Abstract
Objective
The goal of this study was to investigate the role of complement cascade genes in the pathobiology of human abdominal aortic aneurysms (AAAs).
Methods and Results
Results of a genome-wide microarray expression profiling revealed 3,274 differentially expressed genes between aneurysmal and control aortic tissue. Interestingly, 13 genes in the complement cascade were significantly differentially expressed between AAA and the controls. In silico analysis of the promoters of the 13 complement cascade genes showed enrichment for transcription factor binding sites for STAT5A. Chromatin-immunoprecipitation experiments demonstrated binding of transcription factor STAT5A to the promoters of the majority of the complement cascade genes. Immunohistochemical analysis showed strong staining for C2 in AAA tissues.
Conclusions
These results provide strong evidence that the complement cascade plays a role in human AAA. Based on our microarray studies, the pathway is activated in AAA, particularly via the lectin and classical pathways. The overrepresented binding sites of transcription factor STAT5A in the complement cascade gene promoters suggest a role for STAT5A in the coordinated regulation of complement cascade gene expression.
Keywords: Abdominal aortic aneurysm, complement cascade, genetic association study, STAT5, chromatin immunoprecipitation
Abdominal aortic aneurysm (AAA), defined as a dilation greater than 3 cm of the infrarenal abdominal aorta, is a complex disease of the aging population.1 Rupture of AAA is associated with a high mortality rate, making aortic aneurysms the 13th leading cause of death among Caucasian males over the age of 65 (WISQARS Leading Causes of Death Reports, 1999–2007; http://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html). Characteristics of AAA pathogenesis include inflammation, vascular smooth muscle cell (SMC) apoptosis, oxidative stress, and extracellular matrix (ECM) degradation.2 Autoimmunity may also play a role in aneurysm development and progression,3 with one report suggesting increased levels of complement factor 3 (C3) and IgG subclasses in the aneurysmal wall.4
Chronic inflammation plays a role in numerous diseases, especially diseases related to aging. The complement system as part of the innate immunity may contribute to many inflammatory diseases such as age-related macular degeneration (AMD), arthritis, Parkinson’s disease, and Alzheimer’s disease.5 Complement may also have a potential role in the initiation and progression of aneurysms.6 Many different types of immune cells have been reported in AAA tissue such as T-cells, B-cells, macrophages, dendritic cells, mast cells, natural killer cells and natural killer T-cells.3 The complement maintains a link between the innate and adaptive immune system and is known to directly or indirectly associate with many types of immune cells.7
The complement system consists of over 30 proteins, mainly proteases, which create a cascade when triggered.7 Composed of three pathways, the complement system is a complex primary defense to help humans fight against pathogens. The classical pathway is activated by antibodies bound to antigens in immune complexes, while the lectin pathway is activated by microbial sugars, such as mannose-binding lectin. The alternative pathway can be activated in the absence of antibodies by the spontaneous hydrolysis of C3.7,8 All pathways can lead to inflammation, promote antibody production, assist in phagocytosis, and attack cell membranes.7
The aim of the current study was to investigate the role of the complement cascade in the pathogenesis of human AAA. Several members of the complement cascade had increased expression at both the mRNA and protein levels in AAA tissue samples. The results combined with previously published animal studies by other investigators suggest that the complement cascade is involved in AAA pathogenesis.
Materials and Methods
An expanded methods section (including Tables I-IV and Figures I and II) is available in the online supplement (http://atvb.ahajournals.org).
Human samples
Full thickness aortic wall tissue specimens were collected from patients undergoing AAA repair operations at the Geisinger Medical Center, Danville, Pennsylvania, USA, or at the Harper University Hospital, Detroit, Michigan, USA. Non-aneurysmal aortic samples were collected at autopsies. All samples are listed in Supplemental Table I. The collection of the human tissues was approved by the Institutional Review Boards of Geisinger Clinic, Danville, Pennsylvania, USA, and Wayne State University, Detroit, Michigan, USA.
RNA Expression Studies
The details on global mRNA expression profiles for aneurysmal and non-aneurysmal human abdominal aorta (Supplemental Table I) have been described previously9 and the microarray data can be obtained from the Gene Expression Omnibus (GEO) database (Series# GSE7084; http://www.ncbi.nlm.nih.gov/geo/). We used in the current study the microarray results only from the Illumina platform. Pathway information was obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG).10 Gene symbols available from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/) were used.
In Silico Analysis to Identify Transcription Factor Binding Sites
We analyzed the promoter regions of the differentially expressed genes from the complement cascade to identify enriched transcription factor binding sites (TFBSs) using a computational approach implemented in Whole Genome rVISTA11 (http://genome.lbl.gov/vista/index.shtml), a publicly available bioinformatics program.
Chromatin Immunoprecipitation
Chromatin-immunoprecipitation (ChIP) was performed with human monocyte cells (THP-1 cell line; catalog #TIB-202, American Type Culture Collection, Manassas, VA)12 treated with LPS and IFN-γ.13
Immunohistochemical Analysis
Control abdominal aorta samples (N=7; donor ages ranging from 44 to 88 years and mean of 63.6 +/− 15.5 years) were obtained at autopsy (Supplemental Table I). Patient samples (8 AAA patients; ages ranging from 64 to 72 years, mean age 67.9 +/− 2.9) were tissues removed from the aneurysmal sac during open surgical repair operations.
