Abstract
BACKGROUND
The role of maternal zinc nutrition in human oral clefts (OCs) is unclear. We measured plasma zinc concentrations (PZn) of case- and control-mothers to evaluate the associations between PZn and risk of OCs with and without other malformations.
METHODS
Case-mothers were ascertained by the Utah Birth Defects Network and control-mothers were selected from Utah birth certificates by matching for child gender and delivery month and year. Maternal blood was collected > 1 year after the last pregnancy. PZn was available for 410 case-mothers who were divided into four subgroups; isolated cleft lip with or without cleft palate (CL/P-I, n = 231), isolated cleft palate (CP-I, n = 74), CL/P with other malformations (CLP-M, n = 42), and CP with other malformations (CP-M, n = 63). PZn was available for 447 control-mothers. The mean age of children at blood sampling was 3.7 years for all cases combined and 4.3 years for controls.
RESULTS
Mean PZn of all groups were similar, and low PZn (< 11.0 μmol/l) was found in 59% of cases and 62% of controls. Risk of OCs did not vary significantly across PZn quartiles for the four subgroups individually and all OC groups combined.
CONCLUSION
We previously reported that poor maternal zinc status was a risk factor for OCs in the Philippines, where OC prevalence is high and maternal PZn is low. In Utah, however, no such association was found, suggesting that poor maternal zinc status may become a risk factor only when zinc status is highly compromised.
Keywords: zinc status, plasma zinc, cleft lip, cleft palate, malformations, human, Utah
INTRODUCTION
Researchers reported that gestational zinc deficiency in animals leads to various malformations including oral clefts (OCs) (Hurley and Swenerton, 1966; Warkany and Petering, 1972). Despite such a seemingly clear association in animals, only a limited human studies have been conducted, and the results are conflicting (Krapels et al., 2004a; Krapels et al., 2004b; Tamura et al., 2005; Shaw et al., 2006), and there is no consensus on the association between zinc status in pregnancy and the risk of other malformations (Shah and Sachdev, 2006).
We reported that the lower plasma zinc concentrations (PZn) of Filipino mothers were associated with a higher OC risk in their children, where poor zinc status was common in young women (Tamura et al., 2005). We postulated that this association exists also in Utah, a state with the highest birth prevalence of OCs in the US (Gebreab et al., 2008), and evaluated the risk of OCs in relation to maternal PZn in a case-control study. We found no significant association between maternal PZn and OC risk in Utah. In this paper, we offer potential mechanisms as to why our findings differed between Utah and the Philippines.
MATERIALS AND METHODS
Participants
This investigation was a part of a case-control study to identify genetic and environmental factors, including nutrition, that are associated with the risk of OCs with and without other malformations. The study was approved by the Institutional Review Boards of Utah State University, the University of Utah, the Utah Department of Health, and the University of Alabama at Birmingham. Each mother provided informed consent after the detailed study protocol was explained. Case-mothers had a child live-born or still-born with an OC between January 1995 and June 2004. The classification of OCs and other malformations was made after review of the Utah Birth Defects Network records by a geneticist (JCC). Case were categorized into: 1) isolated cleft lip with or without cleft palate (CL/P-I); 2) isolated cleft palate (CP-I); 3) CL/P with other malformations (CLP-M); and 4) CP with other malformations (CP-M). Control-mothers without a history of a cleft-affected pregnancy were randomly selected using Utah birth certificate files by frequency matching births by month and year and child gender to cases. Both case- and control-mothers were Utah residents who spoke either English or Spanish. The interviews of mothers and blood collection occurred between June 2000 and November 2005.
