Abstract
This study was to test the hypothesis that altered IGF2 system in the placental labyrinth zone (LZ) impairs feto-placental growth in response to maternal protein restriction. Rats were fed a 20% protein diet and an isocaloric 6% protein diet (LP) from day 1 to days 14, 18, or 21 of pregnancy. The effects of diet, gender of placenta and fetus, and day of pregnancy on placental weight, fetal weight, and expression of the IGF2 axis in the placental LZ and amino acids in maternal plasma were analyzed. Growth restriction occurred in both female and male fetuses by LP, coincident with impaired LZ growth and efficiency. The expression of Igf2, Igf2P0, Igf1r, Igf2r, Insr, Igfbp1, and Igfbp2 in placental LZ were affected by diet, gender and/or day of pregnancy. Concentrations of total essential amino acids and total nonessential amino acids were reduced and increased, respectively, in maternal plasma of LP-fed rats. These results indicate that adaptation of the IGF2 system in rat LZ occurs in a sex- and time-dependent manner in response to maternal protein restriction; however, these adaptations cannot prevent the growth restriction of both male and female fetuses during late pregnancy.
Keywords: Protein Restriction, Pregnancy, Fetal Growth, IGF2, IGF2P0, IGFBP, Gene Expression, Placenta, Labyrinth Zone
2. INTRODUCTION
Insulin-like growth factor 2 (IGF2), as well as IGF1, plays a critical role in the feto-placental development and growth in mammalian species. They have metabolic, mitogenic, and differentiative actions in a wide range of fetal tissues, including the placenta. However, IGF1 and IGF2 have distinct actions on maternal adaptation to pregnancy and feto-placental growth according to hormone sources, bioavailability, and actions (1–3). Most direct evidence from knockout and overexpression of IGF axis genes support the notion that IGF2 acts as a direct regulator of feto-placental development (4–6). Fetal growth depends mostly on nutrients, including oxygen, obtained from the mother through the placenta. Not being a passive receiver, fetal demand for growth drives placental transport of nutrients partly by regulating expression of imprinted genes in both the fetus and the placenta (7, 8). Among these imprinted genes, IGF2 together with IGF2P0, a placental-specific IGF2, regulates both placental development and nutrient transport (4–6, 9–11).
In rodents, labyrinth zone (LZ), the zone near the chorionic plate, represents the main area of the placenta for maternal-fetal hemotrophic exchange (12). Therefore, LZ is of great importance in fetal survival and development. Maternal blood flows through irregularly shaped spaces, while fetal blood circulates within the fetal capillaries (12). The LZ undergoes rapid and intense angiogenesis, resulting in expanded maternal blood space and enhanced fetal capillary development during mid and late pregnancy, which may underlie the mechanisms responsible for the remarkable increase in placental efficiency in late pregnancy (12, 13). Unlike the extremely low levels of IGF2 in adult rodent circulation, IGF2 is abundantly expressed in the LZ, and its expression is gradually increased with the progress of pregnancy. IGF2P0 expression is restrictively expressed in LZ trophoblast cells. Therefore, local production of IGF2 in the placenta is critical for feto-placental development. In general, IGF2 exerts its effect by binding to IGF receptors (IGF1R and IGF2R) and insulin receptor (InsR). In the LZ, the bioavailability of IGF2 is mainly influenced by IGFBP2, one of the known 6 IGFBPs (14). Accumulating evidence supports the concept that IGF2, select amino acids [specifically L-arginine (Arg) and L-leucine (Leu)], and mammalian target of rapamycin (mTOR) signaling form a regulatory loop in early conceptus development (15–19). Mammalian target of rapamycin is a highly conserved serine/threonine protein kinase that senses and responds to changes in amino acid levels, energy, hormones and mitogens, and has a critical role in cell metabolism and growth (20–22). Thus, nutritional manipulation during the pregnancy may affect feto-placental growth through this regulatory loop.
It has been established that offspring from dams with maternal protein restriction during gestation develop hypertension and cardiovascular diseases in adulthood in a sex- and time-dependent manner with an earlier onset and more severe hypertension in males compared to females (23–29). Pregnant rats subjected to low protein diet, have been widely used in the study of metabolic programming (30). This rodent model also mimics the dietary protein insufficiency in large population due to the poverty especially in developing countries and certain ethnic groups due to varied social-economic limitations in developed countries. In addition, metabolic syndrome caused by maternal protein restriction is relevant to the gender of offspring (31–33). However, the gender-specific effects of maternal protein restriction on fetal growth and placental efficiency have received little attention. Placental Igf2 or Igf2P0 knockout also causes both impaired placental growth and fetal growth retardation (5, 6, 8). The similar outcomes of these manipulations during gestation provide an impetus for us to study interrelationships among these growth-insulting factors. We hypothesize that altered expression of the IGF2 system (IGF2, its relevant receptors, and binding proteins) in the placental LZ impairs feto-placental growth in response to maternal protein restriction. The objectives were to: (a) investigate the gender-specific feto-placental growth retardation in response to maternal protein restriction; (b) explore the alterations in expression of IGF2-signaling-related genes in the placental LZ with maternal protein restriction; and (c) assess the changes in maternal plasma amino acids in response to maternal protein restriction.
3. MATERIALS AND METHODS
3.1. Animals
All procedures were approved by the Animal Care and Use Committee at the University of Texas Medical Branch and were in accordance with those guidelines published by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Virgin female Sprague-Dawley rats (Harlan Sprague Dawley, Houston, TX, USA) weighing between 175 and 225 g (4 months old) were mated with male Sprague-Dawley rats; conception was confirmed by observation of a vaginal copulation plug or the presence of sperm in the vaginal flush. Pregnant rats were randomly divided into 2 dietary groups, housed individually, and fed a control (CT, 20% casein) or low protein (LP, 6% casein) diet until sacrificed on days 14, 18, or 21 of pregnancy (n=10/diet-day of pregnancy). The isocaloric low-protein and normal-protein diets were obtained from Harlan Teklad (Cat. TD.90016 and TD.91352, respectively; Madison, WI, USA). The composition of the diets for the 2 groups, except for the protein content, was identical as previously described (34). The animals were housed in a room with a controlled temperature and a 12-hour light-dark cycle. During 8–10 am on days 14, 18, or 21 of pregnancy, rats were anaesthetized with carbon dioxide. Maternal blood was collected by cardiac puncture into a BD vacuum tube containing K2-EDTA. Whole blood was centrifuged at 3000g for 10 min at 4°C, and the supernatant plasma was aliquoted, snap-frozen in liquid nitrogen, and stored at −80°C until analyzed. Placentas and fetuses were isolated, blotted to remove fluids and blood, and weighed immediately. The LZ and junctional zones (JZ) were dissected as described by Ain et al. (35). LZ and JZ were snap-frozen in liquid nitrogen and stored at −80°C until analyzed.
3.2. DNA extraction from fetal extraembryonic membrane and sex determination
Genomic DNA was extracted from frozen fetal membranes and tails of adult male and female rats with Qiagen DNeasy Blood & Tissue Kit (Cat. 69504; Qiagen Inc., Valencia, CA), and all procedures were performed according to the instruction manual. Sex determination was described by Kwong et al. (36). Males were determined by the presence of the Sry gene in genomic DNA with 1 microgram DNA template added in polymerase chain reactions (PCR) and females by no Sry gene amplification. The sequence of forward primers for the Sry gene was 5′-cacaagttggctcaacagaatc-3′ and reverse primer 5′-agctctactccagtcttgtccg-3′. One microgram genomic DNA from adult males and females was included as either a positive or negative control for the PCR procedure. PCR conditions were as follows: 1) 94°C for 5 min; 2) 94°C for 1 min, 54°C for 2.5 min, and 72°C for 1 min for 36 cycles; and 3) 72°C for 7 min.
3.3. Annotation of rat IGF2P0 transcript for primer design
The primers for rat Igf2P0 transcript and the sequence of rat Igf2P0 promoter region, which is distinct from the other 3 promoters, have not been reported in literature. Using the nucleotide tool published in NCBI (http://www.ncbi.nlm.nih.gov/nuccore), 2 specific pseudo-exons, u1 (8604–8826) and u2 (10682–10915) in mouse Igf2P0 were aligned to the coding sequence of rat Igf2 gene X17012.1. Two sequences localized at 9561–9777 and 11727–11947 in X17012.1 were selected which had 94% and 88% identity with mouse u1 and u2, respectively. Quantitative real-time PCR primers for rat Igf2P0 transcript were designed according to the former sequence.
3.4. RNA extraction and RT-PCR
Total RNAs from frozen LZ tissues (n=6/diet/gender/day of pregnancy) were extracted using Qiagen RNeasy minikit (Cat. 74104; Qiagen Inc., Valencia, CA). The total RNAs were digested with RNA free DNase I (Cat. 79254; Qiagen), followed by a clean-up procedure. All procedures were performed according to the instruction manual. Complementary DNA (cDNA) was synthesized from 1 microgram of total RNA by RT in a total volume of 20 microliter using MyCycler Thermal Cycler (Cat. 170-9703; Bio-Rad Laboratories, Hercules, CA) with the following conditions: 1 cycle at 28°C for 15 min, 42°C for 50 min, and 95°C for 5 min.
3.5. Quantitative real-time PCR
Real-time PCR detection was performed on a CFX96 Real-Time PCR Detection System (Cat. 184-5096; Bio-Rad). The primers were designed using Primer 3 Version 4 and are shown in Table 1. Syber Green Supermix (Cat. 170-8882; Bio-Rad) was used for amplification of Igf2, Igf2p0, Igf1r, Igf2r, Insr, Igfbp1 and Igfbp2. The mixture of reaction reagents was incubated at 95°C for 10 min and cycled according to the following parameters: 95°C for 30 seconds and 60°C for 1 min for a total of 40 cycles. Negative control without cDNA was performed to test primer specificity.
Table 1.