Immunostaining was carried out with formalin-fixed paraffin-embedded tissue sections as described previously.14 The primary antibodies are listed in Supplemental Table III. Double staining was performed with the macrophage/monocyte specific antibody CD6815 and the antibody against STAT5A using EnVision™ G/2 Doublestain System (Dako, Glostrup, Denmark).
Genetic Association Study
A genetic association study was carried out to test for an association with polymorphisms in the complement cascade genes. A complete list of the 16 polymorphisms used in final analyses is in Supplemental Table IV. The DNA samples used for the study consisted of 2 case–control sets: set I had 394 AAA cases and 419 controls; set II comprised of 480 AAA cases and 480 controls, and were described previously.16,17
Power calculations were performed using the Genetic Power Calculator (http://pngu.mgh.harvard.edu/~purcell/gpc/). We assumed that the polymorphism and the disease locus were in a complete linkage disequilibrium and that they had the same allele frequencies, i.e., the polymorphism was the disease locus. Assuming a disease locus with an additive effect and a disease prevalence of 0.02 our initial sample (set I) of 394 cases and 419 controls had an 80% power to detect a susceptibility locus with a genotypic relative risk ≥ 1.2 at a significance level of P<0.05 for a SNP with a minor allele frequency (MAF) ≥ 0.2.
Results
The mRNA Expression of Many Complement Cascade Genes is Altered in AAA
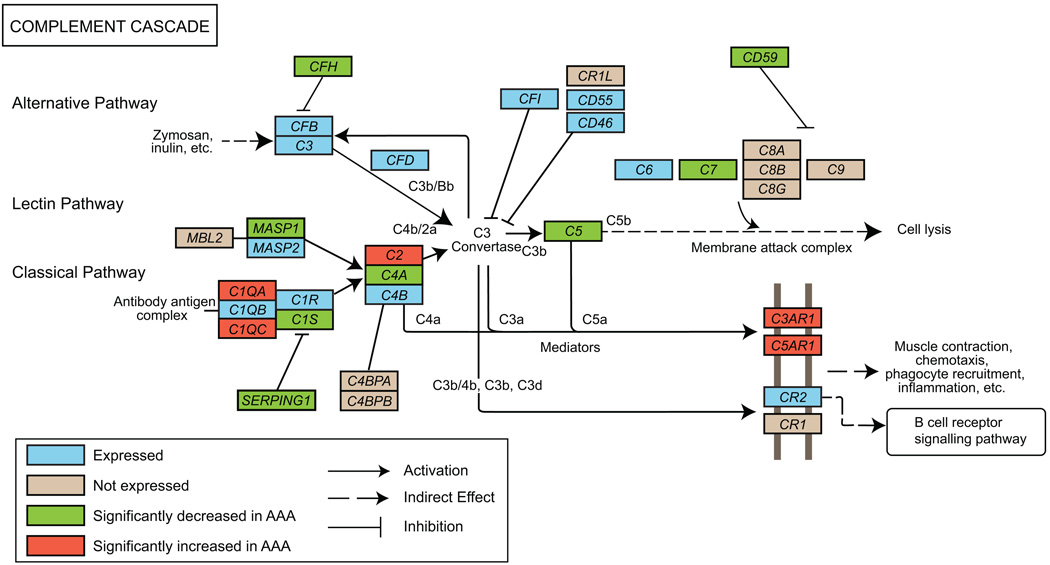
We carried out a microarray-based genome–wide expression profiling of AAA and non-aneurysmal tissue samples previously.9 The study was based on combined analysis using Affymetrix and Illumina data. The Affymetrix array was used with pooled RNA samples and the Illumina array was used with individual RNA samples. We searched the list of differentially expressed genes for those belonging to the KEGG pathway “Complement and Coagulation Cascades” (hsa04610) and found 13 (38%) of the 34 genes of the complement cascade arm of the pathway to have altered expression (Figure 1 and Supplemental Table II). As we describe in the Supplemental Methods, for the current study, we re-analyzed the microarray data using only the Illumina data obtained with individual samples to be able to carry out statistical analyses and assess sample-to-sample variation. Out of the 34 complement cascade genes (Figure 1 and Supplemental Table II) probed for with this array, 25 were considered expressed and are shown in the box-and-whiskers plot in Supplemental Figure I. Nine genes (C4BPA, C4BPB, C8A, C8B, C8G, C9, CR1, CR1L and MBL2) were scored as not expressed since the signals were not statistically different from background noise at the 99% confidence level (Illumina Beadstudio software Detection Score < 0.99). Five of the 13 differentially expressed complement cascade genes had increased and 8 had decreased expression in AAA compared to the control tissues (Figure 1, Supplemental Figure I and Supplemental Table II).
Figure 1.
Modified “Complement and coagulation cascades” (hsa04610) pathway from KEGG. Only the complement cascade part of the pathway is shown. Protein symbols were replaced by gene symbols to reflect gene-centric data. See key for explanation of colors and symbols. Supplemental Table II and Supplemental Figure I provide details on the results.
