Abstract
Background
Despite ongoing controversies surrounding PSA screening, large numbers of men age 65+ undergo screening. However, there are few data quantifying the chain of events following screening in clinical practice to better inform decisions. The objective of this study is to quantify 5-year downstream outcomes following a PSA screening result > 4 ng/ml in older men.
Methods
Longitudinal cohort study of 295,645 men age 65+ who underwent PSA screening in the VA healthcare system in 2003 and were followed for 5 years using national VA and Medicare data. Among men whose index screening PSA was > 4 ng/ml we determined the number who underwent biopsy, were diagnosed with prostate cancer, were treated and survived 5-years, according to baseline characteristics. Biopsy and treatment complications were also assessed.
Results
25,208 (8.5%) men had an index PSA > 4 ng/ml. During 5-year follow-up, 8,313 (33%) men underwent at least one biopsy, 5,220 (63%) of men biopsied were diagnosed with prostate cancer of whom 4,284 (82%) were treated. Receipt of biopsy decreased with advancing age and worsening comorbidity (P<0.001), whereas the percentage treated for biopsy-detected cancer exceeded 75% even among men age 85+, those with Charlson score 3+, and those with low-risk cancer. Among men with biopsy-detected cancer, the risk of dying of non-prostate cancer causes increased with advancing age and comorbidity (P<0.001). 468 (6%) of men had 7-day biopsy complications. Treatment complications included 584 (14%) men with new incontinence and 588 (14%) men with new erectile dysfunction.
Conclusions
Receipt of biopsy is low in older men with abnormal screening PSA and decreases with advancing age and comorbidity. However, once biopsy detects cancer most men undergo immediate treatment regardless of advancing age, comorbidity, or low-risk cancer. Understanding downstream outcomes in clinical practice should better inform individualized decisions among older men considering PSA screening.
INTRODUCTION
The U.S. Preventive Services Task Force (USPSTF), American Cancer Society, and American Urologic Association all recommend against performing prostate-specific antigen (PSA) screening in men with limited life expectancy because of the often indolent nature of screen-detected prostate cancer.1-3 However, PSA screening, which has been covered by Medicare since 2000, continues to be common practice among older men, including those with serious comorbidity.4-7 Even after the 2008 USPSTF recommendation against PSA screening in men over age 75, PSA screening rates have not declined.8 Also, the 2012 USPSTF recommendation against PSA screening in all age groups has been met with criticism from many who believe men deserve the opportunity to make their own informed decisions about the benefits and burdens of PSA screening.9-11
Advising older men about the benefits and burdens of PSA screening is challenging because trials excluded men older than 75 years. Furthermore, trial results were conflicting. While the ERSPC (European Randomized Study of Screening for Prostate Cancer) found that screening reduced prostate cancer mortality by 21% at 11 years, the U.S. PLCO (Prostate, Lung, Colorectal, and Ovarian) Cancer Screening Trial found no reduction in prostate cancer mortality.12,13 In addition, extrapolating trial results to the heterogeneous population of older men seen in clinical practice is challenging, especially since the chain of events following PSA screening in this population is unknown and likely very different from that in trials.
This study makes innovative use of VA and Medicare claims-based data and electronic health records to determine downstream outcomes over 5 years following PSA screening in veterans age 65 years and older. Specifically, we hypothesized that in clinical practice frequencies of abnormal PSA screening results, repeat PSA tests, biopsies, cancer diagnoses, treatment and 5-year survival would differ according to baseline characteristics, such as age and comorbidity. These data are fundamental to informing individualized decisions about PSA screening in older men.
METHODS
Data Sources and Subjects
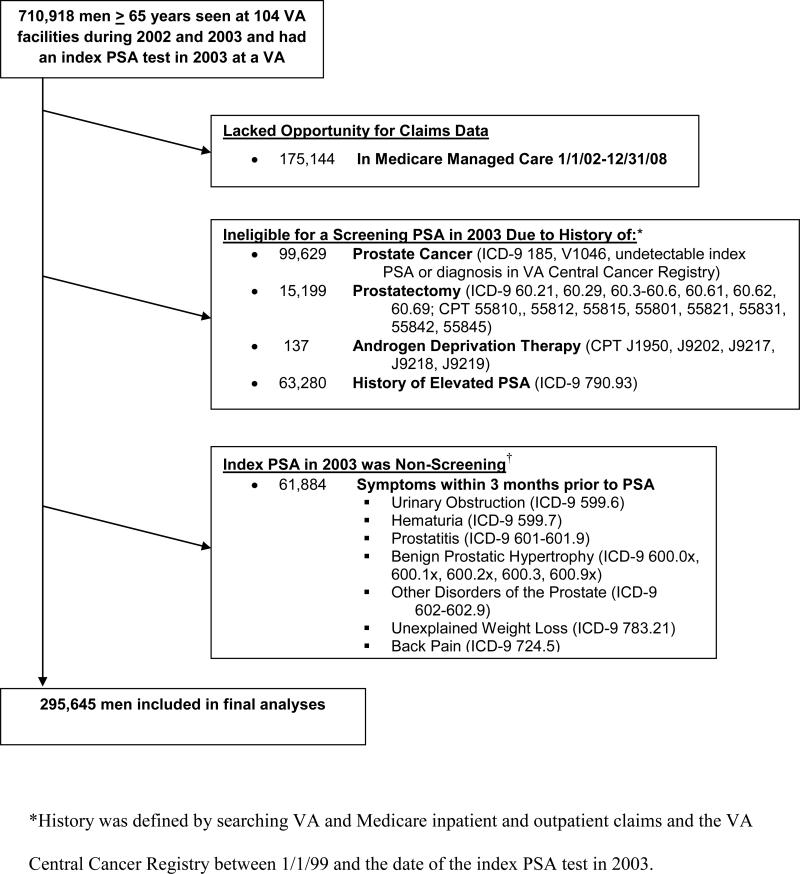
We conducted a longitudinal cohort study of 295,645 men age 65 years and older who underwent PSA screening in the VA healthcare system in 2003 and followed them for 5 years to determine downstream outcomes using national VA and Medicare data. We established a cohort using the VA National Patient Care Database to identify the 710,918 men age 65 years and older who had at least 1 outpatient visit in both 2002 and 2003 and had an index PSA test in 2003 at one of 104 VA facilities (Figure 1).14 An index PSA test was defined as the first outpatient PSA in the 2003 Decision Support System (DSS) National Data Extracts Laboratory Results dataset (which captured PSA results for 104 of the 127 VA facilities).4,15 Also, we used linked Medicare claims to capture services provided to our cohort from Medicare.16 We excluded men enrolled in Medicare managed care and men for whom PSA testing was non-screening due to past history of prostate cancer or elevated PSA, or if they had symptoms within 3 months prior to the test (Figure 1). This left a final PSA screening cohort of 295,645 men.
