Abstract
The etiology of Graves’ orbitopathy (GO) remains enigmatic and thus controversy surrounds its pathogenesis. The role of the TSH receptor (TSHR) and the activating antibodies directed against it in the hyperthyroidism of Graves’ disease (GD) is firmly established. Less well elucidated is what part the TSHR pathway might play in the development of GO. Also uncertain is the participation of other cell surface receptors in the disease. Elevated levels of IGF-1 receptor (IGF-1R) have been found in orbital fibroblasts as well as B and T cells from patients with GD. These abnormal patterns of IGF-1R display are also found in rheumatoid arthritis and carry functional consequences. In addition, activating IgGs capable of displacing IGF-1 from IGF-1R have also been detected in patients with these diseases. IGF-1R forms a complex with TSHR which is necessary for at least some of the non-canonical signaling observed following TSHR activation. Functional TSHR and IGF-1R have also been found on fibrocytes, CD34+ bone marrow-derived cells from the monocyte lineage. Levels of TSHR on fibrocytes greatly exceed those found on orbital fibroblasts. When ligated by TSH or M22, a TSHR-activating monoclonal antibody, fibrocytes produce extremely high levels of several cytokines and chemokines. Moreover, fibrocytes infiltrate both the orbit and thyroid in GD. In sum, based on current evidence, IGF-1R and TSHR can be thought of as “partners in crime”. Involvement of the former probably transcends disease boundaries, while TSHR may not.
Keywords: Graves’ ophthalmopathy, TSH receptor, IGF-1 receptor, orbital fibroblast, fibrocyte
Introduction
The pathogenesis of Graves’ orbitopathy (GO), also known as thyroid associated ophthalmopathy, remains clouded in uncertainty. It is unclear whether the same genetic susceptibility factors implicated In Graves’ disease (GD), namely CD40, thyroglobulin, HLA-DR, CTLA-4, and thyroid stimulating hormone receptor (TSHR) are identical to those contributing to GO (1). Moreover, the environmental factors provoking either GD or GO have yet to be identified. The contribution of tobacco use to disease severity has certainly received substantial attention and smoking cessation remains one of the most important behavior modifications we can suggest to our patients (2). Absence of complete preclinical rodent models of GD has hampered efforts to identify the critical constellation of events underlying GO (3). Encouraging news reporting on the successful generation of a mouse model replete with many of the infiltrative manifestations that occur in human GD were later found to be incorrect (4,5). Thus, most of the insights into the pathogenesis of GO derive from measurements made of serum factors and observations from cell culture-based studies. Unfortunately, the vast majority of opportunities to access orbital tissues occur very late in the disease process when surgical rehabilitation is typically undertaken. Our inability to distinguish primary disease events from secondary tissue reactivity in the orbit has too frequently prevented progress toward specific and targeted therapies. This brief review attempts to outline our current knowledge about the potential roles of IGF-1 receptor (IGF-1R) and its spontaneously generated activating antibodies in the pathogenesis of GO. These will be referred to as GD-IgG. More importantly perhaps is our goal in drawing attention to what remains unknown about GO and to provide a realistic perspective on what might provide answers as research continues.
Orbital fibroblasts exhibit a unique set of phenotypic attributes
The unique characteristics exhibited by orbital fibroblasts, including their particularly robust responses to cytokines (6), may underlie clinically aggressive GO. These cells express high levels of inflammatory mediators such as prostaglandins (7-10), lipoxygenase products (11), and chemokines (12). They display surface receptors for cytokines and produce a diverse array of both Th1 and Th2 cytokine types (13,14). Orbital fibroblasts express multiple enzymes involved in the biosynthesis of the glycosaminoglycan, hyaluronan. These include all three isozymes of hyaluronan synthase (18) and the upstream enzyme, UDP-glucose dehydrogenase (19,20). Orbital fibroblasts, especially those from patients with GO, represent a heterogeneous cell population that can be divided into subsets based on the display of the glycoprotein, Thy-1 (15-17). Expression of this surface marker segregates cells into those which can terminally differentiate into mature adipocytes (Thy-1-) and those which can be provoked into forming myofibroblasts (Thy-1+). A central hallmark of GO, expansion of the orbital connective tissue contents and its extensive fibrosis, can thus be explained, at least in part, on the basis of the Thy-1 display. Yet, many of the studies conducted in efforts to characterize orbital fibroblasts and their roles in disease pathogenesis have ignored their phenotypic diversity, potentially obscuring functional nuance.
A potentially important insight into the cellular makeup of the orbit in GO was provided recently by Douglas and co-workers (21). They identified the increased abundance of a subset of circulating CD34+ cells that infiltrate the orbit in GO and express high levels of functional TSHR. Fibrocytes are CD34+ cells that derive from the bone marrow and appear to partially replace the fibroblasts normally inhabiting orbital connective tissue which are uniformly CD34-. Their potential for differentiation into either fat cells or myofibroblasts might account for the peculiar patterns of tissue remodeling found in GO (21). It appears that fibrocytes also infiltrate the thyroid in GD and may therefore represent an important bridge between the orbit and the thyroid (22).
The strong case for involvement of TSH receptor and its activating antibodies in GO
Glaring deficits in understanding GO have led to considerable debate concerning the potential role of the thyrotropin receptor (TSHR) in this component of GD. While the role of TSHR in the hyperthyroidism associated with GD is well established, whether the stimulatory antibodies directed against TSHR, often referred to as thyroid-stimulating antibodies (TSI or TSAb), play a role in initiating or sustaining orbital tissue remodeling in GO has not been resolved. Many circumstantial pieces of evidence suggest that TSHR and TSAb may be involved. General correlation appears to exist between levels of TSAb and the severity and activity of GO (23-25). Higher cell surface levels of TSHR are found in orbital tissues coming from active disease and are displayed by orbital fibroblasts from these patients, especially following induction of adipogenesis (26). These findings generally support the participation of TSHR. Detection of TSHR mRNA in orbital tissues was first reported by Feliciello et al who found the transcript in healthy tissues and those coming from GO (27). This study was followed shortly thereafter by Heufelder et al who could also identify the mRNA in cultured fibroblasts (28). But these associations between levels of anti-TSHR and disease activity/severity do not constitute proof of a causal relationship. We conclude that the most productive discussions concern not only TSHR and substantiating a role of TSAb in GO. Rather, they should include consideration of additional molecular determinants such as IGF-1R as a participant in the disease process.
