Abstract
STUDY QUESTION
Does fibrin introduced into the extracellular matrix affect the growth and maturation of individual primate follicles during encapsulated three-dimensional (3D) culture?
SUMMARY ANSWER
While not altering follicle survival, fibrin–alginate (FIBRIN) improves macaque primary, but not secondary, follicle development during encapsulated 3D culture in terms of growth, steroidogenesis, anti-Müllerian hormone (AMH)/vascular endothelial growth factor (VEGF) production and oocyte maturation.
WHAT IS KNOWN ALREADY
Efforts to grow non-human primate ovarian follicles from the secondary to the antral stage during encapsulated 3D culture have been successful. However, the growth and maturation of primary follicles in vitro has not been reported in primates, especially in chemically defined conditions.
STUDY DESIGN, SIZE, DURATION
In vitro follicle maturation was investigated using the rhesus macaque (Macaca mulatta). Ovaries (n = 7 pairs) were obtained during the early follicular phase of the menstrual cycle (cycle day 1–4). Primary (80–120 µm diameter) and secondary (125–225 µm diameter) follicles were isolated mechanically, randomly assigned to experimental groups, encapsulated into alginate (0.25% w/v) or FIBRIN (25 mg/ml fibrinogen–0.25% alginate) and cultured for 13 and 5 weeks, respectively.
MATERIALS, SETTING, METHODS
Individual follicles were cultured in alpha minimum essential medium supplemented with FSH. Follicle survival and growth were assessed by microscopy. Follicles that reached the antral stage were treated with recombinant hCG. Metaphase II (MII) oocytes were inseminated via ICSI. Follicle morphology was evaluated by hematoxylin and eosin (H&E) staining. Immunohistochemistry was performed for cytochrome P450 family 17 subfamily A polypeptide 1 (CYP17A1) and 19 subfamily A polypeptide 1 (CYP19A1). Culture medium was analyzed for estradiol (E2) and progesterone by chemiluminescence, androstenedione (A4) by radioimmunoassay, as well as anti-Müllerian hormone (AMH) and vascular endothelial growth factor (VEGF) by enzyme-linked immunosorbent assay.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 105 primary and 133 secondary follicles were collected. The presence of fibrin in the alginate matrix had no effect on either primary or secondary follicle survival. Growing primary and secondary follicles formed an antrum at Weeks 9 and 3, respectively. The percentage of growing follicles was higher (P < 0.05) for primary follicles cultured in FIBRIN than alginate at Week 13. The diameters were larger for the growing secondary follicles cultured in alginate than FIBRIN at Week 5 (P < 0.05). H&E staining revealed the typical morphology for small antral follicles. CPY17A1 immunostaining was detected in theca cells, while CYP19A1 was observed in granulosa cells. E2 increased (P < 0.05) during antrum formation in growing follicles at Week 9 for primary and Week 3 for secondary follicles. AMH levels in medium from growing primary follicles increased (P < 0.05) after Week 4 with peak levels at Weeks 9–11. AMH increased (P < 0.05) in growing secondary follicles at Weeks 3–5. VEGF levels in medium were elevated (P < 0.05) in growing primary follicles at Week 9. VEGF increased (P < 0.05) in medium from growing secondary follicles at Weeks 3–5. E2, AMH and VEGF production was higher (P < 0.05) in primary follicle culture with FIBRIN than alginate alone. One primary follicle cultured in FIBRIN (1 of 5 follicles harvested) and a secondary follicle cultured in alginate alone (1 of 15 follicles harvested) yielded an MII oocyte. The fertilized oocyte from primary follicle culture arrested without cell division after fertilization, while the oocyte from secondary follicle culture cleaved and reached the morula stage.
LIMITATIONS, REASONS FOR CAUTION
The study reports on in vitro development and function of individual macaque follicles, that is limited to the interval from the primary and secondary stage to the small antral stage. The findings await translation to human ovarian follicles.
WIDER IMPLICATIONS OF THE FINDINGS
The 3D model for primate follicle development offers a unique opportunity to investigate the growth and regulation of primate primary, as well as secondary follicles, and their enclosed oocytes, as they grow to the antral stage by monitoring and manipulating factors or signaling pathways in vitro. Since primate primary follicles, in addition to secondary follicles, can be cultured to the antral stage to provide mature oocytes, they represent an additional source of pre-antral follicles for in vitro follicle maturation with the potential to provide gametes for assisted reproductive technology as an option for fertility preservation in women, including patients with cancer.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by The Oncofertility Consortium (NIH U54 RR024347-HD058294, PL1-EB008542), NIH U54-HD18185 (Eunice Kennedy Shriver Specialized Cooperative Centers Program in Reproduction and Infertility Research), NIH ORWH/NICHD 2K12HD043488 (BIRCWH), Oregon National Primate Research Center 8P51OD011092. There are no conflicts of interest.
Keywords: macaque, follicle culture, primary follicle, secondary follicle, fibrin
Introduction
Recent efforts to grow non-human primate ovarian follicles from the secondary to the small antral stage have been successful using encapsulated three-dimensional (3D) culture. In vitro-developed follicles can become functional, as judged by steroidogenesis, paracrine/autocrine factor production and oocyte maturation (Xu et al., 2009, 2010, 2011a,b). In vitro follicle maturation provides a model to help in understanding the process and regulation of folliculogenesis in primates, including gene and protein expression, as well as metabolic pathways during follicular development. The information obtained may be valuable in identifying the optimal conditions for primate follicle culture prior to human application in order to provide options for fertility preservation in women, including patients with cancer (Woodruff, 2007).
Since tissue resources from women are limited, efforts are warranted to utilize the ovarian tissue more efficiently. Smaller and less mature primary follicles represent a larger follicle population than secondary follicles in the ovary (Gougeon, 1986), and are more tolerant of ovarian tissue cryopreservation. Evidence indicates that primary follicles are more likely to survive than secondary follicles during the freeze and thaw procedure, as well as maintain normal morphology and ultrastructure (Hovatta et al., 1996; Nottola et al., 2008; Ting et al., 2011). Stepwise strategies were utilized in mice (Lenie et al., 2004; Jin et al., 2010; Choi et al., 2011) and human (Telfer et al., 2008) ovarian cortical slices to induce transition of the follicle population from primary follicles to secondary follicles for further in vitro maturation. An ovarian stromal cell co-culture system was also attempted for mouse primary follicles (Tingen et al., 2011). Viable embryos were successfully generated through in vitro follicle maturation of mice primary follicles (Jin et al., 2010; Choi et al., 2011). However, efforts to grow and mature primary follicles in vitro in primates have not been reported, especially in chemically defined conditions.
An alginate hydrogel matrix was used to support the 3D architecture and to maintain cell–cell interactions of secondary follicles during in vitro follicle maturation in primates (Xu et al., 2009, 2010, 2011a). Advances in biomaterial engineering resulted in the development of a fibrin–alginate (FIBRIN) interpenetrating network for 3D follicle culture (Shikanov et al., 2009). Fibrinogen is a soluble 340 kDa protein that is polymerized to form fibrin through the action of thrombin in the presence of calcium. While fibrin is degraded by proteases secreted from growing follicles, alginate, a naturally derived polysaccharide produced by brown algae, remains as an inert scaffold to provide mechanical support to the follicle. The combination of fibrin and alginate provides a dynamic mechanical environment for follicle growth. Mouse (Jin et al., 2010) and baboon (Xu et al., 2011b) secondary follicles cultured in FIBRIN grew to the antral stage and achieved oocyte meiotic maturation.
