Abstract
Previous research with substance users has demonstrated, across a variety of psychiatric disorders, significant decreases in psychological symptoms during early substance abstinence. To build on this literature, the current study prospectively assessed trauma symptomatology over 28 days during acute and protracted cocaine and alcohol abstinence. Participants were 162 male and female cocaine and/or alcohol dependent outpatients who reported a history of trauma. Trauma-related symptoms and substance use were assessed at 2, 5, 10, 14, 21, and 28 days following last substance use. For participants who were known to relapse, assessments began again after the last day of substance use. Latent growth modeling was employed to estimate changes in posttraumatic stress disorder (PTSD) symptoms. Consistent with studies of other psychiatric syndromes, PTSD symptoms declined across the 28-day study period regardless of withdrawal substance (i.e., cocaine or alcohol). The majority of change in trauma symptoms occurred within two weeks of last substance use. Moreover, while trauma symptoms for the PTSD participants were more severe than those reported by the non-PTSD participants, trauma symptoms declined across the study period at the same rate irrespective of PTSD status.
Keywords: PTSD, Trauma, Co-morbidity, Withdrawal, Alcoholism, Cocaine Dependence
1. Introduction
Over the past three decades, clear support has been provided that psychiatric symptomatology present at the outset of acute and protracted alcohol abstinence decreases significantly over time (e.g., Brown and Schuckit, 1988; Brown et al., 1995; Driessen et al., 2001; see Lynskey, 1998 and Raimo and Schuckit, 1998 for reviews). A hypothesis put forth to explain this observation is that withdrawal from alcohol is associated with many of the signs and symptoms of depression, anxiety, and other psychiatric conditions and, therefore, as abstinence-related symptoms decrease over time so do the symptoms commonly associated with psychiatric conditions such as depression and anxiety (Schuckit et al., 1997). Likewise, there is evidence that psychiatric symptomatology present at the outset of cocaine abstinence may decrease significantly over time. For example, Brown and colleagues (Brown et al., 1998) found that depressive symptoms decreased significantly during cocaine treatment, a result consistent with the findings of other investigators (e.g., Coffey et al., 2000; Husband et al., 1996; Weddington et al., 1990; Satel et al., 1991;Weiss et al., 1988).
Although numerous studies have documented the reduction of psychiatric symptoms during acute and protracted alcohol and cocaine abstinence, no prospective studies have been conducted to examine changes in posttraumatic stress disorder (PTSD) symptomatology during acute and protracted abstinence in substance dependent patients with a victimization history (e.g., Back et al., 2006; Dansky et al., 1998). This is significant because of the high prevalence rate of victimization in substance dependent populations. For example, results from the Epidemiologic Catchment Area (ECA) study indicate that substance users are almost twice as likely to experience a traumatic event, and three times as likely to develop PTSD as compared to non-substance users (Cottler et al, 1992). Likewise, Dansky and colleagues (Dansky et al., 1995) compared the results of in-person and telephone trauma assessments in substance abusing women and found high rates of victimization in both samples. Specifically, a completed rape was reported by 57% of the participants, sexual molestation was reported by 22–26% of the participants, and attempted sexual abuse was reported by approximately 30% of the sample.
While it is clear that substance use disorders (SUDs) and victimization co-occur at a high rate, the range of the reported rates is fairly large. For example, within treatment-seeking samples of SUD patients, current PTSD rates range from approximately 33–50% (Dansky, et al., 1996; Grice et al., 1995; Triffleman et al., 1995). While a significant portion of this range can be accounted for by different measurement approaches (e.g., self-report versus structured interview, behaviorally specific questions versus general questions), it is possible that some of the variability in prevalence rates may be due to changes in PTSD symptoms during acute and protracted abstinence. The current study was designed to investigate this hypothesis.
With the high rate of PTSD-SUD comorbidity comes the question of causality between the two disorders: are the two disorder causally related? Evidence that a causal relationship exists between PTSD and SUD (e.g., PTSD leads to SUD, SUD leads to PTSD, a third variable leads to both disorders, etc.) comes from a number of different sources (e.g., Kilpatrick et al., 2000; Stewart et al., 2004). Chilcoat and Breslau (1998) suggest that if a causal relationship exists between PTSD and SUD, then as the symptom levels of one disorder increases, so should the symptom levels of the second disorder. This relationship is known as a gradient of effect. Evidence from numerous studies (see Stewart and Conrod, 2003 for a review) supports the notion that a positive correlation exists between symptoms of PTSD and severity of SUD symptoms (e.g., Back et al 2006; Brown et al., 1998; Coffey et al., in press; Stewart et al., 1999). Prospective evidence that PTSD symptoms decrease during acute and protracted substance abstinence would suggest that the two disorders are causally related in some manner.
Determining whether psychiatric symptoms will abate as a function of abstinence from substances of abuse or whether the symptoms will persist despite abstinence from substances of abuse has significant clinical implications. The most obvious pertains to the timing of diagnostic assessments for comorbid psychiatric conditions. Regardless of whether there is a causal pathway between psychiatric symptoms and SUDs or if their comorbidity represents common shared risk factors (e.g., environment, genetics), if psychiatric symptoms decrease during the first 2–4 weeks of sustained abstinence it may be clinically prudent to delay final assessment of psychiatric conditions until acute and protracted abstinence symptoms have decreased.
Therefore, in an attempt to address this void in the literature, substance dependent individuals (cocaine, alcohol, or both cocaine and alcohol) with a trauma history were prospectively assessed during 28-days of monitored abstinence. Participants’ PTSD symptoms and substance use were assessed at days 2, 5, 10, 14, 21, and 28 following last substance use and symptoms of PTSD were compared over the six time-points. It was predicted that trauma symptoms would decrease over time in all three withdrawal groups.
