Abstract
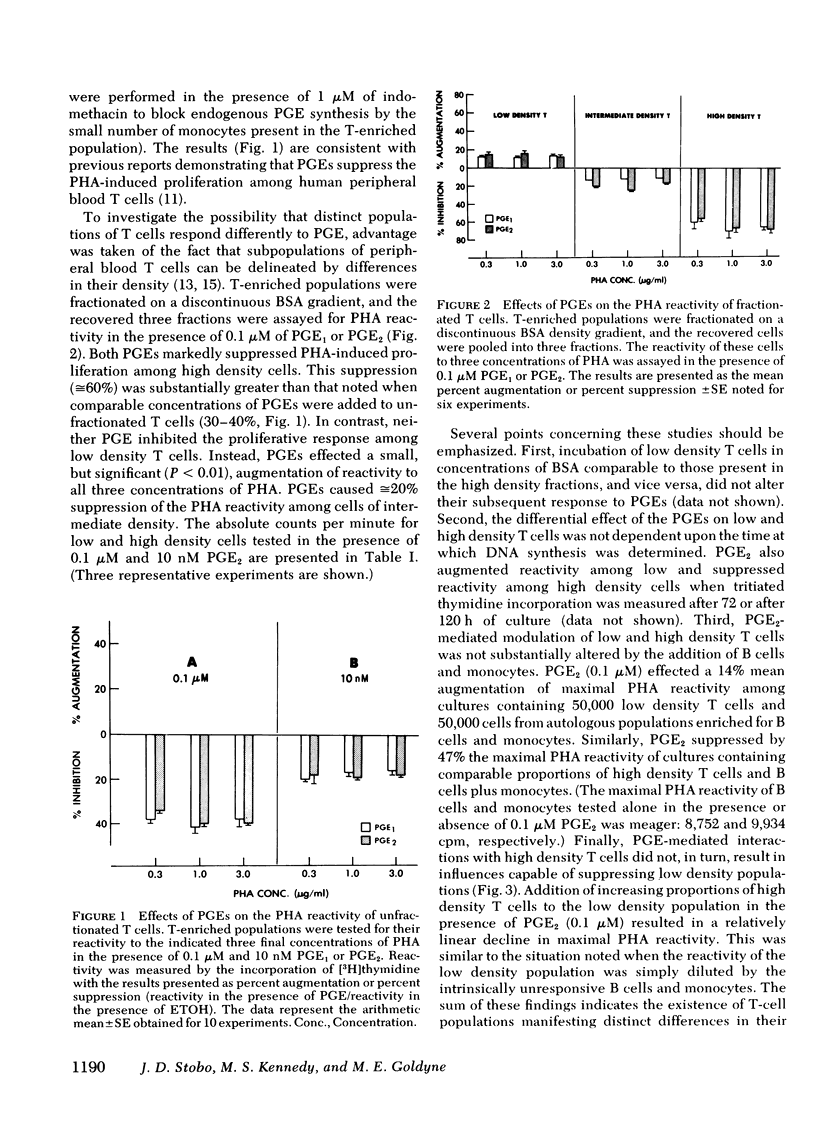
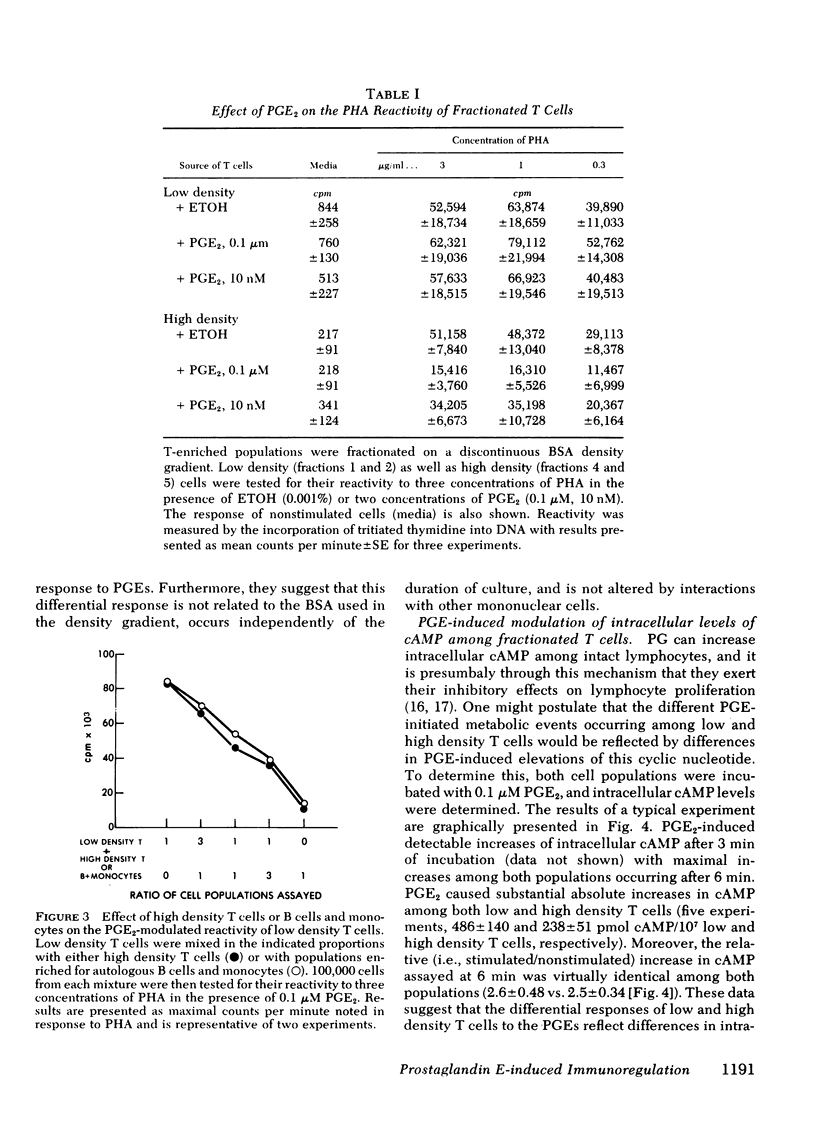
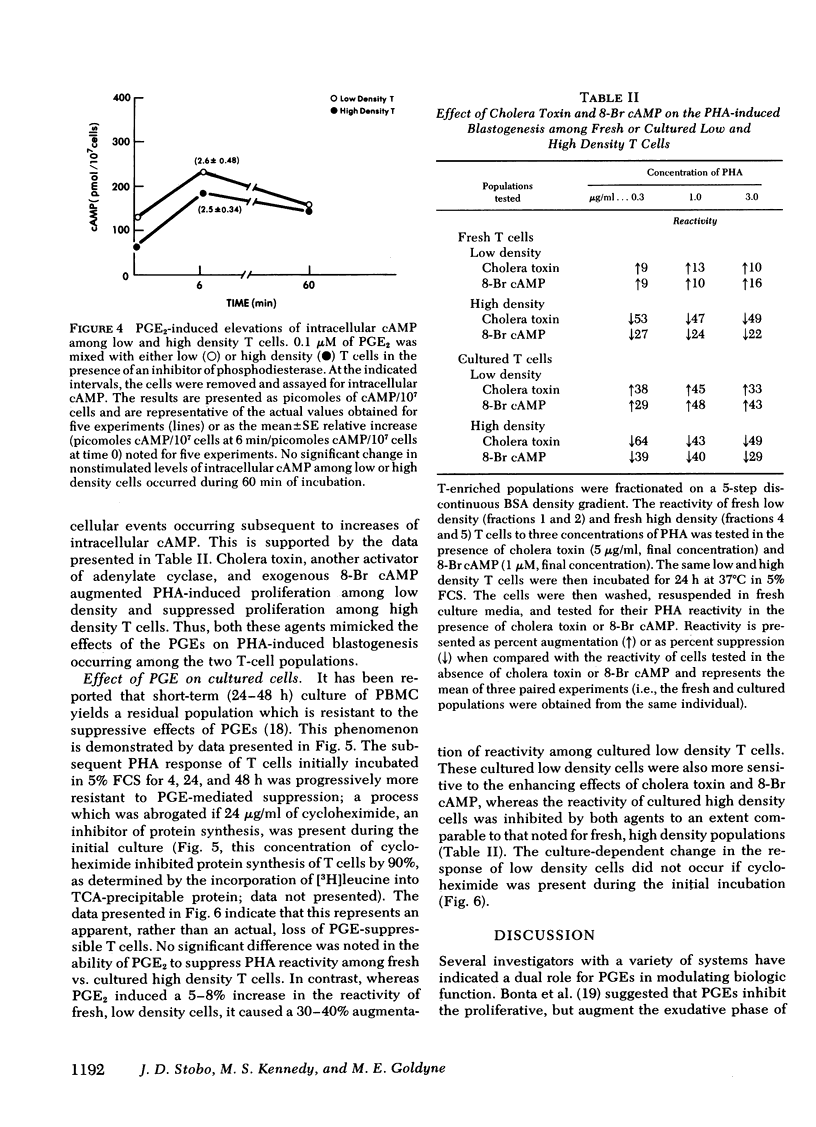
Prostaglandins (PG) of the E series, PGE1 and PGE2 (PGEs), can induce elevations of intracellular cyclic AMP (cAMP) among thymus-derived (T) lymphocytes (T cells) and inhibit their reactivity. For example, 0.1 μM of PGEs induces a two- to threefold increase of intracellular cAMP among human peripheral blood T cells and a 20-30% suppression of their blastogenic response to phytohemagglutinin. However, this suppression actually represents the net reactivity of T-cell populations demonstrating quite different responses to PGEs. Fractionation of T-enriched populations on a discontinuous density gradient yields a population of high density cells whose phytohemagglutinin-induced blastogenic response is suppressed 60%; a population of intermediate density cells whose response is suppressed 20%; and a population of low density T cells whose response is not suppressed, but is enhanced 20% by both of the PGEs. The diametrically opposite responses of low and high density T cells to the PGEs is not