Abstract
Oral contraceptive (OC) use is associated with increased intra-renal renin-angiotensin-aldosterone system (RAA System) activity and risk of nephropathy, though the contribution of progestins contained in the OC in the regulation of angiotensin-dependent control of the renal circulation has not been elucidated. Eighteen OC users (8 non-diabetic, 10 Type 1 diabetic) were studied in high salt balance, a state of maximal RAA System suppression. Progestational and androgenic activity of the progestin in each OC was standardized to that of the reference progestin norethindrone. Renal plasma flow (RPF) was measured by paraaminohippurate clearance at baseline and in response to angiotensin converting enzyme (ACE)-inhibition. There was a positive correlation between OC progestational activity and the RPF response to ACE-inhibition (r=0.52, p=0.03). Similar results were noted with OC androgenic activity (r=0.54, p=0.02). On subgroup analysis, only non-diabetic subjects showed an association between progestational activity and angiotensin-dependent control of the renal circulation (r=0.71, p=0.05 non-diabetic; r=0.14, p=0.7 diabetic; p=0.07 between groups). Similar results were noted with respect to androgenic activity (r=0.88, p=0.005 non-diabetic; r=−0.33, p=0.3 diabetic; p=0.002 between groups). Our results suggest that the OC progestin component is a significant influence on the degree of angiotensin-dependent control of the renal circulation, though these findings may not apply to women with diabetes.
Keywords: Progestational activity, Androgenic activity, Renal plasma flow, Female Oral contraceptive
Introduction
Oral contraceptive (OC) use has been associated with increased intrarenal rennin-angiotensin-aldoesterone system (RAA System) activity1–3 and risk of nephropathy,4–6 though the relative individual contributions of the progestin and estrogen components in the OC have not been elucidated. While most OCs contain ethinyl estradiol as the synthetic estrogen component, they contain different progestins which vary in terms of their chemical composition, potency and formulary dose. As a result, synthetic progestins have varying levels of progestational and androgenic activity,7 and these sex hormones have each been shown to affect RAA System activity.8–10
The importance of the type of progestin contained in hormonal preparations has been previously highlighted.11, 12 Studies have suggested a link between adverse cardiac,13 vascular,14 and thrombotic15 events depending on the type of progestin exposure, and that the type of progestin in a hormonal preparation may play a role in attenuating the vascular effects of estrogen.11, 16 A recent study suggests that OCs containing some newer generation progestins are associated with worsening blood pressure, urinary albumin excretion and renal function;17 though the exact mechanism remains unclear, animal studies have shown an inverse association between exogenous progestin administration and the pressor response to Ang II,18, 19 suggesting a relationship between progestins and activity of the RAA System. While it is clear that ingestion of OCs is associated with an altered state of intrarenal RAA System activity in both non-diabetic2, 3, 6 and diabetic6 women, the influence of the OC progestin component on the degree of angiotensin-dependent control of the renal circulation in humans is not known. Furthermore, as we have previously shown that OC use further enhances the RAA System activation that occurs in diabetes,6 we sought to determine whether the progestin component in the OC influences the response to ACE-inhibition in not only healthy subjects, but also in women with diabetes.
Given the link between progestins and altered RAA System activity, and the strong association between increased renal RAA System activity and OC use, we hypothesized that the level of progestational and androgenic activity of the progestin in the OC was a determinant of the degree of angiotensin-dependent control of the renal circulation in humans. Thus, we examined renal hemodynamic function, both at baseline and in response to the angiotensin converting enzyme (ACE)-inhibitor captopril in 18 women stratified according to diabetic status, as a function of OC progestational and androgenic activity.
Materials and Methods
Subjects
Eighteen female OC users (8 non-diabetic, 10 Type 1 diabetes) participated in the study. Subjects completed an initial medical history, physical examination, electrocardiogram, and laboratory screening. No subject was taking medication other than OCs and insulin. Subjects were questioned as to the specific OC they were ingesting, from which the dosage of ethinyl estradiol and the type and amount of progestin was derived. If the OC was a multiphasic formulation, an average daily concentration was derived unless the exact dosage at the time of the study was known. The progestational and androgenic activity of each progestin was expressed as receptor binding affinity (RBA) and standardized to the activity of the progestin norethindrone, as has been done previously in human studies.20 All subjects gave written informed consent. The study protocol was approved by the local Institutional Review Board.