Sample Collection and Zinc Analysis
Non-fasting blood samples were obtained from mothers using trace-mineral-free tubes with heparin (Preanalytical Solutions, Franklin Lake, NJ). To avoid falsely high zinc values, the tubes were placed on ice immediately until plasma separation that took place within 2 hr after phlebotomy. Plasma samples were shipped on dry ice to Birmingham and stored at −80°C in screw-top tubes until analysis. Samples with visible hemolysis were excluded from the analysis. PZn was measured by the flame-atomic-absorption spectrophotometric method, and the day-to-day coefficient of variation was 4% (Tamura et al., 1994). Blood sampling and zinc analysis for this study were made in the manner as close as possible to those used in the previous Philippine study (Tamura et al., 2005) in order for us to be able to compare PZn values from these two independent studies.
Statistical Analyses
The means of continuous variables were compared between cases and controls using analysis of variance, and categorical variables were evaluated with the Chi-square test. Quartiles of PZn were defined by the distribution of values of all cases and controls combined. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in logistic-regression models with inclusion of potential confounding factors including maternal age, education, smoking, alcohol and multivitamin use during preconceptional period and pregnancy. The multivariate-adjusted ORs for risk of OCs were calculated across increasing PZn quartiles with the lowest quartile as the reference. Tests for linear trends of ORs were performed using the median PZn values of each quartile to score each level. Statistical analyses were performed with the SPSS software programs (SPSS Inc., Chicago, IL).
RESULTS
Demographic information and PZn were available for: 1) 231 CL/P-I case-mothers; 2) 74 CP-I case-mothers; 3) 42 CL/P-M case-mothers; 4) 63 CP-M case-mothers; and 5) 447 control-mothers. Ethnic/racial composition of all subjects was ~89% Caucasian and ~6% Hispanic/Latino, and remaining were Asians, native Americans and African Americans. The overall maternal mean age at delivery of the index child was 27.2 (± 5.5, SD) years old. In general, all five groups of mothers showed similar characteristics including body-mass index that could have affected PZn (Tamura et al., 2004). CL/P-I case-mothers (the largest single OC group) had similar rate of multivitamin use at 3 month before pregnancy, first and third month of pregnancy as well as at the time of blood collection to controls, whereas the other case groups had generally higher rate of multivitamin use than controls. Rates of smoking were higher in case-mothers than control-mothers (P = 0.02), and alcohol use during pregnancy was not significantly different for cases and controls (P = 0.69). Maternal levels of education were lower in case-mothers than controls (P = 0.02). The mean interval between delivery of the index child and blood collection (equivalent to mean age of children) was 3.7 (± 2.2) years for case-mothers, and 4.3 (± 2.3) years for control-mothers (P = 0.02).
The mean PZn was 10.6 ± 2.0 μmol/l for all groups combined. Mean PZn values were similar in the five groups (P > 0.05), including 10.5 ± 2.1 for CL/P-I; 10.7 ± 1.9 for CP-I; 10.9 ± 1.7 for CL/P-M; 10.5 ± 1.9 for CP-M; and 10.6 ± 2.0 for the control group. Using the generally accepted cut-off value (9.9 μmol/l) of PZn for adequate zinc status in non-pregnant women, inadequate zinc status was found in 59.7% of CL/P-I, 59.5% of CP-I, 54.8% of CL/P-M, 60.3% of CP-M, and 62.0% of control-mothers; the differences between groups were not significant (P = 0.90).
After adjusting for maternal age, education, smoking and alcohol use during pregnancy, and interval between delivery and blood collection, the ORs for developing OCs were calculated according to increasing quartiles of PZn with the lowest quartile as the reference. None of the ORs (odds ratios) among the four OC groups or for all OC groups combined were significantly different from 1.0 (all 95% confidence intervals included 1.0). Furthermore, no significant trends were observed across increasing quartiles of PZn (Table 1). Furthermore, we explored narrow and extreme categories (including deciles) of PZn and found no evidence of differences between cases and controls even in the extremes of the zinc distribution (data not shown).
Table 1.
