Abstract
This free-flow micropuncture study examined the dependence of bicarbonate reabsorption in the rat superficial proximal convoluted tubule to changes in filtered bicarbonate load, and thereby the contribution of the proximal tubule to the whole kidney's response to such changes. The independent effects of extracellular fluid (ECF) volume expansion and of acidosis on proximal bicarbonate reabsorption were also examined.
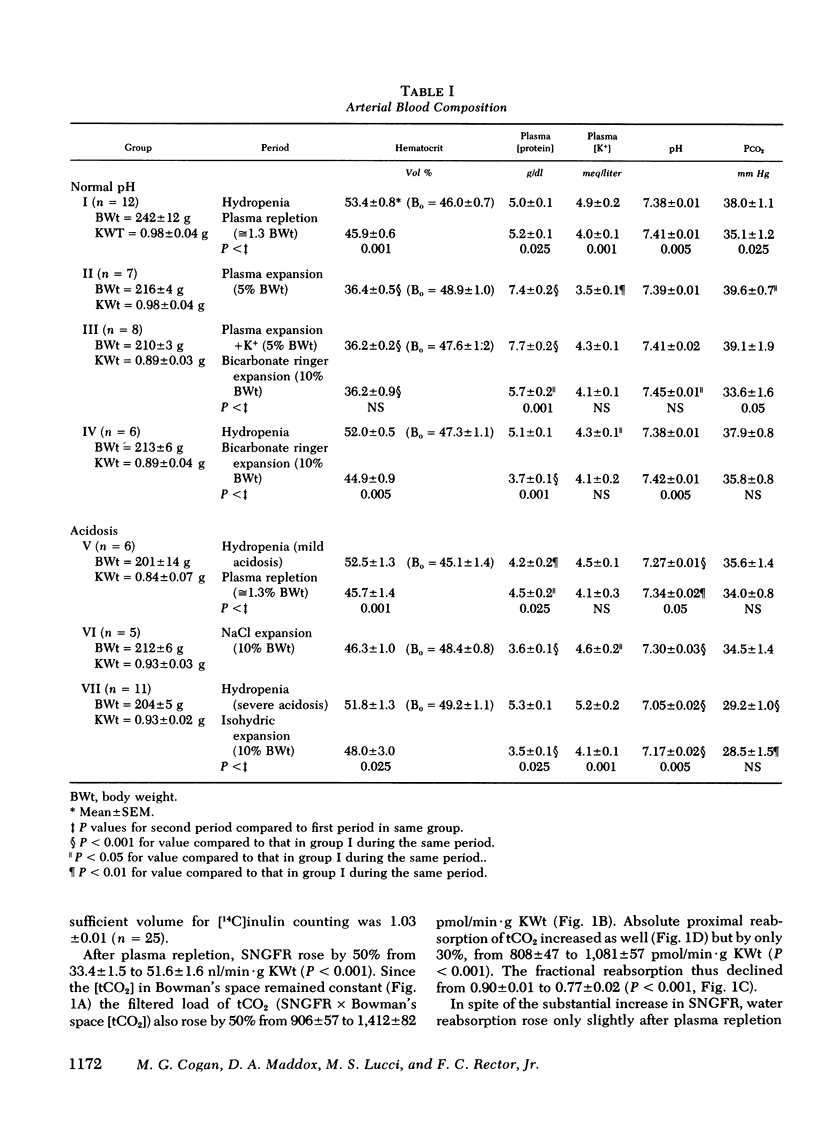
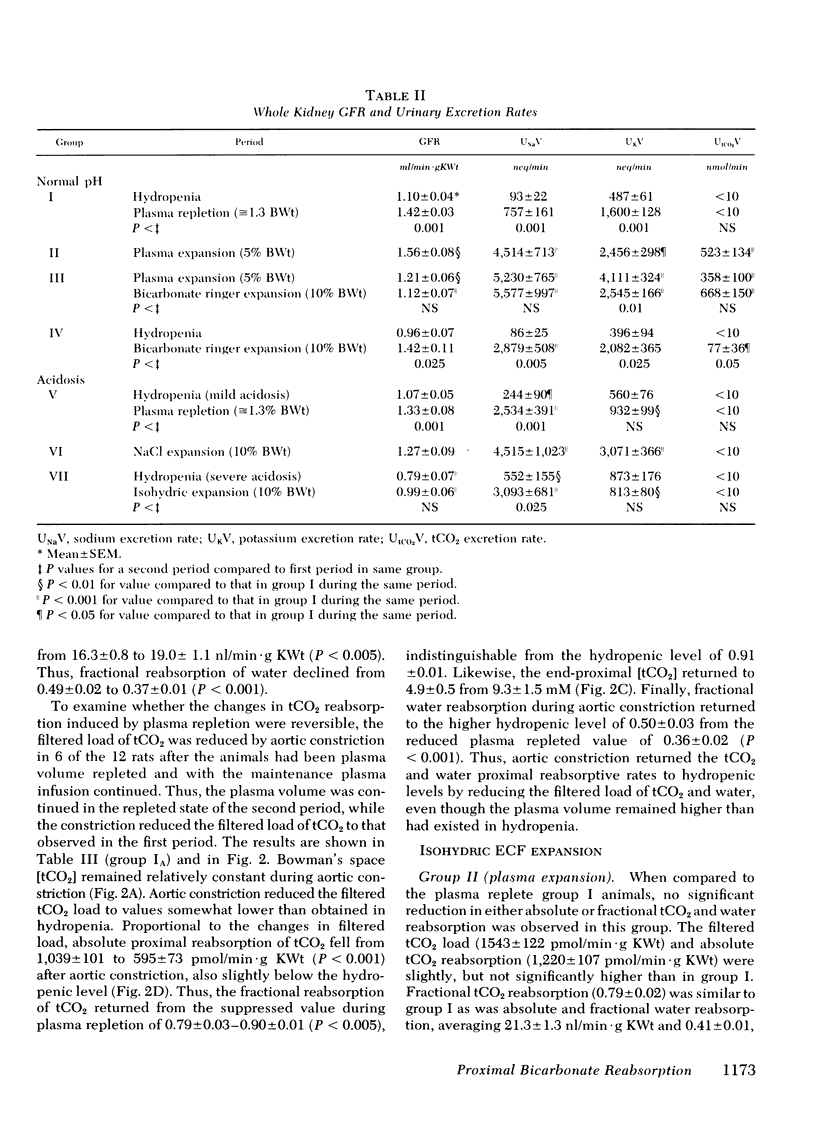
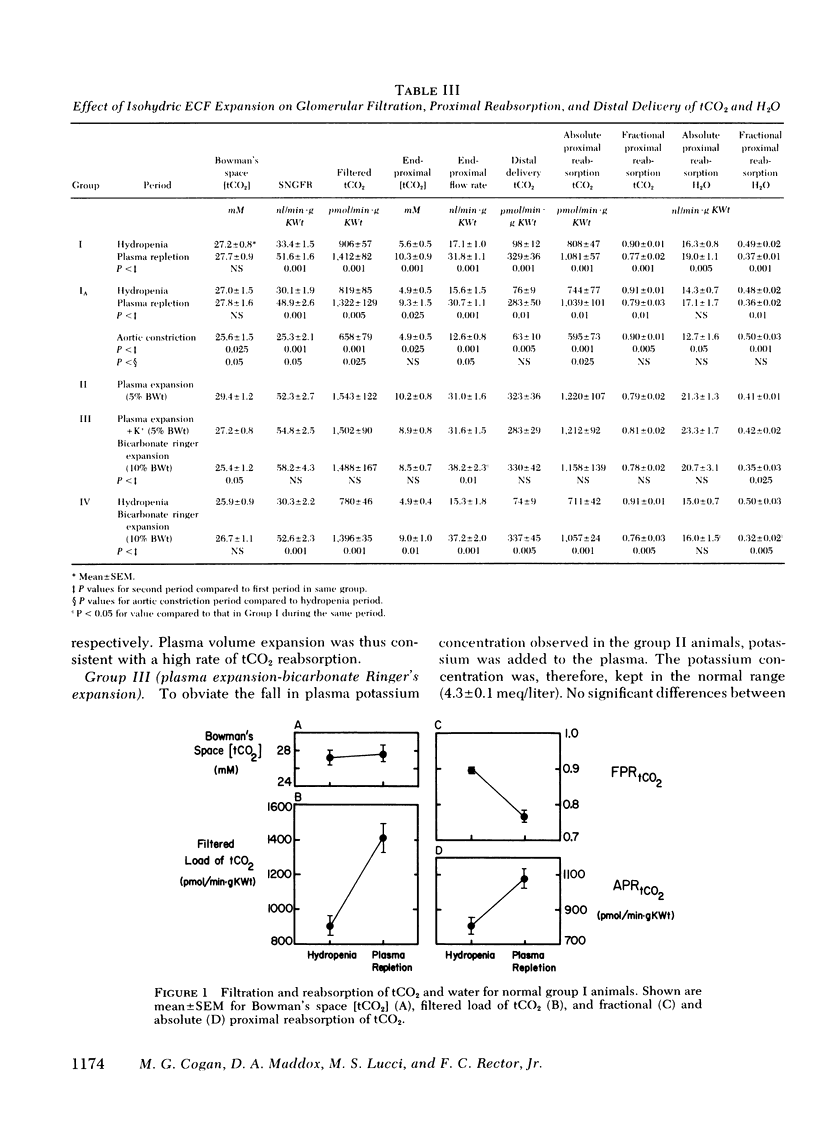
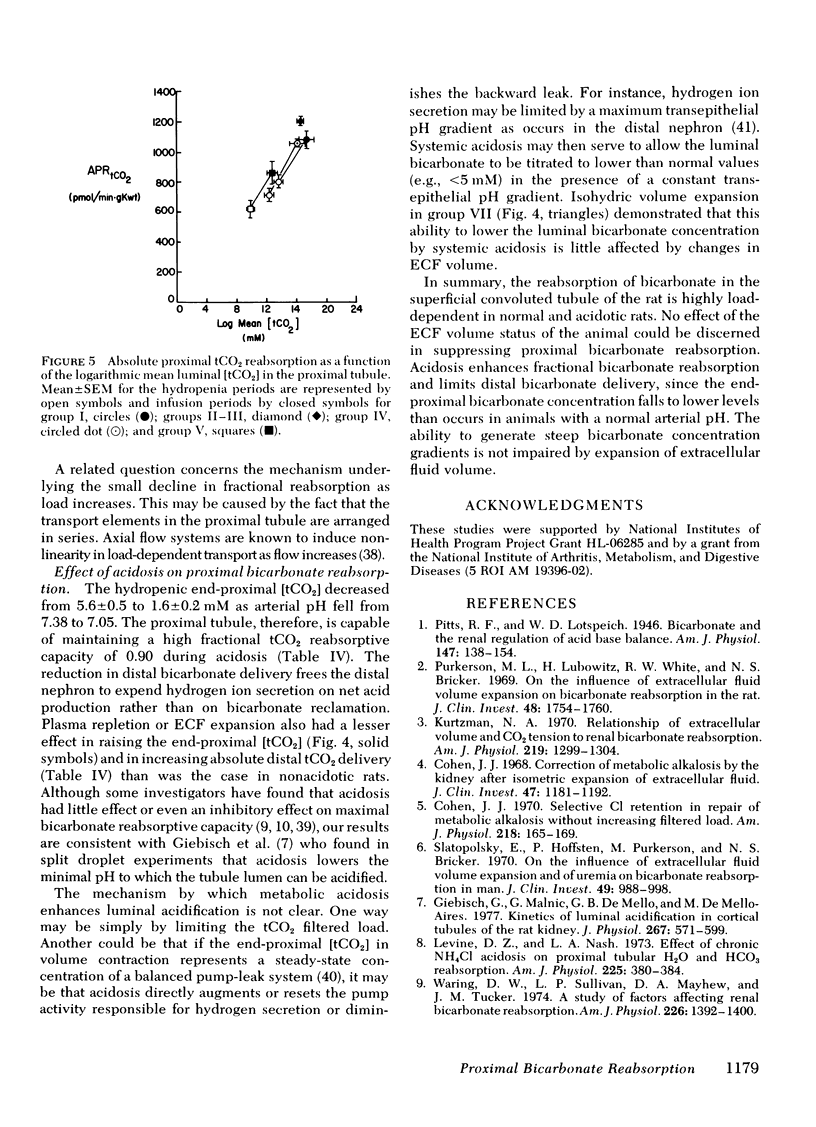
When the plasma volume contraction incurred by the micropuncture preparatory surgery was corrected by isoncotic plasma infusion (≅1.3% body wt), single nephron glomerular filtration rate (SNGFR), and the