Abstract
The circadian system ensures the generation and maintenance of self-sustained ~24 h rhythms in physiology that are linked to internal and environmental changes. In mammals, daily variations in light intensity and other cues are integrated by a hypothalamic master clock that conveys circadian information to peripheral molecular clocks that orchestrate physiology. Multiple immune parameters also vary throughout the day and disruption of circadian homeostasis is associated with immune-related disease. Here we discuss the molecular links between the circadian and immune systems and examine their outputs and disease implications. Understanding the mechanisms that underlie circadian-immune crosstalk may prove valuable for devising novel prophylactic and therapeutic interventions.
Keywords: circadian, immunity, daily rhythms
Time to address the circadian-immune connection
Circadian rhythms refer to autonomous, self-sustained, ~24 h oscillations in physiologic and behavioural processes. These rhythms are entrained by environmental cues, predominantly by daily changes in light intensity. In mammals, light activates a specific group of photoreceptors in the retina connected to the hypothalamic suprachiasmatic nuclei (the body’s circadian master clock), which entrain peripheral circadian clocks via neural and hormonal cues. These rhythms are sustained at the molecular level by a series of interconnected transcription-translation feedback loops that control the expression of clock genes comprising the molecular circadian clock[1].
Several parameters of the immune system demonstrate a time of day-dependent variation evident in many organisms, including humans[2]. Both epidemiologic and clinical data suggest circadian involvement in the predisposition, etiology and progression of immune-related morbidities such as cancer and autoimmune disease [3, 4]. Daily variations in host immune system status might also determine the outcome of an infectious challenge [5, 6]. Data from the last two decades supports the idea that the circadian system is involved in coordination of immune-circadian outputs and that there is active cross-talk with other systems under circadian regulation, such as the neuroendocrine system[2]. The connection between between the sympathetic nervous system and the immune system is highlighted in recent study showing that adrenergic signals modulate adhesion molecule expression on endothelial cells to produce circadian oscillations in leukocyte migration[7]. It has also recently been shown that components of the circadian clock directly regulate expression of innate immune molecules such as proinflammatory cytokines[8] and pattern recognition receptors (PPRs) [9]. These studies provide mechanistic insight into the circadian-immune dialog. Here we review the current understanding of the molecular mechanisms underlying circadian-immune interactions, and discuss the functional outputs and disease implications. Appreciation of the molecular and cellular basis of circadian regulation of immune surveillance mechanisms and the subsequent responses, may allow the circadian-immune connection to be exploited for prophylactic and therapeutic interventions.
Circadian-immune molecular links
Molecular control of circadian rhythms is controlled by a series of interacting transcriptional–translational feedback loops. In mammals, the transcriptional loop is initiated by the bHLH-PAS transcription factors CLOCK and BMAL1[10]. These proteins dimerize and bind to E-boxes present in the promoter region of period genes (Per1, Per2 and Per3) and cryptochrome genes (Cry1 and Cry2) to induce their expression. Upon translation, PER and CRY proteins form a repressor complex that translocates into the nucleus to inhibit CLOCK:BMAL1-mediated activation of Per, Cry, and other clock-controlled genes (CCG), forming a negative feedback loop. The cycle re-starts approximately 24 hours later, upon degradation of PER proteins. The robustness of the circadian molecular clockwork is maintained by several oscillatory mechanisms such as post-translational modifications and chromatin remodeling[11].
Among the core molecular clock components, Per2 is emerging as an important connection point between the circadian and immune systems. Several studies have associated Per2 with cancer, IFN-γ expression and NK cell-mediated cytotoxicity[12, 13]. The precise mechanisms through which Per2 contributes to these processes are unknown. However, Per2 is deregulated in cancer[14] and its over expression leads to suppression of tumor growth in vivo[15], suggesting a role in cell cycle control. Per2 is also necessary for circadian-mediated changes in expression of the cytotoxic receptors Ly49C and Nkg2d in bone marrow cells[16].