IGF-1R represents a multifaceted conduit to signaling involved in mammalian cell regulation from early development
IGF-1, IGF-1R, and IGF-1 binding proteins play many roles in developing and maintaining mammalian tissues (29). Although they are incompletely characterized, multiple aspects of the IGF-I pathway appear to diverge from normal in individuals with autoimmune disease (30). Increasing awareness of these alterations has provoked us to query whether modifying IGF-1 signaling could alter disease course, severity, and activity. Such an impact on the disease might constitute effective therapy. Biological agents continue to be developed routinely for some autoimmune diseases, such as rheumatoid arthritis (31). Many of these agents exhibit highly specific molecular and cellular targeting toward cell surface receptors, cytokines, and growth factors. As a class, many show relatively low toxicity when compared to small molecule drugs such as kinase inhibitors. The use of agents directed at multiple targets has been advocated in rheumatoid arthritis and other allied diseases as a strategy for overcoming potential drug resistance. They are utilized to exploit the potential efficacy of drug combinations.
The notion that some human autoimmune diseases are associated with multiple autoantigens is not novel. Mechanistically, emergence of additional antibodies as candidate participants in GD is consistent with the phenomenon of antigen spread (32). Thus, the detection of multiple autoantibodies in GD, such as those directed at thyroglobulin and thyroid peroxidase, should not be surprising. These well-appreciated examples are not thought to play pathogenic roles in the disease. They are more commonly encountered in other forms of thyroid autoimmunity such as Hashimoto's thyroiditis. A very recent study examined the emerging detectability of antibodies to thyroglobulin, TPO and TSHR in patients prior to and following diagnosis with Hashimoto's thyroiditis and GD (33). Hutfless and colleagues found that the behavior of antibodies to all three exhibited distinct patterns of detectability and that anti-TSHR remained the only one specific for GD.
With specific reference to IGF-1R, antibodies displaying abilities to interrupt IGF-1R signaling were first described more than 20 years ago. The interest at that time was confined to their potential utility in treating cancer. αIR3 is a monoclonal antibody that blocks the activation of IGF-1R. It was first described by Yamashita et al (34) and characterized for blocking the up-regulation by IGF-I of growth hormone synthesis. Later, Li et al (35) described a second IGF-1R blocking antibody, designated 1H7. This monoclonal antibody could block the binding activity of IGF-1 as well as attenuate the activation of IGF-IR. 1H7 could bind IGF-IRα, and inhibit IGF-I and IGF-II binding. 1H7 could inhibit basal and IGF-I- and IGF-II-dependent DNA synthesis in NIH 3T3 cells. These antibodies represent prototypes of those that might be utilized as therapy in GO and perhaps other autoimmune diseases where IGF-1R plays a role in pathogenesis.
Evidence for IGF-1R playing roles in thyroid and orbital tissue function
The importance of TSHR to the pathogenesis of GD was established over the last several decades (36). Yet, the processes that underlie the loss of peripheral immune tolerance to TSHR have yet to be identified. Moreover, the repertoire of cellular proteins that might collaborate with TSHR in thyroid epithelial cells and fibroblasts/fibrocytes expressing TSHR is incompletely identified. In addition to TSH, other factors exert regulatory influence on thyroid tissue growth and function. Among them is the IGF family (37). Importantly, the full impact of TSH requires the addition of IGF-I or insulin to cultures of human and dog thyroid cells. The rationale for examining IGF-1R in the thyroid emerged in large part from early observations of Ingbar and his colleagues (38). They demonstrated the importance of IGF-1 on TSHR signaling. Their important findings, conducted in the rat thyroid epithelial cell line, FRTL-5, suggested that IGF-1 could enhance cell proliferation and promote the impact of TSH on DNA synthesis (38). The same effects could be ascribed to insulin and IGF-II, although the magnitude of these was considerably less than that of IGF-1 (38,39). Moreover, the cell proliferative effects of TSAb were also increased by IGF-1 (36). This same group of investigators found that the growth enhancing effects of both TSH and IGF-1 were mediated by adenosine 3′,5′-monophosphate (40-42). Takahashi and colleagues (43,44) reported that IGF-I rapidly increased tyrosine phosphorylation of a 175-kDa protein and that TSH potentiated this effect. More recently, Clément et al (45) performed a series of studies in transgenic mice over-expressing IGF-1, IGF-1R, or the combination of both. Using constructs driven by the thyroglobulin gene promoter, these mice exhibited thyroid-specific over-expression of both genes. In these animals, thyroid gland weights were increased as were follicular luminal areas, lower serum TSH and minimally elevated thyroxin concentrations. The authors interpreted these results as suggesting that the increased IGF-1/IGF-1R pathway activity might be enhancing thyroid function.
More recently, Tsui et al reported that IGF-1R and TSHR can form a functional complex (26). Specifically, IGF-1Rβ and TSHR can be co-immunoprecipitated from orbital fibroblasts and human thyroid epithelial cells. Moreover, blocking IGF-1R activation with 1H7 can block the signaling initiated by TSH and GD-IgG that results in the phosphorylation of Erk 1/2 (26). Thus, it is possible that the IGF-1R/TSHR complex may account for some of the signaling thus far attributed to TSHR functioning alone. Moreover it may represent the mechanism by which IGF-1 can enhance the impact of TSH on target cells. With regard to GD, the close proximity of IGF-1R to TSHR might make the notion of antigen spread particularly attractive.
Evidence supporting a role for IGF-1 and IgG inducing chemoattractants in orbital fibroblasts
Initial studies, performed almost two decades ago, examined the ability of IgG from patients with GO to displace radiolabeled IGF-1 from their binding sites on fibroblasts. Among the first was the group headed by Kendall-Taylor. Weightman et al (46) found that IgGs from patients with GD could displace 125I IGF-1 binding from the surface of orbital fibroblasts. While the identity of the binding sites was not explored further, these studies suggested the relationship between high affinity cell surface binding sites and antibodies associated with GD.
The nature of this IgG binding to fibroblasts was further explored following the observation by Pritchard and colleagues (47) that circulating antibodies from these patients could activate the Akt/mTOR/FRAP/p70s6K pathway and through that cascade could induce the chemoattractants, IL-16 and RANTES expression in orbital fibroblasts from patients with GD but not those from healthy donors. Auto-antibodies directed against IGF-1R can be detected in almost all patients with GD but in few individuals without the disease (48). Key to these findings was the realization that rapamycin, an antibiotic that specifically targets mTOR, could block the induction of these cytokines (47). This pathway is used extensively by IGF-1R to signal downstream genes, thus this receptor was examined extensively for its potential involvement in the IgG provoked induction of IL-16 and RANTES expression. Initial studies determined that not only IGF-1 but the IGF-1R specific analogue Des1-3 could mimic the action of the GD-IgGs. 1H7, an anti-IGF-1R blocking monoclonal antibody, could block the induction as could transfecting fibroblasts with a dominant negative IGF-1R mutant, designated 486STOP (48). This same pattern of IL-16 and RANTES induction by IGF-I was subsequently described in cultured primary human thyroid epithelial cells (49). Unlike control fibroblasts, thyrocytes from donors without GD or other autoimmune thyroid processes also responded to IGF-I and GD-IgG. These results suggest that fundamental differences might exist in orbital fibroblasts and thyrocytes with regard to IGF-1R signaling. Subsequent work of Pritchard et al implicated IGF-1R and similar disease associated activating antibodies in rheumatoid arthritis (50). Not only could the IgGs from patients with rheumatoid arthritis induce IL-16 and RANTES in synovial fibroblasts, but GD-IgG's could do the same. Thus, the paradigm of activating anti-IGF-1R antibodies provoking the expression of chemoattractants does not appear to be specific to GD. Rather, it suggests a disease mechanism spanning multiple autoimmune diseases. The equivalent effects of GD-IgG and IgGs from patients with rheumatoid arthritis suggest that this mechanism for upregulating chemoattractants might represent a common thread bridging these diseases and could therefore represent a shared therapeutic target.
