Abstract
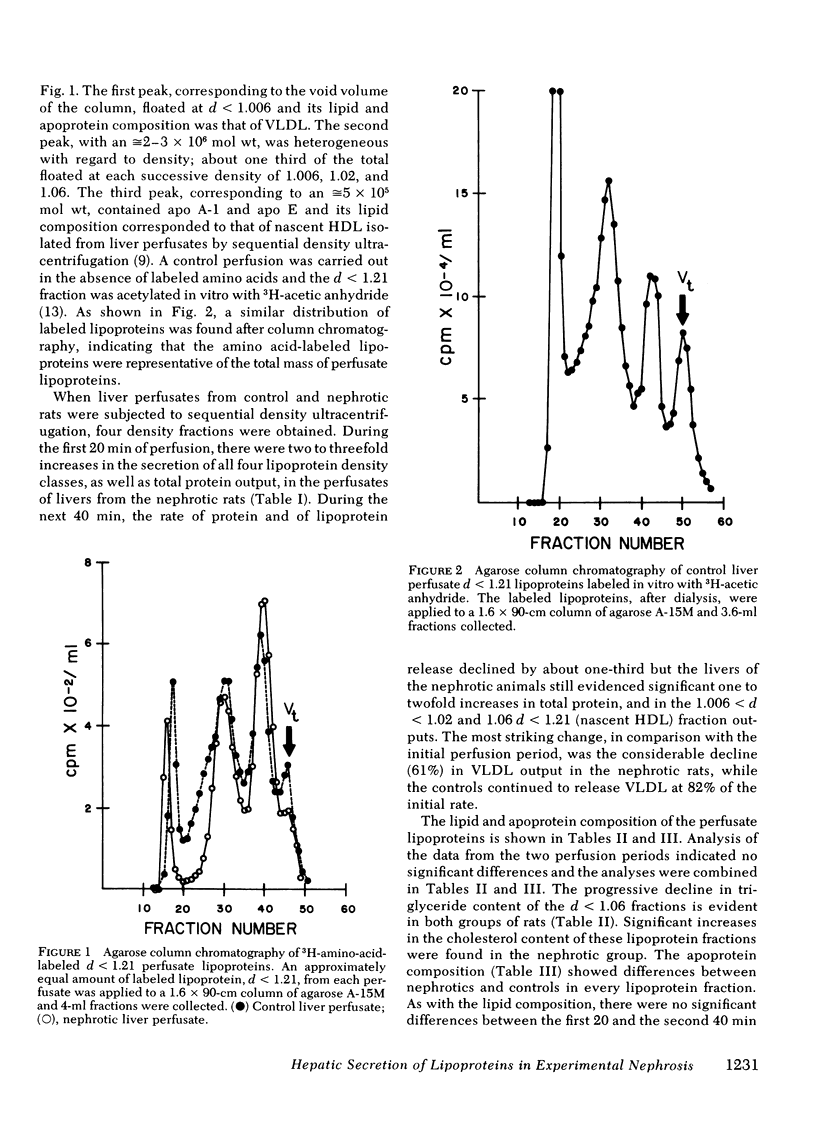
Livers from normal and nephrotic rats were perfused by the nonrecirculating technique. Nephrosis was studied on the 7th d after the injection of puromycin animonucleoside. Amino acid-labeled lipoproteins (d < 1.21) were isolated from the perfusion medium by agarose column chromatography or by sequential density ultracentrifugation. In both groups of animals, in addition to very low density lipoproteins and nascent high density lipoproteins, column chromatography revealed the presence of a peak of 2-3 × 106 daltons. This peak contained lipoproteins of densities corresponding to <1.006, 1.006 < d < 1.02, and 1.02 < d < 1.06, which indicated that rat liver secretes a heterogeneous mixture of triglyceride-rich lipoproteins.
The amount of these lipoprotein density classes was measured and their lipid and apoprotein composition and their apoprotein specific activity were determined. In both groups of rats there was a progressive rise in phospholipid and decrease in triglyceride content as the isolation density increased from 1.006 and 1.06. The lipoproteins from the nephrotics had higher amounts of cholesterol. The livers from the nephrotic rats secreted two to three times as much lipoprotein as controls in all density classes in the first 20 min, but during the next 40 min only the 1.02 < d < 1.06 and nascent high density lipoproteins remained at this high level compared to controls. A larger total liver pool of apolipoproteins in nephrotic livers was inferred from their lower specific activities during the first 20 min.
The apoprotein composition of liver perfusate lipoproteins from nephrotics differed from controls. There was a 40% decrease in the amount of low molecular weight apoproteins in all density classes, with corresponding increases in apo B and apo E in the triglyceride-rich fractions. The apo A-1 content of nascent HDL was increased from 16% in controls to 52% in nephrotics, with corresponding decreases in apo C and apo E. When these results were combined with specific activity measurements of the individual apoproteins and the net secretion rate of total protein in each lipoprotein class, it was possible to estimate the total amount of each apoprotein secreted and the total incorporation of labeled amino acids into each. The incorporation of label gave results similar to those obtained by direct measurement of the amounts of apoproteins. Apo E secretion was increased by a factor of 1.8, apo B by 2.8, and apo A-1 by 8.4, whereas the secretion of apo C was not significantly altered.