2. Method
2.1. Participants
A total of 166 participants were recruited from several local substance abuse treatment programs between the years 1993 to 1999. All participants met DSM-III-R (APA, 1987) criteria for cocaine and/or alcohol dependence. Participants who were dependent on any drug(s) other than cocaine, alcohol, or nicotine were excluded from the study. Of the 166 participants in the sample, 162 participants had complete data for at least one assessment period and were included in our analyses. Participants were recruited from treatment programs associated with a large university-based substance abuse treatment program and a nearby county-funded public hospital. One hundred and twenty-five of the participants (77%) included in the analyses were recruited from outpatient treatment facilities with the remaining participants coming from inpatient units. Individuals recruited from inpatient units (i.e., a university-based or county-funded inpatient unit) spent a portion of their time on inpatient units (i.e., 1–2 weeks) and then were referred to outpatient programs within the same organizations (i.e., intensive outpatient day programs or evening programs within the two referral sites). Participants were then followed as outpatients for the duration of the study. Participants recruited from inpatient sites did not differ on psychiatric or drug symptom severity. However, individuals recruited from inpatient settings (M = .40, SD = .29) reported greater alcohol symptom severity compared to individuals recruited from outpatient settings (M = .29, SD = .27), F(1,159) = 4.35, p = .04. In addition, there was a non-significant trend for individuals recruited from inpatient settings (M = 42.48, SD = 22.81) to report greater PTSD symptom severity compared to individuals recruited from outpatient settings, (M = 33.76, SD = 22.37), F(1,159) = 3.33, p = .07, despite the fact that the two groups did not differ on PTSD diagnostic status.
The total sample consisted of three groups: Individuals withdrawing from cocaine (COC), individuals withdrawing from alcohol (ETOH), and individuals withdrawing from both cocaine and alcohol (COC-ETOH). All of the patients either used crack cocaine or powder cocaine; none used cocaine intravenously. All participants in the study experienced at least one traumatic event in their life that satisfied DSM-III-R Criterion A for PTSD (APA, 1987) and 28% of the final patient group (n = 45) met criteria for current posttraumatic stress disorder (PTSD). Demographic characteristics and trauma history for the final sample are presented in Table 1. Among individuals who reported a particular trauma satisfying DSM-III-R Criterion A, the mean age in which the indexed trauma first occurred was: serious accident 18.5 (SD = 8.1), rape 17.5 (SD = 8.7), forced oral sex 14.4 (SD = 8.9), forced anal sex 13.7 (SD = 5.7), physical attack 19.6 (SD = 7.5), and childhood physical abuse by an adult caretaker 8.4 (SD = 3.8). Individuals maintained on benzodiazapines were excluded from the study. Each participant signed a consent form approved by a university Institutional Review Board and were paid for their participation.
Table 1.
Participant characteristics, substance of withdrawal, posttraumatic stress disorder (PTSD) status, and trauma historya.
| Percent of sample (N = 162) | ||
|---|---|---|
| Ageb | M = 33.6 (7.3) | |
| Gender | Female | 52 |
| Race | White | 44 |
| Black | 54 | |
| Hispanic or other | 2 | |
| Education | No HS diploma | 36 |
| HS diploma or GED | 30 | |
| Some higher education | 34 | |
| Employment | Unemployed | 65 |
| Full or part employment | 33 | |
| Student or retired | 2 | |
| Withdrawal drug | Cocaine only | 30 |
| Alcohol only | 23 | |
| Cocaine and alcohol | 47 | |
| PTSD status | Yes | 28 |
| Trauma historya | Serious accident | 52 |
| Forced sexual intercourse | 27 | |
| Forced anal sex | 16 | |
| Forced oral sex | 7 | |
| Physical attack | 83 | |
| Childhood physical abuse | 36 |
Trauma is defined as satisfying DSM-III-R Criterion A. Total percentage exceeds 100% because many participants experienced multiple traumas over their lifetime.
Mean and standard deviation are presented.
2.2. Materials
Drug dependence was determined at study entry and three methods, as described below, were used to confirm abstinence from alcohol and drugs during the study. PTSD symptomatology and alcohol and drug use were assessed at standard intervals. These measures are described below.
2.2.1. Drug use interviews and abstinence compliance measures
Substance dependence was determined by using the Structured Clinical Interview for the DSM-III-R (SCID, Spitzer and Williams, 1986). The substance use section of the SCID has shown good concurrent, discriminative, and predictive validity for both current and lifetime diagnoses (Kranzler et al., 1996) and has shown high interrater reliability for substance use disorders (Skre et al., 1991).
The Addiction Severity Index (ASI; McLellan et al., 1980) was used to determine alcohol, drug, and psychiatric symptom severity. The ASI is widely used and has been shown be a reliable and valid index of addiction severity (Alterman et al., 1998; Appleby et al., 1997; Kosten et al.,1983). The alcohol, drug, and psychiatric severity composite scores were used in the current study.
The Time Line Follow-Back (TLFB; Sobell and Sobell, 1992) was used to determine daily amounts of drug and alcohol use by employing a calendar to determine subjects’ pattern of drug and alcohol use for the past month. The TLFB was used as a measure of abstinence during the study.
An in vitro diagnostic test (Roche Diagnostic Systems, Inc., Somerville, NJ), conducted at least weekly, provided qualitative detection of illicit substance use. Urine drug screens (UDS) were conducted to test for the presence of THC, cocaine, morphine, and amphetamines at the following respective cutoffs, 50ng/mL, 300 ng/mL, 300 ng.mL, and 1000 ng/mL. To assess alcohol intoxication at each measurement period, breathalyzer values (Intoximeters, St. Louis, MO) also were collected.
2.2.2. Trauma symptomatology and PTSD diagnostic measures
The Modified PTSD Symptom Scale-Self Report (MPSS, Falsetti et al., 1993) is a 17-item self-report measure of PTSD symptom frequency and severity during the two weeks prior to the assessment. The MPSS and the inclusion of PTSD symptom severity represent a modification of the PTSD Symptom Scale-Self Report (Foa et al., 1993). This modification was validated by Falsetti and her colleagues (1993) who reported good overall internal consistency and good concurrent validity with the SCID. The MPSS has been used to assess PTSD in a substance abusing sample (Coffey et al., 1998).
