Abstract
A variety of long chain 1,2-diamines and related compounds were synthesized and tested for their activity on fatty acid amide hydrolase (FAAH) and monoacyglycerol lipase (MGL). (2S,9Z)-Octadec-9-ene-1,2-diamine selectively inhibits MGL (Ki 21.8 μM) without significantly affecting FAAH. This compound exhibited interesting in vivo analgesic and anti-inflammatory properties, suggesting that selective inhibitors of MGL may be valuable novel agents for the treatment of inflammatory pain.
Keywords: 1,2-Diamines; Endocannabinoids; Fatty acid amide hydrolase; FAAH; Inhibitor; Monoacyglycerol lipase; MGL
The discovery of the CB1 and CB2 cannabinoid receptors as well as the endogenous ligand involved in their activation opened a new era in cannabinoid research.1,2 The key endogenous ligands designated as endocannabinoids are anandamide (1) and 2-arachidonoylglycerol (2-AG, 2) (Fig. 1). Of these, 2-AG has been shown to be the most abundant and more potent endocannabinoid.3 2-AG exhibits full agonist efficacy at both CB1 and CB24 and its physiological levels in rat brain exceed those of anandamide by several orders of magnitude.3b Although the endocannabinoid system has received much attention in recent years as a target for the discovery of novel therapeutic agents,5 the role and function of 2-AG are less understood.
Figure 1.
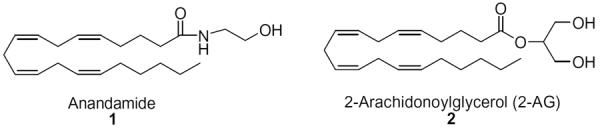
The most important endocannabinoids.
Anandamide and 2-AG are produced by neurons when the need arises, act near their site of biosynthesis, and are rapidly metabolized so that their biological activity is terminated. Both anandamide and 2-AG are hydrolyzed by fatty acid amide hydrolase (FAAH).6 However, existing evidence indicates that the most likely candidate for 2-AG hydrolytic deactivation is monoacylglycerol lipase (MGL), a serine hydrolase that converts 1- and 2-monoacylglycerols into fatty acid and glycerol.7 Dinh et al. cloned MGL from a rat brain cDNA,8 and demonstrated that RNA interference suggested a primary role for MGL in the degradation of 2-AG.9 More recently, Makriyannis and collaborators have characterized the binding domain of this enzyme using a proteomic approach.10 Although a plethora of FAAH inhibitors have been developed,1,2,11 only few MGL inhibiting ligands are available.
Various nonspecific serine hydrolase inhibitors such as methylarachidonoyl fluorophosphonate (3, MAFP) (Fig. 2), hexadecylsulfonyl fluoride (AM374) and phenylmethylsulfonyl fluoride (PMSF) inhibit purified MGL, or 2-AG-degrading enzymatic activity in rat cerebellar membranes.6d,8,12 In addition, sulfhydryl-specific compounds, like N-arachidonylmaleimide (4, NAM) have been shown to inhibit MGL.13 Arachidonoyl trifluoromethyl ketone (5, ATFMK)12 as well as arachidonoyl glycerol derivatives such as compound 6 also interact with MGL.14 It has been reported that MGL activity can be specifically and noncompetitively inhibited by two compounds, URB602 (7) and URB754 (8) (Fig. 2).15 However, a recent report showed that both URB602 and URB754 had no effect on the hydrolysis or signaling capacity of 2-AG in rat brain.16 Most recently, it has been reported that locally injected 2-AG decreased pain behavior in a dose-dependent manner in an inflammatory model of pain.17 It is thought that the antinociceptive effect of 2-AG is mediated through the CB2 receptor.
Figure 2.