In silico analysis of the promoter regions of the 13 differentially expressed complement cascade genes using Whole Genome rVISTA showed enrichment for binding sites for a transcription factor STAT5A when compared to the entire genome (−log10 P=2.4). There were altogether 6 binding sites for STAT5A in the set of these 13 genes, whereas there are a total of 2,785 binding sites in the entire genome. One binding site for STAT5A was present in the promoters of 4 of the 13 genes: MASP1, SERPING1, C7 and C4A, and two bindings sites were present in the C1QC promoter. It is of interest that 4 (MASP1, SERPING1, C7 and C4A) of these 5 genes had decreased expression in AAA tissue and that all but one (MASP1) are part of the so-called classical pathway of the complement cascade. Based on our microarray expression data, STAT5A is expressed in both AAA and non-aneurysmal aorta with no difference in the mRNA levels (not shown).
To establish whether the in silico results reflected true binding, we carried out ChIP followed by quantitative real-time PCR for STAT5A binding on the C1QC promoter in a monocyte cell line activated by LPS and IFN-γ to mimic the inflammatory environment present in the aortic wall. Stimulation with IFN-γ was considered relevant to AAA, since mice lacking IFN-γ are resistant to AAA formation in the CaCl2 model18 and IFN-γ-producing T-cells are present in the blood and aortic wall of most AAA patients.19 STAT5A protein bound to the C1QC promoter at the predicted site with 1000-fold enrichment compared to mock ChIP with mouse IgG.
To identify all the STAT5A binding sites on the promoters of the complement cascade genes, ChIP-chip was carried out using microarrays covering 10 kbp of the promoters of all known genes. STAT5A demonstrated binding to 6,297 distinct genes (FDR < 0.05), 1,095 (17%) of which were differentially expressed in human AAA tissue (431 had increased expression and 664 had decreased expression). In this analysis we concentrated on the results from the complement cascade genes: strong evidence (FDR < 0.05) for STAT5A binding was found on 10 of the 34 complement cascade genes, and moderate evidence (0.05 < FDR < 0.2) on 9 complement cascade genes (Table 1).
Table 1.
STAT5A Binding on the Promoters of Complement Cascade Genes based on ChIP-Chip Analysis
| Significance |
||||
|---|---|---|---|---|
| Gene Symbol |
mRNA expression in AAA vs. control* |
Location of binding site† | FDR < 0.05 (strong) |
0.05 ≤ FDR < 0.2 (moderate) |
| C2 | Up | Chr. 6: 31971437 – 31971781 | 0.0287 | |
| C3AR1 | Up | Chr. 12: 8110970 – 8111501 | 0.185 | |
| C5AR1 | Up | Chr. 19: 52500443 – 52500883 | 0.0231 | |
| C4A | Down | Chr. 6: 32054233 – 32054607 | 0.0364 | |
| CD59 | Down | Chr. 11: 33701293 – 33701742 | 0.0117 | |
| C1QB | Expressed | Chr. 1: 22856663 – 22857102 | 0.0455 | |
| C1R | Expressed | Chr. 12: 7136415 – 7136844 | 0.126 | |
| C3 | Expressed | Chr. 19: 6675788 – 6676062 | 0.0352 | |
| C4B | Expressed | Chr. 6: 32054233 – 32054607 | 0.0364 | |
| C6 | Expressed | Chr. 5: 41248400 – 41248762 | 0.185 | |
| CD46 | Expressed | Chr. 1: 205989938 – 205990302 | 0.056 | |
| CD55 | Expressed | Chr. 1: 205557019 – 205558372 | 0.0012 | |
| CFB | Expressed | Chr. 6: 32020752 – 32021211 | 0.069 | |
| MASP2 | Expressed | Chr. 1: 11032689 – 11033127 | 0.104 | |
| C4BPB | Not expressed | Chr. 1: 205329267 – 205329800 | 0.069 | |
| C8A | Not expressed | Chr. 1: 57092599 – 57093857 | 0.0080 | |
| C8B | Not expressed | Chr. 1: 57203403 – 57203849 | 0.0455 | |
| CR1 | Not expressed | Chr. 1: 205733259 – 205733580 | 0.154 | |
| MBL2 | Not expressed | Chr. 10: 54200747 – 54201083 | 0.185 | |
Results based on microarray analysis.9 See Figures 1 and 2, as well as Supplemental Figure I, for details.
Refers to physical location on the given chromosome, UCSC Golden path hg18.
Immunohistochemical Staining Shows Altered Protein Expression of C2
Differences in tissue architecture were evident between AAA and control aortic tissue after hematoxylin–eosin staining of aortic wall samples (not shown). Aneurysmal aortic wall tissue had signs of ECM degradation and normal vessel architecture was destroyed. Large amount of ECM and loss of elastic fibers in the media were consistent with prior observations.20 AAA tissues also showed neovascularization in the adventitia, media and intima. Most AAA tissue samples showed an increased number of inflammatory cells in the adventitia and media. Some samples showed peripheral nerves with ganglion cells in the adventitia. Three out of 8 AAA samples had thrombus in the lumen and 1 out of 7 controls (Supplemental Table I). The intima layer was thicker in the AAA samples with higher grade of atherosclerosis than in the aortic wall of the controls.
Immunohistological staining with antibodies against CFB, C2, CFH, C3, and STAT5A was evaluated in AAA and control tissue. CFB antibody showed no staining in the control aorta or in the AAA tissue (not shown). The fact that this antibody showed positive staining with a tissue array consisting of cancer tissue samples indicated that the lack of staining in aortic wall was not due to low sensitivity of the antibody but rather due to the absence of this antigen in the aortic tissue (i.e. true negative result).