IGF-1R may mediate the induction of hyaluronan synthesis by GD-IgG in orbital fibroblasts
A hallmark of the orbital tissue remodeling associated with GO is the accumulation of the glycosaminoglycan, hyaluronan (51). Recognition of this component of the disease has prompted examination of the mechanisms that might be involved in its increased synthesis. Orbital fibroblasts in vitro produce remarkably large amounts of hyaluronan when activated by inflammatory cytokines such as IL-1β, interferon γ, leukoregulin, and CD154 (9,19,52,53). Moreover, they express all three hyaluronan synthase isozymes (HAS) (18) as well as extremely high levels of the enzyme, UDP glucose dehydrogenease (19,20). All of these enzymes are up-regulated by IL-1β, actions that are mediated at the pretranslational level. At least some component of the over-determined hyaluronan production that apparently occurs in orbital connective tissue might result from high levels of the gene transcription factor Sp1 (20). In contrast, orbital fibroblasts fail to degrade hyaluronan in vitro (54), findings that are consistent with the phenotype of human dermal fibroblasts (55).
IGF-1 can affect the rates of proteoglycans and hyaluronan synthesis in a wide variety of cell types. For instance, observations made in cultured articular cartilage explants (56), peritubular testicular cells (57), rabbit pericardium (58), and human skin fibroblasts (59) suggest that IGF-1 can influence the rate of hyaluronan synthesis. In contrast, IGF-1 was found to reduce hyaluronan synthesis in breast cancer cells (60) while having no effect in human arterial smooth muscle (61). Many of these studies are rather preliminary in nature and do not contain studies dissecting the molecular mechanisms involved. In aggregate, a substantial body of evidence suggests that the IGF-1/IGF-1R pathway is involved, in at least some tissues, in the regulation of glycosaminoglycan accumulation.
Imai and colleagues reported that both IGF-1 and platelet derived growth factor could upregulate the synthesis of hyaluronan and chondroitin sulfate in orbital fibroblasts (62), a pattern of cellular response that differed from that observed in dermal fibroblast cultures. Subsequently, Smith and Hoa reported some time ago that IGF-1 and GD-IgG could enhance the production of hyaluronan in an anatomic site selective manner (63). Moreover, only those orbital fibroblasts from patients with GO would respond in this manner.
Other laboratory groups have taken different approaches with regard to examining the potential impact of GD-IgG on hyaluronan accumulation by focusing on TSHR. For instance, Zhang et al (64) reported that a gain of function mutant TSHR introduced into orbital fibroblasts resulted in increased levels of cAMP and hyaluronan (65). The impact of GD-IgG on glycosaminoglycan levels was rather modest. van Zeijl et al (66) were unable to demonstrate any effects of GD-IgG or rhTSH on either hyaluronan synthesis or the expression of any of the three hyaluronan synthase (HAS) isozymes. In contrast, they found that the adenylate cyclase activator, forskolin could up-regulate hyaluronan accumulation and the mRNAs encoding HAS1 and HAS3 (66). In a subsequent paper, van Zeijl et al (67) found that if they subjected the orbital fibroblast cultures to a differentiation-provoking medium, they could induce hyaluronan accumulation with GD-IgG and this effect did not appear to be cAMP mediated. rhTSH had no apparent impact on production despite increasing cAMP levels in many of the culture strains examined. Those studies apparently did not include assessment of the potential impact of IGF-1 on hyaluronan production in the differentiated fibroblast cultures. Importantly, the finding of an induction by GD-IgG on hyaluronan production in the face of no response to TSH suggests the potential for GD-IgG activating IGF-1R. In aggregate, the reports of van Zeijl et al (67) may share important similarities with that of Smith and Hoa (63).
IGF-1R plays an important role in defining T and B lymphocyte phenotypes and function
IGF-1 and IGF-1R play important roles in hematopoietic cell growth, differentiation, and normal immune function (68). Peripheral blood T and B cells and monocytes from healthy human donors express low levels of IGF-1R in vivo (69,70). Administration of IGF-1 increases the circulating pool of CD4+ T cells and splenic B cells in mice (71,72), suggesting a role for this growth factor in myelopoietic cell expansion (73). It promotes T cell proliferation during early activation (74) and inhibits apoptosis of both immature and mature T cells through at least three distinct mechanisms (75,76). IGF-1 stimulates inflammatory cytokine production in T cells and monocytes, including IL-2 (77), TNF-α (78) and IL-8 (79). It can bias lymphocyte development toward a Th2 phenotype by enhancing TNF-IL-10 (80), IL-4, and IL-13 synthesis (81) while inhibiting IFN-γ function (82).
Functional display of the IGF-1R also appears critical to B cell expansion from bone marrow CD34+ cells and immunoglobulin production (83,84). IGF-1 selectively increases expression of CD23 (type II IgE receptor, FcεRII) by both primary and established B cells (85). Its administration also enhances IgG production by peripheral B cells and those inhabiting human tonsils and increases circulating Ig levels in mice (72,86-89). Reconstitution of immunodeficient mice with fetal liver cells from IGF-1R-/- mice substantially diminishes humoral responses and immunoglobulin production (90). Thus, a role for IGF-1 and its pathway has been established in both normal and abnormal lymphocyte function.
Potential involvement of IGF-1R in the aberrant behavior of lymphocytes in Graves’ disease
Memory T cells expand in GD (91). Moreover, immune cell infiltration of the thyroid and connective tissue of the orbit represent prominent features of the disease (92). Cytokines synthesized by T and B lymphocytes, monocytes, and mast cells, appear to drive tissue remodeling, including the expansion of extra-ocular muscles and fat (93). It remains unclear why immunocompetent cells are recruited to the orbit (3,5-9). One possibility concerns the finding that fibroblasts from individuals with GO display higher levels of IGF-1R and when treated with IGF-1 or with GD-IgG, synthesize T cell chemoattractants (47,48). Like these orbital fibroblasts, a disproportionately large fraction of peripheral blood T cells also express IGF-1R in patients with GD (94). Subset analysis reveals that these patients demonstrate a relative expansion of IGF-1R+CD4+ and IGF-1R+CD8+ memory (CD45RO+) T cell populations (94). T cells harvested from TAO orbital tissues reflect similar increased proportion of IGF-1R+ T cells as those found in the peripheral circulation. Addition of exogenous IGF-1 enhanced T cell proliferation and inhibited Fas-mediated apoptosis. Memory T cell (CD4 and CD8) populations in the peripheral blood expand in GD (91,94). Identification of increased IGF-1R expression by these cells suggests that IGF-1 and GD-IgG may directly promote survival and/or expansion of antigen specific T cells in GD. These observations may couple with those of exaggerated IGF-1R expression by fibroblasts and the potential consequences of cellular interactions with GD-IgG.