Risk of Oral Clefts by Quartiles of Maternal Plasma Zinc Concentrations
| Number of mothers |
Adjusted odds ratios and 95% confidence intervalsa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile of plasma zinc (μmol/l) |
Control s |
Isolated oral clefts |
Oral clefts with multiple malformations |
Isolated oral clefts | Oral clefts with multiple malformations |
Isolated and multiple malformations |
||||
| CLP | CP | CLP | CP | CLP | CP | CLP | CP | CLP + CP | ||
| Total number | 447 | 231 | 74 | 42 | 63 | |||||
| Quartile 1 (≤ 9.2) | 119 | 64 | 22 | 7 | 16 | 1.00b | 1.00b | 1.00b | 1.00b | 1.00b |
| Quartile 2 | 101 | 38 | 14 | 9 | 15 | 0.64 | 0.75 | 2.51 | 1.03 | 0.79 |
| (9.3 – 10.4) | (0.39-1.06) | (0.36-1.57) | (0.62-10.2) | (0.44-2.40) | (0.52-1.19) | |||||
| Quartile 3 | 119 | 68 | 18 | 15 | 19 | 1.04 | 0.78 | 2.99 | 1.20 | 1.04 |
| (10.4 – 11.6) | (0.67-1.60) | (0.39-1.54) | (0.79-11.4) | (0.55-2.65) | (0.71-1.52) | |||||
| Quartile 4 | 108 | 61 | 20 | 11 | 13 | 0.98 | 0.93 | 2.00 | 0.67 | 0.96 |
| (≥ 11.7) | (0.63-1.53) | (0.47-1.84) | (0.49-8.19) | (0.27-1.67) | (0.65-1.41) | |||||
| P- trend | 0.67 | 0.83 | 0.38 | 0.54 | 0.87 | |||||
Adjusted for maternal age, education, alcohol use smoking and multivitamin use and interval between delivery of the index child and blood collection in logistic regression models.
Reference level. CL/P; cleft lip with or without cleft palate. CP; cleft palate.
Since maternal PZn may be different based on the intervals between the delivery and blood sampling, we calculated the means of PZn based on three intervals (< 36, 36-60, and > 60 months) between all case-mothers combined and control-mothers; however, there was no significant difference in PZn between the two groups. Furthermore, the ORs were calculated for these three interval groups independently based on quartiles of PZn after adjusting for maternal age, education, smoking, alcohol and multivitamin use in logistic-regression models. We acknowledge that sample sizes were small. However, there were no significant differences in ORs between the interval groups (data not shown).
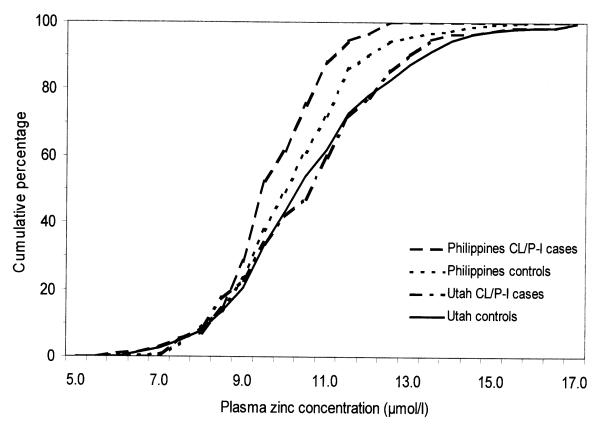
The mean PZn was significantly higher in the Utah mothers than the Filipino mothers among the controls (P < 0.001), the CL/P-I cases (P = 0.002), and the CP-I cases (P = 0.008) (data not shown). We examined the cumulative PZn distributions for CL/PL-I cases and controls in the Utah and Filipino studies (there were too few CP-I cases to examine their distributions in detail). Cumulative-percentage curves revealed a clear difference above the 10th percentile, with higher values (a right-shifted curve) of Filipino controls-mothers than Filipino case-mothers (Fig 1). Curves for the Utah CL/P-I case- and control-mothers were similar over the entire range, and both were distinctly higher (shifted to the right) than the Filipino control-mothers.
Figure 1.
Cumulative distribution of maternal plasma zinc concentrations of control-mothers and CL/P-I mothers by sites (Utah and the Philippines).