Previous studies on monkey secondary follicles indicate that in vitro-developed follicles produce ovarian steroids, including estradiol (E2), androstenedione (A4) and progesterone (Xu et al., 2009, 2010, 2011a). Since A4 production is generally limited to the theca layer (Tamura et al., 1992), this encapsulated 3D system may support theca cell development or function. Theca cell formation was reported following mouse follicle culture (Xu et al., 2006; Itami et al., 2011); however, it has not yet been conclusively demonstrated that theca cells are present in in vitro-developed primate follicles. Besides steroidogenesis, cultured individual macaque follicles also produce local regulatory factors, such as anti-Müllerian hormone (AMH) and vascular endothelial growth factor (VEGF) (Xu et al., 2010, 2011a). AMH and VEGF concentrations in culture medium correlate positively with follicle growth rate in vitro (Xu et al., 2010, 2011a). Moreover, follicles that produced more AMH (Xu et al., 2011a) and VEGF (unpublished data) yielded metaphase II (MII) oocytes during in vitro follicle maturation.
Therefore, we designed experiments to investigate the effect of FIBRIN matrix on supporting the survival and growth of individually cultured rhesus macaque (Macaca mulatta) primary and secondary follicles. Cultured follicles were also assessed for their ability to achieve endocrine (steroid hormones) and paracrine (AMH and VEGF) secretory functions, theca-granulosa cell compartmentalization and function (synthesis of steroidogenic enzymes), as well as oocyte maturation and subsequent embryonic development in vitro.
Materials and Methods
Animals and ovary collection
The general care and housing of rhesus monkeys was provided by the Division of Comparative Medicine, Oregon National Primate Research Center (ONPRC). Animals were caged in pairs in a temperature-controlled (22°C) light-regulated 12L:12D room. Diet consisted of Purina monkey chow (Ralston-Purina, Richmond, IN, USA), provided twice a day, supplemented with fresh fruit or vegetables once a day and water ad libitum. Animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and protocols were approved by the ONPRC Institutional Animal Care and Use Committee (Xu et al., 2010, 2011a).
Adult female rhesus macaques (n = 7; 7–14 years of age) exhibiting regular menstrual cycles of ∼28 days were evaluated daily for menstruation, with the first day of menses termed Day 1 of the cycle. Ovariectomies were conducted on anesthetized monkeys by laparoscopy at early follicular phase, Days 1–4 of the cycle, as previously described (Duffy and Stouffer, 2002). Ovaries were immediately transferred into HEPES-buffered holding media (CooperSurgical, Inc., Trumbull, CT, USA) supplemented with 0.2% (v/v) human serum protein supplement (CooperSurgical, Inc.) and 10 µg/ml gentamicin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C (Xu et al., 2010, 2011a).
Follicle isolation, encapsulation and culture
Follicle isolation and encapsulation were performed as previously described (Xu et al., 2009) with minor modifications. All procedures were conducted at 37°C. Briefly, ovarian cortex was cut into 1 mm × 1 mm × 1 mm cubes. Follicles were mechanically isolated in the holding media using 31-gauge needles. Primary (diameter = 80–120 µm) and secondary (diameter = 125–225 µm) follicles that displayed the following characteristics were selected for encapsulation: (1) an intact basement membrane, (2) healthy (i.e. typical) granulosa cells and (3) a visible, healthy oocyte that was round and centrally located within the follicle, without vacuoles or dark cytoplasm. Follicles from each monkey (mean ± SEM = 15 ± 2 primary follicles/monkey and 19 ± 3 secondary follicles/monkey; n = 7 monkeys) were randomly assigned to two groups for subsequent encapsulation with either alginate or FIBRIN matrix.
Follicles were encapsulated individually into 5 µl alginate or FIBRIN. Alginate matrix contained 0.25% (w/v) sterile sodium alginate (FMC BioPolymers, Philadelphia, PA, USA)–phosphate-buffered saline (PBS) (137 mM NaCl, 10 M phosphate, 2.7 mM KCl, Invitrogen, Carlsbad, CA, USA) and was cross-linked in 50 mM CaCl2, 140 mM NaCl and 10 mM HEPES solution (pH = 7.2). For follicle encapsulation in FIBRIN, fibrinogen (25 mg/ml; Tisseel [Fibrin Sealant], Baxter Healthcare, Inc., Deerfield, IL, USA)–alginate (0.25% w/v) was dropped into the cross-linking solution (as described above) containing 50 mg/ml thrombin (Tisseel [Fibrin Sealant]). Each encapsulated follicle was transferred into an individual well of 48-well plates containing 300 µl alpha minimum essential medium (αMEM, Invitrogen) supplemented with 3 ng/ml recombinant human FSH (NV Organon, Oss, The Netherlands), 0.3% (v/v) human serum protein supplement, 5 µg/ml insulin, 0.5 mg/ml purified bovine fetuin, 5 µg/ml transferrin and 5 ng/ml sodium selenite (Sigma-Aldrich) (Xu et al., 2011a).
Encapsulated follicles were cultured at 37°C in a 5% (v/v) O2 environment (in 6% CO2/89% N2) for 13 (for primary follicles) or 5 (for secondary follicles) weeks. Follicles that reached the antral stage were treated with 100 ng/ml recombinant hCG (Merck Serono, Geneva, Switzerland) for 34 h. Oocytes were retrieved to determine the size and stage of meiotic maturation. Half of the culture media (150 µl) was collected and replaced every other day and stored at −20°C (i.e. three samples per week). The media samples from each week of culture were assayed for ovarian steroids (E2, A4 and progesterone), AMH and VEGF.
Follicle survival and growth
Follicle survival, diameter and antrum formation were assessed weekly using an Olympus CK40 inverted microscope and photographed using an Olympus DP11 digital camera (Olympus Imaging America, Inc., Center Valley, PA, USA) as described previously (Xu et al., 2009). Photographs were imported into ImageJ 1.42 software (National Institutes of Health, Bethesda, MD, USA) and the diameter of each follicle was measured. Follicles were measured from the outer layer of cells and included a measurement at the widest diameter of the follicle and a second measurement perpendicular to the first. The mean of the values was calculated and reported as the follicle diameter. Follicles were considered to be undergoing atresia if the oocyte was dark or not surrounded by a layer of granulosa cells, the granulosa cells became dark and fragmented or the diameter of the follicle decreased (Xu et al., 2009).
Oocyte retrieval, maturation and fertilization
Oocyte retrieval and evaluation were performed on a 37°C warming plate. Cumulus–oocyte complexes were released by breaking the follicle wall (using needles) in Tyrode's albumin lactate pyruvate (TALP)-HEPES-BSA (bovine serum albumin 0.3%) media. Cumulus–oocyte complexes were treated with 2 mg/ml hyaluronidase (Sigma-Aldrich) in TALP–HEPES–BSA for 30 s to dissociate cumulus cells and obtain denuded oocytes. Retrieved oocytes were transferred to TALP medium and photographed. Oocyte diameters (excluding the zona pellucida) and meiotic status were assessed using the same camera and software as described above.
MII oocytes were maintained in TALP medium at 37°C in 6% CO2, 5% O2 and 89% N2 for ICSI within 3 h of oocyte retrieval. Semen collection and ICSI was performed by the ART/Embryonic Stem Cell Support Core at the ONPRC, as reported previously (Lanzendorf et al., 1990; Meng and Wolf, 1997). The resulting zygotes were transferred to 500 μl hamster embryo culture medium-9 with 5% fetal bovine serum and cultured at 37°C in 6% CO2, 5% O2 and 89% N2 (Schramm and Paprocki, 2000). The embryo was photographed daily to document development. Reagents and protocols for embryo culture were provided by the ART Core (Meng and Wolf, 1997).
Follicle histology and immunostaining for steroidogenic enzymes
In vitro-developed antral follicles were fixed in 4% paraformaldehyde–PBS solution for 3 h at room temperature. Follicles were embedded in HistoGel (Thermo Scientific, Kalamazoo, MI, USA) before being dehydrated in ascending concentrations of ethanol (70–100%) and embedded in paraffin. Five micrometer sections were cut by the Imaging and Morphology Support Core at ONPRC, and stained with hematoxylin and eosin (H&E).
