Abstract
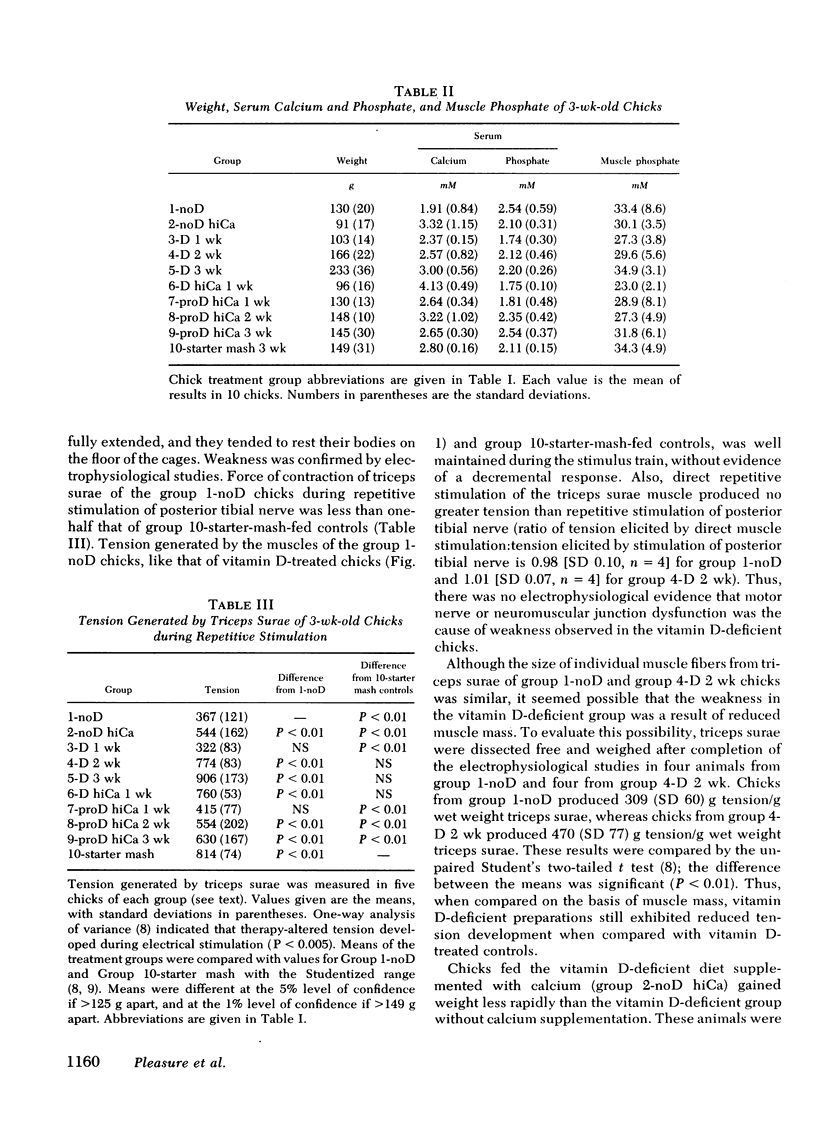
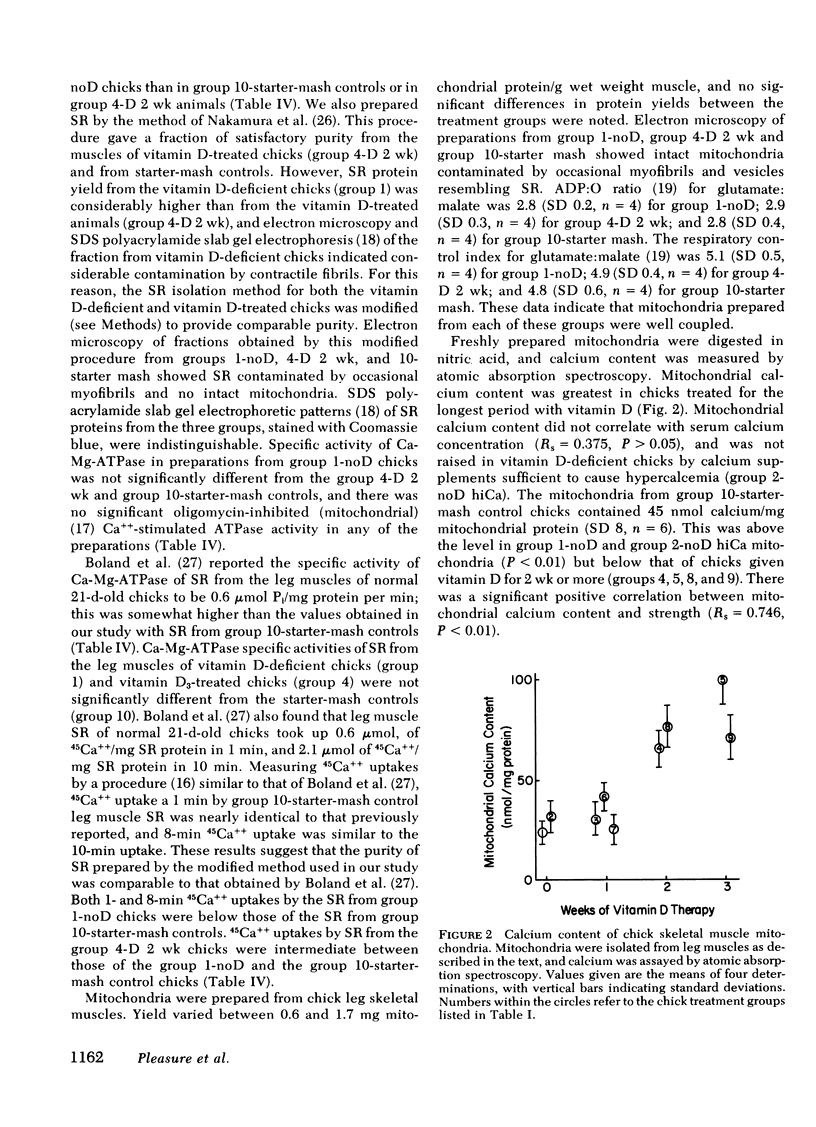
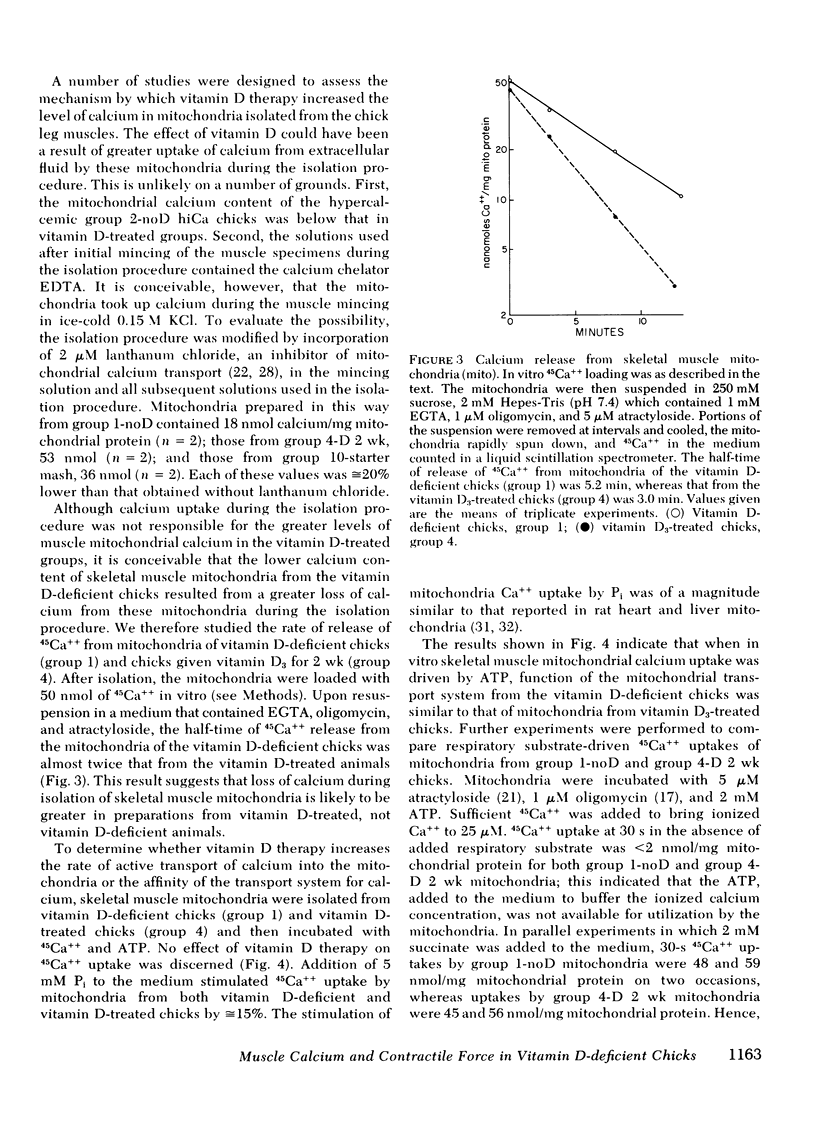
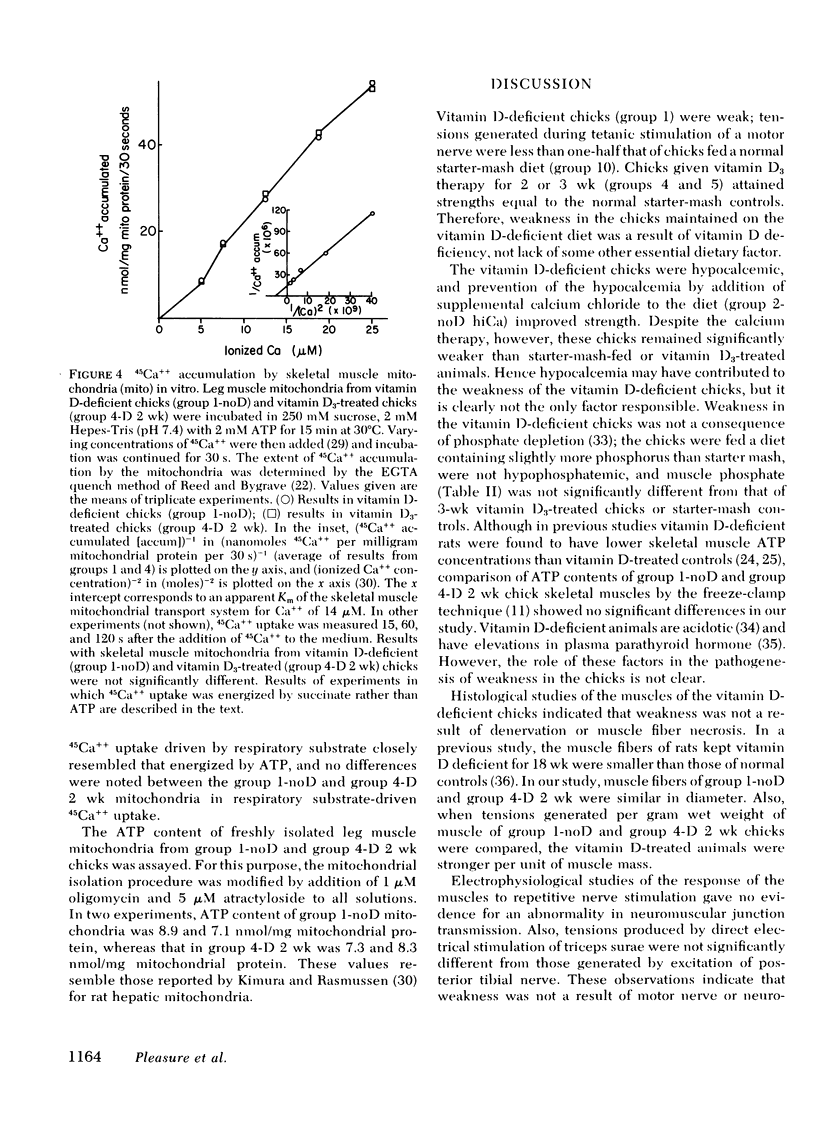
The myopathy associated with vitamin D deficiency has not been well characterized, and it is not known if weakness is a result of a specific effect of vitamin D deficiency on skeletal muscle. Chicks were raised from hatching on a vitamin D-deficient diet, and by 3 wk of age were hypocalcemic and appeared weak. Tension generated by triceps surae during repetitive stimulation of posterior tibial nerve was significantly less than that developed by chicks given vitamin D3 supplements (309 g tension/g wet weight of triceps surae, SD 60, for vitamin D-deficient chicks; 470, SD 77, for vitamin D3-treated chicks, P < 0.01). Histochemical and electron microscopic examination of skeletal muscles of these chicks showed no abnormalities, and there were no electrophysiologic evidences of motor nerve or neuromuscular junction dysfunction. The concentration of ATP in skeletal muscle of the vitamin D-deficient chicks (5.75 μmol/g wet weight, SD 0.17) was not significantly different from that in vitamin D-treated chicks (5.60, SD 0.50). There was no correlation between strength and serum calcium, serum inorganic phosphate, or skeletal muscle inorganic phosphate. Relaxation of tension after tetanic stimulation was slowed in the vitamin D-deficient chicks (20.6 ms, SD 1.7, vs. 15.4, SD 1.3, in vitamin D-treated chicks and 15.3, SD 1.0, in normal control chicks), and in vitro 45Ca++ transport by sarcoplasmic reticulum from the vitamin D-deficient chicks was reduced. Calcium content of mitochondria prepared from leg muscles of vitamin D-deficient chicks (24 nmol/mg mitochondrial protein, SD 6) was considerably lower than that of mitochondria from normal control chicks (45, SD 8) or from chicks treated with vitamin D for 2 wk or more (66-100, depending upon level and duration of therapy). Treatment of the vitamin D-deficient chicks from hatching with sufficient dietary calcium to produce