Abstract
Objective
To determine the effects of fetal sex on aromatase and androgen receptor (AR) expression in the placenta of normal and preeclamptic pregnancies.
Study Design
Placenta from preeclamptic (5-female and 6-male fetus) and healthy pregnancies (7-female and 7-male fetus) were examined by immunofluorescence, Western blotting and quantitative-RT-PCR.
Results
Placental AR levels were significantly higher (P<0.05) in placentae of both male and female fetus compared to their respective sexes in normal pregnancies. The placental aromatase levels varied depending on fetal sex. If the fetus was female, aromatase levels were substantially higher (P<0.05) in preeclamptic than normal placentae. If the fetus was male, the aromatase levels were significantly lower (P<0.05) in preeclamptic than normal placentae. Placental aromatase levels were significantly higher (P<0.05) in male-than in female-bearing normal placentae.
Conclusion
Dysregulation in androgen production and signaling in preeclamptic placentae may contribute to placental abnormalities increasing the frequency of maternal-fetal complications associated with preeclampsia.
Keywords: Preeclampsia, Placenta, Androgen receptor, Aromatase, Fetal sex
Introduction
The placenta is a unique and complex endocrine organ that plays a crucial role during fetal development by allowing rapid exchange of nutrients and wastes between the closely apposed maternal and fetal circulatory systems.1 In addition to the production of a wide variety of hormones and other regulatory factors, the placenta is also an endocrine target tissue, expressing a broad spectrum of hormone receptors and growth factor receptors.2 Thus, a complex interplay of hormones and other regulatory factors produced by the placenta, mother and fetus affect placental development and functions through endocrine, paracrine and autocrine mechanisms.
Preeclampsia is a common pregnancy-specific syndrome that is characterized by hypertension and proteinuria. It is a disorder that affects at least 5% of all pregnancies worldwide.3,4 Preeclampsia is associated with numerous complications including seizures, coma, low birth weight and occasionally death of the mother and/or fetus. Additionally, preeclampsia poses an increased risk for development of cardiovascular dysfunctions later in life for both child and mother.5-8 The cause of preeclampsia is unknown, however it is generally accepted that preeclampsia results from the presence of a placenta9 because the only effective treatment for preeclampsia is the delivery of the placenta. As such, aberrant development and functions of the placenta are implicated as important factors that contribute to preeclampsia associated complications. Indeed, abnormalities in the placenta of women with preeclampsia have been well described; these include poor placental growth, decreased nutrient transport, improper invasion and inadequate angiogenesis and vasculogenesis.10-13 Numerous studies also demonstrate that preeclampsia alters maternal and fetal endocrine profiles including increases in the levels of testosterone and a decrease in estrogen concentrations compared to normal pregnancies.14-25 However, the proportion of increase in testosterone levels in preeclamptic women varies depending of the sex of fetus. Male-bearing preeclamptic women had significantly higher testosterone levels than female-bearing preeclamptic women.21 Whether the placenta directly contributes to some of the fetal sex related abnormal hormonal profiles in preeclampsia or whether preeclampsia affects placental endocrine signaling pathways in relation to fetal sex, remains to be elucidated.
In this study, we evaluated whether there were any fetal sex related differences in the expression of androgen receptor (AR) and aromatase in the placenta between healthy and preeclamptic pregnancies. Our data suggest that fetal sex related differences in placental aromatase exists with increased levels in female and decreased levels in male preeclamptic pregnancies. The AR levels were significantly elevated in placentae with both male and female preeclamptic pregnancies. Dysregulation in androgen production, together with overexpression of their receptors in the placenta, may be associated with abnormalities of placental growth and transport, trophoblast invasion and placental angiogenesis in preeclampsia.
Materials and methods
Placental samples
Eleven placentae from pregnancies with preeclampsia (5 with female and 6 with male fetus) and 14 from normal pregnancies (7 with male and 7 with female fetus) were collected at term (37-41 weeks of gestation). The patients were diagnosed with preeclampsia based on new-onset hypertension after 20 weeks’ gestation such that systolic blood pressures of ≥140 mm Hg, diastolic blood pressures of ≥90 mm Hg, or both were seen on 2 occasions ≥6 hours apart, with significant proteinuria (≥300 mg/24 h). None of the patients had previous history of any known endocrinopathy. Excluders include smokers and alcoholics and women with chronic maternal disease (essential hypertension, connective tissue diseases, hyperthyroidism, hypothyroidism, chronic glomerulonephritis, renal failure, and diabetes mellitus) or gestational diabetes. The mean gestational age in the normal pregnancies were similar to those of the preeclampsia group (Table 1). All samples were obtained from the Department of Obstetrics and Gynecology, University of Texas Medical Branch from January 2009 through June 2011. The study was approved by the Institutional Review Board of the University’s Human Studies Committee; informed written consent was obtained from all subjects. All patients were delivered at term. The placental tissues were collected at delivery and immediately transferred on ice to the laboratory for preparations of protein lysate, RNA isolation and tissue fixation.
Table 1.
Patient Characteristics
| Normal Pregnant | Preeclampsia | |||
|---|---|---|---|---|
| Characteristics | Female | Male | Female | Male |
| Maternal age, y | 23.1±2.18 | 24.8±1.26 | 21.5±2.87 | 22.4±1.43 |
| Gestational age, weeks | 38.8±1.34 | 39.1±1.23 | 37.6±0.91 | 37.1±1.51 |
| BMI (kg/m2)a | 35.2±3.85 | 34.2±1.01 | 36.9±5.53 | 35.7±2.17 |
| Systolic blood pressure, mm Hg | 107.7±1.07 | 118.0±3.67 | 152.7±3.75* | 155.0±5.54* |
| Diastolic blood pressure, mm Hg | 62.1±2.69 | 69.4±3.87 | 83.2±7.33* | 98.8±5.20* |
| Birthweight, g | 3425.7±172.16 | 2927.9±585.29 | 2393.3±413.12* | 2716.0±537.10 |
| Number of placenta (n) | 7 | 7 | 5 | 6 |
|
Ethnicity Hispanic White African-American |
7 - - |
6 1 - |
4 - 1 |
4 1 1 |
Data are expressed as mean ± SEM. Unpaired Student t test (2-tailed) was used for statistical analysis.