Immunohistochemistry was performed on follicle sections for cytochrome P450 family 17 subfamily A polypeptide 1 (CYP17A1) and cytochrome P450 family 19 subfamily A polypeptide 1 (CYP19A1) protein. Follicle sections were deparaffinized and hydrated through xylene (Fisher Scientific, Pittsburgh, PA, USA) and a graded series of ethanol. Sections were rehydrated in PBS, followed by incubation in 3% hydrogen peroxide/60% methanol to quench endogenous peroxidase activity. Sections were incubated at 4°C overnight with primary antibodies (1:750 for rabbit anti-bovine CYP17A1 antibody provided by Dr Alan J. Conley, University of California, Davis, CA, USA; 1:100 for goat anti-human CYP19A1 antibody, sc-14245 from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). As negative controls, antibodies preabsorbed with blocking peptides (Santa Cruz Biotechnology) at 4°C overnight were incubated on adjacent follicle sections. Slides were then incubated with the appropriate secondary antibodies and processed using a VECTASTAIN Elite ABC Kit from Vector Laboratories, Inc. (Burlingame, CA, USA). The antigen–antibody complex was visualized by incubation with 3,3′-diaminobenzidine. The follicles were counterstained using hematoxylin and images were captured via an Olympus BX40 inverted microscope and an Olympus DP72 digital camera (Olympus Imaging America Inc.). Positive control staining was performed on previously archived macaque ovarian tissue sections, containing small antral and pre-ovulatory follicles, during spontaneous menstrual cycles.
Ovarian steroids, AMH and VEGF assays
One medium sample collected weekly was analyzed for E2 and progesterone concentrations by the Endocrine Technology Support Core at ONPRC using an Immulite 2000, a chemiluminescence-based automatic clinical platform (Siemens Healthcare Diagnostics, Deerfield, IL, USA), validated for macaque follicle culture media (Xu et al., 2009). A4 levels in medium were measured by radioimmunoassay using a DSL-3800 kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA) also validated for macaque follicle culture media (Xu et al., 2010).
Another two medium samples collected weekly were analyzed for AMH and VEGF concentrations by enzyme-linked immunosorbent assay using a DSL-10-14400 kit (Diagnostic Systems Laboratories, Inc.) and a Human VEGF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA), respectively, based on the manufacturers' instructions (Christenson and Stouffer 1997; Fréour et al., 2007), and validated for macaque follicle culture media (Christenson and Stouffer 1997; Xu et al., 2010). Owing to cross reaction of fetuin with the AMH antibody, levels assayed in media containing fetuin but no cultured follicles were subtracted from AMH levels in media samples, as previously described (Xu et al., 2010).
Statistical analysis
Statistical significance was analyzed by SigmaPlot 11 software (SPSS, Inc., Chicago, IL, USA) using a two-way analysis of variance (ANOVA) with repeated measures or one-way ANOVA followed by the Student–Newman–Keuls post hoc test for single time points. Comparisons of the two groups of follicles were made at each time point. A Student's t-test was used to compare follicles cultured with alginate and FIBRIN. Differences were considered significant at P < 0.05 and values are presented as mean ± SEM. Follicle survival represents the percentage (mean ± SEM) of seven individual animals in each culture group. Follicle growth, steroid, AMH and VEGF production, and oocyte maturation were analyzed for each individual follicle with total follicle numbers indicated in the figure legends, and represent follicles obtained from the seven individual animals.
Results
Follicle survival
Macaque primary and secondary follicles survived in both alginate and FIBRIN culture (Table I). The presence of fibrin in the alginate matrix (FIBRIN) had no effect on either primary or secondary follicle survival, compared with alginate alone. However, primary follicles had a lower (P < 0.05) survival rate than secondary follicles cultured in FIBRIN.
Table I.
Characteristics of rhesus macaque follicles at Week 13 for primary and Week 5 for secondary follicles in culture
| Follicle culture | Total yield (n) | Percentage of follicles cultured |
Percentage of follicles survived |
|||
|---|---|---|---|---|---|---|
| Degenerated | Survived | No grow | Growing | |||
| Primary follicle | ||||||
| Alginate | 48 | 63 ± 9a | 37 ± 9a | 72 ± 4a | 28 ± 4a | |
| FIBRIN | 57 | 62 ± 14a | 38 ± 14a | 64 ± 3b | 36 ± 3b | |
| Secondary follicle | Slow grow | Fast grow | ||||
| Alginate | 45 | 47 ± 11a,b | 53 ± 11a,b | 40 ± 14c | 34 ± 14a | 26 ± 7b |
| FIBRIN | 88 | 28 ± 10b | 72 ± 10b | 72 ± 12a | 17 ± 8a | 11 ± 7c |
FIBRIN: alginate plus fibrin.
No-grow: surviving follicles which remained at the pre-antral stage throughout the culture were termed ‘no-grow’.
a,b,cDifferent letters indicate significant differences within the column (P < 0.05).
*Values are mean ± SEM of percentage of follicles.
Follicle growth
At the beginning of culture, neither primary (alginate versus FIBRIN = 109 ± 4 versus 112 ± 3 µm) nor secondary (alginate versus FIBRIN = 154 ± 8 versus 170 ± 8 µm) follicle diameters differed between treatment groups.
Primary follicles
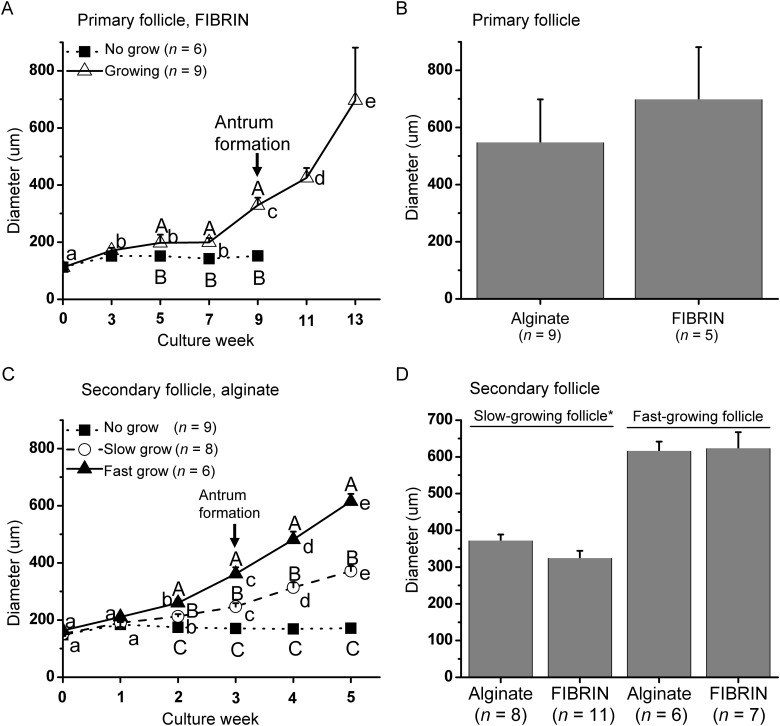
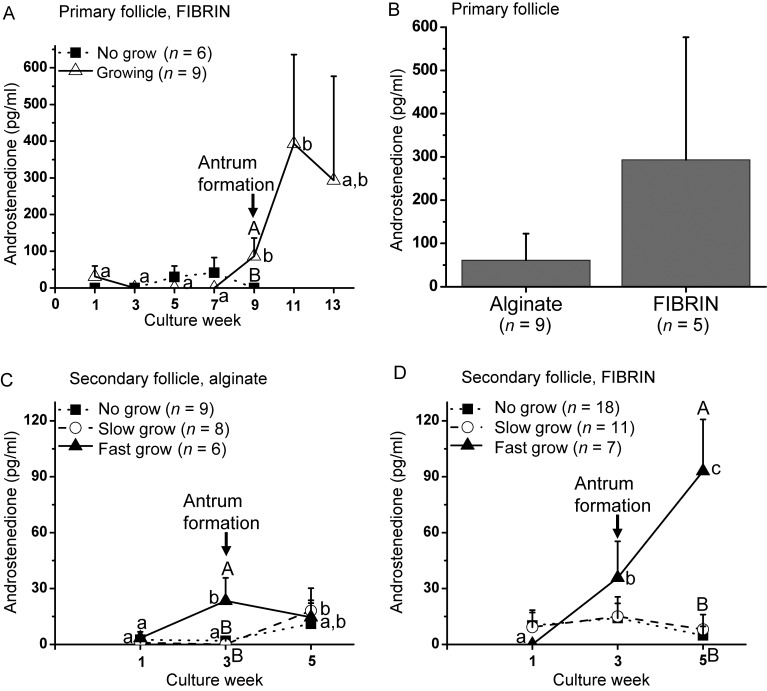
Primary follicles (Fig. 2A) required a long culture interval of 13 weeks to grow in both alginate (data not shown) and FIBRIN (Fig. 1A) matrices. Some of the surviving follicles remained at the pre-antral stage throughout 13 weeks of culture, which were termed ‘no-grow’ (NG) follicles, and did not show signs of atresia. All growing follicles reached the secondary stage at Week 4 (Fig. 2B), formed an antrum at Week 9 (Figs 1A and 2C), and were >500 µm in diameter at Week 13 (Figs 1A and 2D). Fibrin had no effect on the timing of antrum formation (data not shown) or follicle diameters at the end of culture (Fig. 1B). However, the percentage of the growing follicles was higher (P < 0.05) for FIBRIN than alginate culture (Table I).