Protocol
Subjects consumed >200mmol sodium/day for 4 days before the study. Sodium, creatinine, and protein excretion were measured from a 24h urine collection; no data were excluded because of dietary noncompliance. Subjects were studied in the supine position after an 8h fast. At 8:00 A.M., an intravenous catheter was placed in each arm(for infusion and blood sampling). Fasting plasma glucose concentrations were measured at the start of the study. Diabetic subjects received intravenous insulin at 0.015units/kg/h, titrated to maintain blood glucose between 80–150mg/dl (4.4–8.3mmol/L). Blood pressure (BP) was recorded every 15min by an automatic recording device (Dinamap; Critikon, Tampa, FL). A loading dose of 8mg/kg of para-aminohippurate (PAH), followed by a constant infusion of PAH at 12mg/min was given for 90min to establish baseline renal hemodynamic measurements, followed by 25mg captopril taken orally. PAH clearance and plasma renin activity were measured at baseline and up to 240min after captopril ingestion.
Analytical methods
Renal clearance was assessed with PAH (Clinalfa, Läufelfingen, Switzerland) as previously described.21 Serum PAH was measured by an autoanalyzer. Plasma renin activity (PRA) was assayed by radioimmunoassay.21
Statistical analysis
The primary analysis tested associations between OC progestational and androgenic activity and change in RPF in response to ACE-inhibition. Study subject baseline and response to captopril measures were compared using nonparametric methods. The chi-square test was used to compare frequencies. Renal vascular resistance (RVR) was calculated using the equation RVR = [(mean arterial BP–12) ×723 / RPF]. Statistical analyses were performed using Stata (version 9.0; Stata, College Station, TX) with two-tailed significance levels of 0.05.
Results
Baseline characteristics
All subjects were normotensive and none were obese (Table 1). There were no significant differences in baseline characteristics between non-diabetic and diabetic subjects. The OCs ingested by all subjects contained progestins structurally related to testosterone. No subjects were ingesting OCs containing progesterone-derived progestins (Table 2).
Table 1.
Baseline Characteristics*
| All Subjects | Non-Diabetic | Diabetic | |
|---|---|---|---|
| (n=18) | (n=8) | (n=10) | |
| Age (years) | 23±4 | 23±3 | 23±5 |
| Smoker n (%) | 3 (17) | 1 (13) | 2 (20) |
| BMI (kg/m2) | 25±4 | 25±6 | 25±3 |
| MAP (mmHg)† | 83±6 | 84±5 | 83±6 |
| Duration of Diabetes (years) | - | - | 9.8±4.1 |
| Hemoglobin A1c (%) | - | - | 7.6±1.7 |
| Ethinyl estradiol (µg/tablet) | 32±5 | 33±3 | 31±6 |
| Progestin (mg/tablet) | 0.43±0.42 | 0.54±0.49 | 0.34±0.35 |
| Progestational Activity (RBA) | 0.71±0.52 | 0.79±0.59 | 0.64±0.48 |
| Androgenic Activity (RBA) | 0.75±0.56 | 0.75±0.67 | 0.74±0.48 |
| PRA (ng Ang I/mL/h) | 0.5±0.45 | 0.5±0.36 | 0.6±0.53 |
| Fasting Serum Glucose (mg/dL) (mmol/L) | 122±67 (6.8±3.7) | 80±5 (4.4±0.3) | 151±78 (8.4±4.3) |
| Urine Sodium (mmol/24h) | 276±89 | 282±83 | 272±98 |
| RPF (ml/min/1.73m2)† | 614±73 | 646±81 | 588±57 |
| RVR (mmHg/mL/min/1.73m2) | 85±11 | 81±11 | 88±10 |
Abbreviations: BMI, body mass index; MAP, mean arterial pressure; PRA, plasma rennin activity; Ang I, angiotensin I; RPF, renal plasma flow
Data are expressed as mean ± standard deviation unless otherwise indicated
Median of readings at t = −10 min, −5 min and 0
Table 2.