hypercalcemia did not significantly raise skeletal muscle mitochondrial calcium content (31 nmol/mg mitochondrial protein, SD 7) and did not prevent weakness. These studies demonstrate objective weakness as a result of myopathy in vitamin D-deficient chicks, and provide evidence that vitamin D deficiency has effects on skeletal muscle calcium metabolism not secondary to altered plasma concentrations of calcium and phosphate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. H., Rizvi S. N., Vaishnava S. A diagnostic study of 120 cases of nutritional rickets. Indian Pediatr. 1975 Nov;12(11):1141–1147. [PubMed] [Google Scholar]
- Ashley C. C. Calcium ion regulation in barnacle muscle fibers and its relation to force development. Ann N Y Acad Sci. 1978 Apr 28;307:308–329. doi: 10.1111/j.1749-6632.1978.tb41959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski J., Lehninger A. L. Stoichiometric relationships in mitochondrial accumulation of calcium and phosphate supported by hydrolysis of adenosine triphosphate. J Biol Chem. 1966 Oct 10;241(19):4316–4322. [PubMed] [Google Scholar]
- Bikle D. D., Zolock D. T., Morrissey R. L., Herman R. H. Independence of 1,25-dihydroxyvitamin D3-mediated calcium transport from de novo RNA and protein synthesis. J Biol Chem. 1978 Jan 25;253(2):484–488. [PubMed] [Google Scholar]
- Birge S. J., Haddad J. G. 25-hydroxycholecalciferol stimulation of muscle metabolism. J Clin Invest. 1975 Nov;56(5):1100–1107. doi: 10.1172/JCI108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland R., Martonosi A., Tillack T. W. Developmental changes in the composition and function of sarcoplasmic reticulum. J Biol Chem. 1974 Jan 25;249(2):612–623. [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Metabolic acidosis in the vitamin D-deficient chick. Metabolism. 1977 Oct;26(10):1099–1105. doi: 10.1016/0026-0495(77)90036-1. [DOI] [PubMed] [Google Scholar]