filtered total CO2 load increased by 50%. Absolute proximal reabsorption of total CO2 (measured by microcalorimetry) increased by 30%, from 808±47 during volume contraction to 1,081±57 pmol/min·g kidney wt after plasma repletion, as fractional total CO2 reabsorption decreased from 0.90 to 0.77. Aortic constriction in these plasma-repleted rats returned the filtered load and reabsorption of total CO2 to the previous volume contracted levels. In other animals isohydric ECF expansion with plasma (5% body wt) or Ringer's solution (10% body wt), or both, produced no further diminution in fractional proximal total CO2 reabsorption (0.76-0.81).
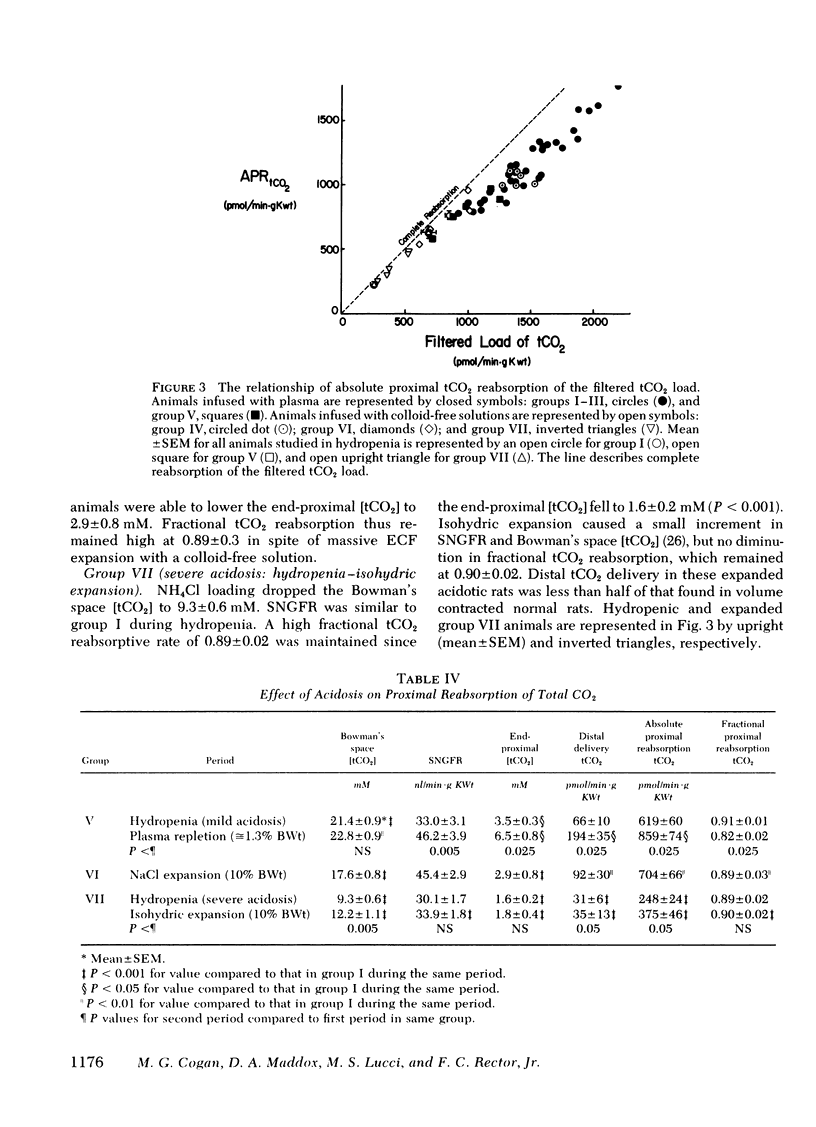
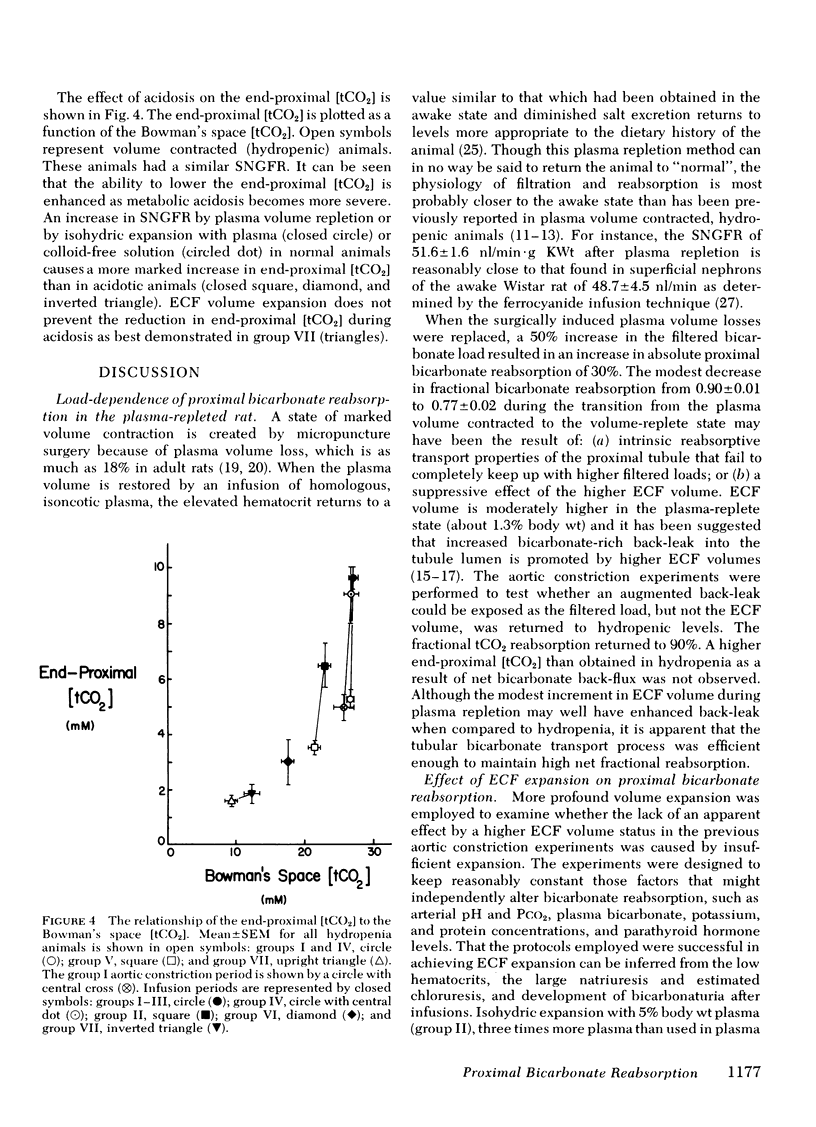
Metabolic acidosis was associated with very high fractional proximal total CO2 reabsorptive rates of 0.82 to 0.91 over a wide range of SNGFR and ECF volumes. At a single level of SNGFR, end-proximal total CO2 concentration progressively decreased from 5.6±0.5 to 1.6 ±0.2 mM as arterial pH fell from 7.4 to 7.1. Expansion of ECF volume in the acidotic rats did not inhibit the ability of the proximal tubule to lower end-proximal total CO2 concentrations to minimal levels.
