Google Scholar]
- 12.Scattish intercollegiate guidelines network Management of Breast Cancer in Women. United Kingdom: Quality Improvement Scotland. Publication Number 84. 2005. Available: www.sign.ac.uk/pdf/sign84.pdf.
- 13.Jenkins J. Cancer Dataset Project: cancer Data Manual based on Cancer Dataset Version 4.5. NHS Health and Social Care Information Centre; 2006. [Google Scholar]
- 14.The National Breast Cancer Centre . Breast cancer specific data items for clinical cancer registration. National Breast and Ovarian Cancer Centre; Australia: 2009. Available from: www.nbcc.org.au. [Google Scholar]
- 15.Cancer Australia 2008. Framework for Specialist MDS Development for Specific Cancers in Clinical Cancer Registry, Australia: Commonwealth of Australia 2008 (Technical monograph1). Available from: www.canceraustralia.gov.au.
- 16.Johnson CH, Adamo M, editors. SEER Program Coding and Staging Manual 2007. National Cancer Institute; NIH; USA: 2008. Publication Number 07-5581. [Google Scholar]
- 17.Breast cancer surveillance consortium . Data dictionary ver. 3.5. The statistical coordination center; USA: 2010. [Google Scholar]
- 18.McDevitt J, Comber H. A Proposed Core National Cancer Dataset: National Cancer Registry. National Cancer Registry Version 1.0; Ireland: 2009. 17, [Google Scholar]
- 19.Reporting Template for Breast Cancer Resection Reports. Faculty of Pathology Royal college of physicians (revise 1); Ireland: 2010. p. 10. [Google Scholar]
- 20.North Trent Breast Cancer Group Guidelines for Treatment & Referral Breast cancer (version 10) 2006.
- 21.CCR Data Dictionary Canadian Cancer Registry – System Guide – Part I 2009.
- 22.Deputy for Research, Breast Cancer Registry Project . Recommendations for the Islamic Republic of Iran. Ministry of health; Iran: 2010. [Google Scholar]
- 23.World Health Organization (WHO) Monitoring reproductive health: selecting a short list of national and global indicators. Geneva: WHO Technical Report; 1997. Ref. WHO/RHT/HRP/97.256. [Google Scholar]
- 24.Mattingly R. Management of health information: functions and applications. Delmar publishers; Florence (KY): 1996. p. 5. [Google Scholar]
- 25.Kowal RP, et al. Creating a MDS on ageing in sub-Saharan Africa. 2010. Kowal free eBook. P: 5.available from: ebook-browse.com/search/kowal?page=5.
- 26.Shokriazde AL. The Comparative study of Legal Medicine organization in Several Courtiers. 2005. [PhD Thesis]. Tehran: School of Allied the Health Sciences, Tehran University of Medical Sciences. [Google Scholar]
- 27.Commission on Cancer . The Rapid Quality Reporting System Overview number 8. American College of Surgeon; USA: 2009. [Google Scholar]
- 28.Ashokkumar AP, et al. The development of common data elements for a multi-institute prostate cancer tissue bank: The Cooperative Prostate Cancer Tissue Resource (CPCTR) experience. BMC Cancer. 2005;5:108. doi: 10.1186/1471-2407-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moje C. Victorian Consensus Data Set. VCDS Governance Committee, Version 1.0; Australia: 2010. Available: www.cancervic.org.au/about-our-research/Victorian_cance_Registry/vcds-project. [Google Scholar]
- 30.Lenti G, et al. Developing a MDS for stroke patient’s assessment: the “Protocollo di Minima per l’Ictus (PMIC) as a starting point towards an Italian stroke registry. Eur J Phys Rehabil Med. 2008;44(3):263–9. [PubMed] [Google Scholar]
- 31.Reilly J, et al. Breast cancer audit: adapting national audit frameworks for local review of clinical practice. Health Bull (Edinb) 2001;59(1):60–2. [PubMed] [Google Scholar]