- Crompton M., Künzi M., Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur J Biochem. 1977 Oct 3;79(2):549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- Curry O. B., Basten J. F., Francis M. J., Smith R. Calcium uptake by sarcoplasmic reticulum of muscle from vitamin D-deficient rabbits. Nature. 1974 May 3;249(452):83–84. doi: 10.1038/249083a0. [DOI] [PubMed] [Google Scholar]
- DELUCA H. F., ENGSTROM G. W., RASMUSSEN H. The action of vitamin D and parathyroid hormone in vitro on calcium uptake and release by kidney mitochondria. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1604–1609. doi: 10.1073/pnas.48.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca H. F. Vitamin D endocrine system. Adv Clin Chem. 1977;19:125–174. doi: 10.1016/s0065-2423(08)60172-9. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Schotland D. L., Bonilla E., Lee C. P., Gambetti P., Rowland L. P. Progressive ophthalmoplegia, glycogen storage, and abnormal mitochondria. Arch Neurol. 1973 Sep;29(3):170–179. doi: 10.1001/archneur.1973.00490270052008. [DOI] [PubMed] [Google Scholar]
- ENGSTROM G. W., DELUCA H. F. THE NATURE OF CA++ BINDING BY KIDNEY MITOCHONDRIA. Biochemistry. 1964 Mar;3:379–383. doi: 10.1021/bi00891a013. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Fuller T. J., Carter N. W., Barcenas C., Knochel J. P. Reversible changes of the muscle cell in experimental phosphorus deficiency. J Clin Invest. 1976 Apr;57(4):1019–1024. doi: 10.1172/JCI108343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Autilio-Gambetti L., Gambetti P., Shafer B. Morphological and biochemical changes in rat synaptosome fractions during neonatal development. J Cell Biol. 1971 Nov;51(21):484–498. doi: 10.1083/jcb.51.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Zaba B. The phosphate requirement for Ca2+-uptake by heart and liver mitochondria. FEBS Lett. 1977 Jul 15;79(2):284–290. doi: 10.1016/0014-5793(77)80804-1. [DOI] [PubMed] [Google Scholar]
- Haussler M., Hughes M., Baylink D., Littledike E. T., Cork D., Pitt M. Influence of phosphate depletion on the biosynthesis and circulating level of 1alpha,25-dihydroxyvitamin D. Adv Exp Med Biol. 1977;81:233–250. doi: 10.1007/978-1-4613-4217-5_24. [DOI] [PubMed] [Google Scholar]
