Abstract
Immunohistochemical loss of the succinate dehydrogenase subunit B (SDHB) has recently been reported as a surrogate biomarker of malignancy in sporadic and familial pheocromocytomas and paragangliomas through the activation of hypoxia pathways. However, data on the prevalence and the clinical implications of SDHB immunoreactivity in ileal neuroendocrine tumors are still lacking. Thirty-one consecutive, advanced primary midgut neuroendocrine tumors and related lymph node or liver metastases from 24 males and seven females were immunohistochemically assessed for SDHB. All patients were G1 tumors (Ki-67 labeling index ≤2%). SDHB immunohistochemistry results were expressed as immunostaining intensity and scored as low or strong according to the internal control represented by normal intestinal cells. Strong positivity for SDHB, with granular cytoplasmatic reactivity, was found in 77% of primary tumors (T), whilst low SDHB expression was detected in 90% of metastases (M). The combined analysis (T+M) confirmed the loss of SDHB expression in 82% of metastases compared to 18% of primary tumors. SDHB expression was inversely correlated with Ki-67 labeling index, which accounted for 1.54% in metastastic sites and 0.7% in primary tumors. A correlation between SDHB expression loss, increased Ki-67 labeling index and biological aggressiveness was shown in advanced midgut neuroendocrine tumors, suggesting a role of tumor suppressor gene.
Keywords: midgut, neuroendocrine tumors, SDHB, immunohistochemistry, Ki-67 antigen
1. Introduction
Ileal neuroendocrine tumors (INETs) are the most common type of neuroendocrine neoplasms in the gastrointestinal tract, with a male prevalence and a median age at the time of diagnosis of 66 years. They are mainly composed of enterochromaffin cells (EC) producing serotonin and substance P [1,2]. A distinctive feature of INETs, especially when involving the liver, is their capability of causing distinct clinical syndromes [1,3,4], which can be faithfully monitored measuring the relevant hormones in the bloodstream. Surgical resection can be curative in early stage patients [5,6], but most of them present with liver involvement at the time of diagnosis, so tumor grading and staging according to WHO/AJCC/ENET criteria are likely to play the most important role in the prognostic and therapeutic assessment of INETs [6]. Accordingly, neuroendocrine carcinomas, which show high proliferative activity as reflected by Ki67 labeling index (LI) over 20%, are treated with cisplatin-etoposide combination chemotherapy similarly to small cell lung cancer (SCLC) [7,8], whereas most ileal neuroendocrine tumors are slowly growing neoplasms, which mainly depend on angiogenesis for their maintenance and growth [9,10,11,12,13,14]. Hence the search for new markers capable of getting new insights into the biological properties of INETs may be clinically warranted.
The succinate dehydrogenase (SDH) enzyme (also known as succinate ubiquinone oxydoreductase) is a highly conserved heterotetrameric protein, with SDHA and SDHB functioning as catalytic subunits, which protrudes into the mitochondrial matrix and is anchored to the inner membrane by means of SDHC and SDHD subunits, the latter also providing the binding site for ubiquinone [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. SDHB is normally ubiquitously expressed with granular cytoplasmic immunostaining reflecting its mitochondrial location [41,42]. It is also been shown that silencing SDHB expression induces tumor-like phenotypic traits in cell cultures [43], and that the loss of any subunit protein, especially B, leads to the loss of SDH expression due to destabilization of its complex [20,44]. However, data on the prevalence of SDHB in INETs and its implications on tumor differentiation and prognosis are still lacking, to the best of our knowledge.
This study was aimed at evaluating the distribution of SDHB by immunohistochemistry (IHC) in 31 INETs and corresponding lymph node or liver metastases in order to explore its diagnostic and prognostic implications.
2. Results and Discussion
There were no differences between functioning (FT) and nonfunctioning (NFT) groups in age, gender, clinical outcome and medical treatment. The only significant association was the greater liver tumor load (p = 0.0000026) and the increased basal chromogranin A (CgA) serum level (p = 0.0113) in the NFT group (Table 1).
Table 1.