Figure 1.

Exclusions used to define the final cohort of elderly men who received a screening PSA test in 2003 at a VA facility.
Baseline Characteristics
Age was determined on the date of the index screening PSA. Charlson-Deyo comorbidity scores were calculated from VA and Medicare inpatient and outpatient claims during the 12 months before the index PSA date.4, 17 Other factors known to influence the use and outcomes of PSA screening were obtained from VA and Medicare data and linkage to the 2000 US census (Appendix).18
Outcomes of Screening
We linked VA National Data Systems, VA Central Cancer Registry, National Death Index, and Medicare claims to capture downstream testing and outcomes during the 5 years after the index screening PSA in 2003. All men were followed until death or to 5 years. The Appendix includes codes for all variables.
Follow-up of an Abnormal PSA Result
We identified men whose index screening PSA value was > 4 ng/ml because this is the most common definition of abnormal for older men in the U.S.19 Among these men we calculated the number of prostate biopsies over the next 5 years or until prostate cancer diagnosis. Men were considered to have undergone more than one biopsy if procedure visit dates were more than 2 weeks apart.20 Among men who received at least one biopsy, we determined the number of repeat PSA tests during the period between the index PSA and first biopsy. Among men who did not receive a biopsy, repeat PSA testing was determined between the index PSA date and prostate cancer diagnosis date or end of the 5-year study period.
Prostate Cancer Diagnosis
Incident prostate cancer was determined during the period between the index PSA and end of the 5-year study period. The VA Central Cancer Registry collects uniformly reported information on all men who receive a diagnosis of prostate cancer or receive their first course of cancer treatment at a VA facility.21 For these men we had access to cancer stage and histologic grade. Outside the VA, prostate cancer was identified from Medicare claims, which do not include prostate cancer characteristics.22, 23
Prostate Cancer Treatment and Survival
Among men diagnosed with prostate cancer we determined: 1) receipt of treatment with curative intent (i.e., radical prostatectomy or radiation therapy), 2) receipt of hormone therapy alone, or 3) no receipt of these treatments during the period between cancer diagnosis and the end of the 5-year study period.21, 24 Men who underwent multiple treatments were assigned to the most aggressive treatment received (radical prostatectomy> radiation> hormone therapy). Among men with prostate cancer who did not receive treatment, we determined repeat PSA testing during the period between cancer diagnosis and end of the 5-year study period. 5-year survival was based on the VA Vital Status file and cause of death was based on the National Death Index.25
Biopsy and Treatment Complications
We determined the number of men who experienced complications (e.g., urinary tract infection, sepsis, urinary retention, and hemorrhage) or death within 7 days of biopsy.26-28 We also calculated the number of men treated for prostate cancer who developed new erectile dysfunction or urinary incontinence based on claims data and receipt of diapers, pads, incontinence and erectile dysfunction medications from VA Pharmacy Benefits Management data.29, 30
Analyses
To determine the frequency of downstream outcomes, we observed men from the date of their index PSA in 2003 until death or to 5 years. Each downstream outcome is presented as the percentage of men who had the event. To determine associations between baseline characteristics and each outcome we used chi-squared tests. We also present the percentages of men who had biopsy or treatment complications. To determine the combined effect of age and comorbidity on risk for dying of non-prostate-cancer causes we categorized men with a positive biopsy into 15 subgroups on the basis of age (5 categories) and Charlson comorbidity score (3 categories) and present percentages and 95% CI of men who died of other causes within 5 years of screening. Differences between percentages in each age-comorbidity subgroup were determined using Cochran-Armitage trend tests. We also conducted a subgroup analysis of men diagnosed with low-risk prostate cancer (clinical stage T1 or T2 without nodes or metastases, Gleason score ≤ 6, and PSA < 10 ng/ml) to determine downstream outcomes.31 We used SAS, version 9.2 (SAS Institute, Cary, NC) for all analyses. The Committee on Human Research at the University of California, San Francisco, and the Committee for Research and Development at the San Francisco VA approved the study.
RESULTS
Participant Characteristics
Our cohort included 295,645 men who had a screening PSA in 2003 at one of 104 VA facilities. Mean age was 73 (range, 65-107), 10% had a Charlson score ≥ 3, and 90% were white (Table 1). 35% received PSA screening in the year prior to their index screening PSA in 2003. 25,208 (8.5%) men had an index PSA > 4ng/ml; 7,399 (2.5%) had a result > 6.5ng/ml and 2,775 (0.9%) had a result > 10ng/ml. The percentage of men with an abnormal PSA result increased with age, from 5.9% for men age 65-69 to 17.3% for men age 85+ (P<0.001)(Table 1). Black men were 1.8 (95% CI, 1.7-1.9) times more likely to have an abnormal result than white men.