Quantitative real-time PCR primers
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) | GenBank accession No. | Product size (bp) |
|---|---|---|---|---|
| Igf2 | tgtctacctctcaggccgtactt | tccaggtgtcgaatttgaagaa | NM_031511.1 | 78 |
| Igf2P0 | gatcatcgtccaggcaattt | gttgcgtagttcccgaagtt | X17012.1 | 96 |
| Igf1r | aaggatggcgtcttcacca | gagtggcgatctcccagag | NM_052807.1 | 70 |
| Igf2r | ctgcaggcgggaaag | ttccactcttatccacagcac | NM_012756.1 | 67 |
| Insr | tgagtcagccagtcttcgagaa | gccatcagttccatcactacca | NM_017071.1 | 141 |
| Igfbp1 | gagcccagagatgacagagg | gcatgctgctgtaggtgcta | NM_013144.1 | 110 |
Gapdh served as an endogenous control to standardize the amount of sample RNA added to a reaction. TaqMan Gene Expression Assays for rat Gapdh (Rn01775763_g1) and supermix reagents were from Applied Biosystems (Carlsbad, CA). The mixture of reaction reagents was incubated at 50°C for 2 min, heated to 95°C for 10 min, and cycled according to the following parameters: 95°C for 30 sec and 60°C for 1 min for a total of 40 cycles. Data were expressed as the ratio of the DNA amplification amount of Gapdh against that of target genes to avoid error from the differences in tissue weight, processing, and loading. The relative amount of every sample was calculated by use of the threshold cycle (CT) Gapdh/CT target gene.
3.6. Western blotting
Rat LZs (n=6/diet/gender/day of pregnancy) were homogenized in lysis buffer (50 mM Tris, 0.1 mM EGTA, 100 mM PMSF, 1.4 microliter of beta-mercaptoethanol, and 1 mini-tablet of protease inhibitor cocktail per 10 mL) with polytran at 15,000 rpm for 10 sec. After centrifugation of the homogenate at 1000 × g for 10 min at 4°C, the supernatant fraction was aliquoted and stored at −80°C until analyzed by Western blotting. Protein concentration was determined by Pierce BCA Protein Assay Kit (Cat. 23225; Pierce Biotechnology, Rockford, IL).
Aliquots of 20 microgram protein were added with 4X nonreducing loading buffer (200mM Tris, 8% SDS, 0.005% bromophenol blue, 20% glycerol) and subjected to electrophoresis with 6% SDS-PAGE (stacking gel) and 15% SDS-PAGE (separating gel). The procedures of Western blotting described by Qiu et al. (37) were followed with minor modifications. Briefly, the separated proteins in SDS-PAGE were transferred to nitrocellulose membrane at 4°C overnight. After blocking in 5% nonfat milk, mouse anti-rat IGF2 monoclonal antibody (Cat. 05-166, clone S1F2; Millipore, Billerica, MA) at 1:1000 dilution was added to nitrocellulose membrane and incubated overnight. After washing, the blots were incubated with HRP-conjugated goat antimouse IgG (Cat. 1030-05; SouthernBiotech, Birmingham, AL) at 1:1500 dilution at room temperature for 1 h. Proteins in blots were visualized with Pierce enhanced chemiluminescence detection (Cat. 32209; Thermo Scientific, Rockford, IL) and Blue Lite Autorad Film (Cat. F9024; BioExpress, Kaysville, UT) according to the manufacturers’ recommendations. Beta-actin was used as an internal control for Western blotting in this study. Blots were incubated with stripping buffer (Cat. 46430; Thermo Scientific) at room temperature for 15 minutes, followed by blocking in 5% nonfat milk for 1 h. Primary antibody, rabbit monoclonal antibody for beta-actin (Cat. 13E5; Cell Signaling, Danvers, MA), and secondary antibody, HRP-conjugated goat anti-rabbit IgG (Cat. 4050-05; SouthernBiotech, Birmingham, AL), were used at 1:10000 and 1:5000 dilution, respectively. Visualization of proteins in blots was the same as aforementioned.
Procedures of Western blotting for rat IGFBP1 and IGFBP2 were similar to those for IGF2, except that proteins were denatured with reducing loading buffer (non-reducing buffer with 2% beta-mercaptoethanol) before being subjected to electrophoresis. Primary antibodies, mouse monoclonal antibody for IGFBP1 (Cat. sc-25257; Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit polyclonal antibody for IGFBP2 (Cat. sc-13096, Santa Cruz) and secondary antibodies, HRP-conjugated goat antimouse IgG (Cat. 1030-05; SouthernBiotech, Birmingham, AL), HRP-conjugated goat anti-rabbit IgG (Cat. 4050-05; SouthernBiotech) were used with 1:1000 and 1:5000 dilutions in 2% milk/TBST, respectively.
The signals in films representing the contents of target proteins IGF2, IGFBP1, IGFBP2 and internal control protein beta-actin were densitometrically quantified by Fluorchem 8000 software (Cell Biosciences, Santa Clara, CA). The relative amount of target protein was calculated by the ratio of total densitometrical value of target protein to that of beta-actin in each rat LZ analyzed by western blotting.
3.7. Analysis of amino acids in maternal plasma
Rat maternal plasma was deproteinized with an equal volume of 1.5 M HClO4, followed by neutralization with 2 M K2CO3 as previously described (38). Amino acids in the extract were determined by a fluorometric HPLC method involving precolumn derivatization with o-phthaldialdehyde (39).
3.8. Statistical analysis
All quantitative data were subjected to least-squares analysis of variance using the general linear models procedures of the Statistical Analysis System (SAS Institute, Cary, NC). Data on fetal-placental weight and gene expression were analyzed for effects of day of pregnancy, diet, gender of placenta, and their interaction. Data on amino acids were analyzed for effects of day of pregnancy, diet, and their interaction. A P value of 0.05 or less was considered significant, whereas a P value greater than 0.05 but less than 0.10 was considered a trend toward significance. Data in tissue weights and amino acids are presented as means with standard errors, while data in gene expression is presented as least-squares means (LSM) with overall standard errors (SE).
4. RESULTS
4.1. Gender determination of placenta and fetus
Partial Sry gene, a single band with a size of 300 bp in electrophoresis, was amplified with template genomic DNA from adult males, while there was no amplification with genomic DNA from adult females (Figure 1). The gender of placenta and fetus was determined by the presence of Sry gene. Samples with ambiguous gender determination due to the unavoidable contamination of fetal blood cells during tissue collection were excluded in the following studies.
Figure 1.
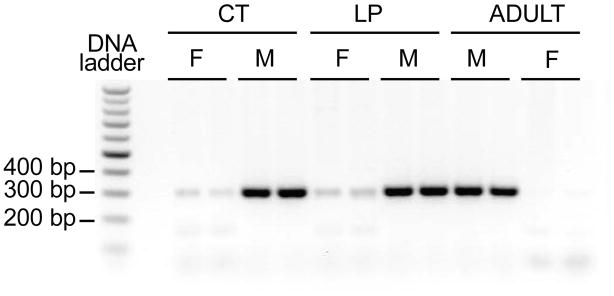
Gender determination by PCR for Sry gene. Partial Sry gene with a size of 300bp was amplified in PCR with genomic DNA from male, but not female, fetal extraembryonic membrane from mothers fed a normal diet (CT) or low protein diet (LP) at day 18 of pregnancy. Genomic DNA from tails of adult males and females were included as positive and negative controls, respectively.
4.2. Feto-placental weights affected by day of pregnancy, diet and gender of fetus and placenta
The fetal weight and placental weight including LZ and JZ, and the ratios among these tissues, were summarized in Table 2. At days 14, 18, and 21 of pregnancy, both female and male fetal weights were significantly lower in the LP group (P < 0.05). Overall, fetal weight was affected by gender, with male fetuses weighing more than females (P < 0.05).
Table 2.
Fetal-placental Weight at Days 14, 18 and 21 of pregnancy
| Day of Pregnancy | Diet | CT | LP | ||
|---|---|---|---|---|---|
| Gender | F | M | F | M | |
| 14 | Placental Weight | 142.0±11.7a | 136.2±7.1a | 99.4±8.7b | 109.8±8.5b |
| LZ Weight | 74.7±6.7a | 76.6±4.6a | 54.2±6.1b | 62.1±5.6b | |
| JZ Weight | 65.7±5.7a | 62.6±3.9a | 42.7±4.4b | 47.6±3.3b | |
| LZ: Placental Weight Ratio | 0.53±0.03 | 0.57±0.02 | 0.54±0.03 | 0.56±0.02 | |
| LZ:JZ Ratio | 1.19±0.12 | 1.35±0.11 | 1.26±0.15 | 1.31±0.08 | |
| Fetal Weight | 98.8±12.1a | 105.8±9.1a | 80.6±3.4b | 79.0±5.2b | |
| Placental efficiency | 0.74±0.06b | 0.80±0.05ab | 1.04±0.15a | 0.86±0.12b | |
| Fetal: LZ Weight Ratio | 1.49±0.05 | 1.39±0.14 | 1.59±0.20 | 1.37±0.18 | |
| 18 | Placental Weight | 333.1±8.9ab | 356.0±11.7a | 303.8±13.2b | 299.8±10.3b |
| LZ Weight | 180.6±8.3a | 205.0±5.0a | 154.7±6.7b | 164.7±8.0b | |
| JZ Weight | 151.3±5.8 | 143.7±7.0 | 145.6±11.4 | 135.15±4.85 | |
| LZ: Placental Weight Ratio | 0.54±0.02ab | 0.58±0.01a | 0.53±0.02b | 0.55±0.01ab | |
| LZ:JZ Ratio | 1.25±0.09ab | 1.40±0.07a | 1.19±0.10b | 1.22±0.06ab | |
| Fetal Weight | 930.0±44.5b | 1092.3±50.8a | 729.4±20.2c | 747.7±23.6c | |
| Placental efficiency | 2.86±0.11ab | 3.26±0.18a | 2.57±0.18b | 2.50±0.05b | |
| Fetal: LZ Weight Ratio | 5.29±0.25 | 5.47±0.30 | 4.82±0.25 | 4.56±0.11 | |
| 21 | Placental Weight | 431.7±19.1 | 401.7±13.6 | 411.0±6.8 | 396.7±11.1 |
| LZ Weight | 249.4±12.4 | 238.2±9.2 | 255.2±6.4 | 245.5±10.9 | |
| JZ Weight | 190.2±10.9a | 163.5±5.8ab | 155.8±5.5b | 151.1±8.6b | |
| LZ: Placental Weight Ratio | 0.56±0.01b | 0.59±0.01ab | 0.62±0.01a | 0.62±0.02a | |
| LZ:JZ Ratio | 1.32±0.07b | 1.47±0.05a | 1.69±0.09a | 1.68±0.14a | |
| Fetal Weight | 3444.9±122.2ab | 3624.6±68.8a | 2950.3±51.6bc | 3121.7±110.5c | |
| Placental efficiency | 8.18±0.31ab | 9.23±0.44a | 7.22±0.17c | 7.88±0.24b | |
| Fetal: LZ Weight Ratio | 14.50±0.74b | 15.89±0.91a | 11.71±0.36c | 12.77±0.27c | |
mg, Mean ± Standard Error
Mean in a row with different superscript letters within the same day of pregnancy are different (P < 0.05).