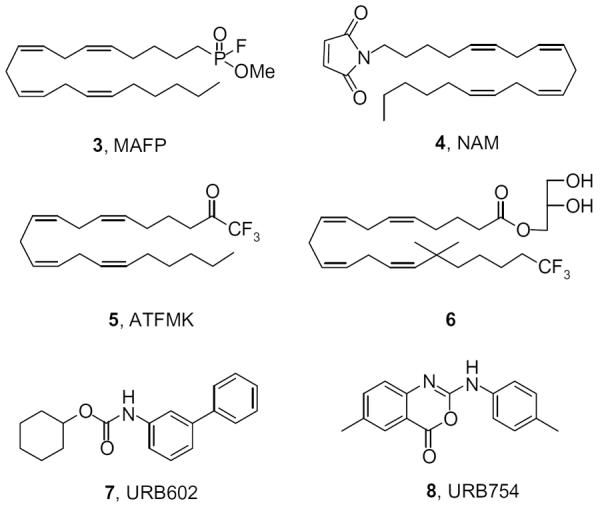
Inhibitors of monoacylglycerol lipase.
The novel finding that the exogeneous application of 2-AG induces peripheral antinociception,17 reveals the therapeutic potential of MGL inhibitors for the treatment of inflammatory pain.18 Since both FAAH and MGL may hydrolyse 2-AG, it is of great importance to develop selective inhibitors of these enzymes in order to fully explore the physiological role for 2-AG. Here, we describe our efforts towards the synthesis of MGL selective inhibitors and the in vivo anti-inflammatory and analgesic properties of such a compound.
During the last years we have developed a variety of inhibitors of lipolytic enzymes (pancreatic and gastric lipases as well as phospholipases cPLA2 and iPLA2),19–25 and we have shown that the cytosolic phospholipase A2 inhibitor AX048 produces a potent anti-hyperalgesic action.26 All of these lipolytic enzymes are serine hydrolases with a mechanism of action involving a serine residue at the catalytic site interacting with a 2-oxoamide functionality of the inhibitors. Since MGL is also a serine hydrolase, we have incorporated the 2-oxoamide functionality in our MGL inhibitor design. Towards this end, we synthesized a lipid analog carrying the 2-oxoamide of ethanolamine. 2-Oxoamide 12 was prepared from the tert-butyl ether of ethanolamine as described in Scheme 1. This compound exhibited weak inhibitory activity towards both FAAH and MGL, with no selectivity (Table 1).
Scheme 1.
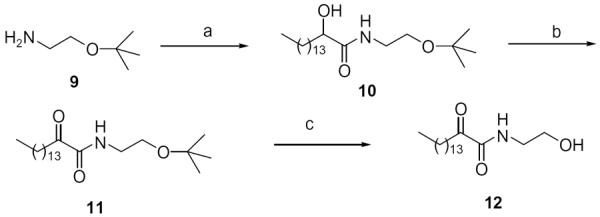
Synthesis of 2-oxoamide 12. Reagents: (a) CH3(CH2)13CHOHCOOH, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, HOBt, Et3N, CH2Cl2; (b) AcNH-TEMPO, NaOCl, NaBr, EtOAc=PhCH3=H2O; (c) 50% TFA=CH2Cl2.
Table 1.
Inhibitory results on FAAH and MGLa
| Structure | FAAH inhibition (%) | MGL inhibition (%) | Ki for MGL (μM) |
|---|---|---|---|
|
23.8 | 27.5 | |
|
33.6 | 31.5 | |
|
31.3 | 30.0 | |
|
31.2 | 30.3 | 58.7 |
|
4.9 | 49.9 | 21.8 |
|
25.9 | 42.2 | 39.0 |
|
0.0 | 0.0 | |
|
|
30.9 | 25.9 | |
|
24.0 | 0.0 |
Percent inhibition of 2-AG hydrolysis using 100 μM concentrations.