Trauma history was assessed using the National Women’s Study (NWS) PTSD module (Kilpatrick et al., 1989; Resnick, 1996), an instrument modified from the Diagnostic Interview Schedule used in the National Vietnam Veterans Readjustment Study (NVVRS; Kulka et al., 1990). Using DSM-III-R criteria, participants were classified as meeting Criterion A, the necessary stressor criterion for PTSD, if they reported the occurrence of at least one traumatic event during their lives and the event was deemed to be outside the range of normal human experience and would be markedly distressing to almost anyone. Open and closed-ended, behaviorally specific questions were used to evaluate a wide range of potentially traumatic events (e.g., sexual assault, physical assault; motor vehicle accidents, combat, etc.). Concurrent validity obtained from a slightly modified version of the PTSD interview with the SCID-PTSD module was good and reliability also was acceptable (see Resnick et al., 1993).
The Clinician Administered PTSD Scale (CAPS; Blake et al., 1995) is a well-established and psychometrically sound structured clinical interview designed to assess the 17 symptoms of PTSD and was used as the diagnostic tool for current PTSD.
2.3. Procedure
Participants were recruited from multiple inpatient and outpatient drug treatment facilities in a mid-sized city in the Southeastern United States. All participants met the following eligibility requirements: 1) dependence on cocaine, alcohol, or both cocaine and alcohol as defined by DSM-III-R, 2) last use of cocaine and/or alcohol within the past 48 hours, 3) no current involvement in psychotherapy for trauma or PTSD, 4) no current psychoses or mania, 5) report and exhibit the ability to read and complete self-report instruments, 6) residence within 60 miles of the assessment site, 7) willingness to participate and remain abstinent from cocaine and alcohol for 28 days, and 8) a history of at least one traumatic event. The NWS-PTSD module, CAPS, MPSS, breathalyzer, and UDS were administered within 48 hours of their last cocaine or alcohol use and at the following time periods: 5 days, 10 days, 14 days, 21 days, and 28 days. If participants relapsed during the study, as evidenced by either self-report, breathalyzer, and/or a cocaine positive UDS, their first assessment was reset to the day following their relapse. That is, if a participant relapsed during the 28-day study period, their first day of abstinence following the relapse was identified as their new “Day 1”. Participants were then monitored for 28 days following their new “Day 1”. One highly trained interviewer conducted all the evaluations. The interviewer was trained by the principal investigator (B.D.C.) in the administration of all structured interviews. The training consisted of viewing video tapes of expert administrations of the interviews, observing the PI administer the interviews to live clinical patients, practicing administration of the interviews to non-clinical volunteers, administering the interviews to clinical patients while being observed by the PI, and when scoring of the instruments exceeded 90% agreement between the interviewer and the PI, the interviewer administered the instruments to study participants without being observed. Weekly supervision was provided to the interviewer by the PI throughout the course of the study. Individuals were compensated up to $100 for their participation in the study. An Institutional Review Board approved all aspects of the study.
2.4. Data analytic strategy
Longitudinal patterns of trauma symptomatology were related to withdrawal symptoms, withdrawal drug, and diagnostic status. Latent growth modeling (LGM; Duncan et al., 1999; McArdle & Epstein, 1987) using AMOS 5.0 (Arbuckle, 2003) was performed to estimate individual patterns of scores on the MPSS across the 28-day study period (i.e., the growth curve). Maximum likelihood estimation with estimation of means and intercepts (i.e., mean structure) was utilized to allow estimation of the model with missing data. The analyses also examined whether type of withdrawal drug (cocaine, alcohol, or cocaine and alcohol) or PTSD status at the outset of treatment, predicted different patterns of trauma symptoms during the study period.
LGM can be regarded as a generalization of the multivariate repeated measures analyses of variance, but offers substantial advantages over traditional repeated measures methods, particularly with regard to the inclusion of individuals with missing data, use of unequally spaced measurement occasions, and the ability to use repeated assessments of independent variables as well as dependent variables. Another advantage of LGM for the current project is that the shape of the growth factor does not have to be specified a priori (Duncan et al., 1999).
3. Results
3.1. Missing data analyses
Missing data is a ubiquitous feature of longitudinal studies (Tabachnick and Fidell, 1996). Therefore, preliminary analyses were conducted to assess the representativeness of the available data. Sixteen of the 162 participants in the study had complete data for all of the six assessments and the mean number of sessions completed was 3.1 (SD = 1.7). Forty-four percent of the sample completed at least 1/2 of the assessments (n = 72). We examined whether the number of assessments participants completed (i.e., 1–6) were associated with their scores on variables of interest in the present study or their demographic characteristics. Chi-square and analysis of variance (ANOVA) tests indicated that the number of assessments completed by participants was unrelated to demographic variables, PTSD diagnosis, PTSD symptom severity at any of the six time points using all available data, or the drug severity composite score on the ASI. However, both psychiatric symptom severity (r = .23, p = .003) and alcohol symptom severity (r = .23, p = .004), as measured by the ASI, were positively associated with the number of sessions completed by participants.
Withdrawal group (e.g., COC, ETOH, or COC-ETOH) was significantly associated with the number of assessments completed F(2, 159) = 5.29, p = .006. Post hoc Bonferroni analyses indicated that participants in the COC group completed significantly fewer assessments (M = 2.57, SD = 1.65) than participants in the ETOH group (M = 3.74, SD = 1.64). Neither the COC group nor ETOH group differed significantly from the COC-ETOH group, whose participants completed an average of 3.04 (SD = 1.68) assessments.
3.2. Addiction, psychiatric, and PTSD severity as a function of withdrawal group and PTSD status
A significant main effect was revealed for withdrawal group on the alcohol severity composite score of the ASI, F(2, 158) = 50.16, p < .001. Specifically, the ETOH (M = .49, SD = .21) and COC-ETOH (M = .40, SD = .27) groups reported greater alcohol addiction severity (both ps < .001) compared to the COC group (M = .06, SD = .13). The ETOH and COC-ETOH groups did not differ significantly on the alcohol addiction severity. When examining the alcohol addiction severity scores as a function of PTSD status, PTSD (M = .38, SD = .28) and non-PTSD (M = .29, SD = .28) groups did not differ significantly.
For the drug severity composite score of the ASI, a main effect for withdrawal group was revealed F(2, 158) = 61.29, p < .001. When comparing the withdrawal groups, the mean scores on drug addiction severity for the three groups (COC-ETOH = .25, SD = .11; COC = .20, SD = .09; ETOH = .04, SD = .09) were all significantly different from one another (ps < .02). Scores on drug addiction severity did not differ between PTSD (M = .22, SD = .14) and non-PTSD ( M = .18, SD = .12) groups.