Placental weights were decreased by LP at days 14 and 18 of pregnancy, but not day 21 (Day x Diet, P = 0.0002). Similarly, LZ weights were also decreased by LP at days 14 and 18 of pregnancy, but not day 21 (Day x Diet, P < 0.0001). JZ weights were affected by day x diet (P < 0.001) with a higher value in the LP group at days 14 and 21 of pregnancy, but not day 18. The LZ:placental weight ratio was affected by gender (P < 0.05), with female-fetal placentae having a higher value, and by day x diet (P < 0.001), with a higher value in the LP group at only day 21 of pregnancy. The LZ: JZ weight ratio was also affected by gender (P < 0.05), with males having a higher value, and by day x diet (P < 0.001), with a higher value in the LPD group at only day 21 of pregnancy.
The ratio of fetal to placental weight is referred to as placental efficiency representing the ability of the placenta to transport nutrients to the fetal side. Placental efficiency was affected by day x diet (P < 0.01), with lower values in the LP group at days 18 and 21 of pregnancy. It was also affected by day x gender (P < 0.05), with higher values in male placentae at day 21 of pregnancy. Overall placental efficiency tended to be greater in male-fetal placentae (P = 0.054). Similarly, the fetal:LZ weight ratio was also affected by day x diet (P < 0.001), with lower values in the LP group at day 21 of pregnancy in both male and females.
4.3. Expression of Igf and placental-specific transcript Igf2P0 in labyrinth zone in response to maternal protein restriction
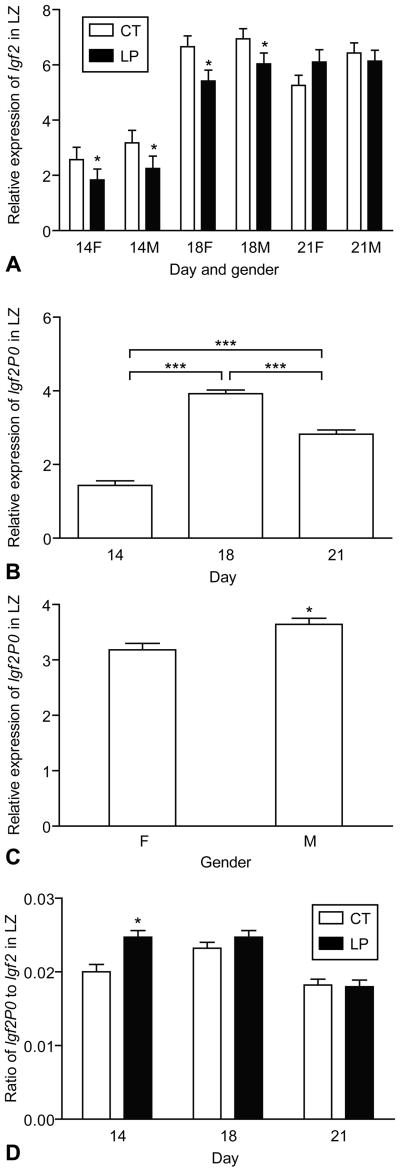
The expression of Igf2 and Igf2P0 is shown in Figure 2. The expression of Igf2 was affected by day x diet (P < 0.05) (Fig. 2A). At days 14 and 18 of pregnancy, expression of Igf2 was decreased by LP in both the male and female LZ, but no changes were observed at day 21 of pregnancy (Day x Diet, P < 0.05). The expression of Igf2 was greater in the male LZ compared to the female LZ (Gender, P < 0.05). The expression of Igf2P0 was affected by day (P < 0.0001), with the greatest value at day 18 of pregnancy, followed by days 21 and 14 (Figure 2B). Similar to IGF2 expression, the expression of Igf2P0 was greater in the male LZ compared to the female LZ (P < 0.01) (Figure 2C). The ratio of Igf2P0 to Igf2 transcripts in LZ was significantly lower at day 21 of pregnancy than that at days 14 and 18 (P < 0.0001), and was increased by LP (P < 0.05) (Figure 2D).
Figure 2.
Expression of Igf2 and placental specific transcript Igf2P0 in the rat labyrinth zone (LZ). A) The expression of Igf2 was affected by day x diet (P < 0.05), with reduced expression in both the male and female LZ at days 14 and 18 of pregnancy. The expression of Igf2 was greater in the male LZ than the female LZ (P < 0.05). B) The expression of Igf2P0 was affected by day (P < 0.0001), with the greatest value at day 18 of pregnancy followed by days 21 and 14. C) The expression of Igf2P0 was greater in the male LZ compared to the female LZ (P < 0.01). D) The ratio of Igf2P0 to Igf2 transcripts in LZ was significantly lower at day 21 of pregnancy than at days 14 and 18 (P < 0.0001) and was increased by LP (P < 0.05). *P < 0.05; ***P < 0.0001
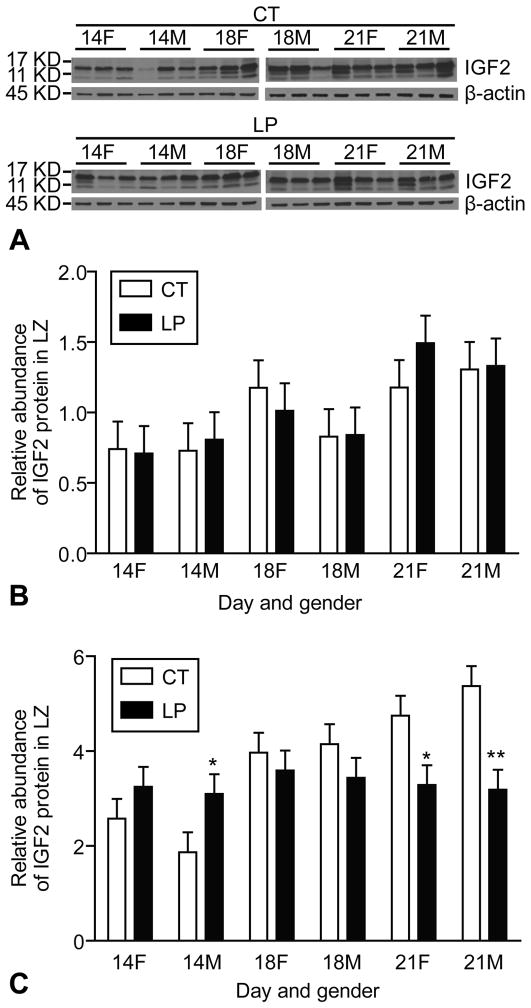
Using Western blot analysis, mature IGF2 (7KD) and big IGF2 (11–17 KD) were detectable in the rat LZ during mid and late pregnancy (Figure 3A). The abundance of mature IGF2 protein was increased in rat LZ at day 21 of pregnancy compared to days 14 and 18 of pregnancy (Day, P < 0.05), but was not affected by diet or gender of the placenta. The abundance of mature IGF2 was increased at Day 21 of pregnancy, but not affected by Diet and Gender of placenta (Figure 3B). The abundance of big IGF2 was increased by LP at day 14 of pregnancy, but decreased by LP at day 21 (Day x Diet, P < 0.001). LP decreased the relative amount of big IGF2 in both the female (Diet, P < 0.05) and male (Diet, P < 0.001) LZ at Day 21 of pregnancy. In contrast, LP increased the amount of big IGF2 in the male LZ at day 14 of pregnancy (P < 0.05) (Figure 3C).
Figure 3.
Western blotting on IGF2 in rat labyrinth zone. Mature IGF2 (7KD) and big IGF2 (11-17KD) were detectable in the rat LZ. The relative amount of mature IGF2 was increased at day 21 of pregnancy. The relative amount of big IGF2 was increased by LP at day 14 of pregnancy, but decreased by LP at day 21 (Day x Diet, P < 0.001). *P < 0.05; **P < 0.001
4.4. Expression of Igf2 receptors in labyrinth zone in response to maternal protein restriction
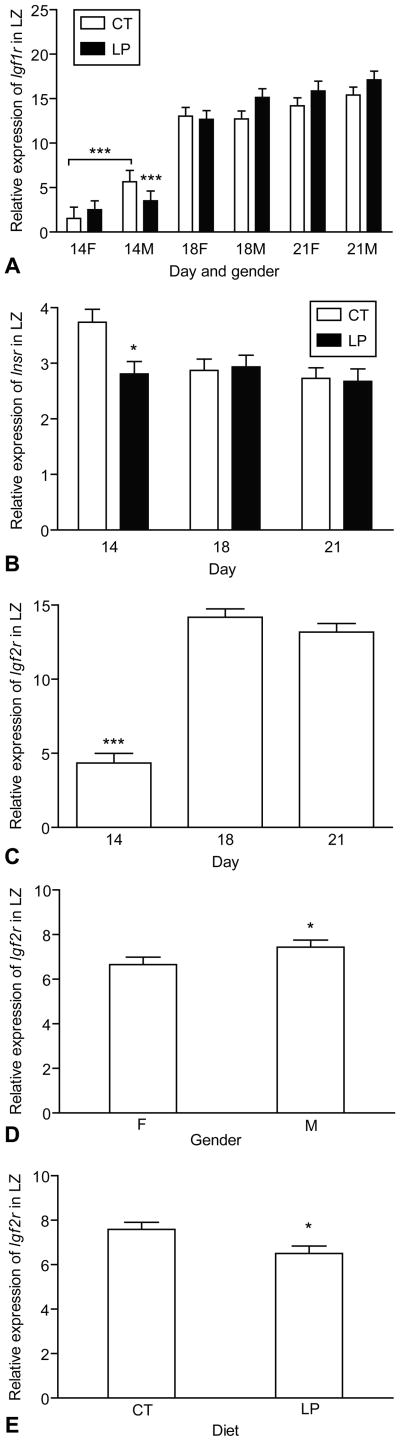
The expression of Igf1r was increased with the advancement of pregnancy (Day, P < 0.01) and was greater in the male LZ compared to the female LZ (Gender, P < 0.05) (Figure 4A). The expression of Insr in LZ was decreased at day 21 of pregnancy compared to days 14 and 18 (Day, P < 0.05) and was not altered by diet and gender of the placenta (Figure 4B). The expression of Igf2r was greater at days 18 and 21 (Day, P < 0.0001) and in the male LZ (Gender, P < 0.05), but was reduced by LP (Diet, P < 0.05) (Figure 4B–4E).