DISCUSSION
We reported that the lower PZn of mothers was associated with a higher OC risk in their children in the Philippines (Tamura et al., 2005). However, we found no association between the risk of developing OCs with and without other malformations and maternal PZn in Utah.
Although our data in Utah and the Philippines conflict, our Utah findings may be analogous to those by Shaw et al. (2006) who reported no significant association between OC risk and maternal zinc intake in case- and control-mothers in the US. Krapels et al. (2004a) reported that mean preconceptional zinc intake (9.9 mg/day) in case-mothers was similar to that (10.7 mg/day) in control-mothers after adjusting for energy intakes in the Netherlands. In contrast, the same Dutch research group reported that mean erythrocyte zinc concentrations of case-mothers having a child with CL/P were significantly lower than control-mothers, where zinc analysis was made at about 14 months after the birth of the index child (Krapels et al., 2004b). This finding indicates that zinc may be involved in normal development of the orofacial structures and is similar to our Filipino data. Considering these results, it remains to be open whether inadequate zinc status is associated with an increased risk OC. There are potentially two reasons for such inconsistencies. One is that only a limited number of mothers having a child with an OC were involved in certain studies. The other reason is the methodological heterogeneity including different time points in assessing maternal zinc status. These points make it difficult, if not impossible, to compare the results between the studies.
The limitations of our present study include that: 1) the sole use of PZn measurement as an indicator of zinc status, and no other analyses were made. It is well-recognized that PZn may not be a dependable indicator of zinc status, since it is affected by many factors (Solomons, 1983); and 2) we extrapolated PZn obtained 3 – 4 years after pregnancy to the critical period of the fetal orofacial development. We assumed that blood nutrient concentrations years after the index pregnancy are correlated with those at the critical time point for orofacial development. This was based on the findings in pregnant women by other researchers. For example, Leck et al. (1983) found that plasma and erythrocyte folate concentrations one year after delivery were significantly correlated with those early in pregnancy. By monitoring dietary habits in pregnant women, Devine et al. (2000) found that only few women changed their dietary habits when compared between prepregnancy and postpartum periods. Despite these limitations, our two studies in Utah and the Philippines provided a unique opportunity to compare our data from two distant locations having distinctive ethnic and cultural differences, because we used identical methodologies for sample collections and zinc analyses. Poor maternal zinc status was an OC risk factor in the Philippines, where OC prevalence is high and maternal PZn was low (Tamura et al., 2005). In contrast, no such association was found in Utah where maternal PZn was not as low as in the Philippines, whereas OC prevalence is high. We conclude that poor maternal zinc status may become a risk factor only when zinc status is compromised to a certain severity. It may be important to add that in the etiology of OCs, zinc status alone may not be a strong risk factor in the US. The interactions between zinc and nutritional status of other nutrients as well as genetic and environmental factors must be taken into account for evaluating such associations.
Finally, the association may be less clear between maternal zinc status and malformations other than OCs as recently reviewed by Shah and Sachdev (2006). The majority of the investigations in the literature are confined to the risk of developing neural-tube defects with small sample sizes with an exception of a few (McMichael et al., 1994; Velie et al., 1999).
ACKNOWLEDGEMENTS
This study was in part supported by a grant (1-R01 HD39061) from the National Institute of Child Health and Human Development and the National Institute of Dental and Craniofacial Research (R. Munger, PI), and the Offices of the Vice-President for Research and the Agricultural Experiment Station at Utah State University. Our thanks to Jane Johnson, Collen Mohr, Amy Nance, Miland Palmer, Dawnya Pearce, Nancy Sassano, Ph.D., Patty Smith and the staff of the Utah Birth Defects Network and the Utah Department of Health. We are grateful for the parents and children of Utah families who participated in this study.
Footnotes
Presented at the Experimental Biology 2007 meeting in April 2007 in Washington, DC.