Table 1.
Baseline characteristics of 295,645 men age 65 years and older who underwent PSA screening and the downstream outcomes that occurred during the 5 years following PSA screening.
| BASELINE CHARACTERISTIC* | DOWNSTREAM CONSEQUENCES† |
|||||
|---|---|---|---|---|---|---|
| Men with Index PSA Result > 4.0 ng/ml | Men who Received Prostate Biopsy | Men Diagnosed with Prostate Cancer | Men Treated for Prostate Cancer | Men Treated and Alive at 5 Years | ||
| All Patients | N | N (%) | N (% of men with index PSA > 4.0 ng/ml) | N (% of men who received prostate biopsy) | N (% of men diagnosed with prostate cancer) | N (% of men treated for prostate cancer) |
| Age, years | ||||||
| 65-69 | 97,251 | 5,731 (5.9) | 2,892 (50.5) | 1682 (58.2) | 1403 (83.4) | 1232 (87.8) |
| 70-74 | 94,422 | 7,163 (7.6) | 2,869 (40.1) | 1808 (63.0) | 1499 (82.9) | 1278 (85.3) |
| 75-79 | 65,501 | 6,789 (10.4) | 1,726 (25.4) | 1150 (66.6) | 920 (80.0) | 718 (78.0) |
| 80-84 | 30,902 | 4,212 (13.6) | 695 (16.5) | 487 (70.1) | 390 (80.1) | 258 (66.2) |
| 85+ | 7,569 | 1,313 (17.3) | 131 (10.0) | 93 (71.0) | 72 (77.4) | 37 (51.4) |
| Charlson Comorbidity Score | ||||||
| 0 | 184,504 | 16,533 (9.0) | 6037 (36.5) | 3812 (63.1) | 3158 (82.8) | 2655 (84.1) |
| 1-2 | 82,808 | 6,482 (7.8) | 1841 (28.4) | 1146 (62.3) | 920 (80.3) | 730 (79.4) |
| 3+ | 28,333 | 2,193 (7.7) | 435 (19.8) | 262 (60.2) | 206 (78.6) | 138 (67.0) |
| Race | ||||||
| White | 264,978 | 21,304 (8.0) | 6951 (32.6) | 4315 (62.1) | 3534 (81.9) | 2916 (82.5) |
| Black | 21,706 | 2,979 (13.7) | 1101 (37.0) | 750 (68.1) | 623 (83.1) | 508 (81.5) |
| Other | 8,961 | 925 (10.3) | 261 (28.2) | 155 (59.4) | 127 (81.9) | 99 (78.0) |
| Married | 208,827 | 16,611 (8.0) | 5741 (34.6) | 3574 (62.3) | 2954 (82.7) | 2495 (84.5) |
| Not Married | 86,818 | 8,406 (9.9) | 2512 (29.9) | 1604 (63.9) | 1295 (80.7) | 999 (77.1) |
| Census region | ||||||
| Midwest | 80,176 | 7,238 (9.0) | 2471 (34.1) | 1550 (62.7) | 1293 (83.4) | 1051 (81.3) |
| Northeast | 36,348 | 3,117 (8.6) | 983 (31.5) | 607 (61.8) | 486 (80.1) | 408 (84.0) |
| South | 132,890 | 10,668 (8.0) | 3500 (32.8) | 2172 (62.1) | 1815 (83.6) | 1502 (82.8) |
| West | 46,231 | 4,185 (9.1) | 1359 (32.5) | 891 (65.6) | 690 (77.4) | 562 (81.5) |
| Lived in ZCTA in which ≥ 25% of Adults Had a College Education‡ | ||||||
| Yes | 81,339 | 6,985 (8.6) | 2343 (33.5) | 1450 (61.9) | 1185 (81.7) | 1002 (84.6) |
| No | 214,306 | 17,433 (8.5) | 5707 (32.7) | 3598 (63.1) | 2960 (82.3) | 2407 (81.3) |
| Median annual income of ZCTA‡ | ||||||
| Highest tertile(>=$41,144) | 95,753 | 8,118 (8.5) | 2678 (33.0) | 1662 (62.1) | 1356 (81.6) | 1134 (83.6) |
| Middle tertile | 95,372 | 7,805 (8.2) | 2543 (32.6) | 1590 (62.5) | 1288 (81.0) | 1044 (81.2) |
| Lowest tertile (<=$32,549) | 95,567 | 8,500 (8.9) | 2830 (33.3) | 1797 (63.5) | 1501 (83.5) | 1231 (82.0) |
| Selected Comorbidities§ | ||||||
| CHF | 18,698 | 1,604 (8.6) | 320 (20.0) | 206 (64.4) | 162 (78.6) | 98 (60.5) |
| COPD | 35,430 | 3,029 (8.5) | 752 (24.8) | 455 (60.5) | 367 (80.7) | 267 (72.8) |
| Diabetes | 52,433 | 3,583 (6.8) | 984 (27.5) | 625 (63.5) | 509 (81.4) | 399 (78.4) |
| Cerebrovascular disease | 19,099 | 1,505 (7.9) | 356 (23.7) | 214 (60.1) | 168 (78.5) | 124 (73.8) |
| Dementia | 1,844 | 222 (12.0) | 18 (8.1) | 13 (72.2) | 10 (76.9) | 6 (60.0) |
Associations between baseline characteristics and downstream outcomes were significant (P<0.001) for all relationships in bold.
