Abstract
Despite remission rates of approximately 85% for children diagnosed with acute myeloid leukemia (AML), greater than 40% will die from relapsed disease. Patients with poor-risk molecular/cytogenetics and/or inadequate response to upfront therapy are typically considered high-risk (HR) and historically have poor outcomes with chemotherapy alone. We investigated whether allogeneic hematopoietic cell transplantation (allo-HCT) with best available donor in first remission (CR1) would abrogate the poor outcomes associated with HR AML in chemotherapy treated children and young adults. We reviewed the outcomes of 50 consecutive children and young adults (ages 0–30 years) with AML who received a myeloablative allo-HCT between 2001 and 2010. Thirty-six patients (72%) were HR, defined as having FLT3-ITD mutations, 11q23 MLL rearrangements, chromosome 5 or 7 abnormalities, induction failure and/or having persistent disease. The majority of patients received cyclophosphamide and total body irradiation conditioning and graft-versus-host-disease (GVHD) prophylaxis was cyclosporine based. Transplant outcomes for HR patients were compared to standard-risk patients with no significant differences observed in overall survival (72% vs. 78%, p=0.72), leukemia-free survival (69% vs. 79%, p=0.62), relapse (11% vs. 7%, p=0.71) or TRM (17% vs. 14%, p=0.89). Children and young adults with HR-AML have comparable outcomes to standard-risk patients following allo-HCT in CR1.
Keywords: AML, High-Risk, transplantation, pediatric
INTRODUCTION
Children diagnosed with acute myeloid leukemia (AML) have not had the same success as children treated for acute lymphoblastic leukemia (ALL). (1, 2) Despite the majority of children with AML achieving complete remission following intensive induction therapy, 40% will die from either disease relapse or treatment related toxicities. (3) Allogeneic hematopoietic cell transplantation (allo-HCT) using a matched sibling donor (MSD) has remained the standard of care for children with AML in first complete remission (CR1), particularly for patients lacking favorable characteristics [e.g. t(8;21) translocation; inv(16) or t(16;16) translocation] (4). In contrast, most cooperative group studies have suggested that those patients with high-risk features (e.g. FLT3/ITD, monosomy 5 or 7, del 5q) (4, 5) or poor response to upfront chemotherapy (e.g. induction failure or having persistent disease) (6, 7) receive transplantation with best available donor since these children have less than a 40% chance of survival when treated with chemotherapy alone. (8, 9) Despite the use of allo-HCT for high-risk (HR) AML patients in first remission, outcomes remain poor with several studies reporting no benefit of HCT compared to chemotherapy alone, questioning the rationale of HCT for HR patients. (4, 10–12) Whether HCT outcomes for these patients have improved in the current era remains in question.
Here we report outcomes for children, adolescents and young adults with HR and standard-risk (SR) AML who received an allo-HCT in first remission (CR1) at our institution between 2001 and 2010. We hypothesized that in the current era of allo-HCT there would be no significant difference in survival for SR and HR patients. This observation would therefore support the practice of recommending allo-HCT in CR1 with best available donor for children, adolescents and young adults with HR AML.