Mean scores for the psychiatric severity composite score of the ASI did not differ between the three withdrawal groups (COC-ETOH = .29, SD = .25; COC = .27, SD = .24; ETOH = .30, SD = .23). In addition, scores on psychiatric severity did not differ between the PTSD (M = .27, SD = .22) and non-PTSD ( M = .29, SD = .25) groups. PTSD symptom severity as measured by the MPSS did not differ as a function of withdrawal group (COC-ETOH = 30.7, SD = 22.4; COC = 40.6, SD = 21.4; ETOH = 40.6, SD = 27.7) but did differ as a function of PTSD status (PTSD M=54.50, SD=14.32; non-PTSD M=25.43, SD=20.37).
3.3. Change in PTSD symptoms
Although a decline in PTSD symptomatology from day 2 to day 28 of abstinence was hypothesized, the exact shape of the growth factor describing this decline was of interest in the present study. To specify a shape function reflecting an optimal pattern of change over the study period, the first and sixth factor loadings for the shape factor were set to 0 and 1 (representing 0% and 100% growth, respectively) and allowed the intervening four loadings to be freely estimated (Anderson, 2004; Ferrer et al., 2004). A linear growth model was also tested to allow for comparison. The full latent growth model provided a good fit to the data (χ2 = 17.051, p = .148, df = 12, CFI = .986, RMSEA = .051), but the linear growth model did not (χ2 = 41.933, p = .000, df = 16, CFI = .929, RMSEA = .099). A nested model comparison indicated that the fit of the full latent growth model was significantly better than the linear model (χ2 = 24.88, p = .000, df = 4).
Examination of the estimated mean intercept value in the full latent growth model indicates that the average MPSS score at the time of the first assessment was 40.85 (SE = 2.45). The estimated mean growth factor value was −.69 (SE = .10). Importantly, there was significant variance in the growth factor, indicating that individual participants’ experiences of PTSD symptoms over the 28-day period were varied. The observed factor loadings for the full models were plotted against the time metric to provide an estimate of the shape of growth (e.g., Duncan et al., 1999). Examination of the estimated factor loadings suggests a faster than expected growth curve through the first four time points. Specifically, the estimates suggest that the majority of reduction in symptoms (80%) occurred between days 2 to 14, and that moreover, no significant change in symptoms occurred between days 21 and 28 (see Figure 1). Examining means from available data at each time point, mean PTSD symptoms for participants with and without PTSD are presented in Table 2.
Figure 1.
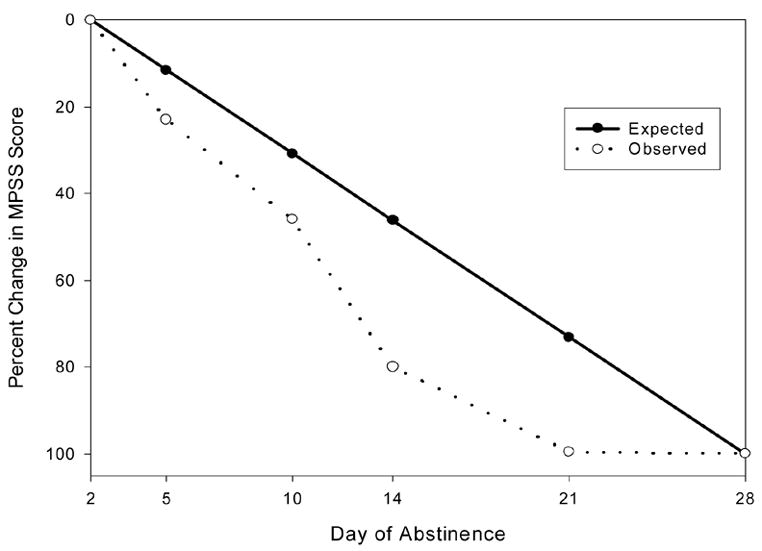
Expected and observed percent change in PTSD symptoms, as measured by the Modified PTSD Symptom Scale – Self Report (MPSS) total score, during 28 days of cocaine and/or alcohol abstinence.
Table 2.
Mean (standard deviation) posttraumatic stress disorder (PTSD) symptoms measured by the Modified PTSD Symptom Scale-Self Report for participants with and without PTSD during the first 28 days of acute withdrawal from alcohol, cocaine, or both cocaine and alcohol using all available data.
| Assessment Visit | |||||||
|---|---|---|---|---|---|---|---|
| Day 2 | Day 5 | Day 10 | Day 14 | Day 21 | Day 28 | ||
| (n=20) | (n=25) | (n=32) | (n=27) | (n=21) | (n=16) | ||
|
|
|||||||
| PTSD Positive | Reexperiencing | 11.30 (5.18) | 11.11 (6.25) | 10.55 (7.21) | 9.46 (6.39) | 9.86 (7.16) | 11.40 (7.57) |
| Avoidance | 23.25 (8.62) | 21.62 (10.37) | 22.06 (9.91) | 117.67 (10.46) | 18.24 (10.41) | 19.27 (11.10) | |
| Hyperarousal | 19.95 (5.46) | 18.59 (6.64) | 17.10 (7.87) | 15.61 (8.05) | 15.19 (8.55) | 18.00 (8.89) | |
| Total | 54.50 (14.32) | 51.28 (20.15) | 49.66 (21.11) | 42.63 (23.72) | 43.29 (23.91) | 46.44 (26.39) | |
|
| |||||||
| (n=47) | (n=66) | (n=76) | (n=63) | (n=53) | (n=50) | ||
|
|
|||||||
| PTSD Negative | Reexperiencing | 5.02 (6.24) | 6.69 (6.47) | 5.19 (6.09) | 4.02 (4.60) | 3.98 (4.30) | 3.62 (4.23) |
| Avoidance | 10.81 (9.98) | 10.13 (8.41) | 9.68 (8.44) | 6.74 (6.66) | 5.85 (5.62) | 5.70 (6.09) | |
| Hyperarousal | 10.06 (7.82) | 10.02 (6.52) | 9.00 (6.49) | 7.54 (5.38) | 6.56 (4.56) | 5.85 (4.67) | |
| Total | 25.43 (20.37) | 26.82 (18.81) | 24.00 (17.57) | 18.00 (14.66) | 16.30 (12.00) | 15.14 (12.79) | |
3.4. Predictors of reductions in PTSD
To examine whether withdrawal substance (cocaine, alcohol, or cocaine and alcohol) or baseline PTSD diagnosis were predictors of changes in PTSD symptoms over time a dichotomized PTSD diagnosis variable, and two dummy variables representing withdrawal substance as predictors of both the latent intercept and growth factors were added to the latent growth model. A visual representation of the model is presented in Figure 2. Factor loadings for the growth factor were constrained at the values established in the initial full latent growth model (see Table 3). The model provided a good fit to the data (χ2 = 30.349, p = .347, df = 28, CFI = .994, RMSEA = .023). As shown in Table 4, examination of the regression weights revealed that baseline PTSD diagnosis was a significant predictor of initial score on the MPSS, but was not a significant predictor of the growth factor. Mean PTSD symptoms did not differ significantly by gender at any time point. Substance of withdrawal was not a significant predictor of either initial score on the MPSS or the growth factor.