The denominator for each row percent is the number of men in the prior column. For example, among men age 65-69, 50.5% of 5,731 men with a PSA result > 4 ng/ml received a biopsy; 58.2% of 2,892 men who received a biopsy were diagnosed with prostate cancer; 83.4% of 1,682 men diagnosed with prostate cancer received treatment (radical prostatectomy, radiation or hormone therapy); and 87.8% of 1,403 men who were treated survived 5 years after their index screening PSA test.
ZCTA=Zip Code Tabulation Area. The following variables had missing data: Marital Status (1%), Income (3%) and Education (3%).
These categories are not mutually exclusive. Reference group for comparisons are men without the specific comorbidity.
Follow-up of an Abnormal PSA Result
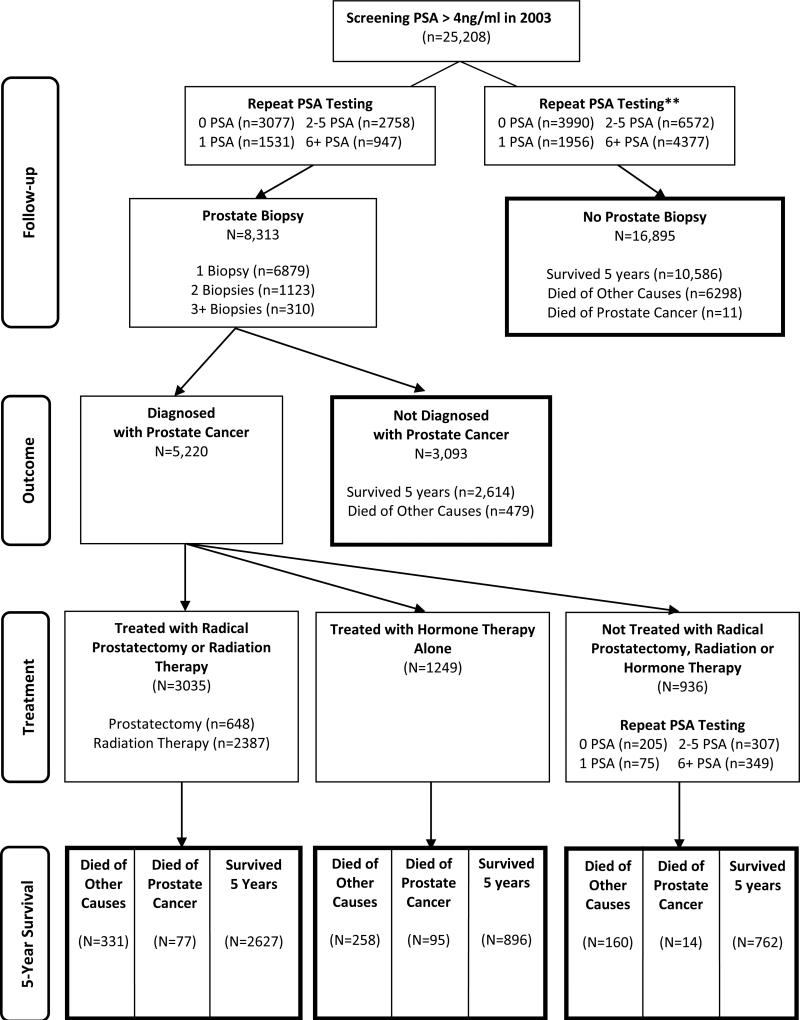
Of 25,208 men with an index screening PSA > 4ng/ml, 3,077 (12%) had a prostate biopsy without repeat PSA testing, 5,236 (21%) had one or more repeat PSA tests prior to biopsy, and 16,895 (67%) never had a biopsy during the 5-year study period (Figure 2). In the subgroup with PSA > 6.5ng/ml, 61% never had a biopsy, while among men with PSA > 10ng/ml, 58.3% never had a biopsy. Age was also a strong predictor of biopsy receipt: 51% of men age 65-69 received a biopsy compared to 10% of men age 85+ (P<0.001)(Table 1). Of men who never had a biopsy, 26% had ≥ 6 repeat PSA tests, 7% underwent transurethral resection of the prostate, and 0.1% died of prostate cancer.
Figure 2.

Flowchart of the 5-Year Outcomes Following a Screening PSA Result > 4ng/ml in Men Age 65 Years and Older.
Prostate Cancer Diagnosis
Of the 8,313 men who had at least one biopsy, 5,220 (63%) were diagnosed with prostate cancer during the study period. Among men diagnosed with cancer, 15% had more than one biopsy prior to diagnosis, and among men not diagnosed with cancer, 21% had more than one biopsy. Age was a strong predictor of prostate cancer diagnosis, ranging from 58% of biopsied men age 65-79 to 71% of men age 85+ (P<0.001)(Table 1). Among the subset of 2,780 (53%) men whose biopsy-detected cancer occurred within the VA, 1,161 (42%) had low risk cancer.
Prostate Cancer Treatment and Survival
Of 5,220 men diagnosed with prostate cancer after biopsy, 58% were treated with curative intent (i.e., radical prostatectomy or radiation therapy), 24% were treated with hormone therapy alone, and 18% were not treated with any of these modalities (Figure 2). Of those treated, 95% started treatment within 1 year of cancer diagnosis. For all strata of characteristics in Table 1, the percentage of men treated for prostate cancer exceeded 75%. Even among the subset of 1,161 men who had low risk cancer diagnosed within the VA, 76% were treated.