Progestin component of oral contraceptive, by subject
| Non-Diabetic Subjects | Diabetic Subjects | ||
|---|---|---|---|
| Progestin Type | Dose | Progestin Type | Dose |
| Desogestrel* | 0.15 mg | Desogestrel* | 0.15 mg |
| Norethindrone* | 0.75 mg | Ethynodiol diacetate* | 1 mg |
| Norethindrone* | 1.5 mg | Levonorgestrel* | 0.1 mg |
| Norethindrone acetate* | 1 mg | Levonorgestrel* | 0.1 mg |
| Norgestimate* | 0.215 mg | Norethindrone* | 1 mg |
| Norgestimate* | 0.215 mg | Norgestimate* | 0.18 mg |
| Norgestimate* | 0.25 mg | Norgestimate* | 0.215 mg |
| Norgestimate* | 0.25 mg | Norgestimate* | 0.215 mg |
| Norgestimate* | 0.215 mg | ||
| Norgestimate* | 0.25 mg | ||
Structurally related to testosterone
Renal and System ic hemodynamic responses to captopril
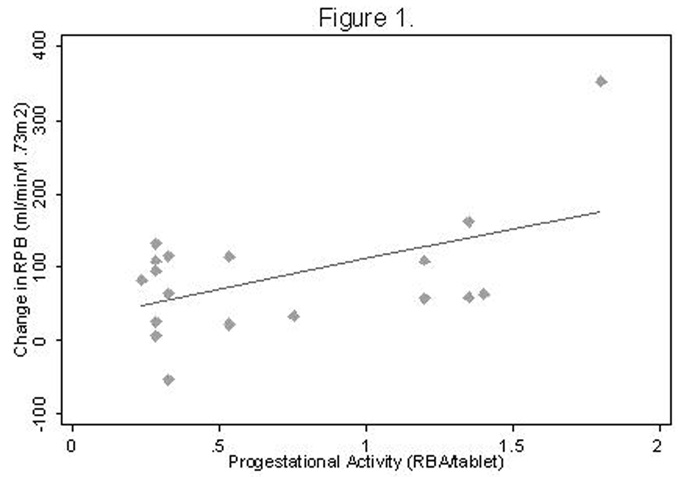
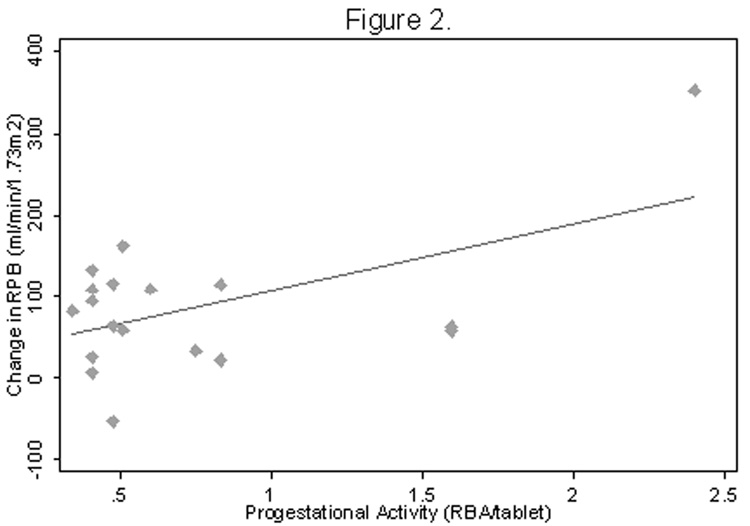
As anticipated, all subjects demonstrated a renal vasodilatory response to ACE-inhibition (p=0.0005), which did not differ by diabetic status (p=0.6)(Table 3). While no association was observed between the dose of ethinyl estradiol and the RPF response to captopril (r=−0.14, p=0.6), there was a positive correlation between increasing OC progestational activity and the RPF response to ACE-inhibition (r=0.52, p=0.03)(Figure 1). Similar results were noted with increasing OC androgenic activity (r=0.54, p=0.02)(Figure 2). A multiplicative effect of increased progestational and androgenic activity on the RPF response to ACE-inhibition was observed (p=0.01). On sub-group analysis, only non-diabetic subjects showed an association between increasing progestational activity of the progestin component of the OC and angiotensin-dependent control of the renal circulation (r=0.71, p=0.05 for non-diabetic subjects; r=0.14, p=0.7 for diabetic subjects; p=0.07 for difference between groups). Similar results were noted with respect to androgenic activity of the OC progestin component (r=0.88, p=0.005 for non-diabetic subjects, r=−0.33, p=0.3 for diabetic subjects; p=0.002 for difference between groups) (Figure 3).
Table 3.
Response to angiotensin-converting enzyme inhibition*
| All Subjects | Non-Diabetic | Diabetic | |
|---|---|---|---|
| (n=18) | (n=8) | (n=10) | |
| MAP Nadir (mmHg)† | 78±7§ | 81±6§ | 76±7§ |
| ΔMAP (mmHg) | −5±7 | −3±5 | −7±8 |
| Peak PRA (ng Ang I/mL/h) | 1.9±2.4§ | 2.6±3.4§ | 1.5±1.3§ |
| ΔPRA (ng Ang I/mL/h) | 1.4±2.2 | 2.1±3.1 | 0.9±1.0 |
| Peak RPF (ml/min/1.73m2)‡ | 699±123§ | 733±152§ | 672±94§ |
| ΔRPF (ml/min/1.73m2) | 85±85 | 87±122 | 84±44 |
| Nadir RVR (mmHg/mL/min/1.73m2) | 70±11§ | 70±16§ | 69±11§ |
| ΔRVR (mmHg/mL/min/1.73m2) | −15±9 | −11±9 | −18±7 |
Abbreviations: MAP, mean arterial pressure; PRA, plasma renin activity; Ang I, angiotensin I; RPF, renal plasma flow.