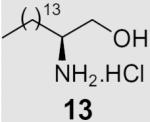
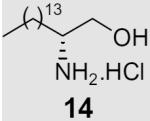
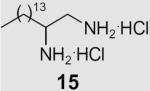
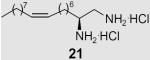
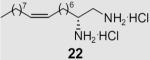
At the same time, we synthesized a series of long chain 1,2-diamines and 1,2-amino alcohols containing a saturated chain or a chain corresponding to that of oleic acid chain. (S)- and (R)-2-aminohexadecanol27 (13, 14) and 1,2-diaminohexadecane28 (15) were prepared as previously described. The synthesis of 1,2-diamine 21 is presented in Scheme 2. l-Glutamic acid was used as starting material and aminoaldehyde 16 was prepared according to literature procedure.29 After a Horner–Wadsworth–Emmons reaction, hydrogenation with simultaneous reduction of the azide group and Boc protection, the key intermediate ester 18 was obtained. Compound 18 was then reduced to aldehyde 19, which was allowed to react with the appropriate ylide to yield diamine 20. Deprotection gave diamine 2130 which encompasses an oleic acid chain. Its enantiomer 22 was prepared correspondingly from d-glutamic acid.
Scheme 2.
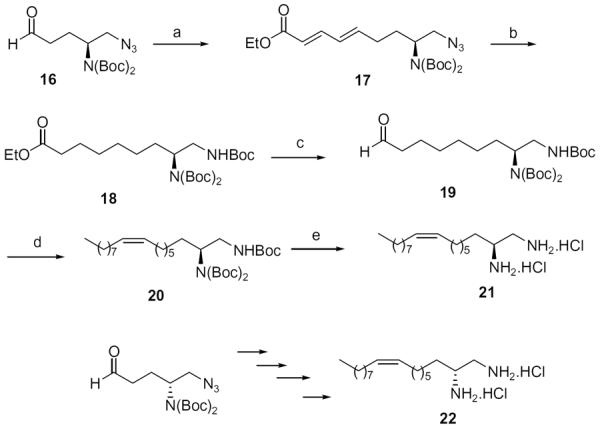
Synthesis of 1,2-diamines 21 and 22. Reagents: (a) EtOOCCH=CHCH2P(=O)(OEt)2, LiOH, THF; (b) H2, 10% Pd/C, Boc2O, MeOH; (c) DIBALH, Et2O; (d) C9H19P+Ph3Br−, KHMDS, toluene; (e) 4 N HCl/Et2O.
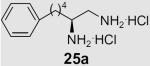
Diamines 25a and 25b were prepared from aminoaldehyde 16 through a Wittig reaction followed by hydrogenation and simultaneous transformation of the azide group into a Boc-protected amine, and deprotection (Scheme 3).
Scheme 3.
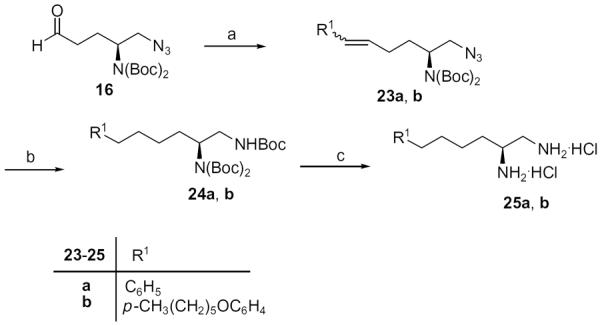
Synthesis of diamines 25a and 25b. Reagents: (a) R1P+Ph3Br−, KHMDS, toluene; (b) H2, 10% Pd/C, Boc2O, MeOH; (c) 4 N HCl=Et2O.
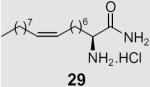
Previously, we used Boc-protected (S)-2-amino oleic31 acid to prepare a potent inhibitor of human pancreatic lipase, which resembled the natural substrate of the enzyme.32 We now used this optically pure unnatural 2-amino acid (26) to prepare the carboxamide of 2-amino oleic acid (29) (Scheme 4).
Scheme 4.