Demographic and clinicopathologic information on the 31 INETs patients under evaluation.
| Variable | NFT | FT | p Value | |
|---|---|---|---|---|
| Age (Years) | <50 | 8 | 4 | 0.106 |
| 51–70 | 7 | 9 | ||
| >70 | 0 | 3 | ||
| Gender | Male | 13 | 12 | 0.653 |
| Female | 2 | 4 | ||
| Outcome | DOD | 5 | 8 | |
| AW | 0 | 0 | 0.3480 | |
| AWD | 7 | 3 | ||
| A.NED | 3 | 3 | ||
| Medical Treatment | SMS | 12 | 13 | |
| CT | 2 | 3 | 0.653 | |
| SMS+CT | 0 | 0 | ||
| NO | 1 | 0 | ||
| Liver Tumor Load | H1 | 13 | 1 | 0.0000026 |
| H2 | 1 | 1 | ||
| H3 | 1 | 14 | ||
| Basal CgA ng/mL | <200 | 10 | 3 | 0.0113 |
| >200 | 5 | 13 | ||
F, female; M, male; DOD, died of disease; AW, alive and well; AWD, alive with disease; A.NED, not evidence of disease; H1, liver involvement <25%; H2, liver involvement between 25 and 50%; H3, liver involvement >50%; INETs, ileal neuroendocrine tumors; SMS, somatostatin analogues; CT, chemotheraphy; CgA, chromogranin A.
SDHB immunoreactivity was found in tumor cells of all cases under assessment, but there was a significant relationship between SDHB intensity and percentage of immunoreactive cells (test for trend) (Table 2). Representative pictures of SDHB and Ki-67 antigen immunoreactivity are depicted in Figure 1. In particular, the more the intensity of immunoreactivity, the more the percentage of positive cells. The percentage of tumor cells was associated significantly with the site of tumors and Ki67 LI, since primary lesions bearing a proliferative activity ≤1.3% showed over 50% SDHB immunoreactive tumor cells (Table 3). Likewise, significant associations were found between the site of tumors (p < 0.0001) or Ki-67 LI (p < 0.0001) and SDHB immunostaining intensity (Table 3). No significant associations were found with age, gender, type of therapy, presence of clinical syndrome, and CgA level.
Table 2.
Relationship between SDH intensity and percentage of positive tumor cells
| All Measures | SDHB intensity | p Value | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| All measures | 39 | 19 | 9 | 11 | |
| SDHB expression | |||||
| 1–25% | 5 | 4 | 0 | 1 | |
| 26–50% | 9 | 6 | 2 | 1 | |
| 51–75% | 15 | 8 | 4 | 3 | 0.076 (Fisher exact) |
| 76–100% | 10 | 1 | 3 | 6 | 0.007 (trend) |
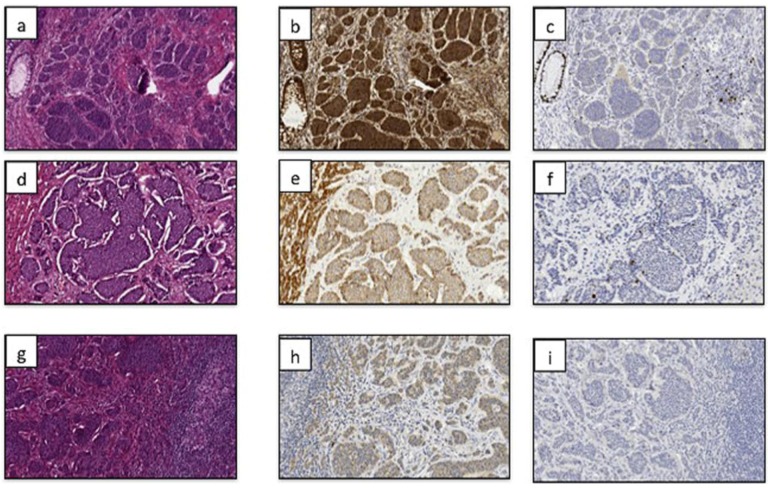
Figure 1.
Representative pictures of primary neuroendocrine tumors of the ileum stained with hematoxylin and eosin (a,d,g), and SDHB (b,e,h) and Ki-67 antigen (c,f,i) immunohistochemistry are shown in primary lesions (a–c), and lymph node (d–f) or liver metastases (g–i). All tumor cells exhibited granular staining due to the mitochondrial accumulation of immunreaction product. A clear gradient of SDHB expression loss can be easily appreciated going from primary tumors (panel “b”) to lymph node (panel “e”) and liver (panel “h”) metastases. The normal epithelial and mesenchimal cells present in the tumor tissue samples served as internal positive controls (for example, the intestinal gland structures detectable on the right side of the panel “b” show a clear and specific decoration for the marker under assessment).
Table 3.