related to any difference in their intrinsic mitogen reactivity nor is it influenced by interactions with other T cells, bone marrow-derived (B) cells, or monocytes. Moreover, the distinct blastogenic response of low and high density T cells to PGEs does not simply correlate with PGE-mediated activation of adenylate cyclase. PGE2 induced comparable absolute and identical relative increases of intracellular cAMP among the low and high density T cells. Cholera toxin, a potent activator of adenylate cyclase, and exogenous 8-bromo cAMP mimicked the effects of the PGEs on these two T-cell populations. These data demonstrate that T cells are heterogeneous with regard to their response to the PGEs. Thus, PGEs should be considered as potential regulators rather than as universal suppressors for T-cell reactivity. Moreover, the effect of PGEs on the blastogenic response of a given T-cell population depends upon intracellular events which occur subsequent to elevations of cAMP.
Full text
PDF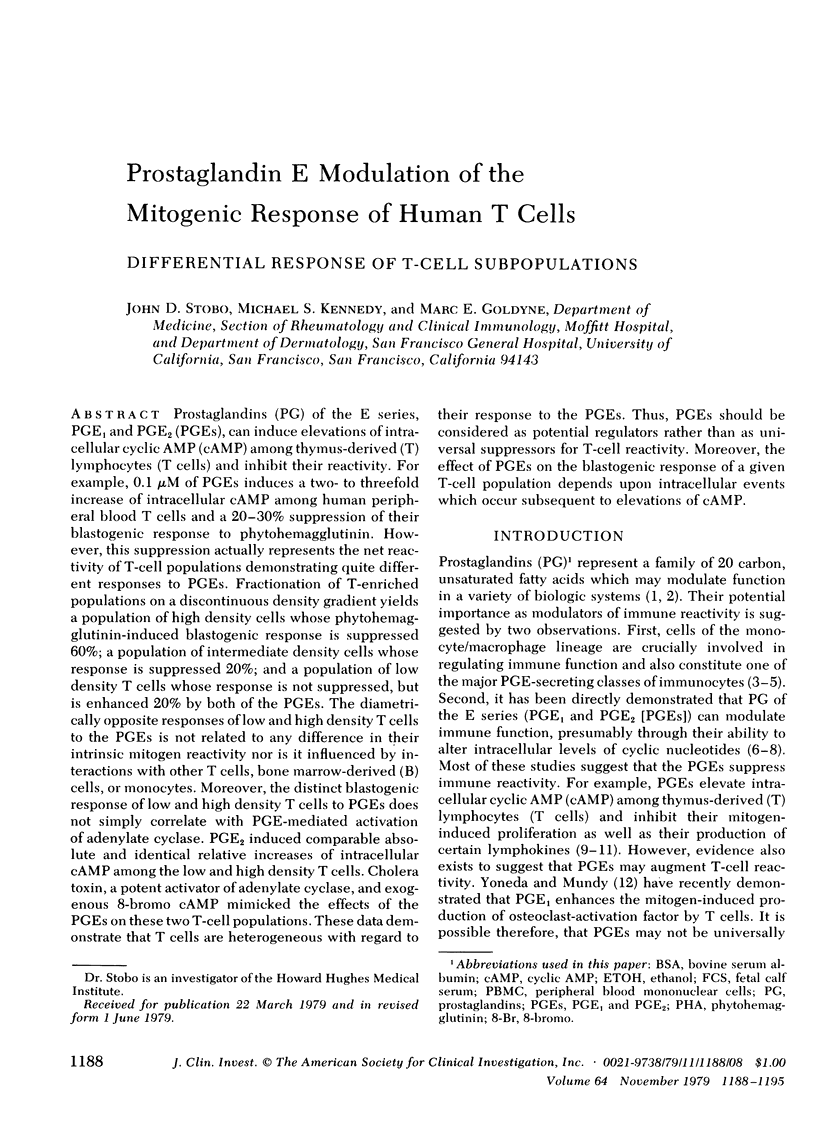