Other transcription-translation feedback loops interact to fine-tune the circadian clock and maintain its oscillations. These additional loops involve other clock-controlled transcription factors such as DBP and REV-ERBα, whose expression is primarily activated by the CLOCK:BMAL1 heterodimer. REV-ERBα represses Bmal1 transcription by directly binding to the ROR elements (ROREs) in the Bmal1 promoter, which adds robustness to the clock [17]. In addition, DBP and REV-ERBα control the expression of genes containing D-box and ROREs in their promoters, acting as molecular relays for the circadian expression of downstream clock-controlled genes. Of note, REV-ERBα modulates the production of inflammatory cytokines (in particular IL-6) in response to endotoxin challenge in murine and human macrophages[18]. Although most CCGs encode essential regulators of hormonal and metabolic control[11], it is possible that the CLOCK:BMAL1 heterodimer binds to E-box elements in of immune-related genes to control their expression. Supporting this idea, CLOCK:BMAL1 drives expression of the PRR Toll-like receptor 9 (TLR9), which is an integral part of the danger/pathogen sensing mechanism that drives innate immune responses and inflammation[9].
Direct interactions between molecular components of the circadian and immune systems might be relevant not only for immune surveillance mechanisms (e.g., TLR expression) and effector functions (e.g., proinflammatory cytokine production) but also for immune cell development and migration/trafficking. The basic leucine zipper transcription factor, E4BP4, regulates circadian rhythm in chick pineal gland and is a pivotal modulator of the period length of the mammalian core circadian expression loop[19]. E4BP4 is also essential for the development of NK cells and CD8α(+) conventional dendritic cells, and is involved in regulation of macrophage activation, CD4+ T cell polarization and IgE class switching[20]. Pleiotropic transcription factors that modulate the expression of genes important for immunity might also be controlled by the circadian system. The circadian clock protein, CRY, regulates proinflammatory cytokine expression [8, 21], and a CRY mutation in p53-null mice delays the onset of cancer by sensitizing the oncogenically transformed cells to TNF-α induced apoptosis by hampering the NF-κB signaling pathway via the GSK3β kinase[22].
There is evidence that the circadian-immune connection is bidirectional. Engagement of the cytokine receptor TNFR1 by TNF-α transiently upregulates expression of molecular circadian clock components of the negative feedback loop (Per1, Per2, Cry1, and Dec1) in a MAPK p38 and calcium signaling-dependent manner [23] as well as a suppression of E-box-driven transcription of Dbp[24]. Thus immune signaling feeds back to the circadian system by modulating its transcription loops.
The molecular connection between the circadian clock and immunity is also observed in non-mammalian systems. Studies in Drosophila reveal that the core circadian protein, Timeless (TIM), mediates resistance against bacterial infection[6]. Wild-type flies show daily oscillations in resistance to S. pneumonia infection that are absent in TIM mutants flies. Mechanistically, the TIM mutation blocked upregulated phagocytosis at night which may explain why mutant flies were more susceptible to infection. In plants, immune responses triggered by pathogen/danger-associated molecular patterns are regulated by the circadian clock and temporal regulation allows plants to anticipate and respond more effectively to pathogen challenge during the day. For example, Arabidopsis thaliana shows circadian-modulated variations in resistance to the virulent bacterial pathogen Pseudomonas syringae pv. tomato DC3000 [25]. A functional link between the circadian clock and plant immunity was also demonstrated in mutagenesis studies showing that circadian clock-associated 1 (CCA1) modulates the expression of R-gene-mediated resistance against downy mildew in Arabidposis, allowing plants to anticipate infection at dawn, when spores are normally dispersed[26].
Circadian outputs in the immune response
Recent work has examined circadian molecular clocks in immune cells and tissues. Analysis of clock gene expression in mouse spleen, thymus, inguinal lymph nodes, and peripheral blood shows that these tissues are equipped with functional circadian clocks [27, 28]. Although the clock gene expression profiles differ in amplitude and phase between the tissues, the spleen, lymph nodes and thymus share similar acrophases for many of the genes analyzed and Rev-erbα mRNA peaks at approximately the same time in all four tissues[27, 28]. This suggests that functional immune outputs of these tissues, such as cytokine expression, are coordinated via systemic circadian signals, as it has been shown for the circadian regulation of the expression of cytotoxic factors and cytokines in splenocytes and NK cells, which is mediated by noradrenergic signals [29]. The data on clock gene expression in immune tissues is complicated by presence of multiple immune cells types in each tissue. The spleen, for example, contains various immune cell types, including dendritic cells, macrophages and B cells [30]. Also, the relative and absolute numbers of different immune cell types in the spleen fluctuates in a circadian fashion [27]. The biological significance of the circadian outputs produced within a specific immune tissue, as well as the contribution of each immune cell type to the overall circadian immune output requires careful investigation.