P < .05 vs respective gender in Normal Pregnant.
BMI: Body mass index
Immunofluorescence
Immunofluorescence was performed on OCT-embedded frozen sections after fixing with ice-cold acetone for 10 min at room temperature. The sections then were incubated with 3% Donkey serum in PBS-Tween (0.01%) for 1h at room temp in a humidified chamber to block nonspecific antibody binding. The first primary antibody, rat anti-AR antibody (Affinity Bioreagents, Golden, CO) or rabbit polyclonal anti-aromatase (Thermo scientific, Rockford, IL) was applied at 1:50 dilution overnight, followed by donkey anti-rat or anti-rabbit IgG Alexa Fluor 488 (Molecular Probes, Eugene, OR). Rat or rabbit IgG (ready to use) (Dako, Glostrup, Denmark) was used as a negative control. Slides were then counterstained with Hoechst 33342 (Molecular Probes). Anti-fade mounting medium (Dako) was applied and slides were viewed and captured with a constant exposure time and aperture using a single threshold value under a Nikon Eclipse E800 epifluorescence microscope using a Nikon Digital camera Dxm1200. Subsequently, images were analyzed using Metamorph software and the numerical output of average 488nm pixel intensity (green fluorescence) per nuclei (blue fluorescence; Hoechst 33342) was calculated.
Western blotting
This procedure was performed with an enhanced chemiluminescence (ECL) detection system. Briefly, placental villi were homogenized in ice-cold 50 mM Tris–HCl, pH 7.4 buffer containing a complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). The protein concentration was measured by Bradford method (Bio-Rad, Hercules, CA). Protein aliquots of 20 μg were electrophoresed in sodium dodecyl sulfate-polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA). The membranes were blocked with 5% (w/v) nonfat dry milk followed by incubation overnight with antibodies against 1:1000 diluted antibodies against AR (Affinity Bioreagents) and aromatase (Themo Scientific). Peroxidase-conjugated anti-rat or anti-rabbit IgG was used as the secondary antibody (Southern Biotech, Birmingham, AL). All the members were re-blotted with 1:1000 diluted polyclonal antibody against β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) to serve as a loading control. Specific protein bands were detected with ECL Western blotting detection reagents (Affinity Bioreagents). One normal female placental sample was used on each gel to normalize density readings between gels. The specific bands were scanned and band intensity was quantified using the Alpha ease image analysis program. Results were expressed as ratios of the intensity of specific band to that of β-actin.
Quantitative RT-PCR
Total placental RNA was isolated with RNeasy kit (Qiagen, Valencia, CA) according to the procedure recommended by the manufacturer. RNA extraction was followed by DNase 1 (Qiagen) treatment to remove DNA contamination. The quality and quantity of the RNA were assessed at 260/280 A, and all samples showed absorbency ratios ranging from 1.8 to 2.0. Total RNA of 1 μg were reverse transcribed into cDNA using avian myeloblastosis virus reverse transcriptase (Promega Corp., Madison, WI) and random oligonucleotide hexamers (Invitrogen, Carlsbad, CA). For the detection of AR and ARO genes, quantitative real-time RT-PCR (q-RT-PCR) was done with CFX96 system (Bio-Rad, Hercules, CA) using published primers for human AR and aromatase. The primer sequences for AR are 5′- CCTGGCTTCCGCAACTTACAC-3′ (forward) and 5′-GGACTTGTGCATGCGGTACTCA -3′ (reverse). The primer sequences for ARO are 5′-GTGGACGTGTTGACCCTTCT -3′ (forward) and 5′-CACGATAGCACTTTCGTCCA -3′ (reverse). A comparative cycle of threshold fluorescence (CT) method was used with GAPDH as an internal control (5′-GGTC TCCT CTGA CTTC AACA-3′ (forward) and 5′-AGCC AAAT TCGT TGTC ATAC-3′(reverse)). The CT value for GAPDH was subtracted from the CT value for the gene of interest to give a ΔCT for each sample. The ΔCT of the calibrator (in this case the mean ΔCT of the placenate from normal pregnant) was then subtracted from each sample to give a ΔΔCT value. This was then inserted into 2−ΔΔCT to give a final expression relative to the calibrator.
Statistical analysis
Results are expressed as mean values ± SE. Comparisons between the data were performed using Graphpad prism Software (San Diego, CA). Criteria for statistical significance were set at P < 0.05 after two-way ANOVA followed by Bonferroni post hoc testing for multiple comparisons. Unpaired student t test (2-tailed) was used for comparisons of biological parameters between normal and preeclamptic pregnancies.
Results
Patient characteristics
In this pilot study, placenta of pregnant women bearing female (n=7) and male fetus (n=7) served as controls for the preeclamptic placentae with female (n=5) and male fetus (n=6). There were no significant differences in mean maternal age, mean gestational age, and body mass index between the groups. The clinical and biological parameters of this aged-matched population group and sociodemographic details are shown in Table 1. The preeclamptic patients with both male and female fetus had a significantly higher systolic (P < .05) and diastolic (P < .05) blood pressure than the respective sex in normal pregnant women.