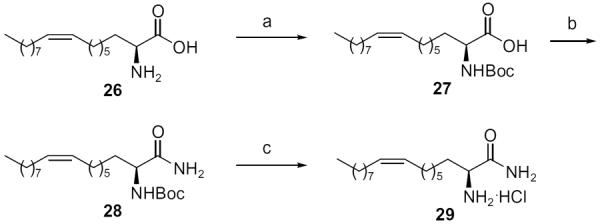
Synthesis of carboxamide 29. Reagents: (a) Boc2O, Et3N, MeOH; (b) DCC, HOBt, 25% aq:NH3, CH2Cl2, DMF; (c) 4N HCl/Et2O.
All new compounds were tested for their abilities to inhibit 2-AG hydrolysis by FAAH and MGL (Table 1). Partially purified FAAH and MGL enzyme preparations were obtained from adult Sprague–Dawley rat brains purchased from Pel-Freeze Biologicals according to previously reported procedures,33,34 while the FAAH and MGL inhibition assays were performed as previously described.34–36
2-Oxoamide 12 presented low inhibition and no selectivity towards the two enzymes. (S)- or (R)-2-aminohexadecanol (13, 14) as well as 1,2-diaminohexadecane (15) inhibit both enzymes equally. However, the unsaturated diamine, (2S,9Z)-octadec-9-ene-1,2-diamine dihydrochloride (21), inhibited MGL selectively with a Ki value of 21.8 μM. This compound did not inhibit FAAH significantly. Compound 21 also exhibited modest stereoselectivity. Its enantiomer (2R,9Z)-octadec-9-ene-1,2-diamine dihydrochloride (22), inhibits MGL with a Ki value of 39 μM, and some FAAH inhibitory activity (25% at a 100 μM).
Diamine 25a was totally inactive towards both enzymes, while 25b, which carries a para alkoxy chain, was weakly active. Thus, it appears that diamines require the presence of a long lipophilic chain in order to successfully inhibit MGL. Our results also indicate that the presence of a long monounsaturated chain corresponding to oleic acid in compound 21 is a key requirement for the selective inhibition of MGL. Recently, an oleoyl-chain phosphonate, UP-101, was reported to be a potent inhibitor of MGL.37 Furthermore, according to a recent article, NAM exhibits approximately a 30-fold higher MGL inhibitory activity than compound 21.10 Conversely, the carboxamide of 2-amino oleic (29) acid does not inhibit MGL, but weakly inhibits FAAH. Thus, it appears that replacement of the CH2NH2 moiety of compound 21 by the CONH2 led to a compound without inhibition towards MGL.
Subsequently, compound 21 was tested for its analgesic and anti-inflammatory activity using models previously described.24 The acetic acid writhing test was used to assess analgesic activity in rats. Acetylsalicylate was used as a reference drug and was administered ip. As shown in Figure 3, 21 exhibited analgesic activity at a dose of 3.6 mg/kg (ip). A more potent effect was observed at a 10-fold higher dose indicating a dose-dependent effect. Furthermore, its enantiomer 22 exhibited similar analgesic activity at the high dose of 36 mg/kg, but had weaker analgesic potency at the lower dose of 3.6 mg/kg.
Figure 3.
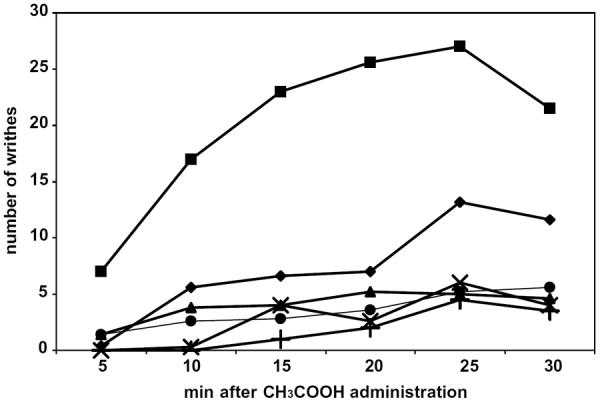
In vivo analgesic activity of inhibitors 21 and 22. Control (■), 22 (3.6 mg/kg, ◆), 21 (3.6 mg/kg, ▴), 22 (36 mg/kg, ●), 21 (36 mg/kg, ×), aspirin (200 mg/kg, +).