The relevance of circadian rhythms for the innate immune inflammatory response has been examined by challenging splenocytes isolated at various times of the day with LPS, which results in a time-dependent TNFα and IL-6 response[27]. Similar results were obtained in vivo when mice were challenged with LPS at different times of the daily cycle[18]. Microarray analysis of peritoneal macrophages revealed changes in transcriptional regulation depending on time of challenge at almost all stages of the LPS-induced immune response [27], further highlighting the complexity of circadian regulation of innate immunity. The trafficking of immune cells constitutes another circadian output. Circulating hematopoietic stem cells fluctuate in a circadian manner, and this fluctuation is regulated by core molecular clock genes through circadian sympathetic signals that regulate chemokine expression [31].
Recent work has investigated circadian clock influences over adaptive immunity. In humans, the number of NK cells, γδTCR expressing cells, T suppressor/cytotoxic cells, T helper cells and total T cells in peripheral blood undergoes circadian fluctuations and these cell types have different acrophases that correlate (positively or negatively) with the blood concentration of several neuro-endocrine humoral factors including cortisol, epinephrine, melatonin, TRH, TSH, and GH[32, 33]. Cell surface molecules on six (CD8+dim, γδTCR+, CD8+, CD16+, CD4+, and CD3d+ cells) of 10 lymphocyte subpopulations analyzed were also reported to show circadian variations in expression[34].
Human CD4+ T cells possess a functional molecular clock and show circadian variations in IFN-γ-, IL-2- and IL-4-production and CD40L expression upon stimulation[35]. Microarray data suggests that the circadian clock regulates T cell responses via the NF-κB pathway[35]. In murine lymph nodes, the relative abundance of B cells and T cell subsets does show circadian variations but the rate of T cell proliferation after stimulation does show such variations [36]. The time of day-dependant T cell proliferation correlated with changes in protein expression of ZAP70, a signaling molecule that acts downstream of the TCR. This study also found that timing of immunization influenced the magnitude of an antigen-specific T cell response.
The work discussed so far demonstrate that circadian system has both direct and indirect control over innate and adaptive immunity. These arms of the immune response, however, are not independent of one another; for example innate immune activation is critical for mounting an effective adaptive immune response. As mentioned earlier, expression and function of TLR9 is directly modulated by the molecular clock and this has implications for the innate inflammatory responses [9], as demonstrated in a TLR-9-dependent model of sepsis, in which disease severity was dependent on the timing of sepsis induction. Circadian control of TLR9 also influences adaptive immunity. Mice immunized with a TLR9 ligand as adjuvant at the time of TLR9 peak responsiveness an enhanced adaptive response weeks after vaccination [9]. These findings highlight the importance of considering the innate/adaptive cross-talk when assessing circadian output in these two branches of the immune system.
Circadian immunity and disease
Daily patterns in disease severity or symptoms are a feature of many diseases with an immune or inflammatory component. For example, rheumatoid arthritis (RA) patients exhibit worse joint inflammation, pain and stiffness in the morning hours (reviewed in [3]), in contrast to patients with osteoarthritis, who exhibit worsening pain through the day. In line with this, nighttime administration of slow release prednisone is a more effective treatment regimen for RA, vs. daytime administration[3, 37, 38]. A recent report found an association between the season of disease onset and short-term outcomes in RA patients[39], suggesting that RA may also be influenced by circannual rhythms, i.e. variations in the length of day by season. Circadian rhythms also seem to play a role in Alzheimer’s disease (AD) (reviewed in [40]). There is a daily fluctuation in cerebrospinal fluid levels of amyloid beta, the main component of amyloid plaques found in the brains of AD patients[41, 42]. Circadian rhythms may also influence the outcome of patients in the ICU with critical illnesses. Patients with sepsis are more likely to die between the hours of 2 and 6 am[43], and as already discussed, in a mouse model of sepsis induced during the nighttime (ZT-19) vs. the daytime (ZT-7) leads to worse disease, coincident with the peak and nadir of TLR9 expression[9]. Although multiple factors probably contribute to changes in disease symptoms throughout the day, circadian regulation of immune function may be one underlying mechanism.