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elattar T. M. Prostaglandins: physiology, biochemistry, pharmacology and clinical applications. J Oral Pathol. 1978 Aug;7(4):175–207. doi: 10.1111/j.1600-0714.1978.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Glatt M., Kälin H., Wagner K., Brune K. Prostaglandin release from macrophages: an assay system for anti-inflammatory drugs in vitro. Agents Actions. 1977 Sep;7(3):321–326. doi: 10.1007/BF01969563. [DOI] [PubMed] [Google Scholar]
- Goldyne M. E. Prostaglandins and the modulation of immunological responses. Int J Dermatol. 1977 Nov;16(9):701–712. doi: 10.1111/j.1365-4362.1977.tb01887.x. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Koopman W. J., Gillis M. H., David J. R. Prevention of MIF activity by agents known to increase cellular cyclic AMP. J Immunol. 1973 Jun;110(6):1609–1614. [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murota S. I., Kawamura M., Morita I. Transformation of arachidonic acid into thromboxane B2 by the homogenates of activated macrophages. Biochim Biophys Acta. 1978 Mar 30;528(3):507–511. [PubMed] [Google Scholar]
- Novogrodsky A., Rubin A. L., Stenzel K. H. Selective suppression by adherent cells, prostaglandin, and cyclic AMP analogues of blastogenesis induced by different mitogens. J Immunol. 1979 Jan;122(1):1–7. [PubMed] [Google Scholar]
- Pelus L. M., Strausser H. R. Prostaglandins and the immune response. Life Sci. 1977 Mar 15;20(6):903–913. doi: 10.1016/0024-3205(77)90274-0. [DOI] [PubMed] [Google Scholar]
- Pénit J., Cantau B., Huot J., Jard S. Adenylate cyclase from synchronized neuroblastoma cells: responsiveness to prostaglandin E1, adenosine, and dopamine during the cell cycle. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1575–1579. doi: 10.1073/pnas.74.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Wahl L. M., Olsen C. E., Sandberg A. L., Mergenhagen S. E. Prostaglandin regulation of macrophage collagenase production. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4955–4958. doi: 10.1073/pnas.74.11.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T., Mundy G. R. Prostaglandins are necessary for osteoclast-activating factor production by activated peripheral blood leukocytes. J Exp Med. 1979 Jan 1;149(1):279–283. doi: 10.1084/jem.149.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]