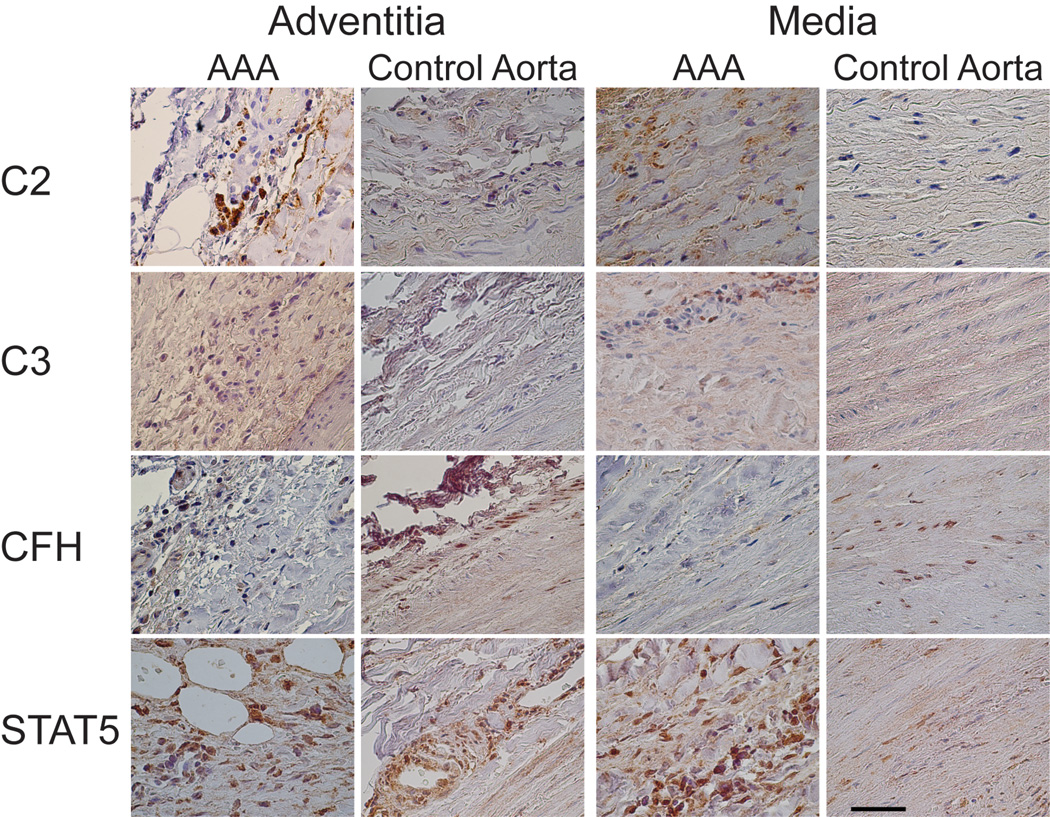
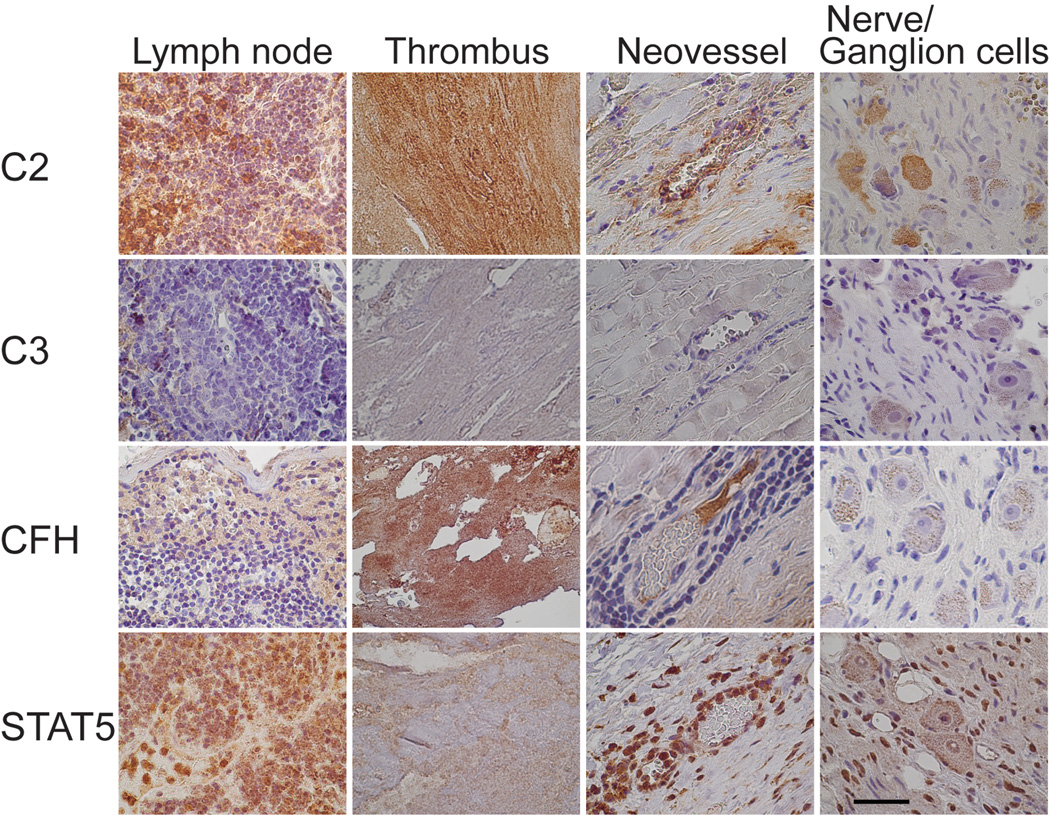
The antibody against C2 showed staining in both the AAA and control aortic tissue with a stronger staining in the AAA samples (Figures 2 and 3). The control aortic tissue had positive staining mainly around the vessels in the adventitia (Figure 2), but the media layer had only weak staining (Figure 2); in the intima layer (not shown) positive staining was seen only in regions of atherosclerotic changes. The AAA tissue showed a stronger staining for C2 in the adventitial layer (Figure 2), surrounding vessels and neovessels (Figure 3). Infiltrating neutrophils and macrophages were also C2–positive. The media layer had staining in the extracellular regions (Figure 2). The staining in the intima depended on the grade of destruction of the tissue, but was always stronger in the AAA samples than in the controls (not shown). Approximately half of the lymphocytes in lymph nodes showed cytoplasmic staining, and cytoplasm of ganglion cells in the peripheral nerve tissue were positive for C2 (Figure 3). Intraluminal thrombus was also strongly positive for C2 (Figure 3).