- Henderson R. G., Russell R. G., Ledingham J. G., Smith R., Oliver D. O., Walton R. J., Small D. G., Preston C., Warner G. T. Effects of 1,25-dihydroxycholecalciferol on calcium absorption, muscle weakness, and bone disease in chronic renal failure. Lancet. 1974 Mar 9;1(7854):379–384. doi: 10.1016/s0140-6736(74)93149-3. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Frommer J. E., McNeill S. C., Richtand N. M., Henley J. W., Potts J. T., Jr Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun. 1977 May 9;76(1):107–114. doi: 10.1016/0006-291x(77)91674-6. [DOI] [PubMed] [Google Scholar]
- Huddart H., Hunt S., Oates K. Calcium movements during contraction in molluscan smooth muscle, and the loci of calcium binding and release. J Exp Biol. 1977 Jun;68:45–56. doi: 10.1242/jeb.68.1.45. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Southard J. H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976 Aug 25;251(16):5069–5077. [PubMed] [Google Scholar]
- Kimberg D. V., Goldstein S. A. Binding of calcium by liver mitochondria: an effect of steroid hormones in vitamin D-depleted and parathyroidectomized rats. Endocrinology. 1967 Jan;80(1):89–98. doi: 10.1210/endo-80-1-89. [DOI] [PubMed] [Google Scholar]
- Kimura S., Rasmussen H. Adrenal glucocorticoids, adenine nucleotide translocation, and mitochondrial calcium accumulation. J Biol Chem. 1977 Feb 25;252(4):1217–1225. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lumb G. A., Stanbury S. W. Parathyroid function in human vitamin D deficiency and vitamin D deficiency in primary hyperparathyroidism. Am J Med. 1974 Jun;56(6):833–839. doi: 10.1016/0002-9343(74)90812-2. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Lee C. P. Biochemical studies of skeletal muscle mitochondria. I. Microanalysis of cytochrome content, oxidative and phosphorylative activities of mammalian skeletal muscle mitochondria. Arch Biochem Biophys. 1968 Jul;126(1):75–82. doi: 10.1016/0003-9861(68)90561-4. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N., Chyn T. L., Schibeci A. The calcium transport of sarcoplasmic reticulum. Ann N Y Acad Sci. 1978 Apr 28;307:148–159. doi: 10.1111/j.1749-6632.1978.tb41940.x. [DOI] [PubMed] [Google Scholar]