Increased frequency of IGF-1R+ T cells has been confirmed in identical twin pairs discordant for GD. The affected twin exhibited an increased frequency of naïve and memory IGF-1R+ T cells compared to the healthy twin (96). In aggregate over-representation of the IGF-1R+ T cell phenotype in individuals with GD appears durable and cannot be attributed to the genetic determinants of GD. Rather, the phenotypic skew toward IGF-1R+ lymphocytes appears to be acquired. These findings underscore the potentially critical role of non-genetic, environmental factors as immunological triggers in genetically susceptible individuals.
TSAbs directed at TSHR are responsible for the metabolic consequences of GD (1). Actions of these immunoglubulins mimic those of TSH resulting in excessive thyroid hormone production. GD-IgG directed at IGF-1R (47,48) also appears to play a pathogenic role in GD. Given the prominent role for B cell function in the pathogenesis of GD, IGF-1R expression was examined in peripheral blood B cells of GD patients and a cohort of control donors. Analogous to their T cells, IGF-1R+ B cells are over-represented in the peripheral blood and orbital tissues in patients with GD relative to controls (95). Increased receptor display has several functional consequences including enhanced B cell expansion and exaggerated Ab production including those directed against the TSHR. Moreover, assessment of B cells from discordant identical twin pairs revealed increased abundance of IGF-1R+ B cells in the affected twin (96).
Where do we stand regarding IGF-1R-targeting antibodies in GD?
Debate has arisen concerning the existence and importance of antibodies targeting IGF-1R in the pathogenesis of GO. Confirmatory reports have yet to appear demonstrating the effects of GD-IgG on T cell chemoattractant synthesis and hyaluronan production. The findings of van Zeijl et al (67) utilizing fibroblasts exposed to an adipocyte differentiation medium may be in agreement with those of Smith and Hoa (63), but further studies to reconcile these studies will be necessary.
Efforts have been made to detect the anti-IGF-1R antibodies using alternate methods. A recent, preliminary report appearing in abstract form described development of an assay based on “luminescent immunoprecipitation” stated that no differences could be detected between serum concentrations of anti-IGF-1R antibodies in control individuals and those with GD (97). Since the apparent design of the assay would not allow discrimination between active and non-active antibodies, the report is probably difficult to interpret and to compare with that of Weightman et al (46) or Pritchard et al (47,48). Additional information about this assay and the results obtained from its implementation should clarify its significance. In another recent study, Zhao and colleagues (98) found that immunization by electroporation of mice with TSHR-encoding plasmids resulted in the generation of anti-IGF-1R. That study failed to identify any phenotypic attributes that could be linked specifically to these antibodies and thus their pathogenic significance remains uncertain.
Concluding comments: A potential way forward-testing the hypothesis
Evidence has been advanced that IGF-1R might play a role in autoimmunity (37). This possibility appears consonant with the established impact that both IGF-1 and IGF-1R exert on multiple components of the mammalian immune system. We have suggested that antibodies generated in GD can bind to the receptor and can initiate signaling that culminates in the production of chemoattractants and hyaluronan. These effects are disease- selective in that only fibroblasts from individuals with GD respond. But the jury has yet to render a verdict with regard to the constellation of molecular participants in the pathogenesis of GO. Moreover, the importance of IGF-1R and the antibodies generated against it might play in GO has yet to be established. Ultimately, personal opinion will neither support nor detract from the necessary empirical evidence that must emerge from thoughtfully conceived and conducted future studies. A multi-center therapeutic trial specifically directed at examining the impact of a fully humanized blocking anti-IGF-1R antibody on the activity and progression of moderate to severe GO is being organized in North America. This therapeutic approach may prove useful in other autoimmune diseases besides GD where the common threads of IGF-I dysfunction might occur. Considering the numerous hurdles that have already been overcome in the development of these IGF-1R-blocking agents for use in cancer, their repurposed application to autoimmunity might represent an attractive prospect for the pharmaceutical industry.
Research agenda.
More detailed examination of TSHR/IGF-1R molecular interactions.
Better understanding of the cellular composition of the human orbit and how this changes in GO.
Characterization of the cell signaling induced through TSHR activation in orbital fibroblasts and fibrocytes.
Development of potential therapeutic agents targeting TSHR and IGF-1R
Identifying the molecular and cellular signatures that distinguish individuals with aggressive versus mild GO
Longitudinal studies following anti-TSHR antibody levels in individuals with GO and assessing how these might respond to therapy.
Potential repurposing of agents initially developed for treating other disease processes
Application of more stringency to research going forward.
Developing consensus about meaningful response metrics in GO as we move into treatment trials
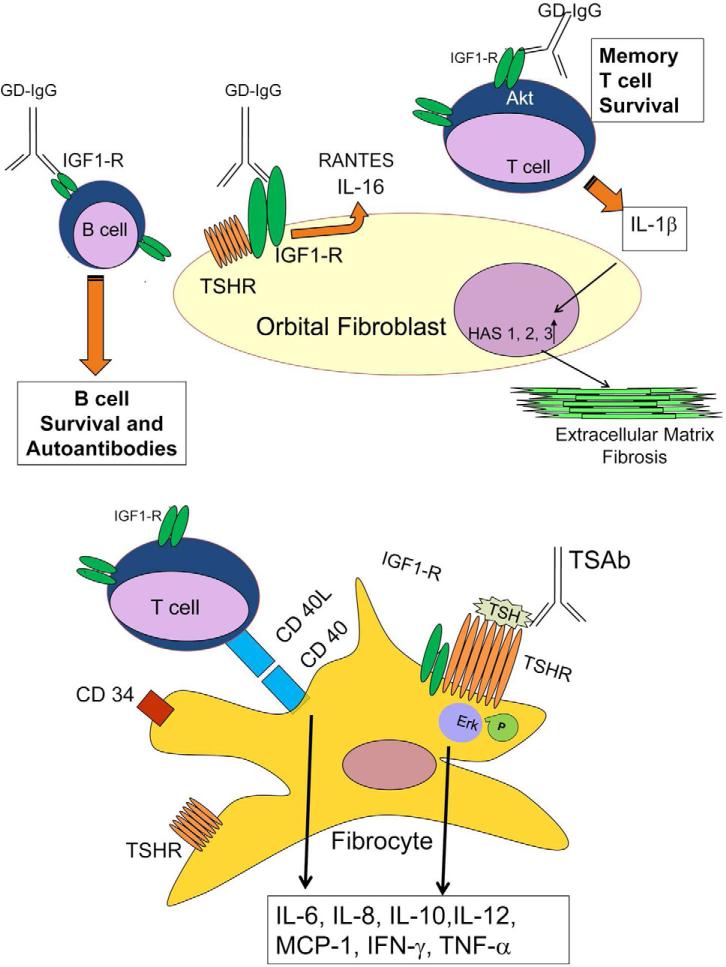
Figure 1.