REFERENCES
- Devine CM, Bove CF, Olson CM. Continuity and change in women’s weight orientations and lifestyle practices through pregnancy and the postpartum period: the influence of life course trajectories and transitional events. Soc Sci Med. 2000;50:567–582. doi: 10.1016/s0277-9536(99)00314-7. [DOI] [PubMed] [Google Scholar]
- Gebreab SY, Gillies RR, Munger RG, Symanzik J. Visualization and interpretation of birth defects data using linked micromap plots. Birth Defects Res (Part A) 2008;82:110–119. doi: 10.1002/bdra.20419. [DOI] [PubMed] [Google Scholar]
- Hurley LS, Swenerton H. Congenital malformations resulting from zinc deficiency in rats. Proc Soc Exp Biol Med. 1966;123:692–696. doi: 10.3181/00379727-123-31578. [DOI] [PubMed] [Google Scholar]
- Krapels IPC, van Rooij IALM, Ocké MC, West CE, van der Horst CMAM, Steegers-Theunissen RPM. Maternal nutritional status and the risk for orofacial cleft offspring in humans. J Nutr. 2004b;134:3106–3113. doi: 10.1093/jn/134.11.3106. [DOI] [PubMed] [Google Scholar]
- Krapels IPC, van Rooij IALM, Wevers RA, et al. Myo-inositol, glucose and zinc status as risk factors for non-syndromic cleft lip with or without cleft palate in offspring: a case-control study. BJOG. 2004a;111:661–668. doi: 10.1111/j.1471-0528.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- Leck I, Iles CA, Sharman IM, Toe T, Waldsworth GR, Dobbin J. Prevention of spina bifida and other neural tube defects. Academic Press; London: 1983. Maternal diet and nutrition during early pregnancy and after delivery in North London; pp. 197–218. [Google Scholar]
- McMichael AJ, Dreosti IE, Ryan P, Robertson EF. Neural tube defects and maternal serum zinc and copper concentrations in mid-pregnancy: a case-control study. Med J Aust. 1994;161:478–482. doi: 10.5694/j.1326-5377.1994.tb127560.x. [DOI] [PubMed] [Google Scholar]
- Shah D, Sachdev HPS. Zinc deficiency in pregnancy and fetal outcome. Nutr Rev. 2006;64:15–30. doi: 10.1111/j.1753-4887.2006.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- Solomons NW. Recent progress in zinc nutrition research. Nutr Update. 1983;1:123–145. [Google Scholar]
- Tamura T, Goldenberg RL, Johnston KE, Chapman VR. Relationship between pre-pregnancy BMI and plasma zinc concentrations in early pregnancy. Br J Nutr. 2004;91:773–777. doi: 10.1079/BJN20041109. [DOI] [PubMed] [Google Scholar]
- Tamura T, Johnston KE, Freeberg LE, Perkins LL, Goldenberg RL. Refrigeration of blood samples prior to separation is essential for the accurate determination of plasma or serum zinc concentrations. Biol Trace Elem Res. 1994;41:165–173. doi: 10.1007/BF02917226. [DOI] [PubMed] [Google Scholar]
- Tamura T, Munger MG, Corcoran C, Bacayao JY, Nepomuceno B, Solon F. Plasma zinc concentrations of mothers and the risk of non-syndromic oral clefts in their children: a case-control study in the Philippines. Birth Defects Res (Part A) 2005;73:612–616. doi: 10.1002/bdra.20179. [DOI] [PubMed] [Google Scholar]
- Velie EM, Block G, Shaw GM, Samuels SJ, Schaffer DM, Kulldorff M. Maternal supplemental and dietary zinc intake and the occurrence of neural tube defects in California. Am J Epidemiol. 1999;150:605–616. doi: 10.1093/oxfordjournals.aje.a010059. [DOI] [PubMed] [Google Scholar]
- Warkany J, Petering HG. Congenital malformations of the central nervous system in rats produced by maternal zinc deficiency. Teratology. 1972;5:319–334. doi: 10.1002/tera.1420050307. [DOI] [PubMed] [Google Scholar]