Data are expressed as mean ± standard deviation unless otherwise indicated
Mean of 2 readings at nadir of response to captopril
Mean of 2 readings at peak response to captopril
p<0.05 compared to baseline
Figure 1. RPF response to ACE-inhibition as a function of progestational activity.
R = 0.52, P = 0.03, N = 18
Abbreviations: RPF, renal plasma flow; ACE, angiotensin converting enzyme; RBA, receptor binding affinity
Figure 2. RPF response to ACE-inhibition as a function of androgenic activity.
R = 0.54, P = 0.02, N = 18
Abbreviations: RPF, renal plasma flow; ACE, angiotensin converting enzyme; RBA, receptor binding affinity
Figure 3. Renal plasma flow response to ACE-inhibition as a function of progestational and androgenic activity of the OC progestin, by diabetic status.
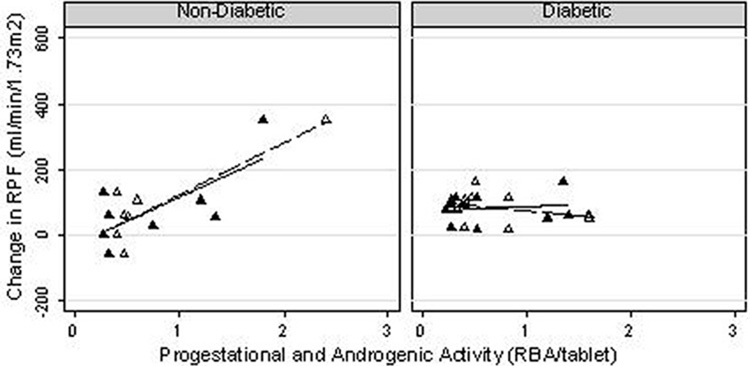
▲ Progestational Activity
△ Androgenic Activity
— Progestational Activity
--- Androgenic Activity
Non-Diabetics:
Progestational Activity: R=0.71, p=0.05; Androgenic Activity: R=0.88, p=0.005
Diabetics:
Progestational Activity: R=0.14, p=0.7; Androgenic Activity: R= −0.33, p=0.3
Mean arterial pressure decreased in response to ACE-inhibition in all subjects, (p=0.004), and the decrease in MAP was similar between non-diabetic and diabetic subjects (p=0.2). Neither OC progestational nor androgenic activity influenced the MAP response to captopril (p=0.5 for OC progestational activity, p=0.4 for OC androgenic activity). There was no correlation between the change in MAP and the change in RPF in response to ACE-inhibition (p=0.1).
Renal vascular resistance (RVR) decreased significantly in all subjects with ACE-inhibition (p=0.003 for all subjects, p=0.03 for non-diabetic subjects, p=0.005 for diabetic subjects). A negative correlation was noted between change in RVR and both progestational activity (r=−0.49, p=0.04 all subjects; r=−0.58, p=0.1 non-diabetic subjects; r=−0.67, p=0.03 diabetic subjects), and androgenic activity (r=−0.49, p=0.04 all subjects; r=−0.53, p=0.2 non-diabetic subjects; r=−0.57, p=0.08 diabetic subjects). The enhanced renovascular response to captopril in OC users with higher progestational or androgenic activity was not reflected in the PRA, thus suggesting a difference in local tissue RAA System activity, such as in the kidney.
Discussion
In this study of 18 women ingesting OCs while in high salt balance, a state of maximal RAA System suppression, a positive correlation between increasing OC progestational activity and RPF response to ACE-inhibition was observed. Similarly, greater OC androgenic activity was also positively associated with the RPF response to captopril, suggesting that increasing progestational and androgenic activity in the OC is associated with enhanced angiotensin-dependent control of the renal circulation, and thus a potential increase in the risk of nephropathy.