Disruption of circadian rhythms can also contribute to disease pathogenesis. Experimental alterations in sleeping and eating patterns of human subjects cause adverse metabolic and cardiovascular effects [44]. Shift work in humans is linked to increased risk and progression of cancer[45], diabetes[46] and stroke[47]. Furthermore, there is a genetic association between certain alleles of the clock gene Cry2 and non-Hodgkin’s lymphoma[48]. A recent report indicates that in a rat model of sepsis, survival is impaired when the normal light-dark cycle is replaced by all-light or all-dark conditions[49]. These studies imply that normal circadian rhythms protect against disease, possibly by anticipating daily changes in susceptibility to external insults and challenges such as exposure to pathogens.
Disease can also result in deregulated circadian homeostasis. AD patients exhibit altered circadian rhythms, glucocorticoid levels and sleep physiology[40]. Circadian rhythms are disrupted in patients with kidney and lung cancer[50-52]. Patients with severe sepsis also exhibit loss of circadian melatonin rhythms, despite diurnal light exposure in the ICU[53, 54], while non-septic patients in the ICU maintain circadian rhythms similar to non-hospitalized controls[53]. Experimental sepsis in rats disturbed the hypothalamic-pituitary adrenal axis[55, 56] and sleep patterns [57]. In some cases, this may reflect a protective mechanism in response to disease (for example, cortisol may be elevated to resolve inflammation), whereas in other cases, this deregulation may be a detrimental component of the disease process, or even a factor contributing to its etiology.
Concluding remarks
Recent work highlights regulation of the immune response by the circadian system, suggesting the feasibility of exploiting circadian-immune links for preventing and fighting disease. For example, understanding the mechanisms that drive daily changes in the responsiveness of PRRs to their corresponding agonists may be beneficial for optimization vaccine immunogenicity and for the treatment of conditions such as multiple sclerosis or asthma[9, 58]. Similarly, dissecting the molecular interplay between core circadian components, immune signaling and cell cycle mechanisms could unveil novel anti-cancer strategies [22].
To fully understand the circadian-immune connection future work should take into account the complexity of circadian regulation. Although circadian-regulated gene expression has been observed in different immune cell types, the extent of circadian regulation may differ depending on the immune cell type, and even within the same cell type [30], and other factors such as cellular developmental stage, location and trafficking status may also determine specific circadian immune outputs. In addition, the absence of a functional circadian molecular clock does not imply that the immune cell type or tissue cannot be modulated by the circadian system. Systemic cues may convey timing information to the cell in order to produce rhythmic immune outputs [29]. Canonical clock components may also modulate immune mechanisms in a circadian-independent manner [59] and at the molecular level circadian regulation can act in different ways e.g., it is not restricted to transcriptional mechanisms[60].
Although the innate immune system seems to be particularly well positioned for circadian regulation due to its surveillance role,(i.e., initial detection of external daily challenges such as exposure to pathogens), the repercussions on subsequent adaptive responses[9] and the intrinsic circadian modulation observed in some lymphocytic lineages[35, 36] deserve careful consideration.
Circadian regulation of immune defense mechanisms seems a conserved feature across several eukaryotic kingdoms. Whether the goal is to explore the teleological and evolutionary significance of immunity rhythms or to devise strategies for pharmaceutical (e.g., target discovery and validation), behavioral (e.g., health education) or procedural (e.g., timing of clinical procedures) intervention, it will be important to establish circadian models that support the translation of experimental findings into humans. For example, a rat model of rotating shift work and jet-jag has revealed how common circadian disruptions cause changes in NK cells that can influence cancer growth[61]. Immune circadian outputs may also become useful biomarkers for immune and inflammatory disease and for identifying the ideal timing for immunomodulation[62]. Conversely, immune outputs that oscillate in a circadian fashion under steady-state conditions may be leveraged to evaluate circadian and sleep homeostasis, as it has been proposed for the human circadian metabolome [63].