Distribution of tumor site (primary vs. metastasis) and Ki-67 labeling index according to SDHB expression (percentage of positive tumor cells) and immunstaining intensity.
| SDHBExpression | SITE | Ki-67 labeling index | ||||
|---|---|---|---|---|---|---|
| Primary Tumor | Metastases | p Value | ≤1.3% | ≥1.3% | p Value | |
| 1–25% | 2 | 3 | 1 | 4 | ||
| 26–50% | 2 | 7 | 0.013 (trend) | 3 | 6 | 0.038 |
| 51–75% | 7 | 8 | 8 | 7 | ||
| 76–100% | 9 | 1 | 7 | 3 | ||
| SDHB Intensity | ||||||
| 1+ | 1 | 18 | 2 | 17 | ||
| 2+ | 8 | 1 | <0.0001(trend) | 7 | 2 | <0.0001(trend) |
| 3+ | 11 | 0 | 10 | 1 | ||
Ki-67 labeling index: 1.3% represented the median value and was chosen as cut-off for distinguishing slower from faster growing tumors within the G1 category.
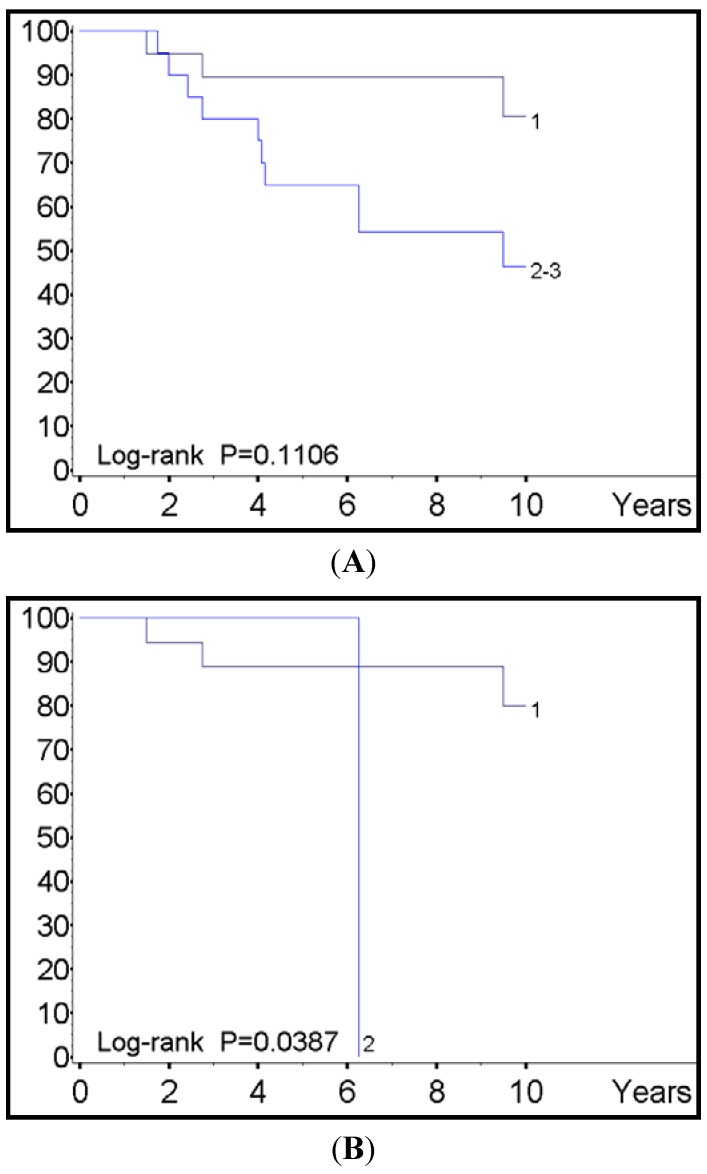
Survival analysis showed that the SDHB intensity but not the SDHB percentage of tumors cells impacted inversely on the patients’ prognosis, with marginal poorer prognosis being observed in individuals loosing SDHB immunoreactivity when considered as a whole (p = 0.1106) and significant shorter survival in the subset of metastatic diseases (p = 0.0387) (Figure 2). Other variables correlating with reduced survival included the lack of transplant treatment (p = 0.0094) and CgA levels >200 (p = 0.001). Although proliferative activity by means of Ki-67 labeling index was not a prognostic factor in this subset of patients, it was however marginally related to survival in metastases rather than primary tumors (p = 0.086). Multivariate analysis according to Cox’s model let emerge CgA level but not SDHB immunostaining intensity as an independent factor of survival (Table 4).
Figure 2.
(A) SDHB immunostining intensity in primary tumors; (B) SDHB immunostining intensity in metastatic sites.
Table 4.