Many factors are associated with RAA System activation. Diabetes22, 23 and acute hyperglycemia are both linked to an activated renal RAA System in healthy24, 25 and diabetic subjects,26 though all of our subjects were euglycemic. Nephron loss ultimately results in increased Ang II production,27 but all subjects had baseline renal hemodynamics in the normal range. A positive correlation between body mass index and the renal response to ACE-inhibition has been shown,28 but all subjects were non-obese. While smoking is hypothesized to lead to increased Ang II production,29 premenopausal women appear to be least affected by the adverse renal effects of smoking.29
Progestin composition and thereby its inherent progestational and androgenic activity vary widely amongst the different OC formulations and thus it may not be possible to completely separate the progestational and androgenic effects of a particular progestin. Furthermore, the evaluation of progestational and androgenic activity of the OC progestin in humans is challenging, but can include measuring the progestin affinity for and binding to the progesterone (PR) and androgen receptor (AR), its effect on sex hormone binding globulin and free testosterone levels, and its degree of binding to sex hormone binding globulin.30–33 However, all progestins bind to the progestin receptor34 and exert the majority of their biological effects through their interactions with specific PRs.35 Testosterone-derived progestins also bind to the AR.34 Both PRs36 and ARs37, 38 have been identified in the kidney, suggesting that it is a target organ for these sex hormones and evaluation of RBA is a suitable method of determining the progestational and androgenic activity of the OC progestin. While this is the first study using progestational and androgenic RBA as a means of evaluating the effects of the OC progestin on renal hemodynamics, other investigators have used a similar method in human studies.20
Animal studies support a positive relationship between progesterone levels and RAA System activity,18, 19, 39, 40 though few studies exist in humans. In postmenopausal women, addition of progesterone to estrogen therapy was associated with an increase in circulating components of the RAA System.41 In the low-salt state, increased endogenous serum progesterone concentrations in hypertensive, postmenopausal women are associated with a blunting of the BP and RVR response to infused Ang II, though these effects were abolished in high-salt balance.10 It is thus conceivable that the lack of association between progestational activity and change in PRA in our study is due to the fact that all of our subjects were in the high-salt state. However, caution must be used in directly applying these findings regarding endogenous progesterone to synthetic progestin as there are clear structural and functional differences between the two compounds.42
Androgens also stimulate synthesis and activity of the RAA System.43–45 In rats, PRA is positively correlated to testosterone levels8, 46, 47 and androgen levels predict angiotensin-dependent control of arterial BP.48 Men demonstrate higher PRA levels compared to women,49, 50 and a sexual dimorphism has been noted in renal RAS activity.51, 52 Androgens play a crucial role in mediating renal injury and hypertension not only in men, but also in women in a physiological state of high androgen levels, such as menopause.53 We are unaware of previous studies looking at the androgenic content of OCs and its effects at the level of the kidney, and one could hypothesize from the results of our study that increased RAA System activity associated with chronic exposure to high androgenic activity from the OC may be detrimental to renal function.
This study has limitations. First, we did not show an association between ethinyl estradiol content and activation of the RAA System in our study as has been previously shown, 2, 3, 6, 54, 55 which may be a reflection of lack of power due to limited sample size and narrow range of ethinyl estradiol in the studied OC formulations. Second, the study sample was not from a target patient population in that participants were selected for normal BP, renal function, and urinalysis. By studying a homogeneous sample with stable ethinyl estradiol exposure, we hoped to examine the impact of the progestin component of the OC, without confounding factors. Third, while we included subjects with Type 1 diabetes, we controlled glycemic status throughout the study and exclusion of diabetic subjects did not alter the results. Previous studies have suggested that OC use has similar renal effects in both non-diabetic and diabetic OC users.3, 4, 6, 17 Fourth, we have used RPF response to captopril as a surrogate marker of renal RAA System activity in our study. While indirect, this approach is one of the few methods available for determining intrarenal RAA System in humans. The RPF response to ACE inhibition is highly correlated with angiotensin receptor blockade in both healthy56 and diabetic57 subjects. The lack of correlation between changes in BP or PRA and the RPF in response to captopril makes it extremely probable that the RPF response to ACE-inhibition reflects reversal of an increase in Ang II–mediated renal vascular tone. Fifth, while there are limited data on the pharmacokinetics of most progestins,58 considerably more is known about testosterone-derived progestins (as used by all study subjects) with the majority of data obtained from studies in which the progestin was a component of an OC formulation.58 Finally, while similar to that of other human studies examining the hemodynamic effects of the OC2, 3, 59–61, our sample size was limited, though this effect was minimized by ensuring all subjects were ingesting similar amounts of salt to minimize RAA System activation.
In our study, women ingesting OCs with higher progestational and androgenic activity demonstrated increased angiotensin-dependent control of the renal circulation, suggesting a role for OC progestins as a determinant of renal RAA System activity. While larger, confirmatory studies are needed prior to recommending changes to clinical practice, the findings of this study merit attention given the strong association between RAA System activity and nephropathy.
Table 4.