Figure 4.
Expression of Igf1r, Igf2r and Insr in the rat labyrinth zone (LZ). A) The expression of Igf1r was increased with the progress of pregnancy (Day, P < 0.01) and greater in the male LZ compared to female (P < 0.05); B) The expression of Insr in LZ was decreased at day 21 of pregnancy compared to days 14 and 18 (P < 0.05) and was not altered by diet and gender of placenta. C) The expression of Igf2r was greater at days 18 and 21 (Day, P < 0.0001). D) The expression of Igf2r was greater in the male LZ (Gender, P < 0.05). E) The expression of Igf2r was reduced by LP (Diet, P < 0.05). *P < 0.05; ***P < 0.0001
4.5. Expression of Igfbp1 and Igfbp2 in labyrinth zone in response to maternal protein restriction
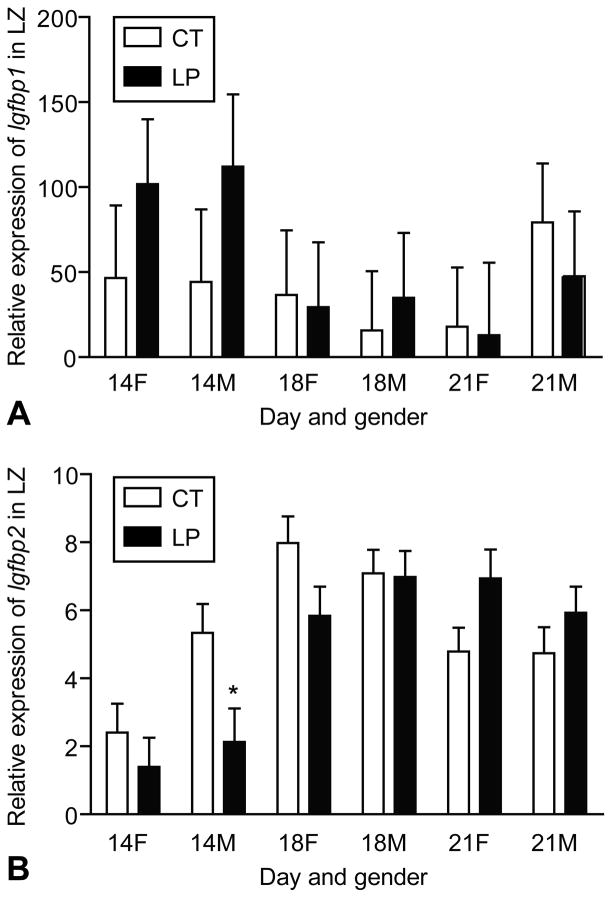
There were large variations in the expression of Igfbp1 within a given group defined by day of pregnancy, diet, and gender. At day 14 of pregnancy, the expression of Igfbp1 tended to be higher compared to days 18 and 21 (P = 0.07), mainly due to the high values in both the female and male LZ treated by LP (Figure 5A). The expression of Igfbp2 was decreased by LP in the male LZ at day 14 of pregnancy, unchanged at day 18, and increased at day 21 (Day x Diet, P < 0.01). At day 21 of pregnancy, LP tended to increase the expression of Igfbp2 in the female LZ (P = 0.06) (Figure 5B).
Figure 5.
Expression of Igfbp1 and Igfbp2 in the rat labyrinth zone (LZ). A) The expression of Igfbp1 was not affected by day of pregnancy, diet or gender due to the huge variations within the group. At day 14 of pregnancy, the expression of Igfbp1 tended to be higher compared to days 18 and 21 (P = 0.07), mainly due to the high values in both the female and male LZ treated by LP. B) The expression of Igfbp2 was decreased by LP in the male LZ at day 14 of pregnancy, not changed at day 18 of pregnancy, and increased at day 21 of pregnancy (Day x Diet, P < 0.01). At day 21 of pregnancy, LP tended to increase the expression of Igfbp2 in the female LZ (P = 0.06). *P < 0.05
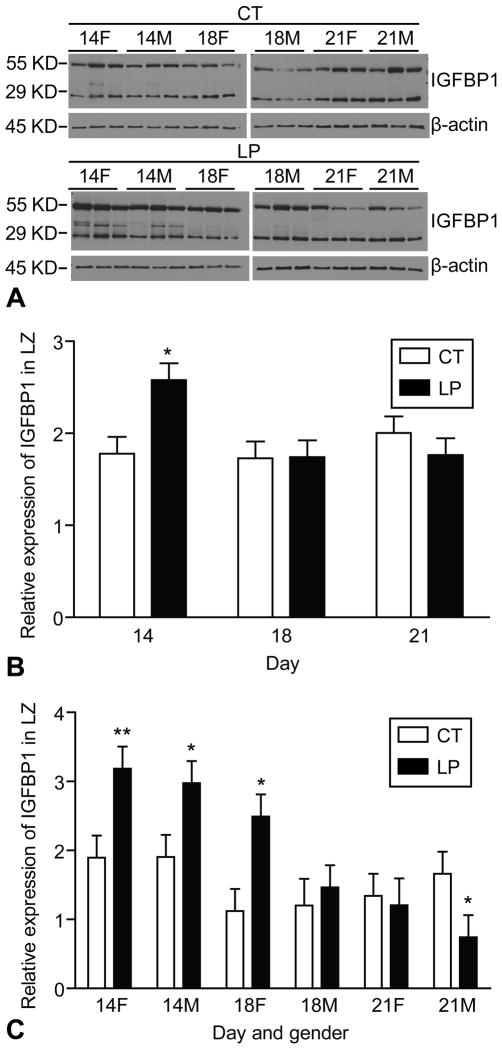
Two main IGFBP1 variants at sizes 29KD and 55KD were detectable by Western blotting (Figure 6A). At day 14 of pregnancy, the amount of 29KD protein was increased by LP (P < 0.05) (Figure 6B). Similarly, at day 14 of pregnancy, the amount of 55KD protein was increased 1.68-fold (P < 0.001) and 1.56-fold (P < 0.05) by LP in the female and male LZ, respectively. At day 18 of pregnancy, the amount of 55KD protein was increased 2.22-fold (P < 0.05) by LP in the female LZ. In contrast, at day 21 of pregnancy, the amount of 55KD protein was decreased 2.24-fold (P < 0.05) by LP in the male LZ (Figure 6C).
Figure 6.
Western blotting analysis of IGFBP1 in the rat labyrinth zone (LZ). A) Two main IGFBP1 variants in the sizes of 29KD and 55KD, respectively, were detectable by Western blotting. B) At day 14 of pregnancy, the amount of 29KD protein was increased by LP (P < 0.05). C) At day 14 of pregnancy, the amount of 55KD protein was increased 1.68-fold (P < 0.001) and 1.56-fold (P < 0.05) by LP in the female and male LZ, respectively. At day 18 of pregnancy, the amount of 55KD protein was increased 2.22-fold (P < 0.05) by LP in the female LZ. In contrast, at day 21 of pregnancy, the amount of 55KD protein was decreased 2.24-fold (P < 0.05) by LP in the male LZ. *P < 0.05; **P < 0.001.
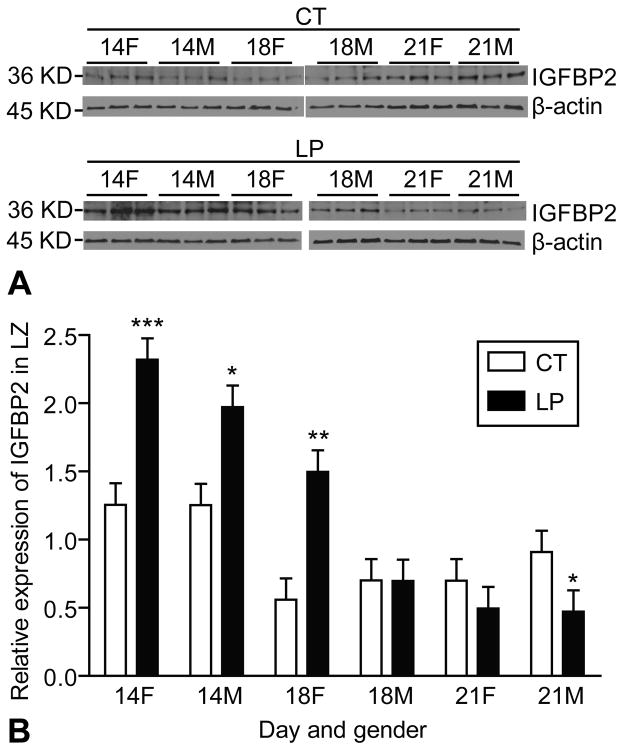
A main IGFBP2 variant at the size of 36KD was detectable by Western blotting (Figure 7A). At day 14 of pregnancy, the amount of IGFBP2 was increased 1.85-fold by LP in the female LZ (P < 0.0001) and 1.57-fold in the male LZ (P < 0.05). At day 18 of pregnancy, the amount of IGFBP2 was increased 2.72-fold (P < 0.05) by LP in the female LZ but remained unchanged in the male LZ. At day 21 of pregnancy, the amount of IGFBP2 was decreased 1.93-fold (P < 0.05) by LP in the male LZ (Figure 7B).