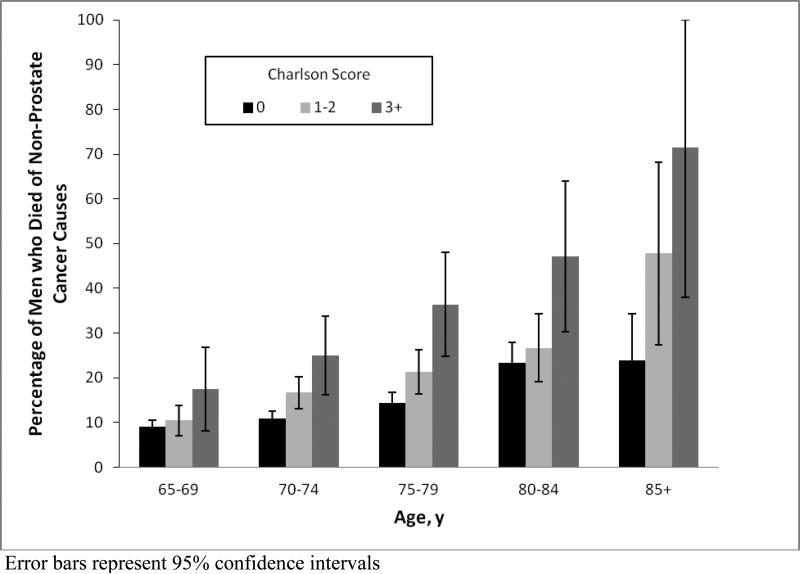
5-year survival for men treated for biopsy-detected prostate cancer was 82% and decreased with advancing age and comorbidity (Table 1). Figure 3 shows that the percentage of men with biopsy-detected prostate cancer who died of other causes increased with advancing age (P < 0.001). Also, within each age group, the percentage of men who died of other causes increased with worsening comorbidity (P < 0.05).
Figure 3.
Impact of age and comorbidity on the likelihood of dying of non-prostate cancer causes within 5 years of screening among men with an index screening PSA result > 4ng/ml and biopsy-detected prostate cancer (N=5,220).
Biopsy and Treatment Complications
Among 8,313 men who underwent biopsy after an index screening PSA > 4ng/ml, 468 (6%) men had complications within 7 days after biopsy, including 131 men who were hospitalized and 9 men who died. Among 4,284 men treated with radical prostatectomy, radiation or hormone therapy, 584 (14%) men had new incontinence and 588 (14%) men had new erectile dysfunction.
DISCUSSION
This study provides frequencies in clinical practice of downstream outcomes during the 5 years following an abnormal screening PSA result among older men, including more than 100,000 men age 75+. The percentage of men with an index screening PSA > 4ng/ml increased with age but only one-third of these men proceeded to biopsy. Receipt of biopsy decreased with advancing age and comorbidity. Prostate cancer detection increased with age and most men diagnosed with cancer received immediate treatment. Therefore, while most men with PSA > 4ng/ml did not undergo biopsy, which reduced biopsy and treatment complications, those who did were often diagnosed with prostate cancer and underwent treatment regardless of advancing age, poor health or low risk cancer.
While many adjustments (e.g., age-specific PSA norms, PSA velocity) have been suggested to better define an abnormal PSA result, >4.0 ng/ml remains the most commonly used cutoff in U.S. practice.19 We found that men age 85+ were nearly three times more likely to have an abnormal screening result than men age 65-69 (17% vs. 6%). These percentages are slightly lower than those from prior studies which did not exclude men with prostate symptoms. For example, in the PLCO trial, which used a PSA cutoff > 4ng/ml, 11% of men age 65-69 had an initial abnormal PSA, while a cross-sectional study using the 2001-2002 National Health and Nutrition Examination Survey found 6% of men age 60-69 and 28% of men age 85+ had PSA values > 4ng/ml.19,32 Consistent with prior studies, black men were more likely to have an abnormal screening PSA result than white men.33
Prostate biopsy is the standard diagnostic procedure after an abnormal screening PSA result.34 In the ERSPC trial, which found screening reduced prostate cancer mortality, 86% of men with an abnormal PSA result underwent biopsy within one year.12,35 In the PLCO trial, which did not show a reduction in prostate cancer mortality, 40% underwent biopsy within one year.9,36 However, biopsy rates in both trials were much higher than what we found. In clinical practice, 33% of men age 65 years and older underwent biopsy within 5 years of a screening PSA > 4ng/ml, which is consistent with prior smaller community studies.37 Even among the 0.9% of men with screening PSA > 10ng/ml, only 42% underwent biopsy within 5 years suggesting that many men in clinical practice do not pursue biopsy even when PSA levels are very high.