Figure 2.
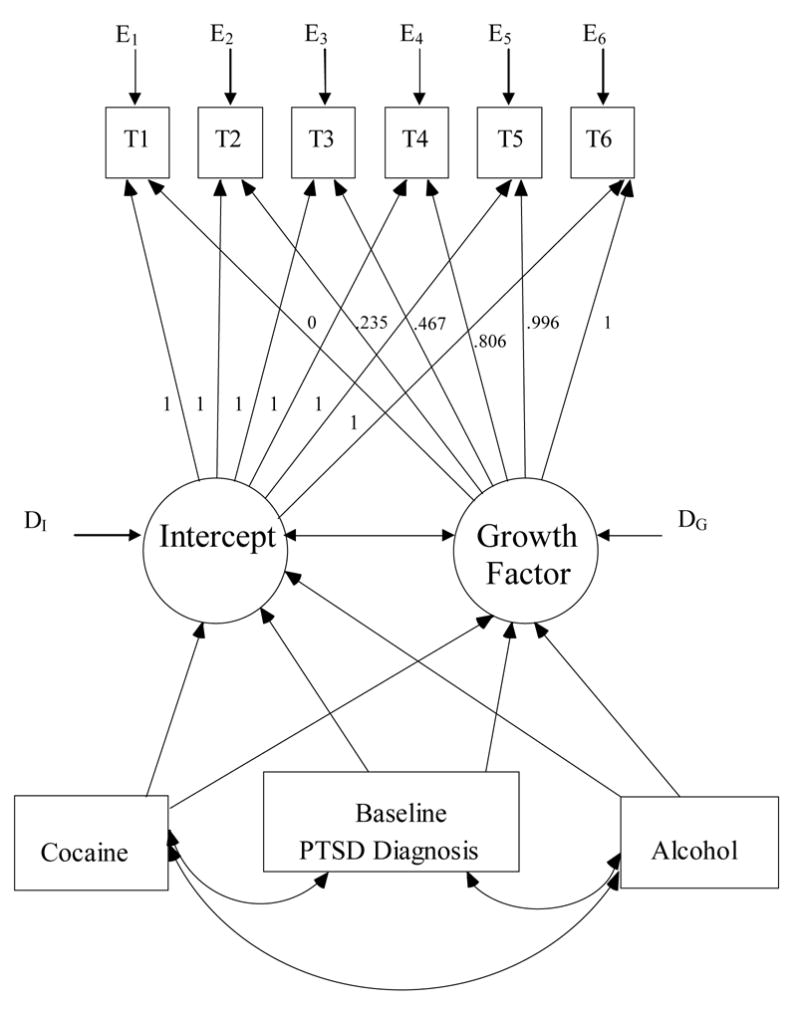
Representation of a latent growth model with mean structure estimation examining change in PTSD symptoms as assessed with the Modified PTSD Symptom Scale – Self Report (MPSS) during 28 days of cocaine and/or alcohol abstinence as a function of withdrawal substance (cocaine, alcohol, or cocaine and alcohol) and baseline PTSD diagnostic status. T1 = MPSS score at day 2; T2 = MPSS score at day 5; T3 = MPSS score at day 10; T4 = MPSS score at day 14; T5 = MPSS score at day 21; T6 = MPSS score at day 28; Cocaine = dummy coded variable (withdrawing from cocaine only = 1; withdrawing from alcohol or alcohol and cocaine = 0); Alcohol = dummy coded variable (withdrawing from alcohol only = 1; withdrawing from cocaine or alcohol and cocaine = 0); Baseline PTSD diagnosis = dichotomous variable (1 = yes; 0 = no); Intercept = latent variable representing baseline MPSS score; Growth Factor = latent variable representing change in MPSS scores over time; E1 − E6 = error variance on T1 − T6 MPSS scores; DI and DG = disturbance terms on latent Intercept and Growth Factor variables.
Table 3.
Comparison of Expected Values for Factor Loadings in a Linear Growth Model and Observed Values in the Full Latent Growth Model of Change in Posttraumatic Stress Disorder (PTSD) Symptoms During 28 Days of Cocaine and Alcohol Abstinence
| Number of Days of Abstinence | Expected Value (Linear Growth Model) | Observed Value (Full Latent Growth Model) |
|---|---|---|
| 2 days | 0/26 = 0.0 | 0.0 (fixed) |
| 5 days | 3/26 = .115 | .235 |
| 10 days | 8/26 = .308 | .467 |
| 14 days | 12/26 = .462 | .806 |
| 21 days | 19/26 = .731 | .996 |
| 28 days | 26/26 = 1.00 | 1.00 (fixed) |
Note: Expected Values for factor loadings range from 0.0 to 1.0 and represent the proportion of change in PTSD symptoms expected to have occurred at each time point if change were linear across time (e.g., .115 = 11.5% of total change). The period between the first assessment (2 days of abstinence) and the final assessment (28 days of abstinence) is 26 days, so the proportion of change expected in a linear model is calculated as number of days elapsed divided by 26 days. Similarly, the initial (2 days) and final (28 days) factor loadings for the growth factor in the Full Latent Growth Model were fixed at 0.0 and 1.0 to set the metric for the growth factor as the proportion of change in PTSD symptoms observed to occur across the 26 day measurement period.
