Fig. 7.
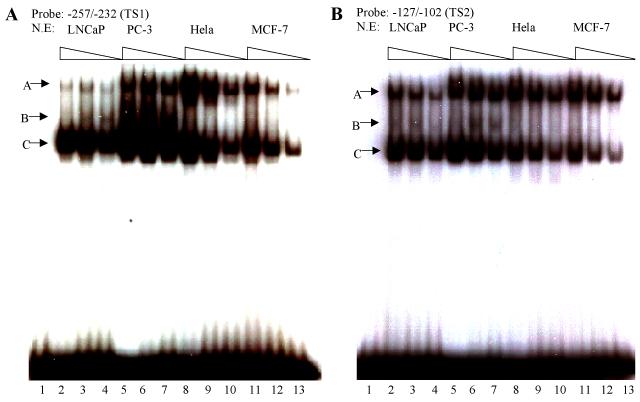
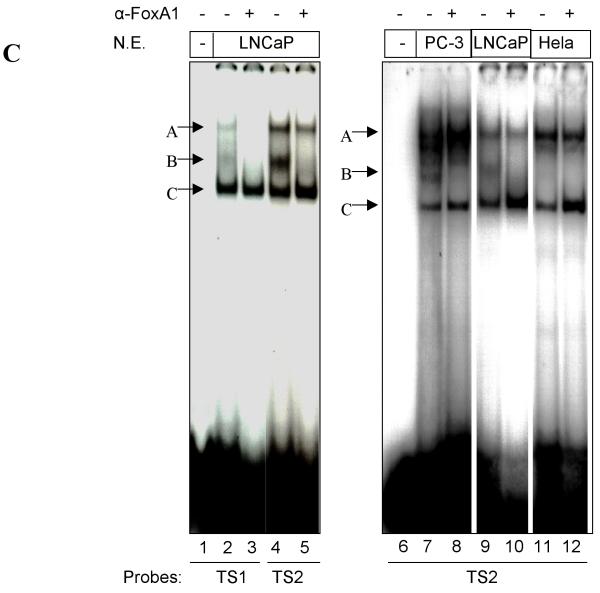
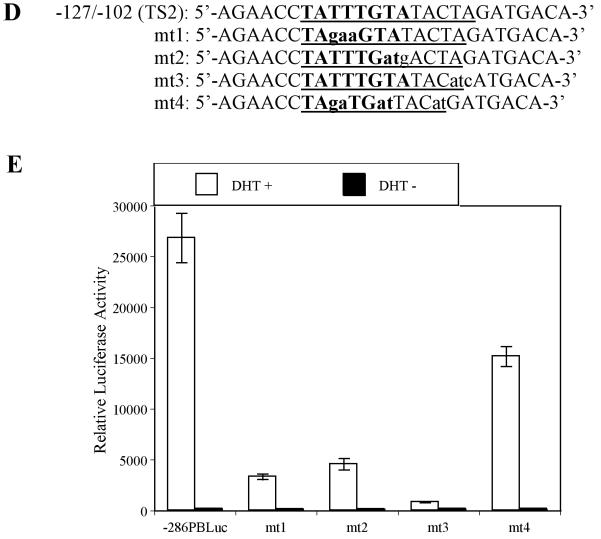
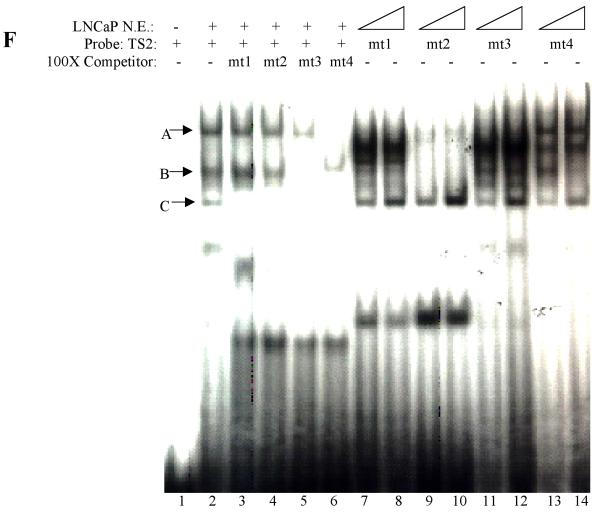
Interaction between cell specific transcription factor(s) and TS1 and TS2 are required for –286PB activation. (A) and (B), Electromobility shift assays of TS1 and TS2 reveal cell specific protein-DNA complexes. The radiolabeled PB –257 to – 232 bp probe containing the TS1 (A) and PB –127 to –102 bp probe containing the TS2 (B) were incubated with 20 μg, 10 μg, and 5 μg of the LNCaP, PC-3, Hela, and MCF-7 nuclear extracts; complexes were resolved on 5% polyacrylimide gels. (C), Radiolabeled TS1 (lanes 2-3) and TS2 probes (lanes 4-5) were used in EMSA with LNCaP nuclear extracts, in the presence (lanes 3 and 5) or absence (lanes 2 and 4) of FoxA1 antibody. Same super shift experiments were performed for PC-3 (lanes 7-8) and Hela cells (lanes 11-12). (D), Wild type PB promoter sequences –127 to –102 bp, containing a FoxA1 motif (bolded) within the TS2 (underlined), was mutated (lowercase characters) as indicated in mt1-mt4. (E), LNCaP cells were transiently transfected with wild type –286PBLuc, and mt1-mt4 reporter constructs together with the AR and Renilla luciferase expression vectors and relative Luc activities were determined in the absence or presence of 10−8 M DHT as described in Materials and Methods. The reporter gene activities were measured in at least three determinations ± SD, and all experiments were adjusted for transfection efficiency. The luciferase activities are expressed as relative luciferase light units/min/μg protein. (F), LNCaP nuclear extracts (10 μg or 20 μg) were incubated with radiolabeled probes corresponding to the wt or mutated TS2 (−127 to −102 bp) in the absence or presence of 100X unlabeled mutated TS2 competitor oligonucleotides; complexes were resolved on 5% polyacrylimide gels.