PATIENTS AND METHODS
Study Design and Patient Characteristics
Patient characteristics are shown in table 1. Fifty patients, ages 0 to 30 years, diagnosed with HR and SR AML underwent myeloablative allo-HCT in first remission (CR1) at the University of Minnesota between 2001 and 2010. Patients with Down syndrome, acute promyelocytic leukemia (APL), relapsed AML, treatment-related AML, as well as those who received a non-myeloablative allo-HCT or a prior allo-HCT were excluded from this analysis. All patients and/or their parents or guardians signed consent to participate on institutional review board approved transplant protocols and outcomes were subsequently reviewed retrospectively. Thirty-six patients (72%) were classified as HR and 14 (28%) as SR. For the purpose of this analysis, HR AML was defined as patients who had either monosomy 5 (n=0) or 7 (n=3), deletion 5q (n=2), FLT3/ITD with a high allelic ratio (>0.4) (n=6), 11q23 MLL gene rearrangement (n=8) (excluding the favorable t(1;11) (q21;q23); MLL AF1q), bi-phenotypic lineage leukemia (n=5), induction failure (>15% blasts prior to the start of the second Induction course) (n=10) or morphological persistent disease after two cycles of induction therapy (n=4). (13) The fourteen patients classified as SR based on their absence of HR features had either a normal karyotype (n=6), translocation involving t(8;21) (n=4), trisomy 8 (n=3), trisomy 4 (n=1) or the presence of monosomy 18 (n=1). These fourteen SR patients were allocated to allo-HCT in CR1 based on their having of a MSD and therefore were not treated with chemotherapy alone.
Table 1.
Patient Characteristics
| Factor | HR AML | SR AML | p-value |
|---|---|---|---|
| N | 36 | 14 | |
| Age | 0.80 | ||
| Median (range) | 15.1 (0.5–30.2) | 14.3 (1.9–25.7) | |
| Gender | 0.02 | ||
| Male | 18 (50%) | 12 (85.7%) | |
| Donor source | <0.001 | ||
| Bone Marrow (BM) | 7 (19.4%) | 11 (78.6%) | |
| Related BM Donor | 4 (57%) | 7 (64%) | |
| Related PBSC | 3 (43%) | 4 (36%) | |
| Umbilical Cord Blood | 29 (80.6%) | 3* (21.4%) | |
| Single | 8 (28%) | 0 | |
| Double | 21 (72%) | 3* (100%) | |
| Recipient CMV | 0.30 | ||
| Positive | 26 (72.2%) | 8 (57.1%) | |
| Negative | 10 (27.8%) | 6 (42.9%) | |
| Conditioning | <0.001 | ||
| CY/Flu/TBI | 24 (66.7%) | 3 (8.3%) | |
| CY/Flu/TBI/ATG | 1 (2.8%) | 0 | |
| CY/TBI | 6 (16.7%) | 5 (35.7%) | |
| Busulfan containing | 5 (13.8%) | 6 (42.9%) | |
| GVHD Prophylaxis | |||
| CSA/MMF | 27 (75.0%) | 3 (21.4%) | |
| CSA/MTX | 6 (16.7%) | 10 (71.4%) | <0.001 |
| CSA/PD/ATG | 3 (21.4%) | 0 | |
| GVHD | 0.13 | ||
| Acute II–IV | |||
| Yes | 13 (36.1%) | 2 (14.3%) | |
| No | 23 (63.9%) | 12 (85.7%) | |
| Acute III–IV | 0.67 | ||
| Yes | 4 (11.1%) | 1 (7.1%) | |
| No | 32 (88.9%) | 13 (92.9%) | |
| Chronic | 0.21 | ||
| Yes | 8 (22.2%) | 1 (7.1%) | |
| No | 28 (77.8%) | 13 (92.9%) |
related umbilical cord blood; PBSC, peripheral blood stem cell; CY, cyclophosphamide; Flu, fludarabine; TBI, total body irradiation; ATG, anti-thymocyte globulin; CSA, cyclosporine; MMF, mycophenolate mofetil; MTX, methotrexate; PD, prednisone
The median age at time of allo-HCT for the entire cohort was 14.9 (range, 0.5–30.2) years with median follow-up of 4.86 (range, 1.10 – 10.19) years. Eighteen (50%) patients with HR AML and 12 (85.7%) with SR AML were male (p=0.02). The median time from diagnosis to allo-HCT was 129.5 (range, 67–277) days for HR patients and 131 (range, 83–219) days for SR patients (p=0.91). The majority of both HR (69%, n=25) and SR (64%, n=9) patients received their allo-HCT between 2005 and 2010 compared to 2001–2004 (30.6%, n=11 and 35.7%, n=5, respectively, p=0.73). The majority of both HR (72.2%) and SR (57.1%) patients were seropositive for cytomegalovirus (CMV) prior to transplant (p=0.30).