Multivariate analysis in 31 nontransplanted patients.
| HR (95% CI) | p Value | |
|---|---|---|
| CgA level | ||
| <200 | 1.00 | |
| >201 | 8.22 (1.02–66.4) | 0.048 |
| SHDB intensity | ||
| 1 | 1.00 | |
| 2–3 | 1.38 (0.49–3.91) | 0.55 |
Interesting findings of our study were that SDHB expression correlated with the tumor cell differentiation and malignant potential of G1 INETs, and that the percentage of immunoreactive cells was associated with the staining intensity (Table 2). As a matter of fact the higher was the loss of SDHB immunoreactivity, the higher the proliferative activity (Table 3), the higher the likelihood of facing with metastatic sites (Table 3), and the shorter the survival (Figure 2a,b). Accordingly, SDHB was likely to behave as tumor suppressor gene in this category of neuroendocrine tumors, in that the lack of this protein was associated with parameters of clinical aggressiveness in metastatic tumors and reduced life expectation.
Although the switch from respiration to glycolysis in tumor cells has often been considered a consequence rather than a cause of cancer [45,46], the discovery that germline, inherited mutations in the genes encoding SDH enzyme subunits may cause paragangliomas and phaeochromocytomas [17,19,45,46,47], whether hereditary or sporadic, has however revolutionized this assumption [45,46]. Interestingly, all SDH subunits have no cytosolic counterpart unlike most Krebs cycle enzymes, but are imported into the mitochondria where they are modified, folded and assembled. Hence, they are able to deeply affect the capability of producing energy, whenever these subunits are downregulated in their expression. In our investigation, we observed that metastatic sites showed loss of SDHB expression along with a higher Ki-67 LI thereby supporting the contention that the development of anaerobic metabolism mechanisms could favor an increase of clinical aggressiveness.
In our paper, we provide evidence for the first time that the assessment of SDHB immunoreactivity in well-differentiated INETs may identify a subset of tumors characterized by reduced life expectation, which are worth treating more aggressively with multimodality therapy. Moreover, this paper challenges the common credence that G1 neuroendocrine tumors of the ileum are uniformly poorly aggressive tumors, while they are likely to be a heterogeneous tumor group harboring different lesions with different degrees of malignancy. The loss of function of SDHB as indicated by its relevant down-expression might be responsible for a pseudo-hypoxic drive via succinate-induced glycolysis and HIF stabilization in normoxic conditions [48], so favoring angiogenesis, tumor growth and progression of malignancy [48]. Recent evidences have corroborated the notion of a possible role for succinate accumulation due to SDHB activity loss also in epigenetic changes of chromatin via the histone H3 methylation in succinate-accumulating tumor cells [48]. Further investigation dealing with somatic mutation analysis of SDHB gene is currently in progress in our laboratory to better clarify additional molecular mechanisms underlying the loss of SDHB expression in this subset of INETs patients.
3. Experimental Section
3.1. Patients
Ileal neuroendocrine tumors from 31 patients (78% males and 22% females, median age 55.5 years, range 19 to 75 years) were retrieved from the archives of the Pathology Department of the National Cancer Institute of Milan. These cases had been surgically treated from 1992 to 2007 at the Department of Surgery of the same Institution. All INETs were G1 neuroendocrine tumors according to WHO/AJCC/ENET criterio for tumor grading (Ki-67 labeling index ≤2%). According to clinical and laboratory findings, two tumor groups were identified: FT group was defined by the occurrence of a compatible clinical syndrome associated with serum elevation and immunohistochemical detection of the relevant hormones, and NFT group by the absence of both clinical symptoms and serum elevation of hormones, regardless of the presence of immunostaining for any hormones [49,50,51,52,53,54,55,56,57,58,59]. All cases were subjected to serum and immunohistochemical assessment for CgA, synapthophisin, serotonin, and somatostatin receptor type 2A. Most of patients underwent surgical primary resection and all of them presented with distant synchronous liver metastases treated with nodule excision in 25 patients and liver transplant in 6 patients according to the so-called Milan criteria [60]. In the patients undergoing liver transplant, three clinical subgroups were considered according to the amount of liver involvement as assessed by surgical staging or CT scan: tumor load <25% (H1), between 25% and 50% (H2), and over 50% (H3) [61]. Clinicopathological data on the INETs under evaluation are shown in Table 1.
3.2. Tumors Specimens, Immunohistochemical Methods and Scoring of Data
The diagnosis of INETs was established by means of the last WHO classification [49,62]. All surgical samples (19 primary tumors, 9 metastases and 11 combined primary and metastatic lesions) had been fixed in 10% buffered formaldehyde solution and embedded in paraffin. To minimize the intratumoral variability because of sampling process, the entire tumor was immunostained if the lesion was up to 2 cm in diameter or at least two representative tissue blocks were immnnostained if the lesion was larger than 2 cm in diameter. Four μm-thick paraffin sections were reacted with monoclonal antibodies against CgA, synaptophysin, serotonin, Ki-67 antigen and SDHB [49] and processed according to previously refined immunohistochemical methods. Internal and external controls were used for all markers as appropriate.