Table 4.
Posttraumatic Stress Disorder (PTSD) Diagnosis and Substance of Withdrawal as Predictors of Initial Status and Changes in PTSD Symptoms (as measured by the Modified PTSD Symptom Scale – Self Report; MPSS) over 28 days of Cocaine and Alcohol Abstinence in a Latent Growth Model: Unstandardized Regression Weights
| Full Latent Growth Model Path | Unstandardized Estimate | SE | z score | p |
|---|---|---|---|---|
| PTSD Diagnosis → Intercept | 16.039 | 2.178 | 2.296 | .022 |
| PTSD Diagnosis → Growth | 2.779 | 7.794 | .357 | .721 |
| Alcohol → Intercept | 2.826 | 4.779 | .591 | .554 |
| Alcohol → Growth | 5.865 | 5.352 | 1.096 | .273 |
| Cocaine → Intercept | .902 | 5.235 | .172 | .863 |
| Cocaine → Growth | 2.059 | 5.862 | .351 | .725 |
Note: PTSD Diagnosis = dichotomously coded variable (1 = met diagnostic criteria for PTSD at first assessment, 0 = did not meet criteria for PTSD at first assessment). Alcohol = dichotomously coded dummy variable representing substance of withdrawal (alcohol = 1, cocaine or cocaine/alcohol = 0). Cocaine = dichotomously coded dummy variable representing substance of withdrawal (cocaine = 1, alcohol or cocaine/alcohol = 0). Together the Alcohol and Cocaine variables represent the 3 drug withdrawal categories (cocaine only, alcohol only, and alcohol and cocaine). Intercept = latent variable representing baseline MPSS score. Growth = latent variable representing change in MPSS scores over time. SE = Standard Error.
4. Discussion
This is the first study to prospectively examine trauma symptoms over time in a substance dependent outpatient sample. Subjects with a history of trauma were assessed prospectively during short-term abstinence from cocaine and alcohol. Subjects were repeatedly assessed over 28-days for symptoms of trauma and symptoms associated with acute and protracted substance abstinence.
As predicted, trauma symptoms declined over the 28-day study period. This prediction was based on the research literature clearly demonstrating reductions in anxiety and depressive disorder symptoms during acute and protracted substance abstinence (e.g., Brown and Schuckit, 1988; Brown et al., 1995; Driessen et al., 2001). Importantly, the analyses indicated that the decline was not consistent with a linear trend. Instead, the estimated factor loadings suggested that the majority of change occurred by day 14, and that essentially no change occurred between days 21 and 28 of abstinence. These findings are consistent with the findings of Brown and Schuckit (1988) regarding the decline in symptoms of depression across the same treatment period (48 hours to 28 days). Although these researchers used a different analytic strategy, they found that depressive symptoms abated quickly with the largest reduction in scores occurring by week two of the four week study. While reductions in trauma symptomatology over time have been observed in non-substance dependent victims of acute sexual (Rothbaum et al., 1992) and acute non-sexual assault (Riggs et al., 1995), this is the first study to prospectively observe a decline in trauma-related symptoms during substance withdrawal.
Although the entire sample of participants reported a history of at least one traumatic event that met DSM-III-R Criterion A for PTSD (APA, 1987), only 28% met criteria for PTSD. To test whether symptoms reported by individuals with PTSD were more resistant to the changes observed in the entire sample, PTSD status was included in the LGM as a predictor of the intercept and growth factors. PTSD diagnostic status was a significant predictor of intercept but not growth, indicating that although the PTSD group reported more PTSD symptomatology, the symptoms of both groups declined at similar rates over the 28-day study period. Given that the PTSD and non-PTSD participants did not differ on alcohol or drug addiction severity, on general psychiatric symptom severity, or on the number of assessment sessions completed suggests that the two groups were relatively similar except for PTSD status. The similarity of the two groups and the fact that PTSD symptoms declined at the same rate regardless of PTSD status, suggests that the construct of PTSD may be viewed clinically as a continuous construct rather than a dichotomous construct (i.e., presence or absence of PTSD) and that regardless of PTSD status, psychiatric symptoms related to trauma will decline during drug and alcohol abstinence.
The current study has important clinical implications. Specifically, the data suggest that trauma-related symptoms in some cocaine and/or alcohol dependent patients may be related to symptoms present during acute and protracted cocaine and alcohol abstinence. If these data are replicated, changes in PTSD diagnostic practices may be warranted. In the absence of replication studies, it would be prudent for clinicians who assess trauma-related symptoms during the first 28 days of cocaine or alcohol abstinence to regularly re-assess trauma symptoms and treatment plans. It is important to note that although PTSD symptoms decreased during the first 28 days of acute abstinence in the PTSD positive participants, at the final study visit (i.e., 28 days post substance use) PTSD symptoms in this group remained quite substantial. To illustrate, when used as a PTSD screener in substance abusing samples, the recommended total score cut off for the MPSS is 28 (Coffey et al., 1998). In contrast, the mean MPSS total score for the PTSD positive participants using all available data was 46.44 indicating the presence of significant PTSD symptoms at the final study visit. Nonetheless, the small but statistically significant reduction in PTSD symptoms in this substance dependent-PTSD sample is inconsistent with clinical lore that PTSD symptoms increase during early abstinence and, thus, this modest reduction in PTSD symptoms is an important finding of this study.