Immunofluorescence
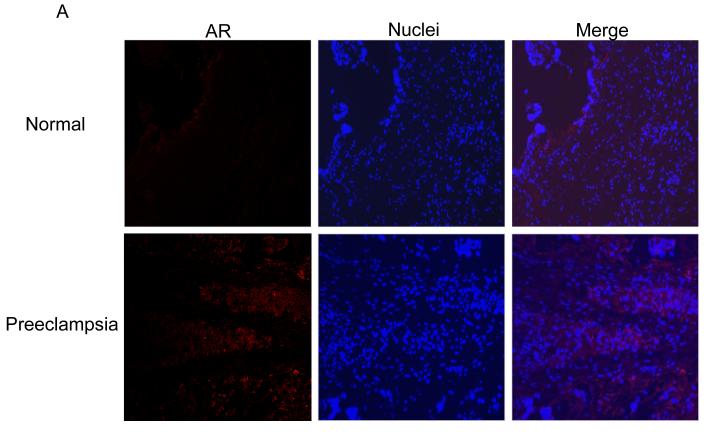
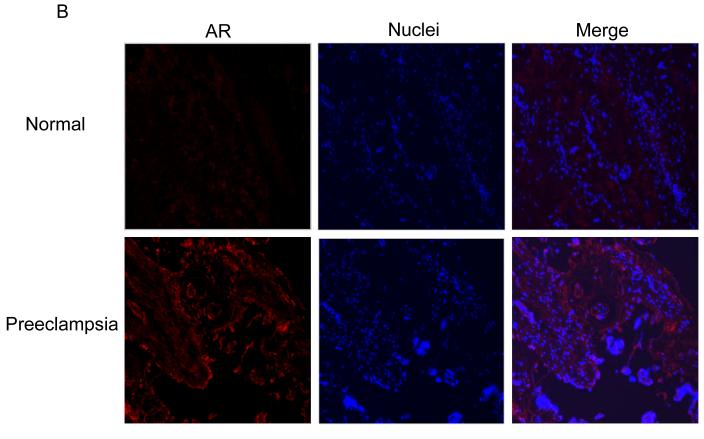
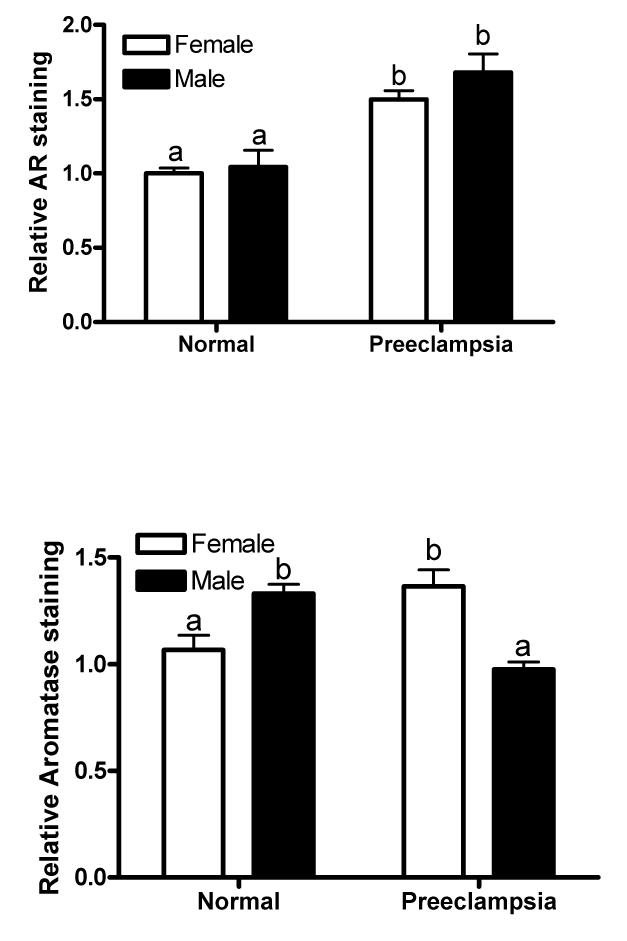
Immunostaining of AR was barely detectable in the placentae of normal pregnancies (Fig. 1A and 1B, top panel). In contrast, the AR was readily detectable in the placentae of preeclamptic pregnancies and was significantly greater in the placentae with both female (P =.002) and male (P =.019) fetus compared to their respective sexes in normal pregnancies (Fig. 1A and 1B, bottom panel and Fig 1E, top panel). The AR staining intensity did not significantly vary between fetal sex when compared within the normal and preeclamptic placentae (Fig. 1E, top panel). Immunostaining of AR in preeclamptic placentae was mainly found in the syncytiotrophoblasts (Fig. 1A and 1B, bottom panel).
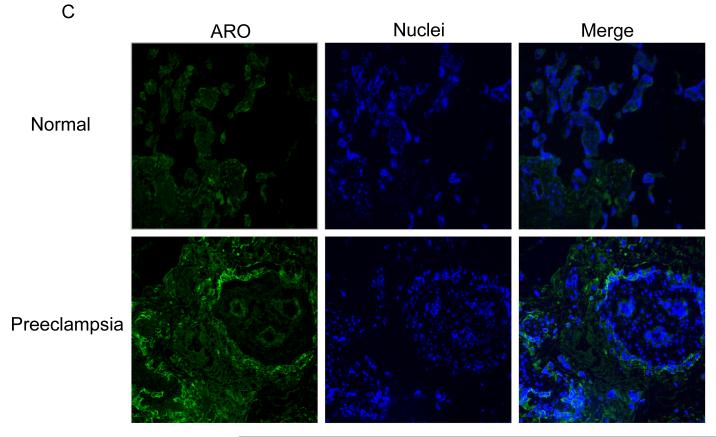
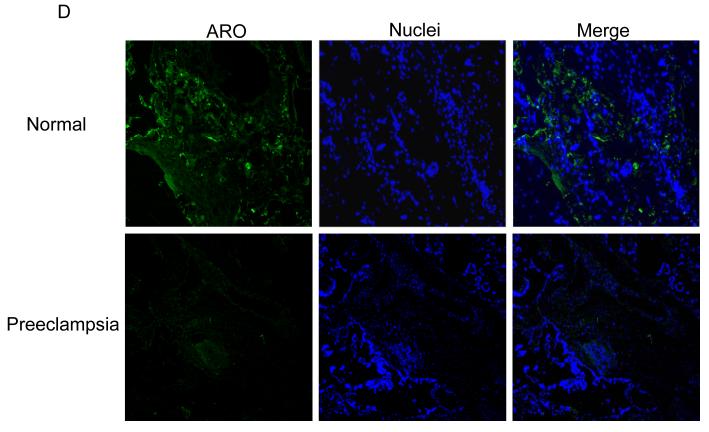
Fig. 1.