- Matthews C., Heimberg K. W., Ritz E., Agostini B., Fritzsche J., Hasselbach W. Effect of 1,25-dihydroxycholecalciferol on impaired calcium transport by the sarcoplasmic reticulum in experimental uremia. Kidney Int. 1977 Apr;11(4):227–235. doi: 10.1038/ki.1977.35. [DOI] [PubMed] [Google Scholar]
- Max S. R., Garbus J., Wehman H. J. Simple procedure for rapid isolation of functionally intact mitochondria from human and rat skeletal muscle. Anal Biochem. 1972 Apr;46(2):576–584. doi: 10.1016/0003-2697(72)90328-4. [DOI] [PubMed] [Google Scholar]
- Meissner G. Isolation of sarcoplasmic reticulum from skeletal muscle. Methods Enzymol. 1974;31:238–246. doi: 10.1016/0076-6879(74)31025-7. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Jilka R. L., Boland R., Martonosi A. N. Mechanism of ATP hydrolysis by sarcoplasmic reticulum and the role of phospholipids. J Biol Chem. 1976 Sep 10;251(17):5414–5423. [PubMed] [Google Scholar]
- Noack E. A., Heinen E. M. A kinetic study of calcium transort by heart mitochondria. Eur J Biochem. 1977 Sep 15;79(1):245–250. doi: 10.1111/j.1432-1033.1977.tb11802.x. [DOI] [PubMed] [Google Scholar]
- Norman A. W. Actinomycin D effect on lag in vitamin D-mediated calcium absorption in the chick. Am J Physiol. 1966 Sep;211(3):829–834. doi: 10.1152/ajplegacy.1966.211.3.829. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Fontaine O., Max E. E., Goodman D. B. The effect of 1alpha-hydroxyvitamin D3 administration on calcium transport in chick intestine brush border membrane vesicles. J Biol Chem. 1979 Apr 25;254(8):2993–2999. [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman J. S., Baker T. Changes in the kinetics of muscle contraction in vitamin D-depleted rats. Kidney Int. 1978 Mar;13(3):189–193. doi: 10.1038/ki.1978.28. [DOI] [PubMed] [Google Scholar]
- Schimrigk K., Lassmann R., Ströder J. Morphological and morphometric studies of the skeletal muscles of rachitic rats. Z Kinderheilkd. 1975;119(4):235–243. doi: 10.1007/BF00443507. [DOI] [PubMed] [Google Scholar]