One potential limitation of the current study is that participants with missing data were included in the analyses and therefore the results are based on estimations of data rather than actual collected data. As stated previously, missing data is a common feature of repeated measures studies (Tabachnick and Fidell, 1996). LGM, the statistical procedure used in the current study, allows for the use of data from participants with missing time points by using maximum likelihood estimation with estimation of means and intercepts. Therefore, LGM allows participants with missing data points to be included in longitudinal analyses, thus substantially increasing the power and utility of longitudinal data sets. A second limitation of the study is that DSM-III-R criteria was used to diagnose PTSD. Although there is good agreement between PTSD diagnoses established with DSM-III-R and DSM-IV classification systems (e.g., Brewin et al., 2000), use of DSM-III-R may reduce somewhat the generalizability of our findings. A third potential limitation of the study is that individuals dependent on cocaine completed fewer assessment sessions compared to participants dependent on both cocaine and alcohol or alcohol alone. Fewer data points in the COC group on which to estimate missing data may introduce greater error in the estimation process compared to error present in the estimation of the COC-ETOH and ETOH groups. Therefore, the findings for the COC group may be less robust than findings reported for the COC-ETOH and ETOH groups. A fourth potential limitation of the study is that the potential interaction of relapse and PTSD symptoms could not be evaluated. Information regarding relapse was collected from participants who remained in the study but is unknown for those who did not remain in the study for the entire 28-day evaluation period. Unfortunately, assuming all who dropped out of the study relapsed would likely overestimate relapse. Moreover, even if relapse was assumed, PTSD symptoms at the time of relapse would remain unknown and, therefore, the relation between relapse and PTSD symptomatology would remain unknown. Lastly, any assumptions made are further confounded by the fact that 23% of the sample spent 1–2 weeks on inpatient units without the opportunity to engage in substance use so the influence of PTSD or withdrawal symptoms on relapse during that time period for that portion of the sample cannot be evaluated. Given the limitations of the current study’s design, future replication studies should be conducted on inpatient samples of substance abusers with PTSD or in other more controlled settings to better evaluate, in a prospective design, the role of PTSD symptoms on substance use relapse.
In summary, our findings that PTSD symptoms decline during acute and protracted substance abstinence mirrors findings from investigators assessing symptoms of other psychiatric disorders. Our results also indicate that PTSD symptoms decline irrespective of substance of abuse. That is, whether an individual is withdrawing from cocaine, alcohol, or cocaine and alcohol, trauma-related symptoms decline at the same rate. Finally, our results also suggest that symptoms of PTSD in victimized substance users should be re-assessed after symptoms of acute substance withdrawal have abated.
Acknowledgments
This research was supported by a scientist development grant from the National Institute on Drug Abuse (K21-DA00243) awarded to Bonnie Dansky Cotton, Ph.D., with Kathleen T. Brady, M.D., Ph.D. serving as mentor. We wish to thank Research Assistant Stephanie Mamay-Gentilin for her invaluable contributions to this project and Tenko Raykov, Ph.D. for consultation on statistical analyses. Correspondence concerning this article should be addressed to Scott F. Coffey, Ph.D., Department of Psychiatry and Human Behavior, The University of Mississippi Medical Center, 2500 North State Street, Jackson, Mississippi 39216.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alterman AI, McDermott PA, Brown LS, Jr, Cook TG, Metzger D, Rutherford MJ, Cacciola JS. New scales to assess change in the Addiction Severity Index for the opioid, cocaine, and alcohol dependent. Psychol Addict Behav. 1998;12:233–246. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- Anderson E. AMOS FAQ #4: Fitting a latent growth curve model. 2004. Retrieved on June 10, 2005 from the University of Texas Information Technology Services Research Consulting Web site: http://www.utexas.edu/its/rc/answers/amos/amos4.html.
- Appleby L, Dyson V, Altman E, Luchins DJ. Assessing substance use in multiproblem patients: reliability and validity of the Addiction Severity Index in a mental hospital population. J Nerv Ment Dis. 1997;185:159–165. doi: 10.1097/00005053-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. AMOS Users’ Guide Version 5.0.1. SmallWaters; Chicago: 2003. [Google Scholar]
- Back SE, Brady KT, Jaanimägi U, Jackson JL. Cocaine dependence and PTSD: a pilot study of symptom interplay and treatment preferences. Addict Behav. 2006;31:351–354. doi: 10.1016/j.addbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J of Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Rose S. Fear, helplessness, and horror in posttraumatic stress disorder: investigating DSM-IV criterion A2 in victims of violent crime. J of Trauma Stress. 2000;13:499–509. doi: 10.1023/A:1007741526169. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Stout RL, Gannon-Rowley J. Substance use disorder-PTSD comobidity: patients’ perceptions of symptom interplay and treatment issues. J of Subst Abuse Treat. 1998;15:445–448. doi: 10.1016/s0740-5472(97)00286-9. [DOI] [PubMed] [Google Scholar]
- Brown RA, Monti PM, Myers MG, Martin RA, Rivinus T, Dubreuil ME, Rohsenow DJ. Depression among cocaine abusers in treatment: relation to cocaine and alcohol use and treatment outcome. Am J of Psychiatr. 1998;155:220–225. doi: 10.1176/ajp.155.2.220. [DOI] [PubMed] [Google Scholar]
- Brown SA, Inaba RK, Gillin C, Schuckit MA, Stewart MA, Irwin MR. Alcoholism and affective disorder: clinical course and depressive symptoms. Am J of Psychiatr. 1995;152:45–52. doi: 10.1176/ajp.152.1.45. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schukit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49:412–417. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Investigations of causal pathways between PTSD and drug use disorders. Addict Behav. 1998;23:827–840. doi: 10.1016/s0306-4603(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Carrigan MH, Brady KT. Acute and protracted cocaine abstinence in an outpatient population: a prospective study of mood, sleep, and withdrawal symptoms. Drug Alcohol Depend. 2000;59:277–286. doi: 10.1016/s0376-8716(99)00126-x. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Falsetti SA, Saladin ME, Brady KT. Screening for PTSD in a substance abuse sample: psychometric properties of a modified version of the PTSD Symptom Scale Self-Report. J Trauma Stress. 1998;11:393–399. doi: 10.1023/A:1024467507565. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Stasiewicz PR, Hughes P, Brimo ML. Trauma-focused imaginal exposure with comorbid PTSD- alcohol dependent individuals: revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psychol Addict Behav. doi: 10.1037/0893-164X.20.4.425. in press. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Compton WM, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. Amer J Psychiatr. 1992;149:664–670. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- Dansky BS, Brady KT, Saladin ME. Untreated symptoms of PTSD among cocaine-dependent individuals: changes over time. J Subst Abuse Treat. 1998;15:499–504. doi: 10.1016/s0740-5472(97)00293-6. [DOI] [PubMed] [Google Scholar]