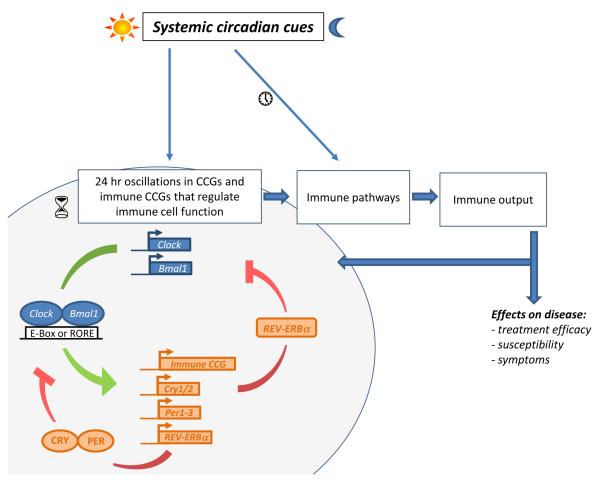
Figure 1. A model depicting the circadian immune connection.
Systemic circadian cues circadian immune outputs can be generated both directly and indirectly . Circadian clock proteins such as CLOCK and BMAL1 bind promoter E-box elements and ROREs to positively regulate expression of clock-controlled genes (CCGs). Several CCGs (e.g. Per2) have been linked to control of immune cell function and immune-related genes might also be under control of clock proteins (immune CCGs, e.g. TLR9). Clock controlled PER and CRY proteins inhibit CLOCK:BMAL1-mediated activation of CCGs by, whereas clock controlled REV-ERBα proteins negatively regulate BMAL1 gene expression. These pathways create a negative feedback look. REV-ERBα proteins have also been implicated in negative control of cytokine gene expression. Daily systemic cues transmitted by the neuroendocrine system might also generate circadian immune outputs by modulating of immune gene expression and signaling or through the entrainment of molecular clock mechanisms. Circadian immune outputs underlie daily variations in immune-mediated disease susceptibility symptoms and treatment efficacy. Immune factors, for example proinflammatory cytokines, can also feedback to the circadian system, either by modulating the circadian molecular clock or the neural and humoral mechanisms that conveying systemic circadian information. Hourglasses depict rhythmic expression of clock genes and CCGs, while the clocks depict oscillations outside the core molecular clockwork.
Acknowledgements
This work was supported by National Institutes of Health Grants AI079360 and AI50031 (to EF), and NIH Training Grant, T32 AI07019 (to AS) and in part by N01 HHSN272201100019C. EF is an Investigator with the Howard Hughes Medical Institute.
Glossary
- Molecular clockwork
network of transcriptional-translational feedback loops involving the circadian expression of core clock genes (Clock, Bmal1, Rev-erbα, Per, Cry).
- Circadian output
a biological process (e.g., gene expression, cytokine secretion) directly or indirectly controlled by the circadian molecular clock.
- Daily resistance oscillations
immune surveillance mechanisms that oscillate in a circadian fashion.
- Acrophase
the time period during which the circadian output peaks
- Zeitgeber
From the German, ‘time giver.’ Any stimulus capable of resetting a pacemaker or synchronizing a self-sustaining oscillation. Usually refers to an external time cue (e.g. light) that synchronizes an organism’s internal circadian clocks.