Immunofluorescence staining of AR in normal and preeclampsia placentae with female (A) and male (B) fetus. Immunostaining of aromatase in placentae of female (C) and male (D) fetus. (100x magnification). (E) Quantification of AR and aromatase staining intensity (green fluorescence) per nuclei (blue fluorescence). The mean staining intensity in the normal placentae with female fetus was assigned a value of 1, and the mean density of the other groups was calculated relative to normal female placentae. Values are given as means ± SEM. Bars with different letter superscripts differ significantly (P<0.05; n=3 in each group)
The immunostaining intensity of aromatase in preeclamptic placentae varied depending on fetal sex. If the fetus was a female, the staining intensity for aromatase was substantially higher (P =.04) in preeclamptic placentae than normal placentae (Fig. 1C and Fig. 1E, lower panel). On the other hand, if the fetus was a male, the staining intensity for aromatase was significantly lower (P =.01) in preeclamptic placentae than normal placentae (Fig. 1D and Fig. 1E, lower panel). Within the placentae of normal pregnancies, aromatase staining intensity was significantly higher (P =.03) in placentae with male than female fetus (Fig. 1E, bottom panel). On the other hand, the aromatase staining intensity was significantly lower (P = .01) in male than preeclamptic placentae (Fig. 1E). Aromatase protein was exclusively localized in the syncytiotrophoblast in the control and preeclamptic placentae (Fig. 1C and D). No detectable staining was observed when primary antibody to AR or aromatase was omitted (procedure control; data not shown).
Western blot
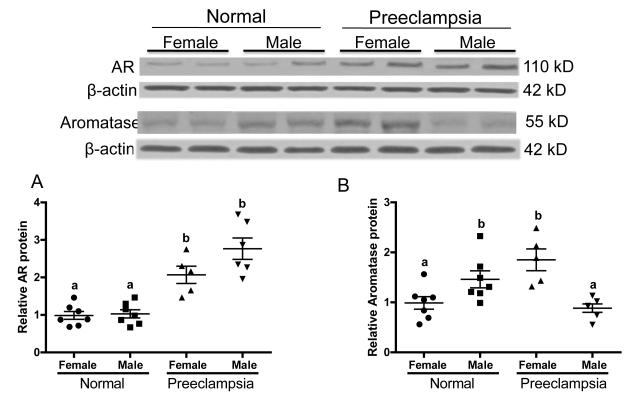
To further verify the changes in AR in the preeclamptic placentae, Western blotting analyses were performed. Consistent with the immunohistochemical observations, the levels of AR protein (110 kDa) are significantly elevated (P=.001) in the placentae of preeclamptic women compared to normal pregnant women and fetal sex had no effect (Fig. 2A). Within the normal and preeclamptic pregnancies there were no significant differences between sexes on levels of AR protein (Fig. 2A).
Fig. 2.
Western blotting analysis of AR and aromatase protein levels in preeclamptic and normal placenta of female and male fetus. Top panels show representative blots of AR and aromatase along with β-actin, and bottom panel is the summary of densitometric analysis of AR (A) and aromatase (B). The mean density in the normal placentae with female fetus was assigned a value of 1, and the mean density of the other groups was calculated relative to normal female placentae. Values are given as means ± SEM. Bars with different letter superscripts differ significantly (P<0.05; n=5-7 in each group)
The levels of aromatase protein (55 kDa) were markedly increased in preeclamptic placentae with female fetus (P =.004) while it is significantly decreased in placentae with male fetus (P =.01) compared to the respective sexes in normal placentae (Fig. 2B). The normal placentae with male fetus have significantly higher (P = 0.04) levels of aromatase protein than normal placentae with female fetus. Conversely, in preeclamptic placentae with male fetus have significantly lower (P = 0.001) levels of aromatase protein than female preeclamptic placentae (Fig. 2B).