Donor selection and Conditioning Regimens
Stem cell sources included HLA matched sibling donor (MSD) bone marrow, matched related peripheral blood stem cell (PBSC), and matched related and unrelated umbilical cord blood (UCB). Patients with HR AML received UCB (80.6%, n=29), PBSC (8.3%, n=3) and MRD (11.1%, n=4), while all the SR patients received MSD grafts comprised of bone marrow (50%, n=7), PBSC (28.6%, n=4) and UCB (21.4%, n=3) (p<0.001). The significant discrepancy in the number of UCB recipients observed between the two groups is likely the result of many of the SR patients being excluded for allo-HCT if they only had a matched related umbilical cord blood donor and our own institutional priority for cord blood for unrelated allo-HCT recipients.
Myeloablative conditioning consisting of cyclophosphamide (120 mg/kg) +/− fludarabine (75 mg/m2) and total body irradiation (TBI; 1320 cGy) was used in 78% (n=39). The remaining 22% (n=11) received myeloablative doses of busulfan/cyclophosphamide or busulfan/melphalan +/−fludarabine. Graft-versus-host-disease (GVHD) prophylaxis was comprised of cyclosporine-based combinations in all patients.
Statistical Methods
Five outcomes were studied: OS, leukemia free survival (LFS), transplant related mortality (TRM), GVHD and relapse. The Kaplan-Meier method was used to estimate OS and LFS, while cumulative incidence was used to estimate TRM, GVHD and relapse. (14, 15) Cox multiple regression models were conducted for OS and LFS. Competing risk regression was employed for TRM, GVHD and risk of relapse. HR versus SR was the primary factor considered for each endpoint in both univariate and multivariate regression. Other covariates used in the models included: gender, CMV status, bone marrow versus UCB, HLA matching (in the case of double cord transplant, the matching of the engrafting cord was used), and year of transplant. The backward method was used to determine the final model with a p-value of ≤0.05 considered significant in all statistical tests. The study had sufficient power to identify a difference in transplant outcomes based on disease risk group. Statistical analysis was performed with Statistical Analysis System statistical software version 9.2 (SAS Institute).
RESULTS
Neutrophil Engraftment
Engraftment by day 42 post-HCT (defined as three consecutive days with an ANC>500/µl) occurred in 90% (n=32) of HR patients and 100% (n=14) of SR patients (p=0.08). The median time to neutrophil engraftment for HR patients was 22 (range, 2–38) days compared to 21 (14–26) days for SR patients (p=0.44). Twenty-seven of the 32 patients who received UCB grafts engrafted by day 42 post-HCT, compared to all 18 recipients of BM and PBSC grafts (88%, 95% CI 73–96% vs. 100%; p<0.01). In multivariate analysis there were no significant differences identified between the probability of engraftment among the SR and HR patients (HR 1.74 95% CI 0.80–3.75; p=0.16).