Among older men who undergo biopsy, many are diagnosed with prostate cancer and undergo treatment. Our cancer detection rate (63%) is higher than in the initial round of the PLCO trial in which 44% of men who underwent biopsy were diagnosed with cancer within 1 year of an abnormal PSA.36 However, our population is substantially older and we followed men for 5 years, including men who underwent more than one biopsy. We also found more than three-quarters of men diagnosed with cancer underwent treatment within a year of diagnosis regardless of age or comorbidity, consistent with trials and observational data in younger men.38,39 Yet, this is the step in the downstream cascade where increasing evidence supports unlinking cancer detection from immediate treatment and pursuing active surveillance with selective, delayed treatment, especially in older men with comorbidities.40-43 Instead, most older men, including those with low risk disease, were immediately treated. 58% underwent potentially curative radical prostatectomy or radiation therapy and 24% of men underwent non-curative hormone therapy alone. These findings suggest a need to better incorporate considerations of advancing age, comorbidity and aggressiveness of screen-detected cancer into treatment decisions
Among those treated for screen-detected prostate cancer, 5-year survival was 82% but decreased with advancing age and comorbidity as deaths from non-prostate-cancer causes increased. Overall, 14% of men with screen-detected prostate cancer died of non-prostate-cancer causes within 5 years and this percentage increased to 71% among men age 85+ with Charlson score 3+. Since PSA screening advances cancer diagnosis between 5-12 years before clinical diagnosis, screening in subgroups with limited life expectancy risks diagnosing cancer that would never have caused symptoms.44, 45
Screening also places men at risk for biopsy and treatment complications, although we found in clinical practice the screening cascade was most frequently stopped prior to biopsy rather than at the treatment decision-making stage. Halting the cascade early on decreases any chance of screening benefit as high-risk cancers will be missed, but also decreases the number of men who will have biopsy- and treatment-related complications. Our complication rates are lower than reported in trials or studies of younger men, who more often pursue follow-up biopsy.1, 46, 47 Also, our estimates of incontinence and erectile dysfunction likely underestimate these problems since these are under-coded in claims and many older men may not seek treatments for these problems.30
Our study has several other limitations. First, laboratory data do not give reasons why a PSA test was ordered such that some tests may have been performed for non-screening reasons. However, chart review and the low number of men with PSA > 4 ng/ml suggests our exclusions selected a cohort in whom PSA tests were primarily sent for screening.4 Second, biopsy detection rates of cancer vary according to number of biopsy cores obtained during a biopsy session but we lacked these data. 12-core biopsy procedures were typical during this period and at present.48 Third, our data do not capture quality-of-life outcomes. However, understanding downstream clinical outcomes are important in their own right to help clinicians and patients have more realistic expectations of screening outcomes and allow men to individually assess their importance. Fourth, this study focuses on 5-year consequences following an index screening PSA test in 2003. Patterns of care might have changed subsequently, although recent data suggest screening and treatment rates remain high.8,38 Fifth, our cohort is veterans so generalizability of our findings to non-veterans is uncertain, although most also were enrolled in Medicare.16
Decisions to pursue PSA screening should include individualized discussion about when to pursue biopsy and treatment because decisions at these steps substantially affect downstream outcomes of screening in clinical practice. This study provides valuable insight into biopsy and treatment practices following PSA screening among men age 65 years and older in the largest U.S. healthcare system. These frequencies of downstream outcomes according to baseline characteristics, such as age and comorbidity, should better inform clinicians and elderly men who are considering PSA screening and want to make more individualized decisions.
ACKNOWLEDGEMENT
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R01 CA134425) to [LW, SF, AP, and RH]; the National Institute on Aging at the National Institutes of Health (grant number K24AG041180) to [LW]; the Veterans Affairs Career Development Award Program (grant number CDA 08-024) to [AP]; and the New Mexico VA Health Care System to [RH].
The funding sources had no role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication.
The corresponding author, Louise Walter, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
APPENDIX
APPENDIX.
Definitions, codes, and data sources for all variables.
| Variable | Data Source | Definition |
|---|---|---|
| Patient Characteristics | ||
| Age | VA NPCDa | Age on date of index screening PSA test in 2003 |
| Comorbidity Index | VA NPCD and Medicareb | Deyo's modification of Charlson index using inpatient and outpatient claims in 12 months prior to index screening PSA test in 2003 |
| Race | Medicare (missing data filled in with VA NPCD) | White, Black, Other |
| Marital Status | VA NPCD | Marital status of patient in 2003 |
| Census Region | VA NPCD | Place of residence according to US Census Regions (Midwest, Northeast, South, West) |
| Income | Census | Place of residence in a zip code tabulation area where median annual income was in top, middle or lower tertile for this cohort |
| Education | Census | Place of residence in a zip code tabulation area where more than 25% of adults have a college education |
| Testing | ||
| PSA results | VA DSSc | DSS LAR code 19 |
| Repeat PSA testing | VA DSS and Medicare | Any of: DSS LAR code 19; CPT codes 84153, G0103 |
| Prostate biopsy | VA NPCD and Medicare | Any of: CPT codes 55700, 55705, 76872, 76873; ICD-9 procedure codes 60.11, 60.12 |
| Transurethral resection of the prostate (TURP) | VA NPCD and Medicare | Any of CPT codes 52601, 52612, 52614, 52620, 52630; ICD-9 procedure code 60.29 |
| Biopsy Complications | ||
| Urinary Tract Infection | VA NPCD and Medicare | ICD-9 diagnosis code 599.0 |
| Sepsis/Septicemia | VA NPCD and Medicare | Any of ICD-9 diagnosis codes 995.91, 038.x, 790.7 |
| Hemorrhage complicating a procedure/rectal hemorrhage | VA NPCD and Medicare | Any of ICD-9 diagnosis codes 998.1x, 569.3 |
| Urinary Retention | VA NPCD and Medicare | ICD-9 diagnosis code 788.2x |
| Prostate Cancer | ||