Schematic diagram of the proposed model in which IGF-1R might participate in concert with TSHR in the pathogenesis of GO.
Acknowledgements
This work was supported in part by National Institutes of Health grants EY008976, EY011708, DK063121, EY016339, EY021197, RR00425, AR053858, an unrestricted grant from Research to Prevent Blindness, a Research to Prevent Blindness Career Development Award, the Bell Charitable Foundation, and an unrestricted grant from the Novo Nordisk Foundation.
Abbreviations
- GD
Graves’ disease
- GO
Graves’ ophthalmopathy or orbitopathy
- TRAb
TSH-receptor antibodies
- TSAb
thyroid stimulating immunoglobulins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors report any conflict of interest.
Contributor Information
Terry J. Smith, Professor of Ophthalmology and Visual Sciences, Professor of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI 48105, U.S.A. Guest Professor, Department of Ophthalmology, University of Southern Denmark, DK-5000 Odense C, Denmark.
Frederick G.L. Huetwell, Professor of Ophthalmology and Visual Sciences, Professor of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI 48105, U.S.A. Guest Professor, Department of Ophthalmology, University of Southern Denmark, DK-5000 Odense C, Denmark
Laszlo Hegedüs, Professor of Internal Medicine and Endocrinology, University of Southern Denmark in Odense. Senior Consultant Physician, Department of Endocrinology and Metabolism, Odense University Hospital, DK-5000 Odense C, Denmark.
Raymond S. Douglas, Associate Professor of Ophthalmology and Visual Sciences, University of Michigan Medical School. Director, Thyroid Eye Disease Center, Kellogg Eye Center, Ann Arbor, MI 48105, U.S.A.
References
- 1.Brent GA. Graves’ Disease. The New England Journal of Medicine. 2008;358:2594–2605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 2.Cawood TJ, Moriarty P, O'Farrelly C, O'Shea D. Smoking and Thyroid-Associated Ophthalmopathy: A Novel Explanation of the Biological Link. The Journal of Clinical Endocrinology & Metabolism. 2007;92:59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- 3.McLachlan S, Nagayama Y, Rapoport B. Insight into Graves’ Hyperthyroidism from Animal Models. Endocrinology Reviews. 2005;26:800–832. doi: 10.1210/er.2004-0023. [DOI] [PubMed] [Google Scholar]
- 4.Many MC, Costagliola S, Detrait M, et al. Development of an Animal Model of Autoimmune Thyroid Eye Disease. The Journal of Immunology. 1999;162:4966–4974. [PubMed] [Google Scholar]
- 5.Baker G, Mazziotti G, von Ruhland C, Ludgate M. Reevaluating Thyrotropin Receptor-Induced Mouse Models of Graves’ Graves and Ophthalmopathy. Endocrinology. 2005;146:835–844. doi: 10.1210/en.2004-1015. [DOI] [PubMed] [Google Scholar]
- 6.Smith TJ. Orbital Fibroblasts Exhibit a Novel Pattern of Responses to Proinflammatory Cytokines: Potential Basis for the Pathogenesis of Thyroid-Associated Ophthalmopathy. Thyroid. 2002;12:197–203. doi: 10.1089/105072502753600133. [DOI] [PubMed] [Google Scholar]
- 7.Wang HS, Cao HJ, Winn VD, et al. Leukoregulin induction of prostaglandin endoperoxide H synthase-2 in human orbital fibroblasts: an in vitro model for connective tissue inflammation. The Journal of Biological Chemistry. 1996;271:22718–22728. [PubMed] [Google Scholar]
- 8.Young DA, Evans CH, Smith TJ. Leukoregulin induction of protein expression in human orbital fibroblasts: Evidence for anatomical-site-restricted cytokine-target cell interactions. Proceeding of the National Academy of Sciences USA. 1998;95:8904–8909. doi: 10.1073/pnas.95.15.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao HJ, Wang HS, Zhang Y, et al. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression: Insights into potential pathogenic mechanisms of thyroid associated ophthalmopathy. The Journal of Biological Chemistry. 1998;273:29615–29625. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 10.Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1α in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent PGE2 synthase expression. The Journal of Biological Chemistry. 2002;277:16355–16364. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Tsui S, Boeglin WE, et al. IL-4 induces 15 lipoxygenase-1 expression in human orbital fibroblasts from patients with Graves’ disease: Evidence for anatomic site-selective action of TH2 cytokines. The Journal of Biological Chemistry. 2006;281:18296–18306. doi: 10.1074/jbc.M603484200. [DOI] [PubMed] [Google Scholar]
- 12.Sciaky D, Brazer W, Center DM, et al. Cultured human fibroblasts express constitutive IL-16 mRNA: Cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. The Journal of Immunology. 2000;164:3806–3814. doi: 10.4049/jimmunol.164.7.3806. [DOI] [PubMed] [Google Scholar]
- 13.Han R, Smith TJ. T helper type 1 and type 2 cytokines exert divergent influence on the induction of PGE2 and hyaluronan synthesis by IL-1β in orbital fibroblasts: Implications for the pathogenesis of thyroid-associated ophthalmopathy. Endocrinology. 2006;147:13–19. doi: 10.1210/en.2005-1018. [DOI] [PubMed] [Google Scholar]
- 14.Han R, Chen B, Smith TJ. Janus kinase 2 dampens the induction by IL-1beta of prostaglandin endoperoxide H synthase-2 expression in human orbital fibroblasts: evidence for divergent influence on the PGE2 biosynthetic pathway. The Journal of Immunology. 2007;179:7147–7156. doi: 10.4049/jimmunol.179.10.7147. [DOI] [PubMed] [Google Scholar]
- 15.Koumas L, Smith TJ, Feldon S, et al. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. The American Journal of Pathology. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1-subpopulations exhibit distinct phenotypes. European Journal of Immunology. 2002;32:477–485. doi: 10.1002/1521-4141(200202)32:2<477::AID-IMMU477>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. The Journal of Clinical Endocrinology & Metabolism. 2002;87:385–392. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 18.Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1ß in human orbital fibroblasts: Potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. The Journal of Clinical Endocrinology & Metabolism. 1999;84:4079–7084. doi: 10.1210/jcem.84.11.6111. [DOI] [PubMed] [Google Scholar]
- 19.Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. The Journal of Biological Chemistry. 1998;273:25117–25124. doi: 10.1074/jbc.273.39.25117. [DOI] [PubMed] [Google Scholar]