In conclusion, bicarbonate reabsorption in the superficial proximal convoluted tubule is highly load-dependent (75-90%) in normal and acidotic rats. No inhibitory effect of ECF volume per se on proximal bicarbonate reabsorption, independent of altering the filtered bicarbonate load, could be discerned. Acidosis enabled the end-proximal luminal bicarbonate concentration to fall below normal values and reduced distal bicarbonate delivery.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGEN A. S. A theoretical treatment of glucose reabsorption in the kidney. Can J Biochem Physiol. 1956 May;34(3):466–474. [PubMed] [Google Scholar]
- Barfuss D. W., Schafer J. A. Active amino acid absorption by proximal convoluted and proximal straight tubules. Am J Physiol. 1979 Feb;236(2):F149–F162. doi: 10.1152/ajprenal.1979.236.2.F149. [DOI] [PubMed] [Google Scholar]
- Barfuss D. W., Schafer J. A. Flow dependence of nonelectrolyte absorption in the nephron. Am J Physiol. 1979 Feb;236(2):F163–F174. doi: 10.1152/ajprenal.1979.236.2.F163. [DOI] [PubMed] [Google Scholar]
- Bonvalet J. P., Champion M., Wanstok F., Berjal G. Nephron filtration rate in conscious rats recovering from anesthesia. Am J Physiol. 1977 Apr;232(4):F329–F334. doi: 10.1152/ajprenal.1977.232.4.F329. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. On the mechanism of inhibition in fluid reabsorption by the renal proximal tubule of the volume-expanded rat. J Clin Invest. 1971 Aug;50(8):1596–1602. doi: 10.1172/JCI106647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassola A. C., Giebisch G., Malnic G. Mechansims and components of renal tubular acidification. J Physiol. 1977 Jun;267(3):601–624. doi: 10.1113/jphysiol.1977.sp011828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Correction of metabolic alkalosis by the kidney after isomertric expansion of extracellular fluid. J Clin Invest. 1968 May;47(5):1181–1192. doi: 10.1172/JCI105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Selective Cl retention in repair of metabolic alkalosis without increasing filtered load. Am J Physiol. 1970 Jan;218(1):165–170. doi: 10.1152/ajplegacy.1970.218.1.165. [DOI] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Nicholas D. P., Brenner B. M. Comparative renal effects of isoncotic and colloid-free volume expansion in the rat. Am J Physiol. 1972 Jan;222(1):225–235. doi: 10.1152/ajplegacy.1972.222.1.225. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of peritubular capillary control of isotonic fluid reabsorption by the renal proximal tubule. Biophys J. 1973 Apr;13(4):340–358. doi: 10.1016/S0006-3495(73)85989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Seldin D. W., Carter N. W. Direct determination of PCO2 in the rat renal cortex. J Clin Invest. 1978 Aug;62(2):338–348. doi: 10.1172/JCI109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., LASSITER W. E., MYLLE M. Localization of urine acidification in the mammalian kidney. Am J Physiol. 1960 Mar;198:581–585. doi: 10.1152/ajplegacy.1960.198.3.581. [DOI] [PubMed] [Google Scholar]
- Garella S., Chazan J. A., Bar-Khayim Y., Cohen J. J. Isolated effect of increased ECF volume on HCO 3 and Cl reabsorption in the dog. Am J Physiol. 1972 May;222(5):1138–1146. doi: 10.1152/ajplegacy.1972.222.5.1138. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Malnic G., De Mello G. B., De Mello Aires M. Kinetics of luminal acidification in cortical tubules of the rat kidney. J Physiol. 1977 Jun;267(3):571–599. doi: 10.1113/jphysiol.1977.sp011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulter H. N., Ilnicki L. P., Harbottle J. A., Sebastian A. Correction of metabolic acidosis by the kidney during isometric expansion of extracellular fluid volume. J Lab Clin Med. 1978 Oct;92(4):602–612. [PubMed] [Google Scholar]