Figure 7.
Western blotting analysis of IGFBP2 in the rat labyrinth zone (LZ). A) A main IGFBP2 variant in a size of 36KD was detectable by Western blotting. B) At day 14 of pregnancy, the amount of IGFBP2 was increased 1.85-fold by LP in the female LZ (P < 0.0001) and 1.57-fold in the male LZ (P < 0.05). At day 18 of pregnancy, the amount of IGFBP2 was increased 2.72-fold (P < 0.05) by LP in the female LZ but not changed in the male LZ. At day 21 of pregnancy, the amount of IGFBP2 was decreased 1.93-fold (P < 0.05) by LP in the male LZ.
4.6. Analysis of amino acids in maternal plasma
The concentrations of 24 free amino acids, total essential amino acids (EAA), total nonessential amino acids (NEAA), total free amino acids in maternal plasma, as well as effects of day of pregnancy, diet and interaction of day and diet on each variable are summarized in Table 3. Total classical EAA (His, Lys, Thr, Tyr, Trp, Met, Val, Phe, Ile and Leu) in maternal plasma were reduced in the LP group at Days 14 and 18 of pregnancy (P < 0.05). In contrast, total classical NEAA (Asp, Glu, Asn, Ser, Gly, Gln, Arg, Ala, Cys and Pro) were increased in the LP group at Days 14, 18 and 21 of pregnancy (P < 0.05). The concentrations of total free amino acids listed in Table 3 were not changed by Diet at Day 14 and 18 of pregnancy, but increased in the LP group at Day 21 of pregnancy (P < 0.05).
Table 3.
Effects of day of pregnancy, diet, and interaction between day and diet on maternal plasma amino acids
| Amino Acids | Day of Pregnancy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 14 | 18 | 21 | Two-way ANOVA | ||||||
| CT | LP | CT | LP | CT | LP | Day | Diet | Day x Diet | |
| Arg | 162 ± 6.0a | 179 ± 9.3a | 174± 13.8a | 133± 7.9b | 129 ± 6.9b | 133± 4.4b | 0.0005 | 0.345 | 0.007 |
| His | 78 ± 5.3ab | 87 ± 4.1a | 65 ± 10.4b | 65± 3.5b | 61± 8.3b | 69 ± 6.3b | 0.012 | 0.314 | 0.746 |
| Orn | 25 ± 2.2abc | 32 ± 3.2a | 26 ± 2.2abc | 23± 3.0bc | 21± 1.6c | 31± 3.1ab | 0.37 | 0.032 | 0.074 |
| Lys | 355 ± 17.7a | 325 ± 18.2a | 469± 37.5a | 378± 55.9ab | 417± 47.0ab | 460± 9.6a | 0.01 | 0.32 | 0.17 |
| Asp | 20 ± 1.4bc | 20 ± 1.8bc | 20 ± 1.5bc | 25± 1.8ab | 13 ± 2.6c | 29 ± 5.8a | 0.63 | 0.003 | 0.01 |
| Glu | 185 ± 26.9 | 193 ± 12.2 | 162 ± 24.7 | 192± 22.6 | 132± 23.2 | 179 ± 26.7 | 0.37 | 0.16 | 0.69 |
| Asn | 48 ± 3.0b | 47 ± 2.1b | 60 ± 4.8ab | 44±1.6b | 66 ±13.8a | 56 ± 2.5ab | 0.079 | 0.071 | 0.39 |
| Ser | 190 ± 9.2c | 381 ± 22.0b | 217 ± 8.5c | 385± 52.8ab | 222 ± 10.8c | 473± 52.9a | 0.033 | < 0.0001 | 0.395 |
| Gly | 116 ± 11.2c | 304 ± 40.6a | 104± 10.8c | 202± 26.6b | 101 ± 17.5c | 328 ± 26.8a | 0.049 | < 0.0001 | 0.064 |
| Thr | 401 ± 49.3b | 280 ± 33.1c | 649 ± 39.8a | 150± 31.0d | 293± 15.6c | 237±24.1cd | 0.01 | < 0.0001 | < 0.0001 |
| Ala | 374 ± 21.3b | 510 ± 6.8ab | 534± 77.8ab | 585± 61.7a | 497 ± 84.0ab | 632±57.9a | 0.045 | 0.03 | 0.722 |
| B-Ala | 297 ± 42.1b | 299± 64.3b | 252± 27.6b | 250± 35.1b | 234 ± 61.4b | 469± 92.3a | 0.272 | 0.111 | 0.095 |
| Tau | 81± 7.7b | 78±14.2b | 126± 7.9a | 131± 18.2a | 70 ± 15.7b | 94±14.9ab | 0.002 | 0.398 | 0.571 |
| Cys | 192± 18.9ab | 175±14.8abc | 213 ± 25.3a | 145± 11.3bc | 205 ± 15.6a | 136± 6.8c | 0.71 | 0.001 | 0.223 |
| Cit | 75± 6.0b | 110±1.8a | 78± 11.9b | 47 ± 3.5c | 39 ± 5.4c | 43±5.1c | < 0.05 | 0.227 | 0.0001 |
| Tyr | 97± 4.5a | 74 ± 4.3a | 80± 5.7a | 78± 3.6a | 85± 6.2ab | 78 ± 3.3a | 0.283 | 0.005 | 0.069 |
| Trp | 164 ± 10.8a | 122 ± 3.9b | 126 ± 10.4b | 67 ± 6.9cd | 95± 22.7bc | 54± 8.6d | < 0.0001 | < 0.0001 | 0.68 |
| Met | 71 ± 8.5abc | 64 ± 5.2bc | 96.3 ± 18.4a | 47.4 ± 2.4c | 83±11.2ab | 50±1.2c | 0.86 | 0.001 | 0.044 |
| Val | 181 ± 6.8a | 140 ± 7.2b | 181 ± 9.1a | 99± 1.7c | 139±7.3b | 99±5.8c | < 0.0001 | < 0.0001 | 0.009 |
| Phe | 74 ± 3.4a | 64 ± 2.3b | 76± 1.4a | 63± 2.2b | 62±2.2b | 68±2.0ab | 0.21 | 0.005 | 0.004 |
| Leu | 132 ± 6.7a | 105 ± 8.0b | 136± 10.0a | 105± 5.1b | 136± 9.3a | 123± 3.9ab | 0.271 | 0.001 | 0.58 |
| Gln | 518 ± 16.9c | 593± 24.8bc | 547± 42.4c | 710± 71.5a | 502± 34.4c | 659±16.0ab | 0.121 | 0.0002 | 0.377 |
| Ile | 101± 5.1a | 80± 6.1b | 98 ±3.9a | 60 ± 4.3c | 75±3.1b | 60±3.9c | 0.0002 | < 0.0001 | 0.094 |
| Pro | 315 ± 6.1ab | 331± 14.7a | 319± 19.5ab | 273± 11.6c | 294± 6.9bc | 274±7.5c | 0.007 | 0.106 | 0.043 |
| Total | 4252±113b | 4590±179ab | 4802±203a | 4257±259ab | 3967.8±147b | 4834±234a | 0.768 | 0.153 | 0.007 |
| EAA | 1654±63b | 1340±39c | 1972±91a | 1111±95d | 1445±21c | 1297±48cd | 0.044 | < 0.0001 | < 0.0001 |
| NEAA | 2120±38b | 2732±88a | 2349±149b | 2694±123a | 2159±110b | 2900±126a | 0.343 | < 0.0001 | 0.199 |
Micromolar; Mean ± Standard Error
Among the 24 free amino acids investigated in this study, the concentrations of Ser, His, Gly, Tau, Ala, Trp, Ile, Orn, and Lys were affected by Day of pregnancy (P < 0.05). The concentrations of Ser, Gln, Gly, Ala and Orn increased in the LP group (P < 0.05), while the concentrations of Trp, Ile, Leu and Cys decreased in the LP group (P < 0.05). The concentrations of Asp, Thr, Cit, Arg, Met, Val, Phe and Pro were affected by interaction of Day and Diet (P < 0.05). In contrast, the concentrations of Glu, Asn and b-Ala were not affected by Day, Diet or their interactions.
5. DISCUSSION
This is the first study to report the effects of maternal protein restriction on the fetal-placental growth as it relates to the diet, fetus gender, and day of pregnancy. Similar to most published reports, we observed that fetal growth was restricted by protein insufficiency in the diet during gestation (40–42). Growth of both male and female fetuses, as well as placentas, was compromised by maternal protein restriction during mid pregnancy (Table 2). Sex and gender matter in development of metabolic diseases and associated complications (31–33); however, the impaired growth in utero may be a common indication and/or possibly underlying mechanism for fetal programming for both males and females. During late pregnancy, placenta, especially the LZ (high LZ:JZ ratio), grew much faster in the LP group than in the control group, and, on average, placental weight increased by 100 mg from day 18 to day 21 of pregnancy (Table 2), so that placental weight was not affected by LP at day 21 of pregnancy. The nutrient partitioning between the uterine-placental unit and fetus is unknown in rats; however, uterine-placental tissue consumes a large proportion of nutrients in sheep (43, 44). Therefore, under maternal protein restriction, the rapid placental growth in late pregnancy may compete with fetal growth for dietary nutrients. The reduced concentrations of essential amino acids including Trp, Ile and Leu (Table 3) in maternal plasma by LP, support this notion. In addition, the disproportionate growth of LZ by LP in late pregnancy was shown by the higher LZ:JZ ratio, and the LZ:placental ratio may compensate for the reduced levels of biologically active IGF2 in the LZ tissue (Figure 3B). This rapid growth in LZ by LP-fed rats may be driven by the increased amount of IGF2P0 transcripts in the overgrowing LZ, as IGF2P0 may act locally to regulate placental growth (6, 10, 11).