- Zeitgeber time (ZT)
in a regular 12 h light/12 h dark cycle, ZT0 is the start of the light phase and ZT12 corresponds to the beginning of the dark phase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dibner C, et al. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 2.Lange T, et al. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 3.Cutolo M. Chronobiology and the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2012;24:312–318. doi: 10.1097/BOR.0b013e3283521c78. [DOI] [PubMed] [Google Scholar]
- 4.Greene MW. Circadian rhythms and tumor growth. Cancer Lett. 2012;318:115–123. doi: 10.1016/j.canlet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Roden LC, Ingle RA. Lights, rhythms, infection: the role of light and the circadian clock in determining the outcome of plant-pathogen interactions. Plant Cell. 2009;21:2546–2552. doi: 10.1105/tpc.109.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone EF, et al. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog. 2012;8:e1002445. doi: 10.1371/journal.ppat.1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christoph Scheiermann YK, Daniel Lucas, Andrew Chow, Jung-Eun Jang, Dachuan Zhang, Daigo Hashimoto, Miriam Merad, Frenette Paul S. ADRENERGIC NERVES GOVERN CIRCADIAN LEUKOCYTE RECRUITMENT TO TISSUES. Immunity. 2012 doi: 10.1016/j.immuni.2012.05.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narasimamurthy R, et al. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver AC, et al. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 11.Bellet MM, Sassone-Corsi P. Mammalian circadian clock and metabolism - the epigenetic link. J Cell Sci. 2010;123:3837–3848. doi: 10.1242/jcs.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J Interferon Cytokine Res. 2006;26:645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- 13.Arjona A, Sarkar DK. Are circadian rhythms the code of hypothalamic-immune communication? Insights from natural killer cells. Neurochem Res. 2008;33:708–718. doi: 10.1007/s11064-007-9501-z. [DOI] [PubMed] [Google Scholar]
- 14.Thoennissen NH, et al. Transcription factor CCAAT/enhancer-binding protein alpha and critical circadian clock downstream target gene PER2 are highly deregulated in diffuse large B-cell lymphoma. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2012.658792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki K, et al. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells. 2010;15:351–358. doi: 10.1111/j.1365-2443.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, et al. Expression profiling reveals a positive regulation by mPer2 on circadian rhythm of cytotoxicity receptors: Ly49C and Nkg2d. Chronobiol Int. 2009;26:1514–544. doi: 10.3109/07420520903553435. [DOI] [PubMed] [Google Scholar]
- 17.Liu AC, et al. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4:e1000023. doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs JE, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamajuku D, et al. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett. 2011;585:2217–2222. doi: 10.1016/j.febslet.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Male V, et al. E4BP4: an unexpected player in the immune response. Trends Immunol. 2012;33:98–102. doi: 10.1016/j.it.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Hashiramoto A, et al. Mammalian clock gene Cryptochrome regulates arthritis via proinflammatory cytokine TNF-alpha. J Immunol. 2010;184:1560–565. doi: 10.4049/jimmunol.0903284. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Sancar A. Regulation of apoptosis by the circadian clock through NF-kappaB signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:120361–2041. doi: 10.1073/pnas.1108125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrzilka S, et al. Clock gene modulation by TNF-alpha depends on calcium and p38 MAP kinase signaling. J Biol Rhythms. 2009;24:283–24. doi: 10.1177/0748730409336579. [DOI] [PubMed] [Google Scholar]
- 24.Cavadini G, et al. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhardwaj V, et al. Defence responses of Arabidopsis thaliana to infection by Pseudomonas syringae are regulated by the circadian clock. PloS one. 2011;6:e26968. doi: 10.1371/journal.pone.0026968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, et al. Timing of plant immune responses by a central circadian regulator. Nature. 2011;470:110–114. doi: 10.1038/nature09766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller M, et al. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzoccoli G, et al. Time-related dynamics of variation in core clock gene expression levels in tissues relevant to the immune system. International journal of immunopathology and pharmacology. 2011;24:869–89. doi: 10.1177/039463201102400406. [DOI] [PubMed] [Google Scholar]