- 20.Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 levels may underlie differential expression of UDP glucose dehydrogenase by fibroblasts: Role in susceptibility to orbital Graves’ disease. The Journal of Biological Chemistry. 2011;286:24487–24499. doi: 10.1074/jbc.M111.241166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas RS, Affiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. The Journal of Clinical Endocrinology & Metabolism. 2010;95:430–438. doi: 10.1210/jc.2009-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie E, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. The Journal of Clinical Endocrinology & Metabolism. 2011 doi: 10.1210/jc.2011-2514. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerding MN, van der Meer WC, Broenink M, et al. Association of thyrotropin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clinical Endocrinology. 2000;52:267–271. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 24.Eckstein AK, Plicht M, Lax H, Neuhauser M, et al. Thyrotropin Receptor Autoantibodies Are Independent Risk Factors for Graves’ Ophthalmopathy and Help to Predict Severity and Outcome of the Disease. The Journal of Clinical Endocrinology & Metabolism. 2006;91:3464–3470. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 25.Lytton SD, Ponto KA, Kanitz M, et al. A Novel Thyroid Stimulating Immunoglobulin Bioassay Is a Functional Indicator of Activity and Severity of Graves’ Orbitopathy. The Journal of Clinical Endocrinology & Metabolism. 2010;95:2123–2131. doi: 10.1210/jc.2009-2470. [DOI] [PubMed] [Google Scholar]
- 26.Tsui S, Naik N, Hoa N, et al. Evidence for an association between thyroid stimulating hormone and insulin-like growth factor 1 receptors: A tale of two antigens implicated in Graves’ disease. The Journal of Immunology. 2008;181:4397–4405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feliciello A, Porcellini A, Ciullo I, et al. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342:337–338. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 28.Heufelder AE, Dutton CM, Sarkar G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297–300. doi: 10.1089/thy.1993.3.297. [DOI] [PubMed] [Google Scholar]
- 29.De Meyts P, Whittaker J. Structural Biology of Insulin and IGF1 Receptors: Implications for Drug Design. Nature Reviews Drug Discovery. 2002;1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- 30.Smith TJ, Tsai CC, Shih MJ, et al. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid. 2008;18:983–988. doi: 10.1089/thy.2007.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breedveld FC, Combe B. Understanding emerging treatment paradigms in rheumatoid arthritis. Arthritis Research & Therapy. 2011;13:53. doi: 10.1186/1478-6354-13-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suciu-Foca N, Harris PE, Cortesini R. Intramolecular and intermolecular spreading during the course of organ allograft rejection. Immunological Reviews. 1998;164:241–246. doi: 10.1111/j.1600-065x.1998.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 33.Hutfless S, Matos P, Taylor MV, et al. Significance of Prediagnostic Thyroid Antibodies in Women with Autoimmune Thyroid Disease. The Journal of Clinical Endocrinology & Metabolism. 2011;96:E1466–E1471. doi: 10.1210/jc.2011-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita S, Weiss M, Melmed S. Insulin-like growth factor-I regulates growth hormone secretion and messenger ribonucleic acid levels in human pituitary tumor cells. The Journal of Clinical Endocrinology & Metabolism. 1986;63:730–735. doi: 10.1210/jcem-63-3-730. [DOI] [PubMed] [Google Scholar]
- 35.Li SL, Kato J, Paz IB, et al. Two new monoclonal antibodies against the α subunit of the human insulin-like growth factor-I receptor. Biochemical and Biophysical Research Communications. 1993;196:92–98. doi: 10.1006/bbrc.1993.2220. [DOI] [PubMed] [Google Scholar]
- 36.Rapoport B, McLachlan SM. The thyroptin receptor in Graves’ disease. Thyroid. 2007;17:911–922. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- 37.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacological Reviews. 2010;62:199–236. doi: 10.1124/pr.109.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-Ig G. Endocrinology. 1986;119:940–942. doi: 10.1210/endo-119-2-940. [DOI] [PubMed] [Google Scholar]
- 39.Maciel RM, Moses AC, Villone G, et al. Demonstration of the production and physiological role of insulin-like growth factor II in rat thyroid follicular cells in culture. The Journal of Clinical Investigation. 1988;82:1546–1553. doi: 10.1172/JCI113764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tramontano D, Moses AC, Picone R, Ingbar SH. Characterization and regulations of the receptor for insulin-like growth factor-I in the FRTL-5 rat thyroid follicular cell line. Endocrinology. 1987;120:785–790. doi: 10.1210/endo-120-2-785. [DOI] [PubMed] [Google Scholar]
- 41.Tramontano D, Moses AC, Veneziani BM, Ingbar SH. Adenosine 3',5'-monophosphate mediates both the mitogenic effect of thyrotropin and its ability to amplify the response to insulin-like growth factor I in FRTL5 cells. Endocrinology. 1988;122:127–132. doi: 10.1210/endo-122-1-127. [DOI] [PubMed] [Google Scholar]
- 42.Tramontano D, Moses AC, Ingbar SH. The role of adenosine 3',5'-monophosphate in the regulation of receptors for thyrotropin and insulin-like growth factor I in the FRTL5 rat thyroid follicular cell. Endocrinology. 1988;122:133–136. doi: 10.1210/endo-122-1-133. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi S, Conti M, Prokop C, et al. Thyrotropin and insulin-like growth factor I regulation of tyrosine phosphorylation in FRTL-5 cells. Interaction between cAMP-dependent and growth factor-dependent signal transduction. The Journal of Biological Chemistry. 1991;266:7834–7841. [PubMed] [Google Scholar]
- 44.Takashashi S, Conti M, Van Wyk JJ. Thyroptropin potentiation of insulin-like growth factor-I dependent deoxribonucleic acid synthesis in FRTL-5 cells: mediation by an autocrine amplification factor(s). Endocrinology. 1990;126:736–745. doi: 10.1210/endo-126-2-736. [DOI] [PubMed] [Google Scholar]
- 45.Clément S, Refetoff S, Robaye B, et al. Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-Ir receptor in the thyroid gland. Endocrinology. 2001;142:5131–5139. doi: 10.1210/endo.142.12.8534. [DOI] [PubMed] [Google Scholar]
- 46.Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16:251–257. doi: 10.3109/08916939309014643. [DOI] [PubMed] [Google Scholar]