Summary Table
| What is known about topic | • | Oral contraceptive use is associated with increased intrarenal RAS activity and risk of nephropathy |
| • | Progestins in the oral contraceptive have been associated with increased blood pressure, albuminuria and loss of kidney function by an unclear mechanism | |
| • | The role of progestins in the oral contraceptive in activation of the RAA System is unknown | |
| What this study adds | • | Progestational activity of the oral contraceptive predicts degree of intrarenal RAA System activity |
| • | Androgenic activity of the oral contraceptive predicts degree of intrarenal RAA System activity | |
| • | Given the strong association between RAA System activity and kidney disease, women at risk of nephropathy may wish to consider oral contraceptives with low progestational and androgenic activity | |
Acknowledgements
Dr. Ahmed is supported by the Kidney Foundation of Canada. This work was supported by grants from the National Institutes of Health (T32 HL-07609, P01AC00059916 and 1P50ML53000-01) to Dr. Hollenberg. The results presented in this paper have not been published previously, but have been presented in abstract form at the Canadian Society of Nephrology Annual General Meeting in London, Ontario, Canada in May 2008, the Mazankowski Alberta Heart Institute Inaugural Congress in Edmonton Alberta, Canada in June 2008, the Canadian Society for Clinical Investigation Annual Meeting in Toronto, Ontario, Canada in September 2008 and the American Society of Nephrology Annual Meeting in Philadelphia, Pennsylvania, USA in November 2008.
Footnotes
Disclosures
None.
References
- 1.Hollenberg NK, Williams GH, Burger B, Chenitz W, Hoosmand I, Adams DF. Renal blood flow and its response to angiotensin II. An interaction between oral contraceptive agents, sodium intake, and the renin-angiotensin system in healthy young women. Circ Res. 1976;38:35–40. doi: 10.1161/01.res.38.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Kang AK, Duncan JA, Cattran DC, Floras JS, Lai V, Scholey JW, et al. Effect of oral contraceptives on the renin angiotensin system and renal function. Am J Physiol Regul Integr Comp Physiol. 2001;280:R807–R813. doi: 10.1152/ajpregu.2001.280.3.R807. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SB, Kang AK, Burns KD, Kennedy CR, Lai V, Cattran DC, et al. Effects of oral contraceptive use on the renal and systemic vascular response to angiotensin II infusion. J Am Soc Nephrol. 2004;15:780–786. doi: 10.1097/01.asn.0000114555.16297.5a. [DOI] [PubMed] [Google Scholar]
- 4.Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161:2000–2005. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- 5.Ribstein J, Halimi JM, du Cailar G, Mimran A. Renal characteristics and effect of angiotensin suppression in oral contraceptive users. Hypertension. 1999;33:90–95. doi: 10.1161/01.hyp.33.1.90. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed SB, Hovind P, Parving HH, Rossing P, Price DA, Laffel LM, et al. Oral contraceptives, angiotensin-dependent renal vasoconstriction, and risk of diabetic nephropathy. Diabetes Care. 2005;28:1988–1994. doi: 10.2337/diacare.28.8.1988. [DOI] [PubMed] [Google Scholar]
- 7.Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47:277–283. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Fortepiani LA, Yanes L, Zhang H, Racusen LC, Reckelhoff JF. Role of androgens in mediating renal injury in aging SHR. Hypertension. 2003;42:952–955. doi: 10.1161/01.HYP.0000099241.53121.7F. [DOI] [PubMed] [Google Scholar]
- 9.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol. 2005;289:F941–F948. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- 10.Szmuilowicz ED, Adler GK, Ricchiuti V, Hopkins PN, Seely EW. Relationships between endogenous sex hormone concentrations and vascular function in postmenopausal women. J Clin Endocrinol Metab. 2007;92:4738–4741. doi: 10.1210/jc.2007-1471. [DOI] [PubMed] [Google Scholar]
- 11.Sitruk-Ware RL. Hormone therapy and the cardiovascular system: the critical role of progestins. Climacteric. 2003;6(Suppl 3):21–28. [PubMed] [Google Scholar]
- 12.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therap. N Engl J Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 13.Tanis BC, van den Bosch MA, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787–1793. doi: 10.1056/NEJMoa003216. [DOI] [PubMed] [Google Scholar]