- Schott G. D., Wills M. R. Muscle weakness in osteomalacia. Lancet. 1976 Mar 20;1(7960):626–629. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- Sjostrom M., Lorentzon R., Larsson S. E., Holmlund D. The influence of 1,25-dihydroxycholecalciferol on the ultrastructural organization of skeletal muscle fibres. Morphometric analyses on vitamin D deficient or calcium deficient growing rats. Med Biol. 1978 Aug;56(4):209–215. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Sloane B., Scarpa A. Electron probe analysis of calcium compartments in cryo sections of smooth and striated muscles. Ann N Y Acad Sci. 1978 Apr 28;307:523–544. doi: 10.1111/j.1749-6632.1978.tb41980.x. [DOI] [PubMed] [Google Scholar]
- Spencer T., Bygrave F. L. The role of mitochondria in modifying the cellular ionic environment: studies of the kinetic accumulation of calcium by rat liver mitochondria. J Bioenerg. 1973 Apr;4(3):347–362. doi: 10.1007/BF01648977. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Tew W. P. Use of the coulombic interactions of the lanthanide series to identify two classes of Ca2+ binding sites in mitochondria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):624–630. doi: 10.1016/0006-291x(77)90225-x. [DOI] [PubMed] [Google Scholar]
- Toffolon E. P., Pechet M. M., Isselbacher K. Demonstration of the rapid action of pure crystalline 1 alpha-hydroxy vitamin D3 and 1 alpha,25-dihydroxy vitamin D3 on intestinal calcium uptake. Proc Natl Acad Sci U S A. 1975 Jan;72(1):229–230. doi: 10.1073/pnas.72.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Warner R. R., Coleman J. R. Electron probe analysis of calcium transport by small intestine. J Cell Biol. 1975 Jan;64(1):54–74. doi: 10.1083/jcb.64.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Bygrave F. L., Lehninger A. L. Characterization of the atractyloside-sensitive adenine nucleotide transport system in rat liver mitochondria. J Biol Chem. 1968 Jan 10;243(1):20–28. [PubMed] [Google Scholar]