Figure 2.
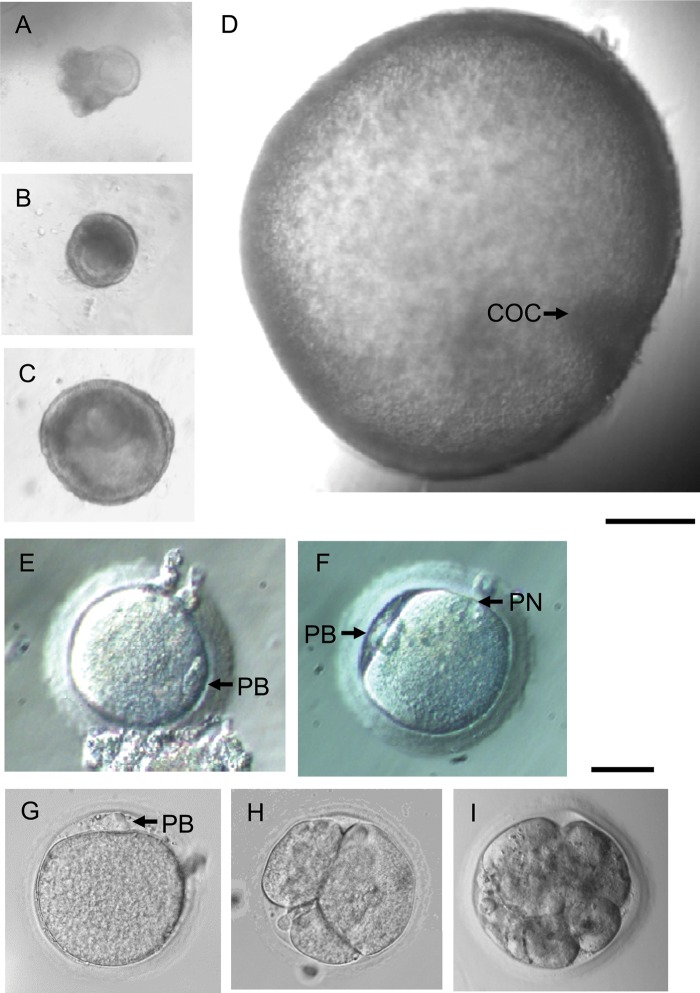
Macaque follicle growth, oocyte maturation, fertilization and embryonic development in vitro. Primary follicle (A), with a single layer of granulosa cells, a centrally located immature oocyte, and attached stromal cells, was encapsulated into FIBRIN. The follicle reached the secondary stage (B) in 4 weeks with granulosa cell proliferation. The follicle maintained its three-dimensional structure and formed an antrum (C) at Week 9. By Week 13, the follicle achieved the small antral stage with a visible cumulus–oocyte complex (COC) (D). Following recombinant hCG treatment, a metaphase II (MII) oocyte was retrieved (E). After ICSI, fertilization was confirmed by the presence of two polar bodies (PB) and two pronuclei (PN) (F). For comparison, a secondary follicle cultured in alginate grew to the small antral stage and yielded an MII oocyte (G), which was inseminated by ICSI. The embryo cleaved to 3 cells (H) 2 days after ICSI, and developed to the morula stage (I) at day 5 post-ICSI. Scale bar = 200 µm for follicles and 50 µm for oocytes and embryos.
Figure 1.
Growth of follicles from rhesus macaque (Macaca mulatta), which survived encapsulated three-dimensional culture: (A) growth patterns of primary follicles cultured in FIBRIN (alginate plus fibrin), (B) diameter of growing primary follicles at Week 13 of culture, (C) growth patterns of secondary follicles cultured in alginate and (D) diameter of slow- and fast-growing follicles at Week 5 for secondary follicle culture. Significant differences over time (lowercase) or between-follicle categories (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 7 animals per group. *P < 0.05.
Secondary follicles
During 5 weeks of culture in either alginate or FIBRIN, three distinct cohorts of surviving secondary follicles were observed based on their growth rate as NG, ‘slow-growing’ (SG), and ‘fast-growing’ (FG) follicles (Xu et al., 2010; 2011a) (Fig. 1C). An antrum was evident within 3 weeks of culture in all SG and FG follicles. Fibrin had no effect on the timing of antrum formation (data not shown). However, diameters were larger (P < 0.05) for SG follicles cultured in alginate than FIBRIN at Week 5 (Fig. 1D). More (P < 0.05) FG follicles were obtained when cultured with alginate alone than FIBRIN (Table I).
Oocyte diameter, maturation and fertilization
Following exposure of antral follicles to hCG, healthy as well as degenerated (dark and condensed cytoplasm) oocytes were retrieved from both primary and secondary follicle cultures regardless of culture matrix. Most of the healthy oocytes remained at the germinal vesicle (GV) stage (Table II).
Table II.
Characteristics of rhesus macaque oocytes retrieved from antral follicles at Week 13 for primary and Week 5 for secondary follicle culture (34 h after addition of hCG)
| Follicle culture |
N |
Diameter (µm)* |
|||||
|---|---|---|---|---|---|---|---|
| Follicles harvested | Oocytes retrieved | Degenerated oocytes | Healthy oocytes |
GV intact oocytes | MII oocytes | ||
| GV intact | MII stage | ||||||
| Primary follicle | |||||||
| Alginate | 3 | 3 | 0 | 3 | 0 | 113 ± 1a | – |
| FIBRIN | 5 | 5 | 1 | 3 | 1 | 106 ± 2a | 108 |
| Secondary follicle | |||||||
| Alginate | 15 | 13 | 5 | 7 | 1 | 102 ± 6a | 118 |
| FIBRIN | 9 | 9 | 4 | 5 | 0 | 81 ± 7b | – |
MII, metaphase II; GV, germinal vesicle.
*Values are mean ± SEM of individual oocyte diameters.
a,bDifferent letters indicate significant differences within the column (P < 0.05).
Primary follicles
There was no difference in the oocyte diameter between the two matrices (Table II). One primary follicle cultured in FIBRIN yielded an MII oocyte (Fig. 2E). Following insemination by ICSI, fertilization was confirmed by the presence of two polar bodies and two pronuclei (Fig. 2F). However, the zygote arrested without cell division.
Secondary follicles
The harvested oocytes were larger (P < 0.05) in secondary follicles cultured in alginate alone than FIBRIN (Table II). One secondary follicle cultured in alginate yielded an MII oocyte (Fig. 2G). After ICSI, the fertilized oocyte cleaved to three cells in 2 days (Fig. 2H). Subsequent cleavage was observed and the embryo developed to the morula stage at Day 5 post-ICSI (Fig. 2I).