- 47.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. The Journal of Immunology. 2002;168:942–950. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 48.Pritchard J, Han R, Horst N, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the IGF-1 receptor pathway. The Journal of Immunology. 2003;170:6348–6354. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 49.Gianoukakis AG, Douglas RS, King CS, et al. IgG from patients with Graves’ disease induces IL-16 and RANTES expression in cultured human thyrocytes: A putative mechanism for T cell infiltration of the thyroid in autoimmune disease. Endocrinology. 2006;147:1941–1949. doi: 10.1210/en.2005-1375. [DOI] [PubMed] [Google Scholar]
- 50.Pritchard J, Tsui S, Horst N, et al. Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves’ disease, express high levels of IL-16 when treated with immunoglobulins against the IGF-1 receptor. The Journal of Immunology. 2004;173:3564–3569. doi: 10.4049/jimmunol.173.5.3564. [DOI] [PubMed] [Google Scholar]
- 51.Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocrine Reviews. 1989;10:366–391. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- 52.Smith TJ, Parikh SJ. HMC-1 mast cells activate human orbital fibroblasts in co-culture: Evidence for up-regulation of prostaglandin E2 and hyaluronan synthesis. Endocrinology. 1999;140:3518–3525. doi: 10.1210/endo.140.8.6881. [DOI] [PubMed] [Google Scholar]
- 53.Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. American Journal of Physiology. 1995;268:C382–C388. doi: 10.1152/ajpcell.1995.268.2.C382. [DOI] [PubMed] [Google Scholar]
- 54.Smith TJ, Bahn RS, Gorman CA. Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: Evidence for differences between retroocular and dermal fibroblasts. The Journal of Clinical Endocrinology & Metabolism. 1989;69:1019–1023. doi: 10.1210/jcem-69-5-1019. [DOI] [PubMed] [Google Scholar]
- 55.Arbogast B, Hopwood JJ, Dorfman A. Absence of hyaluronidase in cultured human skin fibroblasts. Biochemical and Biophysical Research Communications. 1975;67:376–382. doi: 10.1016/0006-291x(75)90326-5. [DOI] [PubMed] [Google Scholar]
- 56.Curtis AJ, Ng CK, Handley CJ, Robinson HC. Effect of insulin-like growth factor-1 on the synthesis and distribution of link protein and hyaluronan in explant cultures of articular cartilage. Biochimica et Biophysica Acta. 1992;1135:309–317. doi: 10.1016/0167-4889(92)90236-5. [DOI] [PubMed] [Google Scholar]
- 57.Thiebot B, Bichoualne L, Langris M, et al. IGF-1 stimulates synthesis of undersulfated proteoglycans and of hyaluronic acid by peritubular cells from immature rat testis. Biochimica et Biophysica Acta. 1997;1358:127–141. doi: 10.1016/s0167-4889(97)00070-0. [DOI] [PubMed] [Google Scholar]
- 58.Honda A, Iwai T, Mori Y. Insulin-like growth factor 1 (IGF-1) enhances hyaluronic acid synthesis in rabbit pericardium. Biochimica et Biophysica Acta. 1989;1014:305–312. doi: 10.1016/0167-4889(89)90227-9. [DOI] [PubMed] [Google Scholar]
- 59.Kuroda K, Utani A, Hamasaki Y, Shinkai H. Up-regulation of putative hyaluronan synthase mRNA by basic fibroblast growth factor and insulin-like growth factor-1 in human skin fibroblasts. Journal of Dermatological Science. 2001;26:156–160. doi: 10.1016/s0923-1811(00)00155-9. [DOI] [PubMed] [Google Scholar]
- 60.Mitropoulou TN, Theocharis AD, Nikitovic D, et al. IGF-I affects glycosaminoglycan/proteoglycan synthesis in breast cancer cells through tyrosine kinase-dependent and –independent pathways. Biochimie. 2004;86:251–259. doi: 10.1016/j.biochi.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Erikstrup C, Pedersen LM, Heickendorff L, et al. Production of hyaluronan and chondroitin sulphate proteoglycans from human arterial smooth muscle – the effect of glucose, insulin, IGF-I or growth hormone. European Journal of Endocrinology. 2001;145:193–198. doi: 10.1530/eje.0.1450193. [DOI] [PubMed] [Google Scholar]
- 62.Imai Y, Odajima R, Inoue Y, Shishiba Y. Effect of growth factors on hyaluronan and proteoglycan synthesis by retroocular tissue fibroblasts of Graves’ ophthalmology in culture. Acta Endocrinologica. 1992;126:541–552. doi: 10.1530/acta.0.1260541. [DOI] [PubMed] [Google Scholar]
- 63.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, IGF-1 receptor. The Journal of Clinical Endocrinology & Metabolism. 2004;89:5076–5080. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 64.Zhang I, Baker G, Janus D, et al. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Investigative Ophthalmology & Visual Science. 2006;47:5197–5203. doi: 10.1167/iovs.06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Bowen T, Grennan-Jones F, et al. Thyrotropin receptor activation increases hyaluronan production in preadipocyte-fibroblasts; contributory role in hyaluronan accumulation in thyroid dysfunction. The Journal of Biological Chemistry. 2009;284:26447–26455. doi: 10.1074/jbc.M109.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Zeijl CJ, Fliers E, van Koppen CJ, et al. Effects of thyrotropin and thyrotropin-receptor-stimulating Graves’ disease immunoglobulin G on cyclic adenosine monophosphate and hyaluronan production in nondifferentiated orbital fibroblasts of Graves’ ophthalmopathy patients. Thyroid. 2010;20:535–544. doi: 10.1089/thy.2009.0447. [DOI] [PubMed] [Google Scholar]
- 67.van Zeijl CJ, Fliers E, van Koppen CJ, et al. Thyrotropin receptor-stimulating Graves’ Disease immunoglobulins induce hyaluronan synthesis by differentiated orbital fibroblasts from patients with Graves’ Ophthalmology not only via cyclic adenosine monophosphate signaling pathways. Thyroid. 2011;21:169–176. doi: 10.1089/thy.2010.0123. [DOI] [PubMed] [Google Scholar]
- 68.Zumkeller W. The insulin-like growth factor system in hematopoietic cells. Leuk Lymphoma. 2002;43:487–491. doi: 10.1080/10428190290011958. [DOI] [PubMed] [Google Scholar]
- 69.Kooijman R, Williems M, DeHaas CJ, et al. Expression of type I insulin-like growth factor receptors on human peripheral blood mononuclear cells. Endocrinology. 1992;131:2244–2250. doi: 10.1210/endo.131.5.1425423. [DOI] [PubMed] [Google Scholar]
- 70.Kooijman RK, Scholtens LE, Rijkers GT, Zegers BJ. Differential expression of type I insulin-like growth factor receptors in different stages of human T cells. Europeaan Journal of Immunology. 1995;25:931–935. doi: 10.1002/eji.1830250411. [DOI] [PubMed] [Google Scholar]
- 71.Clark R, Strasser J, McCabe S, et al. Insulin-like growth factor-1 stimulation of lymphopoiesis. The Journal of Clinical Investigation. 1993;92:540–548. doi: 10.1172/JCI116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jardieu P, Clark R, Mortensen D, Dorshkind K. In vivo administration of insulin-like growth factor-I stimulates primary B lymphopoiesis and enhances lymphocyte recovery after bone marrow transplantation. The Journal of Immunology. 1994;152:4320–4327. [PubMed] [Google Scholar]