We explain these results by postulating that the primary stimulus to hepatic plasma protein synthesis in response to proteinuria is general and that subsequent negative feedback regulation affects individual apolipoprotein synthesis rates. A corollary of this hypothesis is that the biosynthesis and secretion of an apoprotein may be regulated independently of the lipoprotein density class in which it is found.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates S. R., Rothblat G. H. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974 Jul 26;360(1):38–55. doi: 10.1016/0005-2760(74)90178-7. [DOI] [PubMed] [Google Scholar]
- DRABKIN D. L., MARSH J. B., BRAUN G. A. Amino acid mobilization in plasma protein biosynthesis in experimental nephrosis. Metabolism. 1962 Sep;11:967–977. [PubMed] [Google Scholar]
- Derr R. F., Loechler D. K., Alexander C. S., Nagasawa H. T. Inhibition of aminonucleoside nephrosis in rats. IV. Prevention by N6-methyladenosine. J Lab Clin Med. 1968 Sep;72(3):363–369. [PubMed] [Google Scholar]
- Eisenberg S., Bilheimer D. W., Levy R. I. The metabolism of very low density lipoprotein proteins. II. Studies on the transfer of apoproteins between plasma lipoproteins. Biochim Biophys Acta. 1972 Sep 7;280(1):94–104. [PubMed] [Google Scholar]
- Fainaru M., Felker T. E., Hamilton R. L., Havel R. J. Evidence that a separate particle containing B-apoprotein is present in high-density lipoproteins from perfused rat liver. Metabolism. 1977 Sep;26(9):999–1004. doi: 10.1016/0026-0495(77)90017-8. [DOI] [PubMed] [Google Scholar]
- Fainaru M., Glangeaud M. C., Eisenberg S. Radioimmunoassay of human high density lipoprotein apo-protein A-1. Biochim Biophys Acta. 1975 Apr 29;386(2):432–443. doi: 10.1016/0005-2795(75)90286-x. [DOI] [PubMed] [Google Scholar]
- Felker T. E., Fainaru M., Hamilton R. L., Havel R. J. Secretion of the arginine-rich and A-I apolipoproteins by the isolated perfused rat liver. J Lipid Res. 1977 Jul;18(4):465–473. [PubMed] [Google Scholar]
- Gherardi E., Rota E., Calandra S., Genova R., Tamborino A. Relationship among the concentrations of serum lipoproteins and changes in their chemical composition in patients with untreated nephrotic syndrome. Eur J Clin Invest. 1977 Dec;7(6):563–570. doi: 10.1111/j.1365-2362.1977.tb01652.x. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. P., Sata T., Hamilton R. L., Havel R. J. Apoprotein composition of very low density lipoproteins of human serum. J Clin Invest. 1975 Dec;56(6):1622–1634. doi: 10.1172/JCI108245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Brewer H. B., Jr Isolation and characterization of apoLp-Gln-II (apoA-II), a plasma high density apolipoprotein containing two identical polypeptide chains. J Biol Chem. 1972 Dec 10;247(23):7510–7518. [PubMed] [Google Scholar]
- MARSH J. B., DRABKIN D. L. Experimental reconstruction of metabolic pattern of lipid nephrosis: key role of hepatic protein synthesis in hyperlipemia. Metabolism. 1960 Oct;9:946–955. [PubMed] [Google Scholar]
- MARSH J. B., DRABKIN D. L. Metabolic channeling in experimental nephrosis. II. Lipide metabolism. J Biol Chem. 1955 Feb;212(2):633–639. [PubMed] [Google Scholar]
- MARSH J. B., DRABKIN D. L. Metabolic channeling in experimental nephrosis. III. Influence of diet and of adrenalectomy; liver hypertrophy. J Biol Chem. 1958 Feb;230(2):1063–1071. [PubMed] [Google Scholar]
- Marsh J. B. Apoproteins of the lipoproteins in a nonrecirculating perfusate of rat liver. J Lipid Res. 1976 Jan;17(1):85–89. [PubMed] [Google Scholar]
- Marsh J. B. Labeling of high density lipoproteins with [3H] acetic anhydride. J Lipid Res. 1978 Jan;19(1):107–110. [PubMed] [Google Scholar]
- Marsh J. B. Lipoproteins in a nonrecirculating perfusate of rat liver. J Lipid Res. 1974 Nov;15(6):544–550. [PubMed] [Google Scholar]
- Marsh J. B., Weinstein D. B. Simple charring method for determination of lipids. J Lipid Res. 1966 Jul;7(4):574–576. [PubMed] [Google Scholar]
- Mayer M., Shafrir E. Liver and plasma amino acid pattern in glucogenic conditions and in nephrotic syndrome. Isr J Med Sci. 1972 Jun;8(6):859–861. [PubMed] [Google Scholar]
- Nakaya N., Chung B. H., Taunton O. D. Synthesis of plasma lipoproteins by the isolated perfused liver from the fasted and fed pig. J Biol Chem. 1977 Aug 10;252(15):5258–5261. [PubMed] [Google Scholar]
- PETERS T., Jr Evidence of intermediate compounds in serum albumin synthesis. J Biol Chem. 1953 Jan;200(1):461–470. [PubMed] [Google Scholar]
- RADDING C. M., STEINBERG D. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J Clin Invest. 1960 Oct;39:1560–1569. doi: 10.1172/JCI104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafrir E., Brenner T., Gutman A., Orevi M. Hepatic metabolic pattern in experimental nephrotic syndrome: glycolysis, gluconeogenesis, and amino acid metabolism. Am J Physiol. 1974 Jan;226(1):162–167. doi: 10.1152/ajplegacy.1974.226.1.162. [DOI] [PubMed] [Google Scholar]
- Staprans I., Felts J. M. The effect of alpha1-acid glycoprotein (orosomucoid) on triglyceride metabolism in the nephrotic syndrome. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1272–1278. doi: 10.1016/0006-291x(77)91143-3. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Reese H., Eder H. A. Polypeptide composition of rat high density lipoprotein: characterization by SDS-gel electrophoresis. Biochem Biophys Res Commun. 1974 Jul 24;59(2):513–519. doi: 10.1016/s0006-291x(74)80010-0. [DOI] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]