- Kurtzman N. A. Relationship of extracellular volume and CO2 tension to renal bicarbonate reabsorption. Am J Physiol. 1970 Nov;219(5):1299–1304. doi: 10.1152/ajplegacy.1970.219.5.1299. [DOI] [PubMed] [Google Scholar]
- Levine D. Z., Nash L. A., Chan T., Dubrovskis A. H. Proximal bicarbonate reabsorption during Ringer and albumin infusions in the rat. J Clin Invest. 1976 Jun;57(6):1490–1497. doi: 10.1172/JCI108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. Z., Nash L. A. Effect of chronic NH4Cl acidosis on proximal tubular H2O and HCO3 reabsorption. Am J Physiol. 1973 Aug;225(2):380–384. doi: 10.1152/ajplegacy.1973.225.2.380. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Price D. C., Rector F. C., Jr Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol. 1977 Dec;233(6):F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. [DOI] [PubMed] [Google Scholar]
- Malnic G., de Mello-Aires M. Kinetic study of bicarbonate reabsorption in proximal tubule of the rat. Am J Physiol. 1971 Jun;220(6):1759–1767. doi: 10.1152/ajplegacy.1971.220.6.1759. [DOI] [PubMed] [Google Scholar]
- Mathisen O., Raeder M., Sejersted O. M., Kiil F. Effect of acetazolamide on glomerular balance and renal metabolic rate. Scand J Clin Lab Invest. 1976 Nov;36(7):617–625. doi: 10.3109/00365517609054486. [DOI] [PubMed] [Google Scholar]
- Purkerson M. L., Lubowitz H., White R. W., Bricker N. S. On the influence of extracellular fluid volume expansion on bicarbonate reabsorption in the rat. J Clin Invest. 1969 Sep;48(9):1754–1760. doi: 10.1172/JCI106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, CARTER N. W., SELDIN D. W. THE MECHANISM OF BICARBONATE REABSORPTION IN THE PROXIMAL AND DISTAL TUBULES OF THE KIDNEY. J Clin Invest. 1965 Feb;44:278–290. doi: 10.1172/JCI105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. W., Bricker N. S., Gavellas G. Bicarbonate reabsorption in the dog with experimental renal disease. Kidney Int. 1976 Oct;10(4):287–294. doi: 10.1038/ki.1976.111. [DOI] [PubMed] [Google Scholar]
- Seldin D. W., Rector F. C., Jr Symposium on acid-base homeostasis. The generation and maintenance of metabolic alkalosis. Kidney Int. 1972 May;1(5):306–321. doi: 10.1038/ki.1972.43. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Hoffsten P., Purkerson M., Bricker N. S. On the influence of extracellular fluid volume expansion and of uremia on bicarbonate reabsorption in man. J Clin Invest. 1970 May;49(5):988–998. doi: 10.1172/JCI106318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter B. D., Osiecki H. S., Cross R. B., Budtz-Olsen O., Jedrzejczyk H. The regulation of bicarbonate reabsorption. The role of arterial pH, pCO2 and plasma bicarbonate concentration. Pflugers Arch. 1974 May 24;349(1):29–40. doi: 10.1007/BF00587914. [DOI] [PubMed] [Google Scholar]
- Sohtell M., Karlmark B. In vivo micropuncture PCO2 measurements. Pflugers Arch. 1976 May 12;363(2):179–180. doi: 10.1007/BF01062288. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R., Lawson L. R. Effect of luminal pH on ion permeability and flows of Na+and H+ in turtle bladder. Am J Physiol. 1971 Jun;220(6):1573–1580. doi: 10.1152/ajplegacy.1971.220.6.1573. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Radtke H. W., Rumrich G. The role of bicarbonate and other buffers on isotonic fluid absorption in the proximal convolution of the rat kidney. Pflugers Arch. 1971;330(2):149–161. doi: 10.1007/BF00643031. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]
- Waring D. W., Sullivan L. P., Mayhew D. A., Tucker J. M. A study of factors affecting renal bicarbonate reabsorption. Am J Physiol. 1974 Jun;226(6):1392–1400. doi: 10.1152/ajplegacy.1974.226.6.1392. [DOI] [PubMed] [Google Scholar]