- 29.Logan RW, et al. Role of sympathetic nervous system in the entrainment of circadian natural-killer cell function. Brain, behavior, and immunity. 2012;25:101–19. doi: 10.1016/j.bbi.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silver AC, et al. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain, behavior, and immunity. 2012;26:407–43. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendez-Ferrer S, et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–47. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 32.Dimitrov S, et al. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113:5134–143. doi: 10.1182/blood-2008-11-190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzoccoli G, et al. Circadian rhythmicity of lymphocyte subpopulations and relationship with neuro-endocrine system. Journal of biological regulators and homeostatic agents. 2010;24:341–30. [PubMed] [Google Scholar]
- 34.Mazzoccoli G, et al. A timetable of 24-hour patterns for human lymphocyte subpopulations. Journal of biological regulators and homeostatic agents. 2011;25:387–35. [PubMed] [Google Scholar]
- 35.Bollinger T, et al. Circadian clocks in mouse and human CD4+ T cells. PloS one. 2011;6:e29801. doi: 10.1371/journal.pone.0029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortier EE, et al. Circadian variation of the response of T cells to antigen. J Immunol. 2011;187:6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- 37.Alten R. Chronotherapy with modified-release prednisone in patients with rheumatoid arthritis. Expert Rev Clin Immunol. 2012;8:123–13. doi: 10.1586/eci.11.95. [DOI] [PubMed] [Google Scholar]
- 38.Alten R, et al. Hypothalamus-pituitary-adrenal axis function in patients with rheumatoid arthritis treated with nighttime-release prednisone. The Journal of rheumatology. 2010;37:2025–031. doi: 10.3899/jrheum.100051. [DOI] [PubMed] [Google Scholar]
- 39.Mouterde G, et al. Predictors of radiographic progression in the ESPOIR cohort: the season of first symptoms may influence the short-term outcome in early arthritis. Ann. Rheum. Dis. 2011;70:1251–256. doi: 10.1136/ard.2010.144402. [DOI] [PubMed] [Google Scholar]
- 40.Slats D, et al. Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: Focus on the role of hypocretin and melatonin. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Kang JE, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moghekar A, et al. Cerebrospinal fluid Abeta and tau level fluctuation in an older clinical cohort. Arch. Neurol. 2012;69:246–20. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrushesky WJ, Wood PA. Circadian time structure of septic shock: timing is everything. J. Infect. Dis. 1997;175:1283–1284. [PubMed] [Google Scholar]
- 44.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4453–458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straif K, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 46.Pan A, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown DL, et al. Rotating night shift work and the risk of ischemic stroke. Am. J. Epidemiol. 2009;169:1370–377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman AE, et al. Clock-cancer connection in non-Hodgkin’s lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res. 2009;69:3605–3613. doi: 10.1158/0008-5472.CAN-08-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29:127–12. doi: 10.1097/shk.0b013e318142c5a2. [DOI] [PubMed] [Google Scholar]
- 50.Mazzoccoli G, et al. Altered expression of the clock gene machinery in kidney cancer patients. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2012;66:175–179. doi: 10.1016/j.biopha.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Mazzoccoli G, et al. Chronodisruption in lung cancer and possible therapeutic approaches. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2011;65:500–508. doi: 10.1016/j.biopha.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Mazzoccoli G, et al. Altered time structure of neuro-endocrine-immune system function in lung cancer patients. BMC Cancer. 2010;10:314. doi: 10.1186/1471-2407-10-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mundigler G, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit. Care Med. 2002;30:536–540. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Verceles AC, et al. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med. 2012 doi: 10.1007/s00134-012-2494-3. [DOI] [PubMed] [Google Scholar]
- 55.Flierl MA, et al. Disturbances of the hypothalamic-pituitary-adrenal axis and plasma electrolytes during experimental sepsis. Ann Intensive Care. 2011;1:53. doi: 10.1186/2110-5820-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson DE, et al. Cecal ligation and puncture in rats interrupts the circadian rhythms of corticosterone and adrenocortical responsiveness to adrenocorticotrophic hormone. Crit. Care Med. 2006;34:1178–1184. doi: 10.1097/01.CCM.0000207340.24290.3C. [DOI] [PubMed] [Google Scholar]
- 57.Baracchi F, et al. Sepsis-induced alterations in sleep of rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1467–1478. doi: 10.1152/ajpregu.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Logan RW, et al. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol. 2012;188:2583–2591. doi: 10.4049/jimmunol.1102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levi F, Okyar A. Circadian clocks and drug delivery systems: impact and opportunities in chronotherapeutics. Expert Opin Drug Deliv. 2011;8:1535–1541. doi: 10.1517/17425247.2011.618184. [DOI] [PubMed] [Google Scholar]
- 63.Dallmann R, et al. The human circadian metabolome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2625–2629. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]