The rat paw carrageenan-induced edema assay was employed as a model for acute inflammation. Compound 21 exhibited in vivo anti-inflammatory activity (ED50 0.01 mmol/kg) comparable to that of the reference drug indomethacin (47% inhibition of inflammation at 0.01 mmol/kg administered ip).
In conclusion, we synthesized a variety of long chain 1,2-diamines and related compounds and studied their effects on the endocannabinoid deactivating enzymes FAAH and MGL. We demonstrated that (2S,9Z)-octadec-9-ene-1,2-diamine is a novel selective inhibitor of MGL activity (Ki 21.8 μM) with in vivo analgesic and anti-inflammatory properties. Thus, synthetic selective inhibitors of MGL are potential candidates for the development of novel analgesic agents.
Acknowledgments
The project was co-funded by the European Social Fund and National Resources-(EPEAEK II) PYTHAGORAS; Fund for International Collaborations, Northeastern University; and from the National Institutes on Drug Abuse (DA3801). The authors are grateful to Ying Pei and Nikolai M. Zvonok for the biochemical assays.
References and notes
- 1.Kokotos G. Endocannabinoids. In: Kokotos G, Nicolaou A, editors. Bioactive Lipids. The Oily Press; Bridgewater, England: 2004. p. 245. [Google Scholar]
- 2.Lambert DM, Fowler CJ. J. Med. Chem. 2005;48:5059. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- 3.(a) Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminsky NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Biochem. Pharmacol. 1995;50:83. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]; (b) Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem. Biophys. Res. Commun. 1995;215:89. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]; (c) Stella N, Schweitzer P, Piomelli D. Nature. 1997;388:773. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 4.(a) Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. J. Biol. Chem. 1999;274:2794. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]; (b) Gonsiorek W, Lunn C, Fan X, Narula S, Lyndell D, Hipkin RW. Mol. Pharmacol. 2000;57:1045. [PubMed] [Google Scholar]
- 5.(a) Piomelli D. Curr. Opin. Investig. Drugs. 2005;6:672. [PubMed] [Google Scholar]; (b) Di Marzo V, Bifulco M, De Petrocallis L. Nat. Rev. Drug Disc. 2004;3:771. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]; (c) Makriyannis A, Mechoulam R, Piomelli D. 2005. Neuropharmacology. 48:1068. doi: 10.1016/j.neuropharm.2005.03.012. [DOI] [PubMed] [Google Scholar]; (d) Bahr BA, Karanian DA, Makanji SS, Makriyannis A. Expert Opin. Investig. Drugs. 2006;15:351. doi: 10.1517/13543784.15.4.351. [DOI] [PubMed] [Google Scholar]
- 6.(a) Desarnaud F, Cadas H, Piomelli D. J. Biol. Chem. 1995;270:6030. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]; (b) Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. J. Biol. Chem. 1995;270:23823. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]; (c) Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Nature. 1996;384:83. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]; (d) Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. FEBS Lett. 1998;422:69. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]; (e) Lang W, Qin C, Lin S, Khanolkar AD, Goutopoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. J. Med. Chem. 1999;42:896. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- 7.(a) Tornquist H, Belfrage P. J. Biol. Chem. 1976;251:813. [PubMed] [Google Scholar]; (b) Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. J. Biol. Chem. 1997;272:27218. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 8.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10819. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinh TP, Kathuria S, Piomelli D. Mol. Pharmacol. 2004;66:1260. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- 10.Zvonok N, Pandarinathan L, Williams J, Johnston M, Karageorgos I, Janero DR, Krishnan SC, Makriyannis A. Chem. Biol. 2008;15:854. doi: 10.1016/j.chembiol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For selected references, see: Leung D, Du W, Hardouin C, Cheng H, Hwang I, Cravatt BF, Boger DL. Bioorg. Med. Chem. Lett. 2005;15:1423. doi: 10.1016/j.bmcl.2004.12.085.; Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, Hwang I, Hedrick MP, Leung D, Acevedo O, Guimarães CRW, Jorgensen WL, Cravatt BF. J. Med. Chem. 2005;48:1849. doi: 10.1021/jm049614v.; Romero FA, Du W, Hwang I, Rayl TJ, Kimball FS, Leung D, Hoover HS, Apodaca RL, Breitenbucher JG, Cravatt BF, Boger DL. J. Med. Chem. 2007;50:1058. doi: 10.1021/jm0611509.; Hardouin C, Kelso MJ, Romero FA, Rayl TJ, Leung D, Hwang I, Cravatt BF, Boger DL. J. Med. Chem. 2007;50:3359. doi: 10.1021/jm061414r..