Figure 2.
Immunohistochemical staining for C2, C3 (α-chain), CFH, and STAT5A in the adventitia and media of AAA tissue and control aortic tissue. Scale bar = 50 µm.
Figure 3.
Immunohistochemical images of a lymph node, a thrombus, neovessels and nerve or ganglion cells in AAA tissue. Antibodies against C2, C3 (α-chain), CFH, and STAT5A were used for staining. Scale bar = 50 µm.
The antibody against CFH (Figures 2 and 3) showed positive cytoplasmic and nuclear staining in fibroblasts of the control aortic tissue but not in the AAA tissue (Figure 2). Neutrophils in adventitia of AAA tissue were positive for CFH, whereas the lymphocytes were negative (Figure 3). Ganglion cells were CFH–negative, whereas the intraluminal thrombus showed strong staining for CFH in AAA samples (Figure 3).
Antibody against the α-chain of C3b (clone H206) produced only weak staining in the adventitia of the control tissue, and in endothelial cells of the vessels, but none in the media; in the intima staining was evident only in arteriosclerotic plaque lesions. By contrast, the AAA tissue showed an intense extra- and intracellular staining in the adventitia and media, and a weak staining in the intima (not shown). Antibody against the C3 α-chain, C3a, (clone H13) showed cytoplasmic staining in infiltrating neutrophils and monocytes in AAA adventitia and media. The intensity of the staining in the media layer was similar in both the AAA and control tissues (Figure 2). In the intima, both control and AAA tissue showed only weak staining (not shown). There was no staining for this antibody in the lymph nodes, thrombus, neovessels, nerve or ganglion cells (Figure 3).
Both AAA and control samples showed staining for STAT5A in the adventitia and media layers (Figure 2). Cytoplasm of myofibroblasts was positive for STAT5A in both control and AAA tissue (Figure 2). Endothelial cells of vasa vasorum in both controls and AAA, and neovessels in AAA samples, showed strong cytoplasmic staining (Figures 2 and 3). Connective tissue in the adventitia of AAA showed STAT5A–positive adipocytes (Figure 2). STAT5A also showed peripheral staining in the ganglion cells and nuclear staining of Schwann cells (Figure 3). Thrombus stained only weakly for STAT5A in AAA samples (Figure 3).
Double-staining with antibodies against CD6815 and STAT5A demonstrated that some of the monocytes and macrophages present in the aortic wall also stained for STAT5A indicating STAT5A expression (Supplemental Figure II). These findings are consistent with results obtained with human lung tissue where macrophages showed moderately strong staining for STAT5A (The Human Protein Atlas, www.proteinatlas.org).21
The immunohistochemical findings in AAA tissues varied between the AAA samples depending on the grade of destruction of the aortic wall and the extent of inflammation. At least two samples gave the results described above. The control aortic tissues showed more consistency between samples taken from different individuals.
No Evidence of Genetic Association between Complement Cascade Polymorphisms and AAA
In the current study we also tested the hypothesis that a genetic variant in one of the complement cascade genes is associated with AAA and provides genetic susceptibility for AAA. We analyzed all the SNPs associated with AMD22–24 and additional SNPs to cover the entire pathway (Supplemental Table IV). None of the SNPs showed a deviation from HWE in the control populations. The case–control set I (394 cases and 419 controls) showed a borderline significant association for SNP rs9332739 in C2, rs4151667 in CFB, and rs1065489 in CFH. These associations could not be confirmed in the case–control set II (480 cases and 480 controls) or in the combined analyses with 874 AAA cases and 899 controls. Four SNPs (rs1143664, rs547154, rs9332739 and rs4151667) selected based on prior associations with other diseases, and one SNP (rs12146727) selected based on missense substitution, had MAFs substantially below 0.2 in our study population (Supplemental Table IV). In summary, no associations were found, but the SNPs with low MAFs would require larger sample sizes to detect or exclude associations.