Follicle histology and steroidogenic enzyme immunostaining
Primary follicles
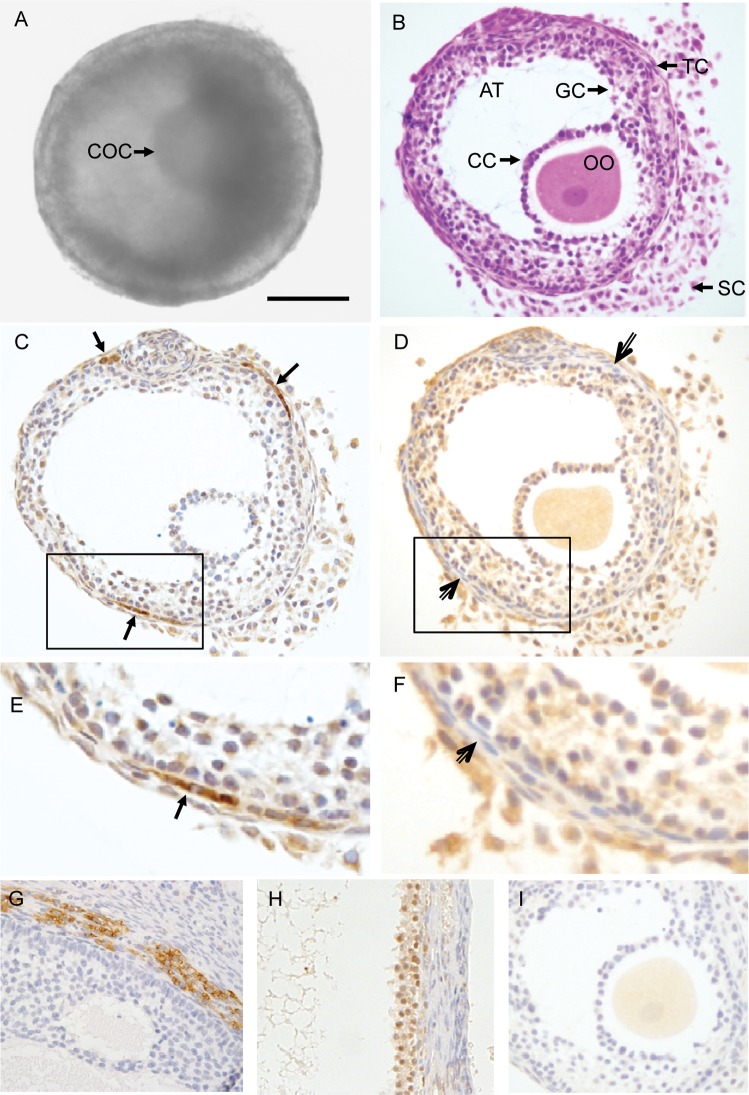
Figure 3A depicts a small antral follicle which developed from a primary follicle cultured in FIBRIN. H&E staining of the follicle revealed a morphology similar to that observed in in vivo-developed small antral follicles in primates (Gougeon, 2004), i.e. a spherical shape with an antrum, a healthy GV oocyte with surrounding cumulus cells, an intact granulosa layer, presumptive theca cells and stroma cells (Fig. 3B).
Figure 3.
Histology and immunohistochemical staining of an in vitro-developed macaque follicle. An early antral follicle (A) developed from a primary follicle cultured in FIBRIN was stained with hematoxylin and eosin (B), and immunostained for cytochrome P450 family 17 subfamily A polypeptide 1 (CYP17A1) (C) and cytochrome P450 family 19 subfamily A polypeptide 1 (CYP19A1) (D). The image in the box of C was enlarged in (E), and D was enlarged in (F). Positive control staining for CYP17A1 and CYP19A1 on previously archived macaque ovarian tissue sections is illustrated in (G) and (H), respectively. The non-specific staining associated with the preabsorbed CYP19A1 antibody is illustrated in (I). Arrows, positive staining for CYP17A1 in theca cells. Open arrows, theca cells negative for CYP19A1 staining. Scale bar = 100 µm. COC, cumulus–oocyte complex; OO, oocyte; AT, antrum; CC, cumulus cells; GC, granulosa cells; TC, theca cells; SC, stroma cells.
Specific cytoplasmic immunostaining for CPY17A1 appeared to concentrate in limited areas of theca cells (Fig. 3C and E), while cytoplasmic staining for CPY19A1 was detected in granulosa, possibly stroma, but not theca, cells (Fig. 3D and F). Positive control staining on ovarian tissue sections localized CPY17A1 in the theca layer of small antral follicles (Fig. 3G) and CYP19A1 in the granulosa layer of the pre-ovulatory follicle (Fig. 3H). Immunostaining for CYP19A1 in the oocyte of cultured follicles (Fig. 3D) appears to be non-specific since similar staining was also observed in the oocyte when the follicle section was incubated with primary antibody preabsorbed with blocking peptide (Fig. 3I).
Secondary follicles
The staining of small antral follicles that developed from secondary follicles was similar to that described for primary follicles, regardless of culture matrix (data not shown).
Follicular steroids
Cultured follicles secreted ovarian steroids into the medium regardless of the initial (primary or secondary) stage of development or culture conditions.
Primary follicles
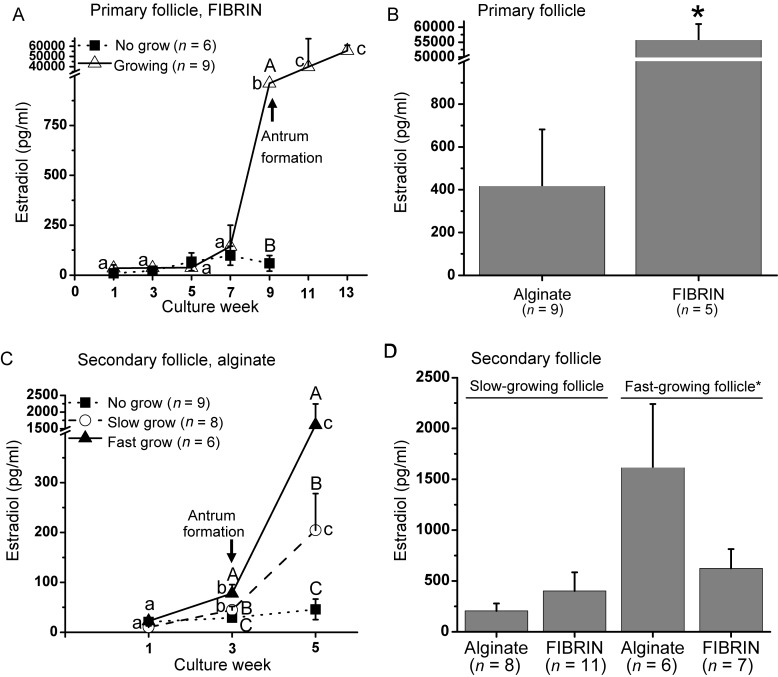
For primary follicles cultured in FIBRIN, E2 levels in medium did not change in NG follicles throughout the culture period (Fig. 4A). In contrast, E2 produced by growing follicles increased (P < 0.05) at Week 9, when levels were greater (P < 0.05) than that of NG follicles, and remained at high levels through Week 13. Similar patterns were observed for E2 released from primary follicles cultured in alginate (data not shown), but levels were lower (P < 0.05) at Week 13 for growing follicles in alginate, compared with FIBRIN (Fig. 4B).
Figure 4.
Estradiol (E2) levels in the culture medium of in vitro-developed macaque follicles: (A) E2 secretion patterns of primary follicles cultured in FIBRIN, (B) E2 levels of growing follicles at Week 13 for primary follicle culture, (C) E2 secretion patterns of secondary follicles cultured in alginate, (D) E2 levels of slow- and fast-growing follicles at Week 5 for secondary follicle culture. Significant differences over time (lowercase) or between-follicle categories (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 7 animals per group. *P < 0.05.
Patterns of A4 production by primary follicles cultured in alginate (data not shown) or FIBRIN (Fig. 5A) were similar to those of E2, except that A4 levels peaked (P < 0.05) at Week 9 without further increase (levels of A4 were similar at Weeks 9 and 11). Mean A4 levels in FIBRIN culture were 5-fold higher than those of alginate culture at Week 13, though variable (Fig. 5B).