- 12.Saario SM, Savinainen JR, Laitinen JT, Jarvinen T, Niemi R. Biochem. Pharmacol. 2004;67:1381. doi: 10.1016/j.bcp.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Saario SM, Salo OMH, Nevalainen T, Poso A, Laitinen JT, Jarvinen T, Niemi R. Chem. Biol. 2005;12:649. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Ghafouri N, Tiger G, Razdan RK, Mahadevan A, Pertwee RG, Martin BR, Fowler CJ. Br. J. Pharmacol. 2004;143:774. doi: 10.1038/sj.bjp.0705948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Nat. Neurosci. 2005;8:1139. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- 16.(a) Saario SM, Palomaki V, Lehtonen M, Nevalainen T, Jarvinen T, Laitinen JT. Chem. Biol. 2006;13:811. doi: 10.1016/j.chembiol.2006.07.008. [DOI] [PubMed] [Google Scholar]; (b) Vandevoorde S, Jonsson K-O, Labar G, Persson E, Lambert DM, Fowler CJ. Br. J. Pharmacol. 2007;150:186. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guindon J, Desroches J, Beaulieu P. Br. J. Pharmacol. 2007;150:693. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohmann AG. Br. J. Pharmacol. 2007;150:673. doi: 10.1038/sj.bjp.0707153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokotos G, Verger R, Chiou A. Chem. Eur. J. 2000;6:4211. doi: 10.1002/1521-3765(20001117)6:22<4211::aid-chem4211>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Kotsovolou S, Chiou A, Verger R, Kokotos G. J. Org. Chem. 2001;66:962. doi: 10.1021/jo005705y. [DOI] [PubMed] [Google Scholar]
- 21.Chiou A, Verger R, Kokotos G. Lipids. 2001;36:535. doi: 10.1007/s11745-001-0754-0. [DOI] [PubMed] [Google Scholar]
- 22.Kokotos G, Kotsovolou S, Verger R. ChemBioChem. 2003;4:101. doi: 10.1002/cbic.200390019. [DOI] [PubMed] [Google Scholar]
- 23.Kokotos G, Kotsovolou S, Six DA, Constantinou-Kokotou V, Beltzner CC, Dennis EA. J. Med. Chem. 2002;45:2891. doi: 10.1021/jm025538p. [DOI] [PubMed] [Google Scholar]
- 24.Kokotos G, Six DA, Loukas V, Smith T, Constantinou-Kokotou V, Hadjipavlou-Litina D, Kotsovolou S, Chiou A, Beltzner CC, Dennis EA. J. Med. Chem. 2004;47:3615. doi: 10.1021/jm030485c. [DOI] [PubMed] [Google Scholar]
- 25.Stephens D, Barbayianni E, Constantinou-Kokotou V, Peristeraki A, Six DA, Cooper J, Harkewicz R, Deems RA, Dennis EA, Kokotos G. J. Med. Chem. 2006;49:2821. doi: 10.1021/jm050993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaksh TL, Kokotos G, Svensson CI, Stephens D, Kokotos CG, Fitzsimmons B, Hadjipavlou-Litina D, Hua X-Y, Dennis EA. J. Pharmacol. Exp. Ther. 2006;316:466. doi: 10.1124/jpet.105.091686. [DOI] [PubMed] [Google Scholar]