Quantitative RT-PCR
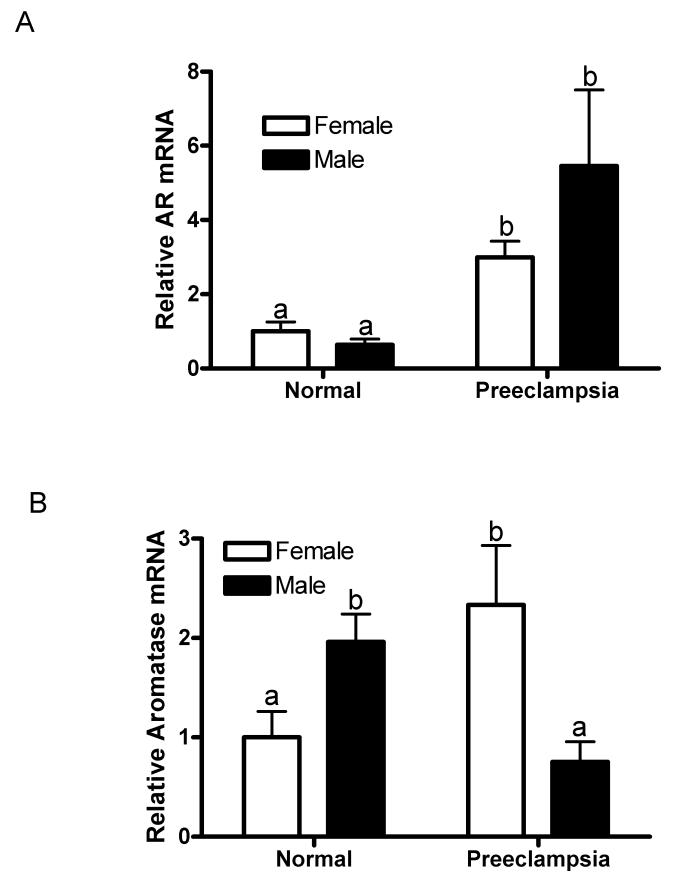
To examine whether preeclampsia also altered the expression of AR and aromatase (also known as CYP19) genes, quantitative RT-PCR analyses were carried out. The results showed that the levels of AR mRNA were significantly elevated in placentae with male (P =.03) and female (P =.001) fetuses in preeclamptic women compared to their respective sexes in placentae from normal women (Fig. 3A). The increase in levels of AR mRNA in the male preeclamptic compared to the female preeclamptic placentae was not statistically significant (P =.33; Fig. 3A).
Fig. 3.
Quantitative RT-PCR analysis of placental gene expression of AR (A) and aromatase (B) in preeclamptic and normal placenta with female and male fetus. The mean mRNA expression of the normal placentae with female fetus was assigned a value of 1 and the mean of the other groups were calculated relative to the normal female placenta. Values are given as means ± SEM. Bars with different letter superscripts differ significantly (P<0.05; n=5-7 in each group)
The levels of aromatase mRNA in preeclamptic placentae were significantly higher if the fetus was a female (P =.04) while it was significantly lower if the fetus was a male (P =.04) compared to their respective sexes in normal placentae Fig. 3B). The levels of aromatase mRNA in the normal placentae were significantly higher in male than female fetus (P = 0.02; Fig. 3B). On the other hand, in the preeclamptic placentae the aromatase mRNA levels were significantly lower in male than female fetus (P < 0.04; Fig. 3B). Changes in AR and aromatase protein levels are consistent with changes in their mRNA abundance suggesting that preeclampsia may influence placental gene expression of the AR and aromatase at the transcriptional level.
Discussion
The placenta is an endocrine organ that plays an important role during pregnancy. Placenta produces numerous hormones that regulate maternal function and fetal growth. There have been a number of reports that women with preeclampsia have higher plasma testosterone levels compared with those of healthy pregnant women.14-25 Although the origin of the increased androgens during pregnancy remains uncertain, studies suggests that elevation of testosterone production during pregnancy is likely of ovarian origin.26,27 Whether the placenta also contributes to the increased testosterone levels in the maternal circulation remains largely unresolved.26,28,29 While the human placenta lacks 17β-hydroxylase and 17,20-desmolase,30 it does express 17β-hydroxysteroid dehydrogenase (17β-HSD)31 and aromatase as well as 3β-hydroxysteroid dehydrogenase (3β-HSD).32 Placenta can therefore synthesize androstenedione from adrenal or ovarian DHEAS and can undertake the onward synthesis of both testosterone and estradiol. Normally, androgens synthesized by the placenta are rapidly converted to estrogens by placental aromatase,33,34 and therefore the placental androgens may contribute only slightly to the increased androgen observed in normal pregnancy. In this study, the placental mRNA and protein levels of aromatase, a rate limiting enzyme converting androgens to estrogens, varied depending on fetal sex. If the fetus was a female, the aromatase expression was higher in preeclamptic than normal placentae. On the other hand if the fetus was a male, the aromatase expression was lower in preeclamptic than normal placentae. Previous studies suggest that maternal testosterone levels are higher in preeclamptic pregnancies irrespective of the sex of the fetus compared to normal pregnancies. However, testosterone levels are significantly higher in male- than in female-bearing preeclamptic pregnancies.35 Based on our findings, it is possible that the increased expression of aromatase in the placentae of female fetus-bearing preeclamptic women could have partially metabolized the excess testosterone contributing for relatively lower maternal testosterone levels. This may be an adaptive protective mechanism to prevent the female fetuses from virilization when the mothers themselves have high circulating testosterone levels. Despite higher aromatase levels in preeclamptic placentae of female fetus, higher maternal circulating levels are observed in female bearing preeclamptic pregnancies36 suggesting that testosterone production is higher such that the increased expression of aromatase may not be sufficient to metabolize androgens to levels that are observed in normal pregnancies. In the placenta with male fetus, the decrease in aromatase levels could have contributed for the relatively higher testosterone levels by altering the equilibrium between estrogens and androgens in favor of androgens. These findings imply that increased testosterone during preeclamptic pregnancies may be primarily of non placental origin but the placenta, depending on fetal sex, may modulate the levels of maternal testosterone. Thus, the fetal sex specific abnormal maternal profile of androgens in preeclampsia may be attributed, at least in part, to altered aromatase levels in the placenta. It would be interesting to assess if such fetal sex-dependent differential expression in aromatase levels also exists in tissues or organs other than placenta.