- Dansky BS, Brady KT, Saladin ME, Killeen T, Becker S, Roitzsch JC. Victimization and PTSD in individuals with substance use disorders: Gender and racial differences. Amer J Drug Alcohol Abuse. 1996;22:75–93. doi: 10.3109/00952999609001646. [DOI] [PubMed] [Google Scholar]
- Dansky BS, Saladin ME, Brady KT, Kilpatrick DG, Resnick HS. Prevalence of victimization and posttraumatic stress disorder among women with substance use disorders: comparison of telephone and in-person assessment samples. Int J Addict. 1995;30:1079–1099. doi: 10.3109/10826089509055829. [DOI] [PubMed] [Google Scholar]
- Driessan M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression, and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Strycker LA, Li F, Alpert A. An Introduction to Latent Variable Growth Curve Modeling: Concepts, Issues, and Applications. Lawrence Erlbaum Associates; Mahwah, NJ: 1999. [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The modified PTSD Symptom Scale: a brief self-report measure of posttraumatic stress disorder. Behav Therapist. 1993;16:161–162. [Google Scholar]
- Ferrer E, Hamagami F, McArdle JJ. Modeling latent growth curves with incomplete data using different types of structural equation modeling and multilevel software. Structural Equation Modeling. 2004;11:452–483. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- Grice DE, Brady KT, Dustan LR, Malcolm R, Kilpatrick DG. Sexual and physical assault history and posttraumatic stress disorder in substance-dependent individuals. Amer J Addict. 1995;4:297–305. [Google Scholar]
- Husband SD, Marlowe DB, Lamb RJ, Iguchi MY, Bux DA, Kirby KC, Platt JJ. Decline in self-reported dysphoria after treatment entry in inner-city cocaine addicts. J Consult Clin Psychol. 1996;64:221–224. doi: 10.1037//0022-006x.64.1.221. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. J Consult Clin Psychol. 2000;68:19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Saunders BE, Best CL. The National Women’s Study PTSD Module. Charleston, SC: Crime Victims Research and Treatment Center, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina; 1989. Unpublished instrument. [Google Scholar]
- Kosten TR, Rounsaville MD, Kleber HD. Concurrent validity of the Addiction Severity Index. J Nerv Ment Dis. 1983;171:606–610. doi: 10.1097/00005053-198310000-00003. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Kadden RM, Babor TF, Tennen H, Rounsaville BJ. Validity of the SCID in substance abuse patients. Addict. 1996;91:859–868. [PubMed] [Google Scholar]
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Readjustment Study. Brunner/Mazel; New York: 1990. [Google Scholar]
- Lynskey MT. The comorbidity of alcohol dependence and affective disorders: treatment implications. Drug Alcohol Depend. 1998;52:201–209. doi: 10.1016/s0376-8716(98)00095-7. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Epstein D. Latent growth curves within developmental structural equation models. Child Dev. 1987;58:110–133. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Raimo EB, Schuckit MA. Alcohol dependence and mood disorders. Addict Behav. 1998;23:933–946. doi: 10.1016/s0306-4603(98)00068-9. [DOI] [PubMed] [Google Scholar]
- Resnick H. Psychometric review of National Women’s Study (NWS) Event History-PTSD Module. In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Sidran Press; Lutherville, MD: 1996. pp. 214–217. [Google Scholar]
- Resnick H, Kilpatrick DG, Dansky BS, Best CL, Saunders BE. Prevalence of civilian trauma and post-traumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Riggs DS, Rothbaum BO, Foa EB. A prospective examination of symptoms of posttraumatic stress disorder in vicitms of nonsexual assault. J Interper Violence. 1995;10:201–214. [Google Scholar]
- Rothbaum BO, Foa EB, Riggs DS, Murdock T, Walsh W. A prospective examination of post-traumatic stress disorder in rape victims. J Trauma Stress. 1992;5:455–475. [Google Scholar]
- Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, Charney DS, Henninger GR, Kleber HD. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Amer J Psychiatr. 1991;148:1712–1716. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- Schukit MA, Tipp JE, Bucholz KK, Nurnberger JI, Hesselbrock VM, Crowe RR, Kramer J. The life-time rates of three major mood disorders and four major anxiety disorders in alcoholics and controls. Addict. 1997;92:1289–1304. [PubMed] [Google Scholar]
- Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the Structured Clinical Interview for DSM-III-R Axis I (SCID-I) Acta Psychiatr Scand. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychological and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Spitzer RL, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York: 1986. Structured Clinical Interview for DSM-III-R-Nonpatient Version. [Google Scholar]
- Stewart SH, Conrod PJ. Psychosocial models of functional associations between posttraumatic stress disorder and substance use disorder. In: Crosby-Ouimette P, Brown P, editors. PTSD and Substance Use Disorder Comorbidity. American Psychological Association; Washington, DC: 2003. pp. 29–56. [Google Scholar]
- Stewart SH, Conrod PJ, Pihl RO, Dongier M. Relationships between posttraumatic stress symptom dimensions and substance dependence in a community-recruited sample of substance-abusing women. Addict Behav. 1999;13:78–88. [Google Scholar]
- Stewart SH, Mitchell TL, Wright KD, Loba P. The relations of PTSD symptoms to alcohol use and coping drinking in volunteers who responded to the Swissair Flight 111 airline disaster. J Anxiety Disord. 2004;18:51–68. doi: 10.1016/j.janxdis.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 3. HarperCollins Publishers; New York: 1996. [Google Scholar]
- Triffleman EG, Marmar CR, Delucchi KL, Ronfeldt H. Childhood trauma and PTSD in substance abuse inpatients. J Nerv Ment Dis. 1995;183:172–176. doi: 10.1097/00005053-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. Arch Gen Psychiatr. 1990;47:861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Mirin SM, Griffin ML, Michael JL. Psychopathology in cocaine abusers: changing trends. J Nerv Ment Dis. 1988;176:719–725. doi: 10.1097/00005053-198812000-00004. [DOI] [PubMed] [Google Scholar]