Figure 5.
Androstenedione (A4) levels in the culture medium of in vitro-developed macaque follicles: (A) A4 secretion patterns of primary follicles cultured in FIBRIN, (B) A4 levels of growing follicles at Week 13 for primary follicle culture, (C) A4 secretion patterns of secondary follicles cultured in alginate, (D) A4 secretion patterns of secondary follicles cultured in FIBRIN. Significant differences over time (lowercase) or between-follicle categories (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 7 animals per group.
Progesterone production by primary follicles during culture followed similar patterns as those of A4 regardless of culture matrix (data not shown).
Secondary follicles
For secondary follicles cultured in alginate, E2 levels remained at baseline throughout culture for NG follicles. E2 concentrations in SG and FG follicles increased (P < 0.05) at Week 3 and reached greater (P < 0.05) levels at Week 5, which were higher (P < 0.05) than those of NG follicles (Fig. 4C). E2 production patterns were similar between follicles cultured with alginate and FIBRIN (data not shown), except that E2 levels of FG follicles were greater (P < 0.05) in alginate versus FIBRIN culture (Fig. 4D).
For secondary follicles cultured in alginate alone, A4 levels of NG follicles did not change throughout the culture. Unlike E2 levels, A4 secreted by SG follicles did not increase (P < 0.05) until Week 5. A4 produced by FG follicles peaked (P < 0.05) at Week 3, which was higher (P < 0.05) than that of SG and NG follicles, without further increase (Fig. 5C). When secondary follicles were cultured in FIBRIN, A4 concentrations from both NG and SG follicles remained at the baseline during 5 weeks of culture. In contrast, A4 levels increased (P < 0.05) at Week 3 for FG follicles and reached higher (P < 0.05) levels at Week 5, which were greater (P < 0.05) than those of NG and SG follicles (Fig. 5D).
Progesterone production by secondary follicles during culture followed similar patterns as those of A4 regardless of culture matrix (data not shown).
AMH
AMH was detectable in medium from both primary and secondary follicles cultured in alginate or FIBRIN.
Primary follicles
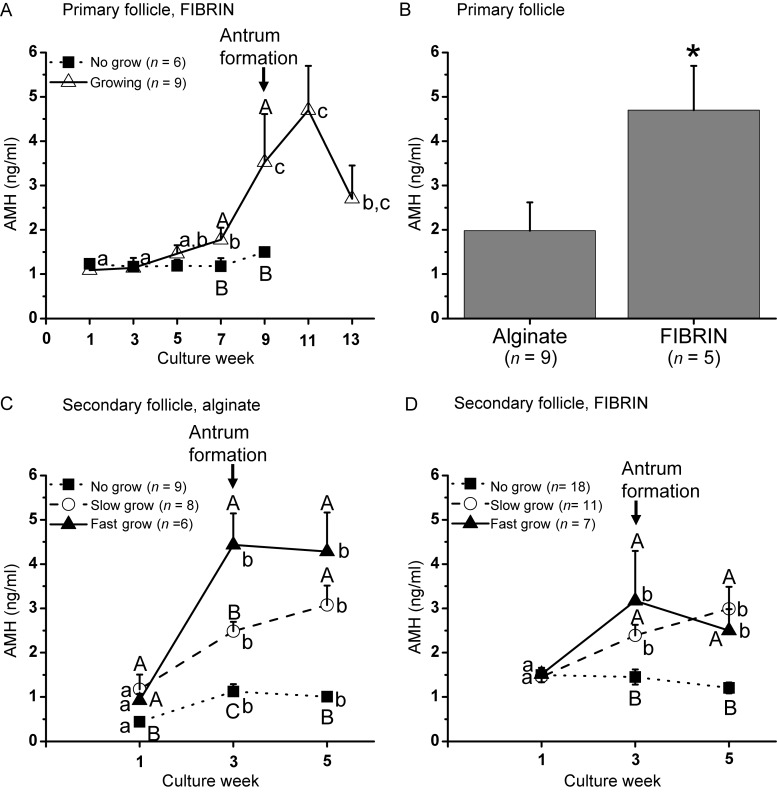
For primary follicles cultured in FIBRIN, AMH levels produced by NG follicles did not change throughout the culture. AMH increased (P < 0.05) in growing follicles at Week 7 with peak levels at Week 9 (AMH levels were similar at Weeks 9 and 11), which were higher (P < 0.05) than those of NG follicles (Fig. 6A). Similar patterns were obtained for AMH produced by primary follicles cultured in alginate alone, except that the levels were lower (P < 0.05) than those of FIBRIN at Week 11 (Fig. 6B).
Figure 6.
Anti-Müllerian hormone (AMH) levels in the culture medium of in vitro-developed macaque follicles: (A) AMH secretion patterns of primary follicles cultured in FIBRIN, (B) AMH levels of growing follicles at Week 11 for primary follicle culture, (C) AMH secretion patterns of secondary follicles cultured in alginate, (D) AMH secretion patterns of secondary follicles cultured in FIBRIN. Significant differences over time (lowercase) or between-follicle categories (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 7 animals per group. *P < 0.05.
Secondary follicles
For secondary follicles cultured in alginate, AMH produced by SG and FG follicles at Week 1 was higher (P < 0.05) than that of NG follicles. AMH levels increased (P < 0.05) at Week 3 and remained at similar levels without further rise at Week 5. Moreover, AMH levels at Week 3 differed (P < 0.05) among all three follicle categories (Fig. 6C). When cultured in FIBRIN, SG and FG follicles produced higher (P < 0.05) levels of AMH during Weeks 3–5 than NG follicles. However, the levels were not distinct between SG/FG and NG at Week 1, or SG and FG at Week 3 (Fig. 6D).
VEGF
Growing primary and secondary follicles cultured in the alginate and FIBRIN matrices produced VEGF.
Primary follicles
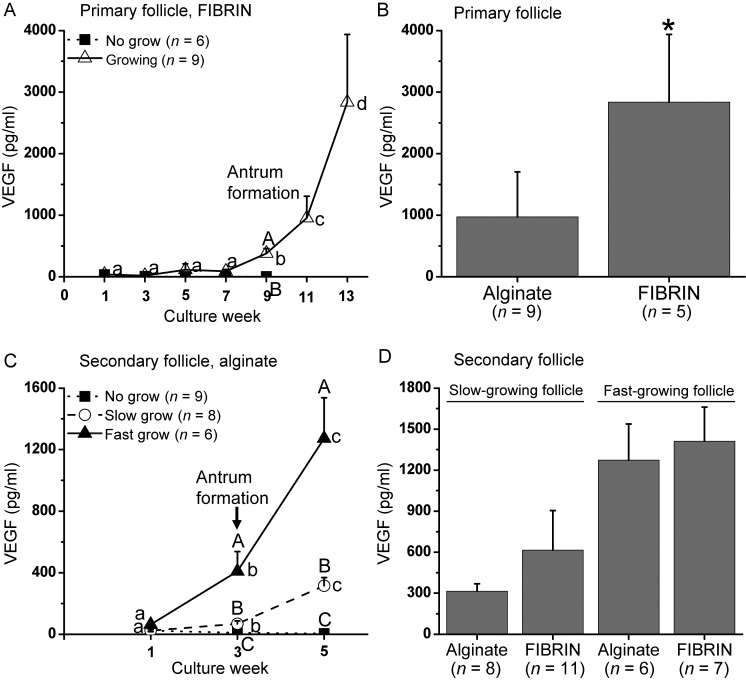
VEGF in culture medium from NG primary follicles was non-detectable. However, VEGF secretion from growing primary follicles increased (P < 0.05) at Week 9 in FIBRIN culture, reaching higher (P < 0.05) levels than those in NG follicles. VEGF levels remained high for growing follicles thereafter (Fig. 7A). Primary follicles cultured in alginate displayed similar patterns for VEGF production as those of FIBRIN (data not shown), except that VEGF production of growing follicles was lower (P < 0.05) at Week 13 than FIBRIN culture (Fig. 7B).