- 27.Magrioti V, Hadjipavlou-Litina D, Constantinou-Kokotou V. Bioorg. Med. Chem. Lett. 2003;13:375. doi: 10.1016/s0960-894x(02)00939-3. [DOI] [PubMed] [Google Scholar]
- 28.Kokotos G, Constantinou-Kokotou V, del Olmo Fernandez E, Toth I, Gibbons WA. Liebigs Ann. Chem. 1992;961 [Google Scholar]
- 29.Markidis T, Kokotos G. J. Org. Chem. 2001;66:1919. doi: 10.1021/jo005677j. [DOI] [PubMed] [Google Scholar]
- 30.All new compounds were fully characterized by 1H and [13]C NMR, MS and elemental analysis. Selected data of final inhibitor: Compound 21: Waxy solid; −8.3(c = 0.7, CH3OH); 1H NMR (200MHz, CD3OD) δ 0.90 (t, J = 5.2Hz, 3H, CH3), 1.29 (br s, 8H, 4× CH2), 1.39 (br s, 12H, 6× CH2), 1.72 (m, 2H, CH2CH), 2.04 (m, 4H, CH2CH = CHCH2), 3.32-3.23 (m, 2H, CH2NH2), 3.52 (m, 1H, CH), 5.35 (m, 2H, CH=CH); 13C NMR (50MHz, CD3OD): δ 14.5, 23.7, 26.0, 28.1, 30.1, 30.3, 30.4, 30.4, 30.6, 30.8, 30.9, 31.7, 39.1, 42.2, 50.9, 130.6, 131.0; MS (ESI) m/z (%) 283.2 (MH+, 100).
- 31.(a) Constantinou-Kokotou V, Magrioti V, Markidis T, Kokotos G. J. Pept. Res. 2001;58:325. doi: 10.1034/j.1399-3011.2001.00910.x. [DOI] [PubMed] [Google Scholar]; (b) Magrioti V, Constantinou-Kokotou V. Lipids. 2002;37:223. doi: 10.1007/s11745-002-0884-4. [DOI] [PubMed] [Google Scholar]
- 32.Magrioti V, Verger R, Constantinou-Kokotou V. J. Med. Chem. 2004;47:288. doi: 10.1021/jm034202s. [DOI] [PubMed] [Google Scholar]
- 33.Lang W, Qin C, Hill WAG, Lin S, Khanolkar AD, Makriyannis A. Anal. Biochem. 1996;238:40. doi: 10.1006/abio.1996.0247. [DOI] [PubMed] [Google Scholar]
- 34.Lang W, Qin C, Lin S, Khanolkar AD, Goutopoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. J. Med. Chem. 1999;42:896. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- 35.Qin C, Lin S, Lang W, Goutopoulos A, Pavlopoulos S, Mauri F, Makriyannis A. Anal. Biochem. 1998;261:8. doi: 10.1006/abio.1998.2713. [DOI] [PubMed] [Google Scholar]
- 36.Vadivel SK, Vardarajan S, Duclos RI, Jr., Wood JT, Guo J, Makriyannis A. Bioorg. Med. Chem. Lett. 2007;17:5959. doi: 10.1016/j.bmcl.2007.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A, Appendino G, Saturino C, Martin B, Razdan R, Di Marzo V. Biochim. Biophys. Acta. 2006;1761:205. doi: 10.1016/j.bbalip.2005.12.009. [DOI] [PubMed] [Google Scholar]