In order to minimize variability in the slide assessment when trying punctual percentages, immunohistochemistry results for SDHB were rendered semiquantitatively on a scale from 1+ to 4+, taking into account a granular labeling product in the cytoplasm. One-plus tumors showed immunoreactivity in up to 25% neoplastic cells, 2+ cases in 26–50% neoplastic cells, 3+ cases in 51–75% neoplastic cells, 4+ cases in 76–100% neoplastic cells. Moreover, the immunostaining intensity was indicated as low (1+), if fainter than that seen in internal controls, or strong (3+), if more intense than the normal internal controls that was in turn indicated as being 2+. As SDHB is ubiquitous in normal cells, internal controls included any type of non-neoplastic cells, whether epithelial or mesenchymal.
3.3. Statistical Analysis
Associations of categorical variables were evaluated by Fisher’s exact t-test, test for trend or chi-square test. Survival estimates were calculated with Kaplan-Maier’s method and compared by Cox-Mantel’s log rank test. The comparative importance of explanatory variables on survival time was evaluated by means of Cox’s proportional hazard regression model. All the analyses were performed using the SAS statistical software (SAS Institute, Inc., Cary, NC, USA). All p-values were based on two-sided testing.
4. Conclusions
Our study provides the first evidence of a down-regulation of SDHB in well-differentiated INETs as likely mechanism contributing to the development of a subset of biologically more aggressive tumors as heralded by increased proliferative activity and reduced survival. Further investigation is currently in progress in our laboratory on a larger cohort of INETs patients, as well as in other types of neuroendocrine tumors, in order to confirm and expand these preliminary data.
Acknowledgments
This work was supported by LILT (Lega Italiana per la Lotta ai Tumori) and is dedicated to the memory of Carlotta, an extraordinarily lively girl who untimely died of cancer in the prime of life.
Conflict of Interest
The Authors declare that they have no conflicts of interest.
References
- 1.Yao J.C., Hassan M., Phan A., Dagohoy C., Leary C., Mares J.E., Abdalla E.K., Fleming J.B., Vauthey J.N., Rashid A., et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Oberg K. Neuroendocrine gastrointestinal tumors—A condensed overview of diagnosis and treatment. Ann. Oncol. 1999;10:S3–S8. doi: 10.1093/annonc/10.suppl_2.s3. [DOI] [PubMed] [Google Scholar]
- 3.Kaltsas G.A., Besser G.M., Grossman A.B. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr. Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 4.Niederle M.B., Hackl M., Kaserer K., Niederle B. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: An analysis based on prospectively collected parameters. Endocr. Relat. Cancer. 2010;17:909–918. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 5.Modlin I.M., Latich I., Kidd M., Zikusoka M., Eick G. Therapeutic options for gastrointestinal carcinoids. Clin. Gastroenterol. Hepatol. 2006;4:526–547. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Modlin I.M., Oberg K., Chung D.C., Jensen R.T., de Herder W.W., Thakker R.V., Caplin M., Delle F.G., Kaltsas G.A., Krenning E.P., et al. Sundin, Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 7.Ahlman H., Nilsson O., McNicol A.M., Ruszniewski P., Niederle B., Ricke J., Jensen R., Kos-Kudla B., Oberg K., O’Connor J.M., et al. Poorly-differentiated endocrine carcinomas of midgut and hindgut origin. Neuroendocrinology. 2008;87:40–46. doi: 10.1159/000109976. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson O., van Cutsem E., Delle F.G., Yao J.C., Pavel M.E., McNicol A.M., Sevilla Garcia M.I., Knapp W.H., Kelestimur F., Sauvanet A., et al. Poorly differentiated carcinomas of the foregut (gastric, duodenal and pancreatic) Neuroendocrinology. 2006;84:212–215. doi: 10.1159/000098013. [DOI] [PubMed] [Google Scholar]