The mechanisms that contribute to the inhibition of aromatase in the preeclamptic placenta with male fetus is not known. In the placenta, the major factors involved in regulating aromatase activity (retinoids,37 tumor-necrosis factor alpha,38 and lipid radicals39) are all dysregulated during preeclampsia in a way that could potentially down-regulate aromatase.40-43 Aromatase expression in the trophoblast is barely detectable under hypoxic conditions44 (which mirror the actual conditions of the placenta in the context of preeclampsia). Moreover, insulin15 and leptin,30 which are significantly increased in preeclamptic patients, has been shown to inhibit aromatase in human cytotrophoblasts. Why the inhibitory effect of placental aromatase is specific to male sex is not known at this time. It is suggested that the placenta with female fetus has greater adaptability to adverse maternal environment that placenta with male fetus.45 Similar findings of fetal sex specific differences in placental cytokine expression, insulin-like growth factor pathways and the placental response to cortisol in relation to the complication of asthma during pregnancy are reported.46-49
Even in the normal pregnancies, the placentae with male fetus have higher aromatase levels than placentae with female fetus. The male fetus produces testosterone starting as early as 8 weeks of age with peak levels reaching upto 150 ng/dl around mid gestation. Despite greater production of testosterone by male fetus, the maternal testosterone levels are not significantly different between the male and female bearing normal pregnant women.35 This suggests that the increased aromatase levels in placentae of male fetus may act as an effective barrier to prevent transfer of testosterone from fetal side to maternal circulation.
Previous studies show inconclusive results regarding the presence of AR in the human placenta.50-53 In this study, we show that the mRNA and protein of AR, are detectable in the syncytiotrophoblasts. The most significant finding of this study is that the expression of AR in the preeclampsia placentae is considerably increased irrespective of fetal sex compared to their respective sexes in normal pregnancies. Although there is no information available at this time regarding the role of androgen signaling in the placenta, androgens are known to be pleiotropic hormones with genomic and non-genomic activity involving a myriad of biological processes. Dysregulation in androgen signaling in the placenta may profoundly interfere with its development and function. It is well known that increase in testosterone levels and altered in utero environment during pregnancy increases the risk of cardiovascular dysfunction in the mothers5-8 as well as program for adult life cardiovascular, metabolic and endocrine dysfunctions in the offspring.54-58 Therefore, it is likely that a combination of overexpression of placental AR and fetal sex-specific dysregulation in placental androgen production in preeclampsia may play a significant role in contributing for development of gender-specific diseases later in life.
Although this pilot study provided initial evidence for increased placental androgen receptor levels in preeclamptic pregnancies and the placental aromatase levels varied in a fetal sex-specific manner in both normal and preeclamptic pregnancies, the limited number of patients is a potential weakness. Our results will have to be confirmed in a larger cohort, with longitudinal data to assess the full impact of our studies.
5. Conclusions
The aromatase levels in placentae vary depending on fetal sex in normal and preeclamptic pregnancies. In preeclamptic pregnancies with male fetuses, the observed increase in circulating testosterone may be associated, at least in part, to due to the decrease in placental aromatase that reduces the conversion of testosterone to estrogens. In preeclamptic pregnancies with female fetuses, the relatively lower circulating testosterone may be due to the increased placental aromatase levels. Placental androgen receptor levels are upregulated in preeclamptic pregnancies with both fetal sexes suggesting that the androgen signaling pathways may be over-activated in the placentae of pregnancies with preeclampsia. Dysregulation of these androgen signaling networks may be involved in the development of placental abnormalities which eventually increase the frequency of maternal and fetal complications associated with preeclampsia.
Acknowledgments
Financial support: is provided in part by grants HD69750, HL58144 and HL72650 from the National Institute of Health.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb.Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Lei ZM, Rao CV. Endocrinology of trophoblastic tissue. In: Becker Kenneth L., editor. Principles and practice of endocrinology and metabolism. 3rd ed Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 180–186. [Google Scholar]
- 3.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet.Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 4.Sibai BM, Caritis S, Hauth J. What we have learned about preeclampsia. Semin.Perinatol. 2003;27:239–246. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 5.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey SG, Sattar N, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–1199. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 7.Luft FC. Pre-eclampsia and the maternal cardiovascular risk. Nephrol.Dial.Transplant. 2003;18:860–861. doi: 10.1093/ndt/gfg062. [DOI] [PubMed] [Google Scholar]
- 8.Laivuori H, Kaaja R, Rutanen EM, Viinikka L, Ylikorkala O. Evidence of high circulating testosterone in women with prior preeclampsia. J Clin Endocrinol Metab. 1998;83:344–347. doi: 10.1210/jcem.83.2.4543. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 10.Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat.Diagn. 2010;30:293–308. doi: 10.1002/pd.2475. [DOI] [PubMed] [Google Scholar]