- 14.Singh M. Progestins and neuroprotection: are all progestins created equal? Minerva Endocrinol. 2007;32:95–102. [PubMed] [Google Scholar]
- 15.Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen components. Lancet. 1995;346:1589–1593. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- 16.Arias-Loza PA, Hu K, Schafer A, Bauersachs J, Quaschning T, Galle J, et al. Medroxyprogesterone acetate but not drospirenone ablates the protective function of 17 beta-estradiol in aldosterone salt-treated rats. Hypertension. 2006;48:994–1001. doi: 10.1161/01.HYP.0000242482.57186.e8. [DOI] [PubMed] [Google Scholar]
- 17.Atthobari J, Gansevoort RT, Visser ST, de Jong PE, de Jong-van den Berg LT. The impact of hormonal contraceptives on blood pressure, urinary albumin excretion and glomerular filtration rate. Br J Clin Pharmacol. 2007;63:224–231. doi: 10.1111/j.1365-2125.2006.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hettiaratchi ES, Pickford M. The effect of oestrogen and progesterone on the pressor action of angiotensin in the rat. J Physiol. 1968;196:447–451. doi: 10.1113/jphysiol.1968.sp008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Matsui K, Ito M, Yoshimura T, Kawasaki N, Fujisaki S, et al. Effects of pregnancy and hormone treatments on pressor response to angiotensin II in conscious rats. Am J Obstet Gynecol. 1988;159:989–995. doi: 10.1016/s0002-9378(88)80186-8. [DOI] [PubMed] [Google Scholar]
- 20.Greer JB, Modugno F, Allen GO, Ness RB. Androgenic progestins in oral contraceptives and the risk of epithelial ovarian cancer. Obstet Gynecol. 2005;105:731–740. doi: 10.1097/01.AOG.0000154152.12088.48. [DOI] [PubMed] [Google Scholar]
- 21.Shoback DM, Williams GH, Hollenberg NK, Davies RO, Moore TJ, Dluhy RG. Endogenous angiotensin II as a determinant of sodium-modulated changes in tissue responsiveness to angiotensin II in normal man. J Clin Endocrinol Metab. 1983;57:764–770. doi: 10.1210/jcem-57-4-764. [DOI] [PubMed] [Google Scholar]
- 22.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 23.Parving HH, Hommel E, Jensen BR, Hansen HP. Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int. 2001;60:228–234. doi: 10.1046/j.1523-1755.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 24.Osei SY, Price DA, Fisher ND, Porter L, Laffel LM, Hollenberg NK. Hyperglycemia and angiotensin-mediated control of the renal circulation in healthy humans. Hypertension. 1999;33:559–564. doi: 10.1161/01.hyp.33.1.559. [DOI] [PubMed] [Google Scholar]
- 25.Osei SY, Price DA, Laffel LM, Lansang MC, Hollenberg NK. Effect of angiotensin II antagonist eprosartan on hyperglycemia-induced activation of intrarenal renin-angiotensin system in healthy humans. Hypertension. 2000;36:122–126. doi: 10.1161/01.hyp.36.1.122. [DOI] [PubMed] [Google Scholar]
- 26.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol. 1999;10:1778–1785. doi: 10.1681/ASN.V1081778. [DOI] [PubMed] [Google Scholar]
- 27.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed SB, Fisher ND, Stevanovic R, Hollenberg NK. Body mass index and angiotensin-dependent control of the renal circulation in healthy humans. Hypertension. 2005;46:1316–1320. doi: 10.1161/01.HYP.0000190819.07663.da. [DOI] [PubMed] [Google Scholar]
- 29.Orth SR. Smoking and the kidney. J Am Soc Nephrol. 2002;13:1663–1672. doi: 10.1097/01.asn.0000018401.82863.fd. [DOI] [PubMed] [Google Scholar]
- 30.Pasinetti E, Falsetti L. Triphasic pills: variability of endocrine parameters and of sex steroid-binding globulins. Acta Eur Fertil. 1993;24:67–70. [PubMed] [Google Scholar]
- 31.Ellis J. Low-dose oral contraceptives: progestin potency, androgenicity, and atherogenic potential. Clin Ther. 1986;8:607–618. [PubMed] [Google Scholar]
- 32.Darney PD. The androgenicity of progestins. Am J Med. 1995;98:104S–110S. doi: 10.1016/s0002-9343(99)80067-9. [DOI] [PubMed] [Google Scholar]
- 33.van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, Thijssen JH. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345–352. doi: 10.1016/0010-7824(90)90034-s. [DOI] [PubMed] [Google Scholar]
- 34.Madauss KP, Stewart EL, Williams SP. The evolution of progesterone receptor ligands. Med Res Rev. 2007;27:374–400. doi: 10.1002/med.20083. [DOI] [PubMed] [Google Scholar]