The expression of IGF2 is complicated, as its regulation may exist at transcriptional, post-transcriptional, translational and post-translational levels. The expression of IGF2 in LZ was reduced by an LP diet at days 14 and 18 of pregnancy, while it was not changed at day 21. In contrast, at the protein levels, the biologically active forms of IGF2, which refer to mature IGF2 and big IGF2 (37, 45), were reduced in both male and female LZ by LP (Figure 3B). In pregnant rats, pro-IGF2, which is biologically inactive, becomes active through proteolytic cleavage (37). Elevated levels of pro-IGF2 were present in sera of patients carrying intrauterine growth restriction (45). We detected the presence of pro-IGF2 in the LZ lysates by Western blotting, but we did not observe an elevation of pro-IGF2 levels in the LZ of LP-fed rats (data not shown). The biologically active forms of IGF2, proteins in the size of 7 KD and 11–17 KD (37), were decreased by LPD only at day 21 of pregnancy. This reduction in active IGF2 in LZ may be responsible, at least partly if not all, for the impaired fetal growth and reduced placental efficiency in late pregnancy. To explore the physiological function of IGF2 in rodents during pregnancy, IGF2 in maternal circulation should not be neglected because its plasma levels increase with the advancement of pregnancy (2), and activation of endoproteolytic processing of IGF2 is elevated in pregnant rats (37).
Igf2P0 transcription may contribute to adaptation to maternal protein restriction, although this strategy is apparently ineffective in promoting fetal growth. Recently, it has been reported that in a mouse model, mild maternal undernutrition reduced the expression of Igf2P0 by 35% in late pregnancy (7). In contrast, isocaloric low protein diet could not alter the Igf2P0 expression in the placental LZ, while the proportion of Igf2P0 transcript in total Igf2 transcripts was increased by LP, which is coincident with the increased proportion of the placental LZ of LP-fed rats. Therefore, placental-specific expression of Igf2P0 may be determined by energy substrates rather than protein itself in maternal nutrition. The ratio of Igf2P0 transcripts to total Igf2 transcripts in rat LZ is in a range of 1.4%–3.4% during mid and late pregnancy, which is similar to that in mice (46). The expression of Igf2P0 is higher in the male LZ compared to the female LZ during the course of pregnancy. Considering that male fetuses are heavier in mid and late pregnancy (Table 2), it may support the proposition that fetal demand promotes placental function by regulating placental gene expression (5, 8). Certainly, the expression of Igf2P0 in LZ also depends on the day of pregnancy (Figure 2B). Knockout of Igf2P0 did not alter the placental efficiency in mice in late pregnancy, but caused compromised placental growth in early pregnancy (8). All these findings indicate that IGF2P0 may play time-dependent roles during the pregnancy. IGF2P0 regulates cell differentiation in all placental layers (5); therefore, IGF2P0 may exert its effect in placental development, mainly in early and mid pregnancy when the placenta is maturing in both structure and function.
The 3 known IGF2 receptors in the placental LZ may play different roles in adaptation to maternal protein restriction. The expressions of IGF2 receptors in the rat LZ show different patterns of changes in response to maternal protein restriction during mid and late pregnancy. Expression of IGF1R increased with the progress of pregnancy, whereas expression of Insr decreased at day 21 of pregnancy, and IGF2R was affected by day of pregnancy, diet and gender of pregnancy (Figure 3). The continuous increase in IGF1R expression in late pregnancy may be responsible for the continuous growth in both placenta and fetus. IGF1R is preferred by IGF2 and IGF1 in mediating their biological functions (14), but the control of fetal and placental growth by IGFs is more complex (1). The functions of IGF receptors are additive; the null Igf1r caused reductions in fetal weight to 45% of the wild type (47). The isocaloric low protein diet does not affect IGF1R expression; therefore, fetal growth continues to increase during the course of pregnancy in LP-fed rats. The impaired fetal growth during mid-late pregnancy may result from other unknown reasons. It has been widely accepted that IGF2R regulates the bioactivity of IGF2 by binding to active IGF2, while not activating signaling transduction intracellularly (48). Unlike the expression of Igr1r and Insr in the rat LZ, LP diet reduced the expression of Igf2r in LZ and, consequently, enhanced the bioactive IGF2 in the rat LZ to overcome the reduced IGF2 levels in response to LP. Moreover, accumulating evidence in recent years indicates that IGF2R may also trigger signal transduction in promoting mitogenesis and survival of trophoblast in humans (49, 50) and guinea pigs (51). Given that the signal transduction via IGF2R is present in the rat LZ and exerts mitogenic effects on trophoblast and fetal vascular cells, the reduced Igf2r expression in the rat LZ by LP could partly explain the impaired LZ growth and consequential retarded fetal growth, while the higher levels of Igf2r in the male LZ may contribute to the male fetal development during mid and late pregnancy.
Changes in Igfbp1 and Igfbp2 mRNA levels apparently did not match those in protein levels as there were different variants resulting from post-translational modifications, including proteolysis, formation of heteropolymers, glycosylation, and phosphorylation (52, 53). For this reason, we tried to analyze the variants which are known to bind to IGF2 in the Western blotting analyses. Overall, LP tends to promote Igfbp1 expression at both mRNA and protein levels at day 14 of pregnancy. Thereafter, at day 18 of gestation, the abundance of the 55 KD IGFBP1 protein in LZ changes in a gender-specific manner, with increased IGFBP1 protein levels in the female LZ and decreased protein levels in the male LZ in response to the LP diet. This sex-related effect in IGFBP1 expression occurs when the fetus is undergoing rapid growth in late pregnancy. Therefore, elevated expression of both IGFBP1 and IGFBP2 in the female LZ reduces the availability of local IGF2 and thus results in more severe growth retardation in the female fetus. In both humans and transgenic mice, elevated IGFBP1 is associated with fetal growth restriction (54–56). In addition, the female-fetal placenta is more vulnerable to stress stimulation during pregnancy in which the expression of Igfbp1 is also elevated (57). Similarly, the 36 KD IGFBP2 protein in LZ was increased by LP in both males and females at day 14 of pregnancy and in the female LZ at day 18 of pregnancy, but decreased in the male LZ at day 21 of pregnancy. The latter may represent a positive adaptation to maternal protein restriction and increases the bioavailability of IGF2 in the male LZ, considering the great abundance of IGFBP2 in the rat placenta (14) and the fact that IGF2 has a greater affinity toward IGFBP2 than IGF1R (48). Although it is widely accepted that IGFBP would reduce IGF bioavailability, increasing evidence indicates that IGFBP has additional functions (53). For instance, IGFBP1 acts as a prosurvival factor (58), which may help prevent apoptosis in the placenta (59). This may explain why overexpressed Igfbp1 promotes placental growth in transgenic mice (54). In general, the regulation of IGFBP expression and physiological functions are complicated and must be addressed in the context of placental development during pregnancy in addition to the routine functional study in vitro.
A rat model with maternal protein restriction during gestation has been widely used in metabolic programming especially the fetal programming on adulthood hypertension. Although the elevated blood pressure in offspring was frequently reported in literature, it is noteworthy that there are differences in the percentages of protein in diets, time frame of dietary treatment and even strains of rats among the different research groups (24–27, 29, 36, 41, 42, 60, 61). These differences may be responsible for the inconsistent and even contradictory findings in the published studies. For instance, the higher Met in diet may result in the increased fetal and placental weights (25, 61). In addition, the protein insufficiency may not be the sole determinant in this model. It has been reported that the amount of food intake is variable in animals fed LP diets (24–27, 29, 36, 41, 42, 60, 61), and, therefore, the different intake of components in diets other than proteins may also play a role in this model. To compare with similar studies on fetal programming, we chose to use this widely accepted model and isocaloric low-protein and normal-protein diets. The present study reveals low protein diet and/or day of pregnancy changes the concentrations of most of amino acids in maternal plasma during pregnancy, except Glu, Asn and b-Ala, probably due to reduced catabolism in the whole body as an adaptive mechanism. Thus, it is not reasonable to narrow down the temporal window to only time point in study of the effects of nutritional manipulation on feto-placental growth (42) or to combine the physiological data such as amino acids during the pregnancy (41).
This is the first study to report that the maternal protein restriction reduces the total essential amino acids at day 14 and day 18 of pregnancy in rats, but not day 21 of pregnancy. The former may be responsible at least partly for the impaired fetal and placental growth by maternal protein restriction (Table 2), while the later may represent the maternal metabolic adaptation to meeting the demands of rapid fetal growth in late pregnancy. The reduced levels of circulating essential amino acids in response to maternal low protein diet or hypercholesterolemia were also seen in mice (62). Additionally, concentrations of EAA (including Trp, Ile, Leu and Val) in maternal plasma were reduced by LP during late pregnancy. The reduced Trp levels together with reduced Met, Thr, Leu and Ile may affect the production of serotonin in that they compete with large neutral amino acid transporters in brain and peripheral organs (63, 64), thereby affecting the maternal metabolism and feto-placental development via the versatile functions of serotonin (65–67). The reduced Leu in maternal plasma by LP may attribute to the impaired placental and fetal growth. Leu can promote the placental and fetal growth by stimulating the mTOR signaling pathway (68) and dietary Leu supplementation reverses the reduction of maternal insulin levels by LP (41, 69). The plasma Thr levels in the LP group were reduced to 69% and 23% of controls at days 14 and 18 of pregnancy, respectively. This is consistent with reports by others (70–72). The remarkable decrease of Thr is coincident with a 2- to 3-fold increase in Gly levels and a 1.8–2.1-fold increase in Ser in the LP group at days 14, 18 and 21 of pregnancy (Table 3). This may indicate that Thr can be converted into Gly in pregnant rats as reported for young pigs (73). Ser is formed from Gly by serine hydroxymethyltransferase. However, the underlying mechanism remains unknown except that Thr oxidation in LP rats does not account for the changes in Thr (71). Also, the elevation in maternal plasma Gly and/or Ser levels in LP-fed rats seems insufficient to support normal fetal growth. We propose that Gly plays an important role in regulating fetal programming of hypertension in adults because (a) Gly is considered to be limited in mammalian pregnancy due to the large demand for fetal growth (16, 74); (b) Gly participates in multiple metabolic pathways essential to whole-body homeostasis (75); and (c) dietary supplementation of Gly can reverse the hypertension in LP offspring (30).