Discussion
The exact underlying pathobiology of AAA remains unknown, but several characteristic features have been recognized in the aortic wall including chronic inflammation.2,3 The complement cascade is at the interface between innate and adaptive immunity by augmenting antibody responses and enhancing immunologic memory.7 It contributes to opsonization, chemotaxis and activation of leukocytes and can directly destroy bacteria and cells. Additionally, complement factors are part of the disposal of cellular waste by clearing tissue from immune complexes and apoptotic cells.7
Our results provide multiple lines of evidence that complement cascade plays an important role in AAA pathogenesis. Based on our microarray studies, the pathway is activated in AAA, particularly via the lectin and classical pathways. First, expression of C2, which is at the intersection between the lectin and classical pathways, is elevated. Second, C1QA and C1QC, which can be activated by an antibody–antigen complex in the first step of the classical pathway, also had increased mRNA expression in AAA. Third, three inhibitors of the complement cascade, CFH (acting in the alternative pathway by inhibiting CFB activation), SERPING1 (inhibitor of C1S in the classical pathway), and CD59 (inhibits the membrane attack complex), all have decreased expression in AAA. Finally the receptors C3AR and C5AR located on the cell membrane have increased expression.
Two previous studies on complement cascade carried out in mouse models also demonstrated a significant role of complement in the development of AAA. In the elastase-induced AAA model with C57BL/6 mice complete depletion of complement activity protected the mice from AAA development.25 Complement inhibition done 24 hours before elastase infusion prevented AAA formation and provided evidence that the complement cascade is important in the initiation of AAA. Furthermore, the investigators showed that mice deficient in Cfb were resistant to AAA development in the elastase-model. In addition, antagonism of C3a also blocked AAA development completely.25
In our results increased gene expression of C1QA, C1Q, and C2 as well as decreased expression of the inhibitor SERPING1 in human AAA tissue suggests that the classical pathway plays a more prominent role in human AAA. The apparent inconsistency between the previously published mouse studies and the current human study could be due to mechanistic differences in the mouse model and human AAA or due to the late–stage disease samples of human AAA tissue studied here whereas in knockout mice earlier stages of AAA development were studied.
Of particular interest to AAA is the fact that the classical pathway can be activated independently of antibodies by pentraxins, a family of several pentameric proteins that bind to various lipids.7 One of the best known pentraxins is the C-reactive protein (CRP); serum levels of CRP have been reported to be elevated in AAA patients,26 providing another potential mechanism for the increased activity of the complement cascade in AAA.
Only two studies were published previously on the complement system in human AAA. The first study showed that C3 measured by ELISA had a 125–fold increase in AAA tissue compared to the control aortic tissue. Additionally IgG1, 2 and 3 levels, which can initiate the classical pathway, were increased in AAA patients.4 The fact that C2 mRNA levels are increased in AAA may explain the elevated C3 levels without having an increased C3 mRNA expression, since C2 is converted to C2a and with C4b forms the C3 convertase (C4b2a) which activates C3.4 C3 has been linked to autoimmune diseases in that C3 activation appears to promote autoimmunity. This was observed in a mouse model of type II collagen-induced arthritis characterized by joint inflammation and destruction.27 It is possible that complement activation contributes to AAA via a similar mechanism.
The second, more recent study on complement cascade in human AAA was published while the current study was under review and investigated polymorphisms in genes of the alternative pathway in a case–control study of 434 AAA patients and 378 controls.28 No evidence for association was found, including polymorphism rs2230199 in C3, rs17611 in C5, rs13157657 in C7, and rs4151667 in CFB, which were also investigated in the current study.28
The components of the early events in complement cascade activation were not the only differentially expressed genes in our microarray study. mRNA levels of the membrane receptors C3AR1 and C5AR1 were also significantly increased in AAA tissue. These receptors interact with the complement cascade components and can induce inflammation,7 a characteristic feature of the AAA tissue. Both C3AR1 and C5AR1 are G-protein-coupled receptors of the seven-transmembrane type and are similar to chemokine receptors.7 C3AR1 is present on basophils, mast cells and SMCs.7 It is noteworthy that a previous study demonstrated mast cell invasion in AAA tissue29 and activation of the C3AR1 receptor can induce inflammation and SMC contraction. C5AR1 is expressed on inflammatory cells such as basophils, mast cells, monocytes, and neutrophils as well as on endothelial cells and SMCs.7 Activation of C5AR1 induces inflammation and chemotaxis, and increases vascular permeability.7 The invasion of neutrophils into the AAA tissue has been shown in mouse and human studies.30,31
SERPING1, CFH and CD59, three of the four naturally occurring inhibitors in complement cascade, had decreased mRNA levels in AAA tissue based on our microarray results. The importance of CD59 as an inhibitor of the final steps in forming the membrane attack complex was demonstrated in Cd59-deficient mice in the angiotensin II-induced mouse model (mCd59ab−/−Apoe−/−) of AAA which showed accelerated development of AAA.32
To elucidate the mechanisms of transcriptional control of complement cascade genes a search for transcription factor binding on the promoters was carried out in silico followed by ChIP-chip analysis. Both methods showed overrepresented binding sites or strong binding of transcription factor STAT5A on the promoters of complement cascade genes. Interestingly, the in silico analysis demonstrated 5 genes with overrepresented STAT5A binding sites, of which 4 belong to the classical pathway. ChIP-chip analysis on monocyte cell cultures showed strong binding of STAT5A on 10 of the 34 complement cascade genes and moderate binding on 9 of the 34 complement genes, consistent with involvement of STAT5A transcription factor in the regulation of these genes. STAT5A was found to be present in both AAA and non-aneurysmal abdominal aorta as mRNA and protein. Doublestaining showed that some macrophages present in the tissue were positive for STAT5A (Supplemental Figure II). The role of STAT5A in vascular diseases was also investigated in a recently published study carried out in a mouse model.33 The study used the model where angiotensin II is infused into Apoe−/− deficient mice and a dominant negative form of monocyte chemoattractant protein, named MCP1-7ND, was administered to these mice.33 Immunohistochemical staining of phosphorylated STAT5 was decreased in SMCs in the MCP1-7ND group and these animals tended to have fewer AAAs, although the results were not statistically significant.33 The authors speculated that the findings suggest that AAA development is due to decreased proliferative and migratory response, but point out that their results were not consistent with another study using a mouse model where AAAs are induced with calcium chloride.