- 9.Ballian N., Loeffler A.G., Rajamanickam V., Norstedt P.A., Weber S.M., Cho C.S. A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB (Oxford) 2009;11:422–428. doi: 10.1111/j.1477-2574.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couvelard A., O’Toole D., Turley H., Leek R., Sauvanet A., Degott C., Ruszniewski P., Belghiti J., Harris A.L., Gatter K., et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: Negative correlation of microvascular density and VEGF expression with tumour progression. Br. J. Cancer. 2005;92:94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinke A., Muller H.H., Schade-Brittinger C., Klose K.J., Barth P., Wied M., Mayer C., Aminossadati B., Pape U.F., Blaker M., et al. Placebocontrolled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 12.Moertel C.G., Lefkopoulo M., Lipsitz S., Hahn R.G., Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 13.Moertel C.G., Kvols L.K., O’Connell M.J., Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::AID-CNCR2820680202>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Kulke M.H., Siu L.L., Tepper J.E., Fisher G., Jaffe D., Haller D.G., Ellis L.M., Benedetti J.K., Bergsland E.K., Hobday T.J., et al. Future directions in the treatment of neuroendocrine tumors: Consensus report of the National Cancer Institute Tumor clinical trials planning meeting. J. Clin. Oncol. 2001;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenders J.W., Eisenhofer G., Mannelli M., Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 16.Pasini B., Stratakis C.A. SDH mutations in tumorigenesis and inherited endocrine tumours: Lesson from the phaeochromocytoma-paraganglioma syndromes. J. Intern. Med. 2009;266:19–42. doi: 10.1111/j.1365-2796.2009.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimm O., Armanios M., Dziema H., Neumann H.P., Eng C. Somatic and occult germ-line mutations in SDHD, a mitochondrial complex II gene, in nonfamilial pheochromocytoma. Cancer Res. 2000;60:6822–6825. [PubMed] [Google Scholar]
- 18.Astuti D., Douglas F., Lennard T.W., Aligianis I.A., Woodward E.R., Evans D.G., Eng C., Latif F., Maher E.R. Germline SDHD mutation in familial phaeochromocytoma. Lancet. 2001;357:1181–1182. doi: 10.1016/S0140-6736(00)04378-6. [DOI] [PubMed] [Google Scholar]
- 19.Van Nederveen F.H., Korpershoek E., Lenders J.W., de Krijger R.R., Dinjens W.N. Somatic SDHB mutation in an extraadrenal pheochromocytoma. N. Engl. J. Med. 2007;357:306–308. doi: 10.1056/NEJMc070010. [DOI] [PubMed] [Google Scholar]
- 20.Gimenez-Roqueplo A.P., Favier J., Rustin P., Mourad J.J., Plouin P.F., Corvol P., Rotig A., Jeunemaitre X. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am. J. Hum. Genet. 2001;69:1186–1197. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gimenez-Roqueplo A.P., Favier J., Rustin P., Rieubland C., Kerlan V., Plouin P.F., Rotig A., Jeunemaitre X. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J. Clin. Endocrinol. Metab. 2002;87:4771–4774. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- 22.Pasini B., McWhinney S.R., Bei T., Matyakhina L., Stergiopoulos S., Muchow M., Boikos S.A., Ferrando B., Pacak K., Assie G., et al. Clinical and molecular genetics of patientswith the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur. J. Hum. Genet. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 23.Levitas A., Muhammad E., Harel G., Saada A., Caspi V.C., Manor E., Beck J.C., Sheffield V., Parvari R. Familial neonatal isolated cardiomyopathy caused by a mutation in the flavoprotein subunit of succinate dehydrogenase. Eur. J. Hum. Genet. 2010;18:1160–1165. doi: 10.1038/ejhg.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stratakis C.A., Carney J.A. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): Molecular genetics and clinical implications. J. Intern. Med. 2009;266:43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann H.P., Pawlu C., Peczkowska M., Bausch B., McWhinney S.R., Muresan M., Buchta M., Franke G., Klisch J., Bley T.A., et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 26.Ricketts C.J., Forman J.R., Rattenberry E., Bradshaw N., Lalloo F., Izatt L., Cole T.R., Armstrong R., Kumar V.K., Morrison P.J., et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum. Mutat. 2010;31:41–51. doi: 10.1002/humu.21136. [DOI] [PubMed] [Google Scholar]
- 27.Vanharanta S., Buchta M., McWhinney S.R., Virta S.K., Peczkowska M., Morrison C.D., Lehtonen R., Januszewicz A., Jarvinen H., Juhola M., et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am. J. Hum. Genet. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricketts C., Woodward E.R., Killick P., Morris M.R., Astuti D., Latif F., Maher E.R. Germline SDHB mutations and familial renal cell carcinoma. J. Natl. Cancer Inst. 2008;100:1260–1262. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 29.Schimke R.N., Collins D.L., Stolle C.A. Paraganglioma, neuroblastoma, and a SDHB mutation: Resolution of a 30-year-old mystery. Am. J. Med. Genet. 2010;152A:1531–1535. doi: 10.1002/ajmg.a.33384. [DOI] [PubMed] [Google Scholar]