Figure 7.
Vascular endothelial growth factor (VEGF) levels in the culture medium of in vitro-developed macaque follicles: (A) VEGF secretion patterns of primary follicles cultured in FIBRIN, (B) VEGF levels of growing follicles at Week 13 for primary follicle culture, (C) VEGF secretion patterns of secondary follicles cultured in alginate, (D) VEGF levels of slow- and fast-growing follicles at Week 5 for secondary follicle culture. Significant differences over time (lowercase) or between-follicle categories (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 7 animals per group. *P < 0.05.
Secondary follicles
For secondary follicles cultured in alginate, VEGF increased (P < 0.05) at Weeks 3 and 5 in SG and FG follicles. VEGF levels were greater (P < 0.05) in SG and FG than NG follicles at Weeks 3 and 5, and differed (P < 0.05) among all three follicle categories (Fig. 7C). The presence of fibrin did not alter VEGF production patterns or concentrations during secondary follicle culture (Fig. 7D).
Discussion
Addition of fibrin to the alginate matrix improves the growth of primary follicles from macaques, as well as increases follicular E2, AMH and VEGF production in vitro. Studies in rodent follicle 3D culture revealed a direct link between the biomechanical environment and follicle function, and suggested a non-hormonal mechanism regulating follicular development (West et al., 2007). The FIBRIN matrix may better mimic the natural ovarian environment, in which primary follicles reside in a rigid cortex relative to the more permissive medulla, as they increase in size. For the first time, we have shown that a primate oocyte retrieved from an in vitro-developed primary follicle displayed the competence to reinitiate meiosis for fertilization. Effort is now required in order to improve the competence of oocytes for further embryonic development.
In contrast to primary follicles, fibrin has a negative impact on secondary follicle development during culture. Secondary follicles cultured in FIBRIN had a lower growth rate, produced lower levels of E2 and AMH, and generated oocytes with a smaller diameter compared with the alginate alone. FIBRIN was developed to provide dynamic cell-responsive mechanical properties for cultured follicles through degradation of fibrin by follicle-secreted proteases (Shikanov et al., 2009). Since secondary follicles experience a more rapid volume expansion during in vitro growth, forming an antrum at Week 3 versus 9 for primary follicles, a lower compressive force from the embedding matrix may be needed within a relatively shorter culture period. It could be that the solid content in FIBRIN is not reduced rapidly enough by growing secondary follicles to provide an appropriate physical environment for further follicle growth.
Three groups of surviving secondary follicles with different growth rates were observed in the current study, as well as our previous (Xu et al., 2010, 2011a) studies, in macaques. Interestingly, culture of primary follicles generated growth patterns which differed from those of secondary follicles. Surviving primary follicles either remained at the small, secondary stage without antrum formation throughout the culture, or formed an antrum and achieved a diameter of >500 µm. Our previous studies indicated that the population of secondary follicles in the primate ovary at the early follicular phase of the cycle is heterogeneous in their capacity to grow in an FSH-replete milieu (Xu et al., 2010, 2011a). Their growth rate may depend upon their ability, at initial retrieval, to recognize or respond to FSH (Kreeger et al., 2005) or other hormones (e.g. insulin) (Xu et al., 2010), or to synthesize and respond to other local factors that modulate follicular growth (e.g. AMH and VEGF) (Xu et al., 2010, 2011a). In vitro follicular development from the primary to secondary stage in a less complex environment than that in vivo may reduce variation in characteristics of the follicle population, which could result in different growth patterns subsequently. This is also reflected in the observation that fewer degenerated oocytes were obtained from primary than secondary follicle culture. In the current study, it took 4 weeks for primary follicles to reach early secondary stage, and an additional 5 weeks for the growing follicles to develop an antrum. The results agree with a human in vivo study that proposed basal follicular growth from the primary to antral stage is a month-long process (Gougeon, 1986).
Follicle growth rate followed that of E2 production during 3D culture. For both primary and secondary follicles, E2 produced by growing follicles increased around the time of antrum formation and continued to increase during further growth. It is notable that A4 production in FG follicles cultured in alginate alone remained low at Week 5, which is different from increased A4 levels produced by those cultured in FIBRIN. This may be due to the active E2 synthesis that consumed A4 substrate in FG follicles cultured in alginate versus FIBRIN. The change in E2 and A4 production patterns is consistent with the 2-cell, 2-gonadotrophin model wherein theca cells produce A4 for subsequent E2 production in the granulosa cells, which allows steroidogenic maturation of the follicles (McNatty et al., 1980). Since the steroidogenic enzymes CYP17A1 and CYP19A1 were detected in theca and granulosa cells, respectively, of cultured follicles by immunostaining, this encapsulated 3D system supports theca cell development, as well as the steroidogenic function in both theca and granulosa cells in macaque follicles, which is consistent with mouse studies (Xu et al., 2006; Itami et al., 2011).
For the first time, an MII-stage oocyte was retrieved from a primate antral follicle following growth from a primary follicle under chemically defined conditions. However, most of the healthy oocytes obtained remained at the GV stage, and the MII oocyte that fertilized arrested without subsequent embryonic development. Nevertheless, the MII oocyte retrieved from the secondary follicle culture fertilized, and the embryo cleaved and reached the morula stage. In rhesus monkeys, the transition from the maternal to embryonic genome occurs at the 6- to 8-cell stage (Schramm and Bavister, 1999). Therefore, the oocyte must contain the appropriate instructions, involving the expression of new protein-coding genes (Kocabas et al., 2006), to drive the first few divisions and the awakening of the embryonic genome. It is clear that the MII oocyte from secondary follicle culture achieved not only nuclear maturation but also some aspects of cytoplasmic maturation, which needs to be improved in the primary follicle culture.
In the present study, levels of AMH in the culture medium were non-detectable until the primary follicles reached the secondary stage, and the levels were higher in growing than the NG follicles. The results are consistent with our previous observation that AMH levels were higher in the secondary follicles that would grow and form an antrum later during culture (Xu et al., 2010, 2011a). AMH immunostaining in marmoset ovarian sections also suggested an increase in AMH protein in secondary follicles compared with primary follicles (Thomas et al., 2007). AMH produced by in vitro-developed follicles increased in line with the growth rate during secondary follicle culture, as reported previously (Xu et al., 2010, 2011a). While information in primates is absent, there is one report (McGee et al., 2001) that AMH promoted growth of rat secondary follicles during culture, suggesting a stimulatory role of AMH on the early development of pre-antral follicles. Thus, early AMH production may be a potential marker for predicting further development of pre-antral follicles with different growth rates during in vitro follicle maturation. Notably, AMH levels remained high at Week 5 in small antral follicles, instead of decreasing as observed in our previous study (Xu et al., 2010, 2011a). This may be due to improvements in follicle retrieval and culture conditions which promote follicle health for further growth in vitro.
The finding that growing primary follicles began to produce VEGF during antrum formation, with levels higher than the NG follicles, is consistent with secondary follicle culture in the present and previous studies (Xu et al., 2010). While the onset of VEGF production is likely related to the stage of follicle growth (i.e. antrum formation), the magnitude of VEGF production may be influenced by follicle size and activity as reported in rats (Danforth et al., 2003), cattle (Greenaway et al., 2004) and sheep (Chowdhury et al., 2010). In both primary and secondary follicle culture, VEGF levels paralleled the subsequent rate of follicle growth, which is consistent with the conclusion that the onset of VEGF production permits its purported angiogenic actions as the antrum forms to create an extensive surrounding vasculature for the increased transport of nutrients and hormones to/from the growing follicle (Taylor et al., 2004, Abramovich et al., 2009). Thus, VEGF may play a role during follicular development and may be a potential marker for follicle quality during in vitro follicle maturation.