In humans, the liver is the major source of complement proteins,34 but other tissues also express complement cascade proteins. Glial cells and neurons have been shown to express complement proteins after stimulation by inflammatory cytokines,35–37 but their expression has not been described previously in peripheral nerves. In another study, human cerebrovascular SMCs expressed mRNA of many complement cascade genes including C1QB, C1R, C1S, C2, C3 and C4 from the classical pathway.38 Our results demonstrated the presence of the complement proteins C2, CFH, C3, and transcription factor STAT5A in the cells of the aortic wall. Although CFB-mRNA was expressed in AAA tissues, CFB protein was not detectable in aortic wall tissue. An interesting finding was that ganglion cells in peripheral nerve expressed C2; this might be inflammation-induced. Production of CFH in myoblasts has been reported in a previous in vitro study on human myoblast cell lines.39 CFH protein was present in the cytoplasm of myofibroblasts of the control aortic tissue in our study, but myofibroblasts in AAA wall were negative for CFH. This could be due to downregulation of CFH mRNA in the AAA tissue or due to apoptosis of SMCs.
In conclusion, our results provide strong evidence that complement cascade plays a role in the pathophysiology of human AAA. Furthermore, the overrepresented binding sites of transcription factor STAT5A on the promoter regions of the complement cascade genes suggest coordinated regulation of their gene expression.
Supplementary Material
Acknowledgments
Sources of Funding
This project was funded in part by grants from the National Heart, Lung, and Blood Institute (HL064310 to H.K.), NIH, and the American Heart Association, Great Rivers Affiliate (D.J.C.), as well as by Geisinger Clinic and Elizabeth C. King Trust (L.A.D.). I.H. is a recipient of a Fellowship from Deutsche Forschungsgemeinschaft (Hi 1479/2-1) and J.H.L. is a recipient of a Predoctoral Fellowship (AG030900) from the National Institute on Aging, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was previously published in abstract form at the Annual Meeting of Arteriosclerosis, Thrombosis, and Vascular Biology of the American Heart Association (April 2010).
Disclosures
None.
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Boddy AM, Lenk GM, Lillvis JH, Nischan J, Kyo Y, Kuivaniemi H. Basic research studies to understand aneurysm disease. Drug News Perspect. 2008;21:142–148. [PubMed] [Google Scholar]
- 3.Kuivaniemi H, Platsoucas CD, Tilson MD., 3rd Aortic aneurysms: an immune disease with a strong genetic component. Circulation. 2008;117:242–252. doi: 10.1161/CIRCULATIONAHA.107.690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capella JF, Paik DC, Yin NX, Gervasoni JE, Tilson MD. Complement activation and subclassification of tissue immunoglobulin G in the abdominal aortic aneurysm. J Surg Res. 1996;65:31–33. doi: 10.1006/jsre.1996.0339. [DOI] [PubMed] [Google Scholar]
- 5.McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26(Suppl 1):94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Donoso LA, Vrabec T, Kuivaniemi H. The role of complement Factor H in age-related macular degeneration: a review. Surv Ophthalmol. 2010;55:227–246. doi: 10.1016/j.survophthal.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 7.DeFranco AL, Locksley RM, Robertson M. Innate Immunity. In: Freeland K, editor. Immunity: The immune response in infections and inflammatory disease. London, UK: New Science Press Ltd; 2007. [Google Scholar]
- 8.Gros P, Milder FJ, Janssen BJ. Complement driven by conformational changes. Nat Rev Immunol. 2008;8:48–58. doi: 10.1038/nri2231. [DOI] [PubMed] [Google Scholar]
- 9.Lenk GM, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genomics. 2007;8:237. doi: 10.1186/1471-2164-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, Insel PA. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci U S A. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroecksnadel S, Jenny M, Kurz K, Klein A, Ledochowski M, Uberall F, Fuchs D. LPS-induced NF-kappaB expression in THP-1Blue cells correlates with neopterin production and activity of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2010;399:642–646. doi: 10.1016/j.bbrc.2010.07.134. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 14.Tromp G, Gatalica Z, Skunca M, Berguer R, Siegel T, Kline RA, Kuivaniemi H. Elevated expression of matrix metalloproteinase-13 in abdominal aortic aneurysms. Ann Vasc Surg. 2004;18:414–420. doi: 10.1007/s10016-004-0050-5. [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Hussien H, Hanemaaijer R, Kleemann R, Verhaaren BF, van Bockel JH, Lindeman JH. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J Vasc Surg. 2010;51:1479–1487. doi: 10.1016/j.jvs.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 16.Elmore JR, Obmann MA, Kuivaniemi H, Tromp G, Gerhard GS, Franklin DP, Boddy AM, Carey DJ. Identification of a genetic variant associated with abdominal aortic aneurysms on chromosome 3p12.3 by genome wide association. J Vasc Surg. 2009;49:1525–1531. doi: 10.1016/j.jvs.