- 11.Roberts CT. IFPA Award in Placentology Lecture: Complicated interactions between genes and the environment in placentation, pregnancy outcome and long term health. Placenta. 2010;31:S47–S53. doi: 10.1016/j.placenta.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Foidart JM, Schaaps JP, Chantraine F, Munaut C, Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia--a step forward but not the definitive answer. J Reprod.Immunol. 2009;82:106–111. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Furuya M, Ishida J, Aoki I, Fukamizu A. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc.Health Risk Manag. 2008;4:1301–1313. doi: 10.2147/vhrm.s4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laivuori H, Kaaja R, Rutanen EM, Viinikka L, Ylikorkala O. Evidence of high circulating testosterone in women with prior preeclampsia. J Clin.Endocrinol.Metab. 1998;83:344–347. doi: 10.1210/jcem.83.2.4543. [DOI] [PubMed] [Google Scholar]
- 15.Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet.Gynecol. 1999;180:60–63. doi: 10.1016/s0002-9378(99)70150-x. [DOI] [PubMed] [Google Scholar]
- 16.Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur.J Obstet.Gynecol.Reprod.Biol. 2006;126:16–19. doi: 10.1016/j.ejogrb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol.Pol. 2008;59:390–392. [PubMed] [Google Scholar]
- 18.Serin IS, Kula M, Basbug M, Unluhizarci K, Gucer S, Tayyar M. Androgen levels of preeclamptic patients in the third trimester of pregnancy and six weeks after delivery. Acta Obstet.Gynecol.Scand. 2001;80:1009–1013. doi: 10.1034/j.1600-0412.2001.801107.x. [DOI] [PubMed] [Google Scholar]
- 19.Carlsen SM, Romundstad P, Jacobsen G. Early second-trimester maternal hyperandrogenemia and subsequent preeclampsia: a prospective study. Acta Obstet.Gynecol.Scand. 2005;84:117–121. doi: 10.1111/j.0001-6349.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- 20.Baksu A, Gurarslan H, Goker N. Androgen levels in pre-eclamptic pregnant women. Int J Gynaecol.Obstet. 2004;84:247–248. doi: 10.1016/S0020-7292(03)00318-7. [DOI] [PubMed] [Google Scholar]
- 21.Steier JA, Ulstein M, Myking OL. Human chorionic gonadotropin and testosterone in normal and preeclamptic pregnancies in relation to fetal sex. Obstet.Gynecol. 2002;100:552–556. doi: 10.1016/s0029-7844(02)02088-4. [DOI] [PubMed] [Google Scholar]
- 22.Hsu TY, Lan KC, Tsai CC, Ou CY, Cheng BH, Tsai MY, et al. Expression of androgen receptor in human placentas from normal and preeclamptic pregnancies. Taiwan.J Obstet.Gynecol. 2009;48:262–267. doi: 10.1016/S1028-4559(09)60301-6. [DOI] [PubMed] [Google Scholar]
- 23.Gerulewicz-Vannini D, Camero Y, Salas J, Hernandez-Andrade E. [High plasmatic androgen levels in women affected with pregnancy-induced hypertension] Rev.Invest Clin. 2006;58:228–233. [PubMed] [Google Scholar]
- 24.Atamer Y, Erden AC, Demir B, Kocyigit Y, Atamer A. The relationship between plasma levels of leptin and androgen in healthy and preeclamptic pregnant women. Acta Obstet.Gynecol.Scand. 2004;83:425–430. doi: 10.1111/j.0001-6349.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- 25.Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, et al. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol. 2003;32:455–460. doi: 10.1093/ije/dyg094. [DOI] [PubMed] [Google Scholar]
- 26.Dokras A, Spaczynski RZ, Behrman HR, Duleba AJ. Testosterone levels in pregnant women correlate with the insulin response during the glucose tolerance test. Fertil.Steril. 2003;79:492–497. doi: 10.1016/s0015-0282(02)04764-7. [DOI] [PubMed] [Google Scholar]
- 27.Steier JA, Ulstein M, Myking OL. Human chorionic gonadotropin and testosterone in normal and preeclamptic pregnancies in relation to fetal sex. Obstet.Gynecol. 2002;100:552–556. doi: 10.1016/s0029-7844(02)02088-4. [DOI] [PubMed] [Google Scholar]
- 28.Nestler JE. Modulation of aromatase and P450 cholesterol side-chain cleavage enzyme activities of human placental cytotrophoblasts by insulin and insulin-like growth factor I. Endocrinology. 1987;121:1845–1852. doi: 10.1210/endo-121-5-1845. [DOI] [PubMed] [Google Scholar]
- 29.Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet.Gynecol. 1999;180:60–63. doi: 10.1016/s0002-9378(99)70150-x. [DOI] [PubMed] [Google Scholar]
- 30.Christensen A. Hormone and enzyme assays in pregnancy. I. Studies on the placental and the tissue cystine-aminopeptidase activity in peripheral plasma from non-pregnant and pregnant women, and in plasma from the umbilical cord. Acta Endocrinol (Copenh) 1974;76:189–200. [PubMed] [Google Scholar]
- 31.Takeyama J, Sasano H, Suzuki T, Iinuma K, Nagura H, Andersson S. 17Beta-hydroxysteroid dehydrogenase types 1 and 2 in human placenta: an immunohistochemical study with correlation to placental development. J Clin Endocrinol Metab. 1998;83:3710–3715. doi: 10.1210/jcem.83.10.5212. [DOI] [PubMed] [Google Scholar]
- 32.Mason JI, Ushijima K, Doody KM, Nagai K, Naville D, Head JR, et al. Regulation of expression of the 3 beta-hydroxysteroid dehydrogenases of human placenta and fetal adrenal. J Steroid Biochem.Mol.Biol. 1993;47:151–159. doi: 10.1016/0960-0760(93)90069-9. [DOI] [PubMed] [Google Scholar]
- 33.Gant NF, Hutchinson HT, Siiteri PK, MacDonald PC. Study of the metabolic clearance rate of dehydroisoandrosterone sulfate in pregnancy. Am J Obstet.Gynecol. 1971;111:555–563. doi: 10.1016/0002-9378(71)90472-8. [DOI] [PubMed] [Google Scholar]
- 34.Buster JE, Chang RJ, Preston DL, Elashoff RM, Cousins LM, Abraham GE, Hobel CJ, Marshall JR. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, delta 5-androstenediol, delta 4-androstenedione, testosterone, and dihydrotestosterone. J Clin Endocrinol Metab. 1979;48:139–142. doi: 10.1210/jcem-48-1-139. [DOI] [PubMed] [Google Scholar]