- 30.Zantour B., Guilhaume B., Tissier F., Louvel A., Jeunemaitre X., Gimenez-Roqueplo A.P., Bertagna X. A thyroid nodule revealing a paraganglioma in a patient with a new germline mutation in the succinate dehydrogenase B gene. Eur. J. Endocrinol. 2004;151:433–438. doi: 10.1530/eje.0.1510433. [DOI] [PubMed] [Google Scholar]
- 31.Galera-Ruiz H., Gonzalez-Campora R., Rey-Barrera M., Rollon-Mayordomo A., Garcia-Escudero A., Fernandez-Santos J.M., DeMiguel M., Galera-Davidson H. W43X SDHD mutation in sporadic head and neck paraganglioma. Anal. Quant. Cytol. Histol. 2008;30:119–123. [PubMed] [Google Scholar]
- 32.Astuti D., Morris M., Krona C., Abel F., Gentle D., Martinsson T., Kogner P., Neumann H.P., Voutilainen R., Eng C., et al. Investigation of the role of SDHB inactivation in sporadic phaeochromocytoma and neuroblastoma. Br. J. Cancer. 2004;91:1835–1841. doi: 10.1038/sj.bjc.6602202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grau E., Oltra S., Orellana C., Hernandez-Marti M., Castel V., Martinez F. There is no evidence that the SDHB gene is involved in neuroblastoma development. Oncol. Res. 2005;15:393–398. doi: 10.3727/096504005776449671. [DOI] [PubMed] [Google Scholar]
- 34.Selak M.A., Armour S.M., MacKenzie E.D., Boulahbel H., Watson D.G., Mansfield K.D., Pan Y., Simon M.C., Thompson C.B., Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Pollard P.J., Briere J.J., Alam N.A., Barwell J., Barclay E., Wortham N.C., Hunt T., Mitchell M., Olpin S., Moat S.J., et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 37.Pollard P., Wortham N., Barclay E., Alam A., Elia G., Manek S., Poulsom R., Tomlinson I. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J. Pathol. 2005;205:41–49. doi: 10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- 38.Dahia P.L., Ross K.N., Wright M.E., Hayashida C.Y., Santagata S., Barontini M., Kung A.L., Sanso G., Powers J.F., Tischler A.S., et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Jimenez E., Gomez-Lopez G., Leandro-Garcia L.J., Munoz I., Schiavi F., Montero-Conde C., de Cubas A.A., Ramires R., Landa I., Leskela S., et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol. Endocrinol. 2010;24:2382–2391. doi: 10.1210/me.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Favier J., Briere J.J., Burnichon N., Riviere J., Vescovo L., Benit P., Giscos-Douriez I., de Reynies A., Bertherat J., Badoual C., et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS ONE. 2009;4:e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill A.J., Benn D.E., Chou A., Clarkson A., Muljono A., Meyer-Rochow G.Y., Richardson A.L., Sidhu S.B., Robinson B.G., Clifton-Bligh R.J. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromo-cytoma syndromes. Hum. Pathol. 2010;34:636–644. doi: 10.1016/j.humpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Gill A.J., Chou A., Vilain R., Clarkson A., Lui M., Jin R., Tobias V., Samra J., Goldstein D., Smith C., et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am. J. Surg. Pathol. 2010;34:805–814. doi: 10.1097/PAS.0b013e3181d6150d. [DOI] [PubMed] [Google Scholar]
- 43.Cervera A.M., Apostolova N., Crespo F.L., Mata M., McCreath K.J. Cells silenced for SDHB expression display characteristic features of the tumor phenotype. Cancer Res. 2008;68:4058–4067. doi: 10.1158/0008-5472.CAN-07-5580. [DOI] [PubMed] [Google Scholar]
- 44.Janeway K.A., Kim S.Y., Lodish M., Nosé V., Rustin P., Gaal J., Dahia P.L., Liegl B., Ball E.R., Raygada M., et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baysal B.E., Ferrell R.E., Willett-Brozick J.E., Lawrence E.C., Myssiorek D., Bosch A., van der Mey A., Taschner P.E., Rubinstein W.S., Myers E.N., et al. Mutations in SDHD, amitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 46.Burnichon N., Briere J.J., Libe R., Vescovo L., Riviere J., Tissier F., Jouanno E., Jeunemaitre X., Benit P., Tzagoloff A., et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Astuti D., Latif F., Dallol A., Dahia P.L., Douglas F., George E., Skoldberg F., Husebye E.S., Eng C., Maher E.R. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am. J. Hum. Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amar L., Baudin E., Burnichon N., Peyrard S., Silvera S., Bertherat J., Bertagna X., Schlumberger M., Jeunemaitre X., Gimenez-Roqueplo A.P., et al. Succinate dehy-drogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J. Clin. Endocrinol. Metab. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 49.Capella C., Heitz P.U., Hofler H., Solcia E., Kloppel G. Revised classification of neuroendocrine tumours of the lungg, pancreas and gut. Virchows Arch. 1995;425:547–560. doi: 10.1007/BF00199342. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki H., Christofides N.D., Ghiglione M., Ferri G.L., Chretien M., Seidah N.G., Polak J.M., Bloom S.R. Distribution of a novel pituitary protein (7B2) in mammalian gastrointestinal tract and pancreas. Dig. Dis. Sci. 1998;33:718–723. doi: 10.1007/BF01540436. [DOI] [PubMed] [Google Scholar]
- 51.Al-Khafaji B., Noffsinger A.E., Miller M.A., DeVoe G., Stemmermann G.N., Fenoglio-Preiser C. Immunohistologic analysis of gastrointestinal and pulmonary carcinoid tumors. Hum. Pathol. 1998;29:992–999. doi: 10.1016/S0046-8177(98)90206-4. [DOI] [PubMed] [Google Scholar]
- 52.Barbareschi M., Girlando S., Mauri F.A., Arrigoni G., Laurino L., Dalla Palma P., Doglioni C. Tumour suppressor gene products, proliferation, and differentiation markers in lung neuroendocrine neoplasms. J. Pathol. 1992;166:343–350. doi: 10.1002/path.1711660405. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham R.T., Pogue K.M., Curry W.J., Johnston C.F., Sloan J.M., Buchanan K.D. Immunostaining for vasostatin I distinguishes between ileal and lung carcinoids. J. Pathol. 1999;187:321–325. doi: 10.1002/(SICI)1096-9896(199902)187:3<321::AID-PATH258>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Pelosi G., Bresaola E., Bogina G., Pasini F., Rodella S., Castelli P., Iacono C., Serio G., Zamboni G. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: A comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum. Pathol. 1996;27:1124–1134. doi: 10.1016/S0046-8177(96)90303-2. [DOI] [PubMed] [Google Scholar]
- 55.Tonnies H., Toliat M.R., Ramel C., Pape U.F., Neitzel H., Berger W., Wiedenmann B. Analysis of sporadic neuroendocrine tumours of the enteropancreatic system by comparative genomic hybridisation. Gut. 2001;48:536–541. doi: 10.1136/gut.48.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panzuto F., Nasoni S., Falconi M., Corleto V.D., Capurso G., Cassetta S., Di Fonzo M., Tornatore V., Milione M., Angeletti S., et al. Prognostic factors and survival in endocrine tumour patients: Comparison between gastrointestinal and pancreatic localization. Endocr. Relat. Cancer. 2005;12:1083–1092. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 57.Rigaud G., Missiaglia E., Moore P.S., Zamboni G., Falconi M., Talamini G., Pesci A., Baron A., Lissandrini D., Rindi G., et al. High resolution allelotype of nonfunctional pancreatic endocrine tumours: Identification of two molecular subgroups with clinical implications. Cancer Res. 2001;61:285–292. [PubMed] [Google Scholar]
- 58.Furlan D., Cerutti R., Uccella S., La Rosa S., Rigoli E., Genasetti A., Capella C. Different molecular profiles characterize well-differentiated endocrine tumours and poorly differentiated endocrine carcinomas of the gastroenteropancreatic tract. Clin. Cancer Res. 2004;10:947–957. doi: 10.1158/1078-0432.ccr-1068-3. [DOI] [PubMed] [Google Scholar]
- 59.Honegger J., Prettin C., Feuerhake F., Petrick M., Schulte-Mönting J., Reincke M. Expression of Ki-67 antigen in nonfunctioning pituitary adenomas: Correlation with growth velocity and invasiveness. J. Neurosurg. 2003;99:674–679. doi: 10.3171/jns.2003.99.4.0674. [DOI] [PubMed] [Google Scholar]
- 60.Mazzaferro V., Bhoori S., Sposito C., Bongini M., Langer M., Miceli R., Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 61.Belli L.S., Caccamo L., Mazzaferro V., Silini E., Alberti A., Melada E., Regalia E., Gridelli B., Rubino A., Gennari L., et al. Milan multicenter experience in liver transplantation for hepatitis C-related cirrhosis: Report of 105 cases. Transplant. Proc. 1994;26:3582–3584. [PubMed] [Google Scholar]
- 62.Bosman F.T., Carneiro F., Hruban R.H., Theise N.D., editors. World Health Organization Classification of Tumours of the Digestive System. 4th. International Agency for Research on Cancer (IARC); Lyon, France: 2010. [Google Scholar]