| Prostate cancer diagnosis | VACCRd and VA NPCD and Medicare | From VACCR coding of cancer site using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) code for prostate cancer: C61.9 or any of the following from VA or Medicare claims: ICD-9 diagnosis code 185 within six months of a prostate biopsy or TURP or by the initiation of androgen deprivation therapy (“hormone therapy”) at any point irrespective of biopsy or TURP. We did not include men diagnosed at autopsy or by death certificate only. |
| Stage | VACCR | American Joint Committee on Cancer (AJCC) clinical stages T1 (incidental or biopsy finding), T2 (confined to prostate), T3 (extracapsular extension), T4 (invades structures other than seminal vesicles) |
| Histologic grade | VACCR | Well (Gleason 2-6), Moderately to Poorly/Undifferentiated (Gleason 7-10) |
| Treatment Received | ||
| Radical Prostatectomy | VACCR and VA NPCD and Medicare | From VACCR coding of site-specific surgery or any of the following from VA or Medicare claims: CPT codes 55801, 55810, 55812, 55815, 55821, 55831, 55840, 55842, 55845; ICD-9 procedure codes 60.2-60.69. |
| Radiation Therapy (e.g., external beam, 3D radiation, and brachytherapy) | VACCR and VA NPCD and Medicare | From VACCR coding of radiation therapy or any of the following from VA or Medicare claims: CPT codes 55859, 55860, 77261-77370, 77401-77499 and 77750-77799, and ICD-9 procedure codes 92.21-92.29, and ICD-9 diagnosis codes V58.0, V66.1 and V67.1 and HCPCS codes C1715-C2636, Q3001. |
| Hormone Therapy (e.g., orchiectomy and GnRH agonists) | VACCR and VA NPCD and Medicare and VA PBMe | From VACCR coding of hormone therapy or any of the following from VA or Medicare claims for orchiectomy: CPT codes 54520-54535, 54690 and ICD-9 procedure codes 62.4-62.42 and for GnRH agonists: HCPCS codes J1950, J9217, J9218, J9219, J9202 and VA Pharmacy data for leuprolide and goserelin acetate. |
| Treatment Complications | ||
| Urinary Incontinence | VA NPCD and Medicare and PBM | From PBM coding of (supplies): diaper/underpants, pad, underpad, incontinence device, incontinence skin kit; (medications) oxybutynin, tolterodine; or any of the following from VA and Medicare claims: CPT codes (repair) 51715, 11950, 11951, 11952, 11954, 51840, 51841, 53440, 53442, 53445, 53447, 53431, 53449; (diagnostic tests) 51725, 51726, 51772, 51784, 51785, 51792, 51795, 51797; ICD-9 procedure codes 59.4, 59.5, 59.6, 59.7x, 58.93, 59.3, 89.22, 89.21, 89.23, 89.25; ICD-9 diagnosis codes 788.3x, 599.82 ; HCPCS codes L8603, E0740, A4335. At baseline, 2.6% of men (213/8,313) had incontinence and were excluded from this variable |
| Erectile Dysfunction | VA NPCD and Medicare and PBM | From PBM coding of medications: vardenafil, sildenafil, tadalafil, alprostadil, yohimbine; or any of the following from VA and Medicare claims: CPT codes 54231, 54235, 54400, 54401, 54415, 54416, 54405, 54406, 54408, 54410, 54411, 54415-54417; ICD-9 procedure codes 64.94-64.97; ICD-9 diagnosis code 607.84; HCPCS codes C1007, C1813, C2622, C3500, C8514, C8516, C8534, L7900, J0270, J0275, J2760. At baseline, 11.6% of men (960/8,313) had erectile dysfunction and were excluded from this variable. |
| Mortality | ||
| Cause of Death | National Death Index | Either died of prostate cancer (ICD-10 code C61) or died of other causes. Cause of death was obtained for all men diagnosed with prostate cancer. |
Veterans Affairs National Patient Care Database (NPCD) which includes inpatient and outpatient claims
Medicare inpatient and outpatient claims
Veterans Affairs Decision Support System (DSS) National Data Extracts Laboratory Results dataset
Veterans Affairs Central Cancer Registry (VACCR)
Veterans Affairs Pharmacy Benefits Management (PBM) data
Footnotes
The authors report no conflicts of interest related to the work described in this manuscript.
REFERENCES
- 1.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;157 doi: 10.7326/0003-4819-157-2-201207170-00459. (published on-line) [DOI] [PubMed] [Google Scholar]
- 2.Smith RA, Cokkinides V, Brooks D, Saslow D, Shah M, Brawley OW. Cancer screening in the United States, 2011: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2011;61(1):8–30. doi: 10.3322/caac.20096. [DOI] [PubMed] [Google Scholar]
- 3.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182(5):2232–2241. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 4.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 5.Bynum J, Song Y, Fisher E. Variation in prostate-specific antigen screening in men aged 80 and older in fee-for service Medicare. J Am Geriatr Soc. 2010;58(4):674–680. doi: 10.1111/j.1532-5415.2010.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29(13):1736–1743. doi: 10.1200/JCO.2010.31.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollack CE, Platz EA, Bhavsar NA, et al. Primary care providers’ perspectives on discontinuing prostate cancer screening. Cancer. 2012;118:5518–5524. doi: 10.1002/cncr.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad SM, Drazer MW, Huo D, Hu JC, Eggener SE. 2008 US Preventive Services Task Force recommendations and prostate cancer screening rates. JAMA. 2012;307(16):1692–1694. doi: 10.1001/jama.2012.534. [DOI] [PubMed] [Google Scholar]
- 9.Catalona WJ, D'Amico AV, Fitzgibbons WF, et al. What the U.S. Preventive Services Task Force missed in its prostate cancer screening recommendation. Ann Intern Med. 2012 doi: 10.7326/0003-4819-157-2-201207170-00463. (published on-line) [DOI] [PubMed] [Google Scholar]
- 10.Miller DC, Hollenbeck BK. Missing the mark on prostate-specific antigen screening. JAMA. 2011;306(24):2719–2720. doi: 10.1001/jama.2011.1879. [DOI] [PubMed] [Google Scholar]
- 11.Volk RJ, Wolf AMD. Grading the new US Preventive Services Task Force prostate cancer screening recommendation. JAMA. 2011;306(24):2715–2716. doi: 10.1001/jama.2011.1893. [DOI] [PubMed] [Google Scholar]
- 12.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andriole GL, Crawford ED, Grubb RL, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VA Information Resource Center . VHA Medical SAS® Outpatient Datasets and Inpatient Encounters Dataset FY2009: VIReC Research User Guide. U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; Hines, IL: Apr, 2011. [July 17, 2012]. http://www.virec.research.va.gov/DataSourcesName/Medical-SASDatasets/MedSAS-Outpt-RUG/MedSAS-RUG-Outpt.htm. [Google Scholar]
- 15.VA Information Resource Center . VIReC Research User Guide: VHA Decision Support System Clinical National Data Extracts. 2nd ed. U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; Hines, IL: Sep 1, 2009. [July 17, 2012]. http://www.virec.research.va.gov/References/RUG/RUG-DSS-2nd-Ed-er.pdf. [Google Scholar]
- 16.Hynes DM, Koelling K, Stroupe KT, et al. Veterans’ Access to and Use of Medicare and Veterans Affairs Health Care. Med Care. 2007;45(3):214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.US Census Bureau [July 17, 2012];Census 2000 summary file 3—United States/prepared by the US Census Bureau. 2002 http://www2.census.gov/census_2000/datasets/Summary_File_3/0_National/.