- 73.Alpdogan O, Muriglan SJ, Kappel BJ, et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 74.Hettmer S, Dannecker L, Foell, et al. Effects of insulin-like growth factors and insulin-like growth factor binding protein-2 on the in vitro proliferation of peripheral blood mononuclear cells. Human Immunology. 2005;66:95–103. doi: 10.1016/j.humimm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 75.Liu E, Law HK, Lau YL. Insulin-like growth factor I promotes maturation and inhibits apoptosis of immature cord blood monocyte-derived dendritic cells through MEK and PI 3-kinase pathways. Pediatric Research. 2003;54:919–925. doi: 10.1203/01.PDR.0000088067.04673.1B. [DOI] [PubMed] [Google Scholar]
- 76.Navarro M, Baserga R. Limited redundancy of survival signals from the type 1 insulin-like growth factor receptor. Endocrinology. 2001;142:1073–1081. doi: 10.1210/endo.142.3.7991. [DOI] [PubMed] [Google Scholar]
- 77.Kooijman R, Rijkers GT, Zegers BJ. IGF-I potentiates interleukin-2 production in human peripheral T cells. Journal of Endocrinology. 1996;149:351–356. doi: 10.1677/joe.0.1490351. [DOI] [PubMed] [Google Scholar]
- 78.Renier G, Clement I, Desfaits AC, Lambert A. Direct stimulatory effect of insulin-like growth factor-I on monocyte and macrophage tumor necrosis factor-alpha production. Endocrinology. 1996;137:4611–4618. doi: 10.1210/endo.137.11.8895324. [DOI] [PubMed] [Google Scholar]
- 79.Kooijman R, Coppens A, Hooghe-Peters E. IGF-I stimulates IL-8 production in the promyelocytic cell line HL-60 through activation of extracellular signal-regulated protein kinase. Cell Signal. 2003;15:1091–1098. doi: 10.1016/s0898-6568(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 80.Kooijman R, Coppens A. Insulin-like growth factor-I stimulates IL-10 production in human T cells. Journal of Leukocyte Biology. 2004;76:862–867. doi: 10.1189/jlb.0404248. [DOI] [PubMed] [Google Scholar]
- 81.Wynes MW, Riches DW. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. The Journal of Immunology. 2003;171:3550–3559. doi: 10.4049/jimmunol.171.7.3550. [DOI] [PubMed] [Google Scholar]
- 82.Bernabei P, Bosticardo M, Losana G, et al. IGF-1 down-regulates IFN-gamma R2 chain surface expression and desensitizes IFN-gamma/STAT-1 signaling in human T lymphocytes. Blood. 2003;102:2933–2939. doi: 10.1182/blood-2003-01-0100. [DOI] [PubMed] [Google Scholar]
- 83.Kim JH, Park HH, Lee CE. IGF-1 potentiation of IL-4-induced CD23/Fc(epsilon)RII expression in human B cells. Molecules and Cells. 2003;15:307–312. [PubMed] [Google Scholar]
- 84.Landreth KS, Narayanan R, Dorshkind K. Insulin-like growth factor-I regulates pro-B cell differentiation. Blood. 1992;80:1207–1212. [PubMed] [Google Scholar]
- 85.Stuart CA, Meehan RT, Neale LS. Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphoctyes. The Journal of Clinical Endocrinology & Metabolism. 1991;72:1117–1122. doi: 10.1210/jcem-72-5-1117. [DOI] [PubMed] [Google Scholar]
- 86.Kimata H, Fujimoto M. Growth hormone and insulin-like growth factor I induce immunoglobulin (Ig)E and IgG4 production by human B cells. The Journal of Experimental Medicine. 1994;180:727–732. doi: 10.1084/jem.180.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimata H, Yoshida A. Differential effect of growth hormone and insulin-like growth factor-I, insulin-like growth factor-II, and insulin on Ig production and growth in human plasma cells. Blood. 1994;83:1569–1574. [PubMed] [Google Scholar]
- 88.Kimata H, Yoshida A. Effect of growth hormone and insulin-like growth factor-I on immunoglobulin production by and growth of human B cells. The Journal of Clinical Endocrinology & Metabolism. 1994;78:635–641. doi: 10.1210/jcem.78.3.8126135. [DOI] [PubMed] [Google Scholar]
- 89.Robbins K, McCabe S, Schneiner T. Immunological effects of insulin-like growth factor-I--enhancement of immunoglobulin synthesis. Clinical & Experimental Immunology. 1994;95:337–342. doi: 10.1111/j.1365-2249.1994.tb06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baudler S, Baumgartl J, Hampel B. Insulin-like growth factor-1 controls type 2 T cell-independent B cell response. The Journal of Immunology. 2005;174:5516–5525. doi: 10.4049/jimmunol.174.9.5516. [DOI] [PubMed] [Google Scholar]
- 91.Bossowski A, Urban M, Stasiak-Barmuta A. Analysis of changes in the percentage of B (CD19) and T (CD3) lymphocytes, subsets CD4, CD8 and their memory (CD45RO), and naive (CD45RA) T cells in children with immune and non-immune thyroid diseases. Journal of Pediatric Endocrinology & Metabolism. 2003;16:63–70. doi: 10.1515/jpem.2003.16.1.63. [DOI] [PubMed] [Google Scholar]
- 92.Akamizu T, Mori T, Nakao K. Pathogenesis of Graves’ disease: molecular analysis of anti-thyrotropin receptor antibodies. Endocrine Journal. 1997;44:633–646. doi: 10.1507/endocrj.44.633. [DOI] [PubMed] [Google Scholar]
- 93.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocrinology Reviews. 2003;24:802–835. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 94.Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the IGF-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. The Journal of Immunology. 2007;178:3281–87. doi: 10.4049/jimmunol.178.5.3281. [DOI] [PubMed] [Google Scholar]
- 95.Douglas RS, Naik V, Hwang CJ, et al. B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: Implications for disease pathogenesis. The Journal of Immunology. 2008;181:5768–5774. doi: 10.4049/jimmunol.181.8.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Douglas RS, Brix TH, Hwang CJ, et al. Divergent frequencies of IGF-I receptor- expressing blood lymphocytes in monozygotic twin pairs discordant for Graves’ disease: Evidence for a phentotypic signature ascribable to nongenetic factors. The Journal of Clinical Endocrinology & Metabolism. 2009;95:1797–1802. doi: 10.1210/jc.2008-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Minich WB, Eckstein A, Dehina N. Detection of autoantibodies against the insulin-like growth factor-1 receptor in patients with Graves’ orbitopathy by luminescent immunoprecipitation analysis. Proceedings of the 14th International Thyroid Congress, Paris, France. 2010;39 (Abstract OC-074) [Google Scholar]
- 98.Zhao SX, Tsui S, Cheung A, et al. Orbital fibrosis in a mouse model of Graves’ disease induced by genetic immunization of thyrotropin receptor cDNA. Journal of Endocrinology. 2011;210:369–377. doi: 10.1530/JOE-11-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]