Maternal protein restriction consistently increases the concentrations of total nonessential amino acids in maternal plasma during late pregnancy. As a result, total amino acids levels in maternal plasma in LP rats are maintained at Days 14 and 18 of pregnancy at control levels, and are higher compared to control rats at day 21 of pregnancy (Table 3). This metabolic adaptation may help to meet the large demands on amino acids for rapid fetal growth (76). Unlike the consistent increase in plasma Gly and Ser levels by LP, plasma Gln levels were increased by LP only at days 18 and 21 of pregnancy while one of its precursors, Glu was not changed by day of pregnancy and diet. The increased levels of Gln in maternal plasma by LP were coincident with the decreased levels of branch-chained amino acids, including Leu, Ile and Val which can serve as the precursors for Gln synthesis (77). Gln is the most abundant free amino acid in pregnant rats in both control and LP groups; however, the physiological significance of elevated Gln levels in maternal plasma in response to protein restriction remains unknown and warrants further investigation. In addition, plasma levels of Arg and its precursors Cit and Pro are affected by day of pregnancy and/or diet. Interestingly, the plasma levels of these three amino acids are decreased with the advancement of pregnancy, but at day 18 of pregnancy when rat fetus starts exponential growth, these amino acid levels are reduced by LP diet. Arginine, an essential amino acid for fetal-placental growth and development (68), is required for synthesis of substances, including nitric oxide (NO) and polyamines that have versatile functions (78). As a major regulator of angiogenesis (79) and utero-placental-fetal blood flows, NO determines the rate of transfer of nutrients and oxygen from mother to fetus (80). In addition, arginine regulates key metabolic pathways critical for nutrient utilization and protein deposition through FKBP12-rapamycin complex-associated protein 1 and NO signaling pathways (19, 81). Arg supplement ameliorates fetal growth retardation in rats (82) and sheep (83). More importantly, prenatal Arg supplementation reduces the blood pressure in offspring in spontaneously hypertensive rats (84). Therefore, Arg is of great importance in fetal programming on adulthood hypertension in that Arg insufficiency may play a role in the etiology of hypertension and Arg supplementation may provide a potential postnatal therapeutic remedy and/or preventative treatments during pregnancy.
In this study, the relationship between the altered placental IGF2 expression and maternal plasma amino acid levels is still unclear. It has been reported that reduced placental transport of amino acids may be responsible for fetal growth retardation in response to maternal protein restriction (41, 42). The reduced levels of placental Arg and Leu in LP rats, presumably similar to those in maternal plasma, may inhibit the mTOR signaling pathway, thereby reducing expression of placental amino acid transporters (41, 60) as well as placental IGF2. Reduced expression of the placental mTOR protein may exacerbate the reduction in expression of placental amino acid transporter (41). We propose that a positive feedback loop exists between mTOR and IGF2 involving translation of one mature transcript of IGF2 in exponentially growing cells. This loop may be upregulated by the RPS6KB1 kinase signaling pathway which is under the control of mTOR (85). If this positive feedback between mTOR and IGF2 also occurs in placenta, the link between maternal plasma amino acids and placental IGF2 expression could be easily established by the mediation of mTOR signaling pathway.
Taken together, results of the present study indicate: 1) maternal protein restriction causes growth retardation in both female and male fetuses during mid and late pregnancy, which is correlated with the impaired growth of the placenta of both male and female fetuses (particularly the LZ) and with reduced placental efficiency; 2) placental and fetal growth restriction caused by maternal protein restriction may result from reduced expression of Igf2 and elevated expression of Igfbp1 and Igfbp2 in the placental LZ in a gender-and/or gestational-day-specific manner; 3) the adaptations of the placenta to maternal protein restriction include an overgrown LZ in late pregnancy and reduced expression of Igf2r in LZ during mid and late pregnancy; 4) in LZ, higher expression of Igf2, placental-specific Igf2P0 and Igf1r during mid and late pregnancy, and reduced expression of Igfbp2 in late pregnancy favor the growth of male fetuses compared to female fetuses; 5) most of amino acids in maternal plasma are affected by maternal protein deficiency during pregnancy with reduced levels of total EAA but elevated levels of NEAA. These findings suggest that the IGF2 axis in the placenta and maternal amino acid metabolism play an important role in LPD-induced fetal programming in rats.
Acknowledgments
This research was supported by National Institutes of Health grants R01HL102866 and R01HL58144.
Abbreviations
- IGF2
insulin-like growth factor
- IGF2P0
IGF2 transcript with promoter 0
- IGFBP
insulin-like growth factor binding protein
- IGF1R
insulin-like growth factor receptor type 1
- IGF2R
insulin-like growth factor receptor type 2
- Insr
insulin receptor
- LZ
labyrinth zone
- JZ
junctional zone
References
- 1.Roberts CT, Owens JA, Sferruzzi-Perri AN. Distinct actions of insulin-like growth factors (IGFs) on placental development and fetal growth: lessons from mice and guinea pigs. Placenta. 2008;29(Suppl A):S42–S47. doi: 10.1016/j.placenta.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol. 2010;589:7–20. doi: 10.1113/jphysiol.2010.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sferruzzi-Perri AN, Owens JA, Pringle KG, Robinson JS, Roberts CT. Maternal insulin-like growth factors-I and -II act via different pathways to promote fetal growth. Endocrinology. 2006;147:3344–3355. doi: 10.1210/en.2005-1328. [DOI] [PubMed] [Google Scholar]
- 4.Coan PM, Fowden AL, Constancia M, Ferguson-Smith AC, Burton GJ, Sibley CP. Disproportional effects of Igf2 knockout on placental morphology and diffusional exchange characteristics in the mouse. J Physiol. 2008;586:5023–5032. doi: 10.1113/jphysiol.2008.157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 6.Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GJ, Fowden AL, Constancia M. Placental-specific insulin-like growth factor 2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proc Natl Acad Sci U S A. 2004;101:8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588:527–538. doi: 10.1113/jphysiol.2009.181214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl A):S98–102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm Res. 2006;65(Suppl 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- 11.Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 13.Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70:1806–1813. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- 14.Han VK, Carter AM. Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals. Placenta. 2000;21:289–305. doi: 10.1053/plac.1999.0498. [DOI] [PubMed] [Google Scholar]
- 15.Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. Select nutrients in the ovine uterine lumen. VI. Expression of FK506-binding protein 12-rapamycin complex-associated protein 1 (FRAP1) and regulators and effectors of mTORC1 and mTORC2 complexes in ovine uteri and conceptuses. Biol Reprod. 2009;81:87–100. doi: 10.1095/biolreprod.109.076257. [DOI] [PubMed] [Google Scholar]
- 16.Gao H, Wu G, Spencer TE, Johnson GA, Li X, Bazer FW. Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod. 2009;80:86–93. doi: 10.1095/biolreprod.108.071597. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Song G, Gao H, Farmer JL, Satterfield MC, Burghardt RC, Wu G, Johnson GA, Spencer TE, Bazer FW. Insulin-like growth factor II activates phosphatidylinositol 3-kinase-protooncogenic protein kinase 1 and mitogen-activated protein kinase cell Signaling pathways, and stimulates migration of ovine trophectoderm cells. Endocrinology. 2008;149:3085–3094. doi: 10.1210/en.2007-1367. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Burghardt RC, Wu G, Johnson GA, Spencer TE, Bazer FW. Select nutrients in the ovine uterine lumen. VII. Effects of arginine, leucine, glutamine, and glucose on trophectoderm cell signaling, proliferation, and migration. Biol Reprod. 2011;84:62–69. doi: 10.1095/biolreprod.110.085738. [DOI] [PubMed] [Google Scholar]
- 19.Martin PM, Sutherland AE. Exogenous amino acids regulate trophectoderm differentiation in the mouse blastocyst through an mTOR-dependent pathway. Dev Biol. 2001;240:182–193. doi: 10.1006/dbio.2001.0461. [DOI] [PubMed] [Google Scholar]
- 20.Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16:6242–6251. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao XH, Majithia A, Huang X, Kimmel AR. Growth control via TOR kinase signaling, an intracellular sensor of amino acid and energy availability, with crosstalk potential to proline metabolism. Amino Acids. 2008;35:761–770. doi: 10.1007/s00726-008-0100-3. [DOI] [PubMed] [Google Scholar]
- 23.Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol. 2005;192:952–960. doi: 10.1016/j.ajog.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 25.Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr. 1996;126:1578–1585. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- 26.McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension. 2005;46:1374–1380. doi: 10.1161/01.HYP.0000188702.96256.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- 28.Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, Gray WP, Hanson MA, Fleming TP. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol. 2008;586:2231–2244. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–74. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 30.Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond) 2002;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- 31.Kautzky-Willer A, Handisurya A. Metabolic diseases and associated complications: sex and gender matter! Eur J Clin Invest. 2009;39:631–648. doi: 10.1111/j.1365-2362.2009.02161.x. [DOI] [PubMed] [Google Scholar]