- 19.Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions of abnormal. J Natl Cancer Inst. 2005;97(15):1132–1137. doi: 10.1093/jnci/dji205. [DOI] [PubMed] [Google Scholar]
- 20.Merrill RM, Feuer EJ, Warren JL, Schussler N, Stephenson RA. Role of transurethral resection of the prostate in population-based prostate cancer incidence rates. Am J Epidemiol. 1999;150(8):848–860. doi: 10.1093/oxfordjournals.aje.a010090. [DOI] [PubMed] [Google Scholar]
- 21.Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector. Ann Intern Med. 2011;154(11):727–736. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 22.Howrey BT, Kuo Y, Lin Y, Goodwin JS. The impact of PSA screening on prostate cancer mortality and over-diagnosis of prostate cancer in the United States. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls135. (published on-line) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzman D, Penberthy L, Whittemore M, et al. Ability of Medicare claims data and cancer registries to identify cancer cases and treatment. Am J Epidemiol. 1997;145(3):227–233. doi: 10.1093/oxfordjournals.aje.a009095. [DOI] [PubMed] [Google Scholar]
- 24.VA Information Resource Center . VIReC Research User Guide: VHA Pharmacy Prescription Data. 2nd ed. U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; Hines, IL: Sep 1, 2008. [July 17, 2012]. http://www.virec.research.va.gov/RUGs/RUG-PBM-2nd-Ed-CY08-ER.pdf. [Google Scholar]
- 25.Sohn M, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin K, Lipsitz R, Miller T, Janakiraman S. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update from the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(3):192–199. doi: 10.7326/0003-4819-149-3-200808050-00009. [DOI] [PubMed] [Google Scholar]
- 27.Rosario DJ, Lane JA, Metcalfe C, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within Protec T study. BMJ. 2012;344 doi: 10.1136/bmj.d7894. (published on-line Jan. 9, 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183(3):963–969. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 29.Taylor KL, Luta G, Miller AB, et al. Long-term disease-specific functioning among prostate cancer survivors and noncancer controls in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.41.2767. (published on-line June 25, 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(8 supp):IV-62–IV-68. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 31.Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 32.Wolters T, Roobol MJ, Steyerberg EW, et al. The effect of study arm on prostate cancer treatment in the large screening trial ERSPC. Int J Cancer. 2009;126(10):2387–2393. doi: 10.1002/ijc.24870. [DOI] [PubMed] [Google Scholar]
- 33.Morgan TO, Jacobsen SJ, McCarthy WF, et al. Age-specific reference ranges for prostate-specific antigen in black men. New Engl J Med. 1996;335(5):304–310. doi: 10.1056/NEJM199608013350502. [DOI] [PubMed] [Google Scholar]
- 34.Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99(18):1395–1400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 35.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 36.Grubb RL, Pinsky PF, Greenlee RT, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU Int. 2008;102(11):1524–1530. doi: 10.1111/j.1464-410X.2008.08214.x. [DOI] [PubMed] [Google Scholar]
- 37.Zeliadt SB, Buist DSM, Reid RJ, Grossman DC, Ma J, Etzioni R. Biopsy follow-up of prostate-specific antigen tests. Am J Prev Med. 2012;42(1):37–43. doi: 10.1016/j.amepre.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boevee SJ, Venderbos LDF, Tammela TLJ, et al. Change in tumour characteristics and treatment over time in both arms of the European Randomized study of screening for prostate cancer. Eur J Cancer. 2010;46(17):3082–3089. doi: 10.1016/j.ejca.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 40.Chou R, LeFevre ML. Prostate cancer screening—the evidence, the recommendations, and the clinical implications. JAMA. 2011;306(24):2721–2722. doi: 10.1001/jama.2011.1891. [DOI] [PubMed] [Google Scholar]
- 41.Carroll PR. Early stage prostate cancer—do we have a problem with over-detection, overtreatment or both? J Urol. 2005;173(4):1061–1062. doi: 10.1097/01.ju.0000156838.67623.10. [DOI] [PubMed] [Google Scholar]
- 42.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 44.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esserman L, Shieh T, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 46.Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomized trial. Lancet Oncol. 2011;12(9):891–899. doi: 10.1016/S1470-2045(11)70162-0. [DOI] [PubMed] [Google Scholar]
- 47.Wilt TJ, MacDonald R, Rutks I, Shamilyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 48.Abd TT, Goodman M, Hall J, et al. Comparison of 12-core versus 8-core prostate biopsy: multivariate analysis of large series of US veterans. Urology. 2011;77(3):541–547. doi: 10.1016/j.urology.2010.06.008. [DOI] [PubMed] [Google Scholar]