In summary, FIBRIN improves macaque primary, but not secondary, follicle development during encapsulated 3D culture. This 3D model for primate follicle development offers a unique opportunity to investigate the growth and regulation of primate primary, as well as secondary ovarian follicles, and their enclosed oocytes, as they grow to the antral stage by monitoring and manipulating factors or signaling pathways in vitro. As primate primary follicles, in addition to secondary follicles, can be cultured to the antral stage to provide mature oocytes, they represent an additional source of pre-antral follicles for in vitro follicle maturation with the potential to provide gametes for assisted reproductive technology as an option for preservation of fertility in women, including patients with cancer.
Authors' roles
J.X. provided contributions to (1) experimental design, (2) follicle collection, culture and oocyte retrieval, (3) follicle growth recording and diameter measurement, (4) histology staining, (5) data analysis and interpretation on follicle development, oocyte maturation, steroid and AMH/VEGF production, (6) manuscript drafting and critical revising and (7) final approval of the version to be submitted for publication. M.S.L. contributed to (1) animal health and reproduction record, (2) follicle collection, (3) immunohistochemistry staining and (4) final approval of the version to be submitted for publication. R.R.Y. contributed to (1) follicle collection and culture and (2) final approval of the version to be submitted for publication. T.A.M. contributed to (1) VEGF assays and (2) final approval of the version to be submitted for publication. A.Y.T. contributed to (1) histology/immunohistochemistry staining and (2) final approval of the version to be submitted for publication. R.L.S. provided contributions to (1) conception and design of the experiments, (2) data interpretation on follicle development, oocyte maturation, steroid and AMH/VEGF production, (3) critical manuscript revising for important intellectual content and (4) final approval of the version to be submitted for publication. M.B.Z. provided contributions to (1) conception and design of the experiments, (2) follicle collection, (3) data interpretation on follicle development, oocyte maturation, steroid and AMH/VEGF production, (4) critical manuscript revising for important intellectual content and (5) final approval of the version to be submitted for publication.
Funding
This work was supported by the National Institute of Health (NIH) U54 RR024347, RL1HD058294, PL1EB008542 (the Oncofertility Consortium), NIH-NICHD through cooperative agreement as part of the Specialized Cooperative Center Program in Reproduction and Infertility Research U54HD18185, NIH ORWH/NICHD 2K12HD043488 (Building Interdisciplinary Research Careers in Women's Health), and ONPRC 8P51OD011092.
Conflict of interest
The authors have no conflict of interests to disclose.
Acknowledgements
We are grateful to members of the Division of Comparative Medicine, the Endocrine Technology Support Core, the Imaging and Morphology Support Core and the Assisted Reproductive Technology/Embryonic Stem Cell Support Core at ONPRC, as well as Drs Ariella Shikanov, Lonnie Shea, Min Xu and Teresa Woodruff at the Northwestern University, for their valuable expertise and technical assistance. We also thank Baxter Healthcare, Inc. for the generous donation of Tisseel [Fibrin Sealant] used in this study.
References
- Abramovich D, Rodriguez Celin A, Hernandez F, Tesone M, Parborell F. Spatiotemporal analysis of the protein expression of angiogenic factors and their related receptors during folliculogenesis in rats with and without hormonal treatment. Reproduction. 2009;137:309–320. doi: 10.1530/REP-08-0130. [DOI] [PubMed] [Google Scholar]
- Choi JH, Kim GA, Park JH, Song G, Park JW, Kim DY, Lim JM. Generation of viable embryos and embryonic stem cell-like cells from cultured primary follicles in mice. Biol Reprod. 2011;85:744–754. doi: 10.1095/biolreprod.110.084137. [DOI] [PubMed] [Google Scholar]
- Chowdhury MW, Scaramuzzi RJ, Wheeler-Jones CPD, Khalid M. The expression of angiogenic growth factors and their receptors in ovarian follicles throughout the estrous cycle in the ewe. Theriogenology. 2010;73:856–872. doi: 10.1016/j.theriogenology.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Stouffer RL. Follicle-stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J Clin Endocrinol Metab. 1997;82:2135–2142. doi: 10.1210/jcem.82.7.4169. [DOI] [PubMed] [Google Scholar]
- Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod. 2003;68:1736–1741. doi: 10.1095/biolreprod.101.000679. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- Fréour T, Mirallié S, Bach-Ngohou K, Denis M, Barrière P, Masson D. Measurement of serum anti-Müllerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375:162–164. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Dynamics of human follicular growth: morphologic, dynamic, and functional aspects. In: Leung PCK, Adashi EY, editors. The Ovary. San Diego, CA, USA: Elsevier Academic Press; 2004. pp. 25–43. [Google Scholar]
- Greenaway J, Connor K, Pedersen HG, Coomber BL, Lamarre J, Petrik J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145:2896–2905. doi: 10.1210/en.2003-1620. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11:1268–1272. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- Itami S, Yasuda K, Yoshida Y, Matsui C, Hashiura S, Sakai A, Tamotsu S. Co-culturing of follicles with interstitial cells in collagen gel reproduce follicular development accompanied with theca cell layer formation. Reprod Biol Endocrinol. 2011;9:159. doi: 10.1186/1477-7827-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–2639. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, Tam WL, Rosa GJ, Halgren RG, Lim B, et al. The transcriptome of human oocytes. Proc Natl Acad Sci USA. 2006;103:14027–14032. doi: 10.1073/pnas.0603227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzendorf SE, Gliessman PM, Archibong AE, Alexander M, Wolf DP. Collection and quality of rhesus monkey semen. Mol Reprod Dev. 1990;25:61–66. doi: 10.1002/mrd.1080250111. [DOI] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71:1730–1738. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- McGee EA, Smith R, Spears N, Nachtigal MW, Ingraham H, Hsueh AJ. Müllerian inhibitory substance induces growth of rat preantral ovarian follicles. Biol Reprod. 2001;64:293–298. doi: 10.1095/biolreprod64.1.293. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Makris A, Osathanondh R, Ryan KJ. Effects of luteinizing hormone on steroidogenesis by thecal tissue from human ovarian follicles in vitro. Steroids. 1980;36:53–63. doi: 10.1016/0039-128x(80)90067-7. [DOI] [PubMed] [Google Scholar]
- Meng L, Wolf D. Sperm-induced oocyte activation in the rhesus monkey: nuclear and cytoplasmic changes following intracytoplasmic sperm injection. Hum Reprod. 1997;12:1062–1068. doi: 10.1093/humrep/12.5.1062. [DOI] [PubMed] [Google Scholar]
- Nottola SA, Camboni A, Van Langendonckt A, Demylle D, Macchiarelli G, Dolmans MM, Martinez-Madrid B, Correr S, Donnez J. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil Steril. 2008;90:23–32. doi: 10.1016/j.fertnstert.2007.05.069. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. Onset of nucleolar and extranucleolar transcription and expression of fibrillarin in macaque embryos developing in vitro. Biol Reprod. 1999;60:721–728. doi: 10.1095/biolreprod60.3.721. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM. Birth of rhesus monkey infant after transfer of embryos derived from in-vitro matured oocytes: short communication. Hum Reprod. 2000;15:2411–2414. doi: 10.1093/humrep/15.11.2411. [DOI] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Kitawaki J, Yamamoto T, Osawa Y, Kominami S, Takemori S, Okada H. Immunohistochemical localization of 17 alpha-hydroxylase/C17–20 lyase and aromatase cytochrome P-450 in the human ovary during the menstrual cycle. J Endocrinol. 1992;135:589–595. doi: 10.1677/joe.0.1350589. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Hillier SG, Fraser HM. Effects of GnRH antagonist treatment on follicular development and angiogenesis in the primate ovary. J Endocrinol. 2004;183:1–17. doi: 10.1677/joe.1.05685. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Telfer EE, Fraser HM. Expression of anti-Mullerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology. 2007;148:2273–2281. doi: 10.1210/en.2006-1501. [DOI] [PubMed] [Google Scholar]
- Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461–2472. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, Woodruff TK. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809–820. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young and older adult, rhesus monkeys during encapsulated three-dimensional (3D) culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotropins, oxygen, and fetuin. Hum Reprod. 2011a;26:1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011b;84:689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]