- 32.Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 35.Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. In: Soares MJ, Hunt JS, editors. Methods in Molecular Medicine, Vol. 121: Placenta and Thophoblast: Methods and Protocols. Vol. 1. Humana Press; NJ: 2005. [DOI] [PubMed] [Google Scholar]
- 36.Kwong WY, Miller DJ, Wilkins AP, Dear MS, Wright JN, Osmond C, Zhang J, Fleming TP. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol Reprod Dev. 2007;74:48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]
- 37.Qiu Q, Jiang JY, Bell M, Tsang BK, Gruslin A. Activation of endoproteolytic processing of insulin-like growth factor-II in fetal, early postnatal, and pregnant rats and persistence of circulating levels in postnatal life. Endocrinology. 2007;148:4803–4811. doi: 10.1210/en.2007-0535. [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Meininger CJ. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008;440:177–189. doi: 10.1016/S0076-6879(07)00810-5. [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Davis PK, Flynn NE, Knabe DA, Davidson JT. Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr. 1997;127:2342–2349. doi: 10.1093/jn/127.12.2342. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Twinn DS, Ozanne SE, Ekizoglou S, Doherty C, James L, Gusterson B, Hales CN. The maternal endocrine environment in the low-protein model of intra-uterine growth restriction. Br J Nutr. 2003;90:815–822. doi: 10.1079/bjn2003967. [DOI] [PubMed] [Google Scholar]
- 41.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malandro MS, Beveridge MJ, Kilberg MS, Novak DA. Effect of low-protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol. 1996;271:C295–C303. doi: 10.1152/ajpcell.1996.271.1.C295. [DOI] [PubMed] [Google Scholar]
- 43.Wallace JM, Aitken RP, Milne JS, Hay WW., Jr Nutritionally mediated placental growth restriction in the growing adolescent. consequences for the fetus. Biol Reprod. 2004;71:1055–1062. doi: 10.1095/biolreprod.104.030965. [DOI] [PubMed] [Google Scholar]
- 44.Wallace JM, Milne JS, Aitken RP. Maternal growth hormone treatment from day 35 to 80 of gestation alters nutrient partitioning in favor of uteroplacental growth in the overnourished adolescent sheep. Biol Reprod. 2004;70:1277–1285. doi: 10.1095/biolreprod.103.023853. [DOI] [PubMed] [Google Scholar]
- 45.Qiu Q, Basak A, Mbikay M, Tsang BK, Gruslin A. Role of pro-IGF-II processing by proprotein convertase 4 in human placental development. Proc Natl Acad Sci U S A. 2005;102:11047–11052. doi: 10.1073/pnas.0502357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore T, Constancia M, Zubair M, Bailleul B, Feil R, Sasaki H, Reik W. Multiple imprinted sense and antisense transcripts, differential methylation and tandem repeats in a putative imprinting control region upstream of mouse Igf2. Proc Natl Acad Sci U S A. 1997;94:12509–12514. doi: 10.1073/pnas.94.23.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 48.Forbes K, Westwood M. The IGF axis and placental function. a mini review. Horm Res. 2008;69:129–137. doi: 10.1159/000112585. [DOI] [PubMed] [Google Scholar]
- 49.Harris LK, Crocker IP, Baker PN, Aplin JD, Westwood M. IGF2 Actions on Trophoblast in Human Placenta Are Regulated by the Insulin-Like Growth Factor 2 Receptor, Which Can Function as Both a Signaling and Clearance Receptor. Biol Reprod. 2011;84:440–446. doi: 10.1095/biolreprod.110.088195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zygmunt M, McKinnon T, Herr F, Lala PK, Han VK. HCG increases trophoblast migration in vitro via the insulin-like growth factor-II/mannose-6 phosphate receptor. Mol Hum Reprod. 2005;11:261–267. doi: 10.1093/molehr/gah160. [DOI] [PubMed] [Google Scholar]
- 51.Sferruzzi-Perri AN, Owens JA, Standen P, Roberts CT. Maternal insulin-like growth factor-II promotes placental functional development via the type 2 IGF receptor in the guinea pig. Placenta. 2008;29:347–355. doi: 10.1016/j.placenta.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Clemmons DR, Busby W, Clarke JB, Parker A, Duan C, Nam TJ. Modifications of insulin-like growth factor binding proteins and their role in controlling IGF actions. Endocr J. 1998;4(Suppl):S1–S8. doi: 10.1507/endocrj.45.suppl_s1. [DOI] [PubMed] [Google Scholar]
- 53.Rechler MM, Clemmons DR. Regulatory Actions of Insulin-like Growth Factor-binding Proteins. Trends Endocrinol Metab. 1998;9:176–183. doi: 10.1016/s1043-2760(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 54.Crossey PA, Pillai CC, Miell JP. Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J Clin Invest. 2002;110:411–418. doi: 10.1172/JCI10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Struwe E, Berzl G, Schild R, Blessing H, Drexel L, Hauck B, Tzschoppe A, Weidinger M, Sachs M, Scheler C, Schleussner E, Dotsch J. Microarray analysis of placental tissue in intrauterine growth restriction. Clin Endocrinol (Oxf) 2010;72:241–247. doi: 10.1111/j.1365-2265.2009.03659.x. [DOI] [PubMed] [Google Scholar]
- 56.Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VK. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology. 2006;147:1175–1186. doi: 10.1210/en.2005-0606. [DOI] [PubMed] [Google Scholar]
- 57.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leu JI, George DL. Hepatic IGFBP1 is a prosurvival factor that binds to BAK, protects the liver from apoptosis, and antagonizes the proapoptotic actions of p53 at mitochondria. Genes Dev. 2007;21:3095–3109. doi: 10.1101/gad.1567107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta. 2009;30:411–417. doi: 10.1016/j.placenta.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Langley-Evans SC, Gardner DS, Jackson AA. Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fertil. 1996;106:307–312. doi: 10.1530/jrf.0.1060307. [DOI] [PubMed] [Google Scholar]
- 62.Bhasin KK, van NA, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009;58:559–566. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernstrom JD. Diet-induced changes in plasma amino acid pattern: effects on the brain uptake of large neutral amino acids, and on brain serotonin synthesis. J Neural Transm. 1979;Suppl:55–67. doi: 10.1007/978-3-7091-2243-3_5. [DOI] [PubMed] [Google Scholar]
- 64.Yokogoshi H, Iwata T, Ishida K, Yoshida A. Effect of amino acid supplementation to low protein diet on brain and plasma levels of tryptophan and brain 5-hydroxyindoles in rats. J Nutr. 1987;117:42–47. doi: 10.1093/jn/117.1.42. [DOI] [PubMed] [Google Scholar]
- 65.Davidson S, Prokonov D, Taler M, Maayan R, Harell D, Gil-Ad I, Weizman A. Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes. Pediatr Res. 2009;65:236–41. doi: 10.1203/PDR.0b013e318193594a. [DOI] [PubMed] [Google Scholar]
- 66.Salas SP, Giacaman A, Romero W, Downey P, Aranda E, Mezzano D, Vio CP. Pregnant rats treated with a serotonin precursor have reduced fetal weight and lower plasma volume and kallikrein levels. Hypertension. 2007;50:773–9. doi: 10.1161/HYPERTENSIONAHA.107.094540. [DOI] [PubMed] [Google Scholar]
- 67.Templeton AG, Kingdom JC, Whittle MJ, McGrath JC. Contractile responses of the human umbilical artery from pregnancies complicated by intrauterine growth retardation. Placenta. 1993;14:563–70. doi: 10.1016/s0143-4004(05)80209-7. [DOI] [PubMed] [Google Scholar]
- 68.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 69.Amaral AG, Rafacho A, hado de Oliveira CA, Batista TM, Ribeiro RA, Latorraca MQ, Boschero AC, Carneiro EM. Leucine supplementation augments insulin secretion in pancreatic islets of malnourished mice. Pancreas. 2010;39:847–855. doi: 10.1097/MPA.0b013e3181d37210. [DOI] [PubMed] [Google Scholar]
- 70.Metcoff J, Cole TJ, Luff R. Fetal growth retardation induced by dietary imbalance of threonine and dispensable amino acids, with adequate energy and protein-equivalent intakes, in pregnant rats. J Nutr. 1981;111:1411–1424. doi: 10.1093/jn/111.8.1411. [DOI] [PubMed] [Google Scholar]
- 71.Rees WD, Hay SM, Buchan V, Antipatis C, Palmer RM. The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br J Nutr. 1999;81:243–250. [PubMed] [Google Scholar]
- 72.Rees WD, Hay SM, Cruickshank M. An imbalance in the methionine content of the maternal diet reduces postnatal growth in the rat. Metabolism. 2006;55:763–770. doi: 10.1016/j.metabol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Ballevre O, Cadenhead A, Calder AG, Rees WD, Lobley GE, Fuller MF, Garlick PJ. Quantitative partition of threonine oxidation in pigs: effect of dietary threonine. Am J Physiol. 1990;259:E483–E491. doi: 10.1152/ajpendo.1990.259.4.E483. [DOI] [PubMed] [Google Scholar]
- 74.Kwon H, Spencer TE, Bazer FW, Wu G. Developmental changes of amino acids in ovine fetal fluids. Biol Reprod. 2003;68:1813–1820. doi: 10.1095/biolreprod.102.012971. [DOI] [PubMed] [Google Scholar]
- 75.Jackson AA. The glycine story. Eur J Clin Nutr. 1991;45:59–65. [PubMed] [Google Scholar]
- 76.Jackson AA. Salvage of urea-nitrogen and protein requirements. Proc Nutr Soc. 1995;54:535–547. doi: 10.1079/pns19950022. [DOI] [PubMed] [Google Scholar]
- 77.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 78.Wu G, Morris SM., Jr Arginine metabolism. nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meininger CJ, Wu G. Regulation of endothelial cell proliferation by nitric oxide. Methods Enzymol. 2002;352:280–295. doi: 10.1016/s0076-6879(02)52026-7. [DOI] [PubMed] [Google Scholar]
- 80.Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- 81.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Vosatka RJ, Hassoun PM, Harvey-Wilkes KB. Dietary L-arginine prevents fetal growth restriction in rats. Am J Obstet Gynecol. 1998;178:242–246. doi: 10.1016/s0002-9378(98)80007-0. [DOI] [PubMed] [Google Scholar]
- 83.Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J Nutr. 2010;140:1242–1248. doi: 10.3945/jn.110.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Queiroz DB, Ramos-Alves FE, Fernandes RL, Zuzu CP, Duarte GP, Xavier FE. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure but not ameliorate the altered vascular function in spontaneously hypertensive rats. J Physiol Biochem. 2010;66:301–309. doi: 10.1007/s13105-010-0036-4. [DOI] [PubMed] [Google Scholar]
- 85.Nielsen FC, Ostergaard L, Nielsen J, Christiansen J. Growth-dependent translation of IGF-II mRNA by a rapamycin-sensitive pathway. Nature. 1995;377:358–362. doi: 10.1038/377358a0. [DOI] [PubMed] [Google Scholar]