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Ogata T, Gregoire L, Goddard KA, Skunca M, Tromp G, Lancaster WD, Parrado AR, Lu Q, Shibamura H, Sakalihasan N, Limet R, MacKean GL, Arthur C, Sueda T, Kuivaniemi H. Evidence for association between the HLA-DQA locus and abdominal aortic aneurysms in the Belgian population: a case control study. BMC Med Genet. 2006;7:67. doi: 10.1186/1471-2350-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 19.Duftner C, Seiler R, Klein-Weigel P, Gobel H, Goldberger C, Ihling C, Fraedrich G, Schirmer M. High prevalence of circulating CD4+CD28− T-cells in patients with small abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2005;25:1347–1352. doi: 10.1161/01.ATV.0000167520.41436.c0. [DOI] [PubMed] [Google Scholar]
- 20.Holmes DR, Liao S, Parks WC, Thompson RW. Medial neovascularization in abdominal aortic aneurysms: a histopathologic marker of aneurysmal degeneration with pathophysiologic implications. J Vasc Surg. 1995;21:761–771. doi: 10.1016/s0741-5214(05)80007-2. [DOI] [PubMed] [Google Scholar]
- 21.Berglund L, Bjorling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, Persson A, Ottosson J, Wernerus H, Nilsson P, Lundberg E, Sivertsson A, Navani S, Wester K, Kampf C, Hober S, Ponten F, Uhlen M. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7:2019–2027. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thakkinstian A, Han P, McEvoy M, Smith W, Hoh J, Magnusson K, Zhang K, Attia J. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet. 2006;15:2784–2790. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 24.Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 25.Pagano MB, Zhou HF, Ennis TL, Wu X, Lambris JD, Atkinson JP, Thompson RW, Hourcade DE, Pham CT. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation. 2009;119:1805–1813. doi: 10.1161/CIRCULATIONAHA.108.832972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badger SA, Soong CV, O'Donnell ME, Mercer C, Young IS, Hughes AE. C-reactive protein (CRP) elevation in patients with abdominal aortic aneurysm is independent of the most important CRP genetic polymorphism. J Vasc Surg. 2009;49:178–184. doi: 10.1016/j.jvs.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 27.Hietala MA, Jonsson IM, Tarkowski A, Kleinau S, Pekna M. Complement deficiency ameliorates collagen-induced arthritis in mice. J Immunol. 2002;169:454–459. doi: 10.4049/jimmunol.169.1.454. [DOI] [PubMed] [Google Scholar]
- 28.Bradley DT, Badger SA, Bown MJ, Sayers RD, Hughes AE. Coding polymorphisms in the genes of the alternative complement pathway and abdominal aortic aneurysm. Int J Immunogenet. 2011 doi: 10.1111/j.1744-313X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 29.Mäyränpää MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JR, Parikh S, Grella L, Sarfati I, Corbie G, Danna D, Wise L. Role of the neutrophil in abdominal aortic aneurysm development. Cardiovasc Surg. 1993;1:373–376. [PubMed] [Google Scholar]
- 31.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, Pham CT. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci U S A. 2007;104:2855–2860. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu G, Chen T, Shahsafaei A, Hu W, Bronson RT, Shi GP, Halperin JA, Aktas H, Qin X. Complement regulator CD59 protects against angiotensin II-induced abdominal aortic aneurysms in mice. Circulation. 2010;121:1338–1346. doi: 10.1161/CIRCULATIONAHA.108.844589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Waard V, Bot I, de Jager SC, Talib S, Egashira K, de Vries MR, Quax PH, Biessen EA, van Berkel TJ. Systemic MCP1/CCR2 blockade and leukocyte specific MCP1/CCR2 inhibition affect aortic aneurysm formation differently. Atherosclerosis. 2010;211:84–89. doi: 10.1016/j.atherosclerosis.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer's disease brain. Am J Pathol. 1999;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasque P, Fontaine M, Morgan BP. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J Immunol. 1995;154:4726–4733. [PubMed] [Google Scholar]
- 36.Levi-Strauss M, Mallat M. Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J Immunol. 1987;139:2361–2366. [PubMed] [Google Scholar]
- 37.Thomas A, Gasque P, Vaudry D, Gonzalez B, Fontaine M. Expression of a complete and functional complement system by human neuronal cells in vitro. Int Immunol. 2000;12:1015–1023. doi: 10.1093/intimm/12.7.1015. [DOI] [PubMed] [Google Scholar]
- 38.Walker DG, Dalsing-Hernandez JE, Lue LF. Human postmortem brain-derived cerebrovascular smooth muscle cells express all genes of the classical complement pathway: a potential mechanism for vascular damage in cerebral amyloid angiopathy and Alzheimer's disease. Microvasc Res. 2008;75:411–419. doi: 10.1016/j.mvr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legoedec J, Gasque P, Jeanne JF, Fontaine M. Expression of the complement alternative pathway by human myoblasts in vitro: biosynthesis of C3, factor, B, factor H and factor I. Eur J Immunol. 1995;25:3460–3466. doi: 10.1002/eji.1830251238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.