- 35.Steier JA, Ulstein M, Myking OL. Human chorionic gonadotropin and testosterone in normal and preeclamptic pregnancies in relation to fetal sex. Obstet.Gynecol. 2002;100:552–556. doi: 10.1016/s0029-7844(02)02088-4. [DOI] [PubMed] [Google Scholar]
- 36.Vlkova B, Vavrova S, Szemes T, Minarik G, Turna J, Celec P. Testosterone and estradiol in maternal plasma and their relation to fetal sex. Prenat.Diagn. 2010;30:806–807. doi: 10.1002/pd.2535. [DOI] [PubMed] [Google Scholar]
- 37.Zhu SJ, Li Y, Li H, Wang YL, Xiao ZJ, Vihko P, Piao YS. Retinoic acids promote the action of aromatase and 17beta-hydroxysteroid dehydrogenase type 1 on the biosynthesis of 17beta-estradiol in placental cells. J Endocrinol. 2002;172:31–43. doi: 10.1677/joe.0.1720031. [DOI] [PubMed] [Google Scholar]
- 38.Diaz L, Noyola-Martinez N, Barrera D, Hernandez G, Avila E, et al. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J Reprod.Immunol. 2009;81:17–24. doi: 10.1016/j.jri.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Milczarek R, Sokolowska E, Hallmann A, Kaletha K, Klimek JN. J Steroid Biochem.Mol.Biol. 2008;110:230–235. doi: 10.1016/j.jsbmb.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Williams MA, Sanchez SE, King IB, Ware-Jauregui S, Larrabure G, et al. Plasma concentrations of carotenoids, retinol, and tocopherols in preeclamptic and normotensive pregnant women. Am J Epidemiol. 2001;153:572–580. doi: 10.1093/aje/153.6.572. [DOI] [PubMed] [Google Scholar]
- 41.Williams MA, Woelk GB, King IB, Jenkins L, Mahomed K. Plasma carotenoids, retinol, tocopherols, and lipoproteins in preeclamptic and normotensive pregnant Zimbabwean women. Am J Hypertens. 2003;16:665–672. doi: 10.1016/s0895-7061(03)00897-5. [DOI] [PubMed] [Google Scholar]
- 42.Cackovic M, Buhimschi CS, Zhao G, Funai EF, Norwitz ER, Kuczynski E, et al. Fractional excretion of tumor necrosis factor-alpha in women with severe preeclampsia. Obstet.Gynecol. 2008;112:93–100. doi: 10.1097/AOG.0b013e31817c4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur G, Mishra S, Sehgal A, Prasad R. Alterations in lipid peroxidation and antioxidant status in pregnancy with preeclampsia. Mol.Cell Biochem. 2008;313:37–44. doi: 10.1007/s11010-008-9739-z. [DOI] [PubMed] [Google Scholar]
- 44.Jiang B, Kamat A, Mendelson CR. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2) Mol.Endocrinol. 2000;14:1661–1673. doi: 10.1210/mend.14.10.0539. [DOI] [PubMed] [Google Scholar]
- 45.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Hodyl NA, Wyper H, Osei-Kumah A, Scott N, Murphy VE, Gibson P, Smith R, Clifton VL. Sex-specific associations between cortisol and birth weight in pregnancies complicated by asthma are not due to differential glucocorticoid receptor expression. Thorax. 2010;65:677–683. doi: 10.1136/thx.2009.123091. [DOI] [PubMed] [Google Scholar]
- 47.Clifton VL, Hodyl NA, Murphy VE, Giles WB, Baxter RC, Smith R. Effect of maternal asthma, inhaled glucocorticoids and cigarette use during pregnancy on the newborn insulin-like growth factor axis. Growth Horm.IGF.Res. 2010;20:39–48. doi: 10.1016/j.ghir.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Scott NM, Hodyl NA, Murphy VE, Osei-Kumah A, Wyper H, Hodgson DM, Smith R, Clifton VL. Placental cytokine expression covaries with maternal asthma severity and fetal sex. J.Immunol. 2009;182:1411–1420. doi: 10.4049/jimmunol.182.3.1411. [DOI] [PubMed] [Google Scholar]
- 49.Engel PJ, Smith R, Brinsmead MW, Bowe SJ, Clifton VL. Male sex and pre-existing diabetes are independent risk factors for stillbirth. Aust.N.Z.J.Obstet.Gynaecol. 2008;48:375–383. doi: 10.1111/j.1479-828X.2008.00863.x. [DOI] [PubMed] [Google Scholar]
- 50.Macaulay JO, Warne GL, Krozowski ZS. Human placenta contains a high affinity R1881 binding site that is not the androgen receptor. J Steroid Biochem. 1988;29:497–503. doi: 10.1016/0022-4731(88)90184-7. [DOI] [PubMed] [Google Scholar]
- 51.Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum.Reprod. 1992;7:1461–1466. doi: 10.1093/oxfordjournals.humrep.a137595. [DOI] [PubMed] [Google Scholar]
- 52.Iwamura M, Abrahamsson PA, Benning CM, Cockett AT, di Sant’Agnese PA. Androgen receptor immunostaining and its tissue distribution in formalin-fixed, paraffin-embedded sections after microwave treatment. J Histochem.Cytochem. 1994;42:783–788. doi: 10.1177/42.6.8189040. [DOI] [PubMed] [Google Scholar]
- 53.Chan CC, Lao TT, Ho PC, Sung EO, Cheung AN. The effect of mifepristone on the expression of steroid hormone receptors in human decidua and placenta: a randomized placebo-controlled double-blind study. J Clin Endocrinol Metab. 2003;88:5846–5850. doi: 10.1210/jc.2003-030958. [DOI] [PubMed] [Google Scholar]
- 54.King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am.J.Physiol Endocrinol.Metab. 2007;292:E1837–E1841. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- 55.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Elevated Androgen Levels During Pregnancy Impair Fetal Growth Due to Placental Insufficiency and Programs for Adult Hypertension in Rats. Biology of Reproduction. 2009;83:250. (Abstract) [Google Scholar]
- 56.Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137:865–877. doi: 10.1530/REP-08-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am.J.Physiol Endocrinol.Metab. 2008;295:E262–E268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol.Reprod. 2008;78:636–647. doi: 10.1095/biolreprod.107.065904. [DOI] [PubMed] [Google Scholar]
- 59.Stark MJ, Clifton VL, Wright IM. Neonates born to mothers with preeclampsia exhibit sex-specific alterations in microvascular function. Pediatr.Res. 2009;65:292–295. doi: 10.1203/pdr.0b013e318193edf1. [DOI] [PubMed] [Google Scholar]