- 35.Winneker RC, Fensome A, Zhang P, Yudt MR, McComas CC, Unwalla RJ. A new generation of progesterone receptor modulators. Steroids. 2008;73:689–701. doi: 10.1016/j.steroids.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Lemale J, Bloch-Faure M, Grimont A, El Abida B, Imbert-Teboul M, Crambert G. Membrane progestin receptors alpha and gamma in renal epithelium. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamcr.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem. 1993;41:671–678. doi: 10.1177/41.5.8468448. [DOI] [PubMed] [Google Scholar]
- 38.Boulkroun S, Le Moellic C, Blot-Chabaud M, Farman N, Courtois-Coutry N. Expression of androgen receptor and androgen regulation of NDRG2 in the rat renal collecting duct. Pflugers Arch. 2005;451:388–394. doi: 10.1007/s00424-005-1410-x. [DOI] [PubMed] [Google Scholar]
- 39.Winer BM. Renin in pregnancy and the menstrual cycle. J Clin Invest. 1965;44:1112. [Google Scholar]
- 40.Chesley LC, Tepper IH. Effects of progesterone and estrogen on the sensitivity to angiotensin II. J Clin Endocrinol Metab. 1967;27:576–581. doi: 10.1210/jcem-27-4-576. [DOI] [PubMed] [Google Scholar]
- 41.Seely EW, Walsh BW, Gerhard MD, Williams GH. Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension. 1999;33:1190–1194. doi: 10.1161/01.hyp.33.5.1190. [DOI] [PubMed] [Google Scholar]
- 42.Oelkers WH. Drospirenone in combination with estrogens: for contraception and hormone replacement therapy. Climacteric. 2005;8(Suppl 3):19–27. doi: 10.1080/13697130500330341. [DOI] [PubMed] [Google Scholar]
- 43.Reckelhoff JF. Sex steroids, cardiovascular disease, and hypertension: unanswered questions and some speculations. Hypertension. 2005;45:170–174. doi: 10.1161/01.HYP.0000151825.36598.36. [DOI] [PubMed] [Google Scholar]
- 44.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and rennin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 46.Reckelhoff JF, Zhang H, Granger JP. Decline in renal hemodynamic function in aging SHR: role of androgens. Hypertension. 1997;30:677–681. doi: 10.1161/01.hyp.30.3.677. [DOI] [PubMed] [Google Scholar]
- 47.Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol. 1999;26:127–131. doi: 10.1046/j.1440-1681.1999.02996.x. [DOI] [PubMed] [Google Scholar]
- 48.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan NM, Kem DC, Holland OB, Kramer NJ, Higgins J, Gomez-Sanchez C. The intravenous furosemide test: a simple way to evaluate renin responsiveness. Ann Intern Med. 1976;84:639–645. doi: 10.7326/0003-4819-84-6-639. [DOI] [PubMed] [Google Scholar]
- 50.James GD, Sealey JE, Muller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl. 1986;4:S387–S389. [PubMed] [Google Scholar]
- 51.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55:278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- 52.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, et al. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17:2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 53.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 54.Gordon MS, Chin WW, Shupnik MA. Regulation of angiotensinogen gene expression by estrogen. J Hypertens. 1992;10:361–366. doi: 10.1097/00004872-199204000-00007. [DOI] [PubMed] [Google Scholar]
- 55.De Lignieres B, Basdevant A, Thomas G, Thalabard JC, Mercier-Bodard C, Conard J, et al. Biological effects of estradiol-17 beta in postmenopausal women: oral versus percutaneous administration. J Clin Endocrinol Metab. 1986;62:536–541. doi: 10.1210/jcem-62-3-536. [DOI] [PubMed] [Google Scholar]
- 56.Gansevoort RT, de Zeeuw D, de Jong PE. Is the antiproteinuric effect of ACE inhibition mediated by interference in the renin-angiotensin system? Kidney Int. 1994;45:861–867. doi: 10.1038/ki.1994.113. [DOI] [PubMed] [Google Scholar]
- 57.Lansang MC, Price DA, Laffel LM, Osei SY, Fisher ND, Erani D, et al. Renal vascular responses to captopril and to candesartan in patients with type 1 diabetes mellitus. Kidney Int. 2001;59:1432–1438. doi: 10.1046/j.1523-1755.2001.0590041432.x. [DOI] [PubMed] [Google Scholar]
- 58.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Cherney DZ, Scholey JW, Cattran DC, Kang AK, Zimpelmann J, Kennedy C, et al. The effect of oral contraceptives on the nitric oxide system and renal function. Am J Physiol Renal Physiol. 2007;293:F1539–F1544. doi: 10.1152/ajprenal.00351.2007. [DOI] [PubMed] [Google Scholar]
- 60.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol. 2008;294:H1630–H1637. doi: 10.1152/ajpheart.01314.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol. 2007;292:H2874–H2880. doi: 10.1152/ajpheart.00762.2006. [DOI] [PubMed] [Google Scholar]