Overall Survival and Leukemia Free Survival
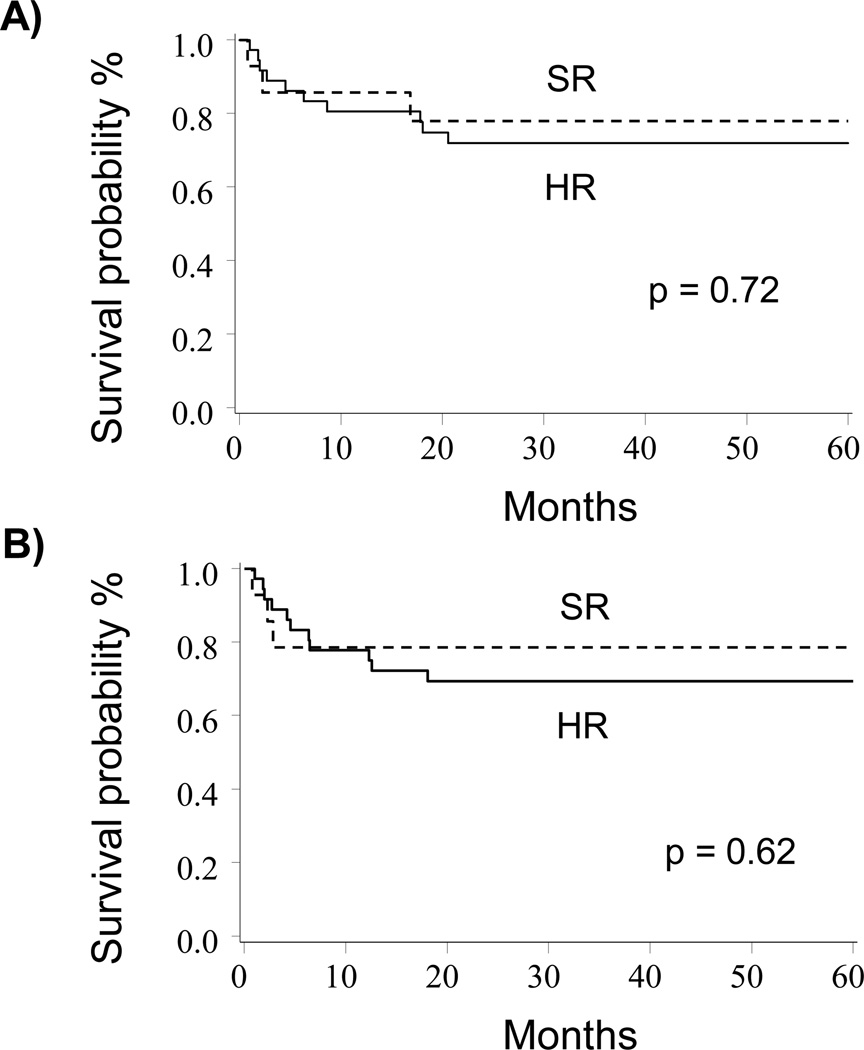
Comparing survival outcomes between HR and SR groups, there was no significant difference in OS (72%, 95% CI 54–84% vs. 78%, 95% CI 46–92%; p=0.72) or LFS (69%, 95% CI 51–82% vs. 79%, 95% CI 47–93%, p=0.62) at 5-years in univariate analysis (Figure 1A and 1B). This finding was confirmed in a multivariate analysis that demonstrated similar OS (HR 0.64, 95% CI: 0.17–2.36; p=0.50) and LFS (HR 0.56, 95% CI: 0.15–2.04; p=0.38) between the two groups (Table 2).
Figure 1. OS and LFS for HR and SR patients.
A) OS of HR vs. SR patients (72% vs. 78%, p=0.72). B) The cumulative incidence of LFS for HR vs. SR patients (69% vs. 79%, p=0.62).
Table 2.
Multivariate Analysis SR vs. HR AML
| Factor | Relative Risk 95% CI | p-value |
|---|---|---|
| Overall Survival | 0.64 (0.17–2.36) | 0.50 |
| Leukemia-Free Survival | 0.56 (0.15–2.04) | 0.38 |
| Transplant Related Mortality | 0.50 (0.04–5.46) | 0.57 |
| Relapse | 0.79 (0.08–8.07) | 0.84 |
| Engraftment | 1.71 (0.85–3.46) | 0.13 |
| Acute GVHD (Grade II–IV) | 0.35 (0.08–1.58) | 0.17 |
| Acute GVHD (Grade III–IV) | 1.10 (0.14–8.75) | 0.93 |
| Chronic GVHD | 0 (0 -0) | <0.01 |
Treatment Related Mortality and GVHD
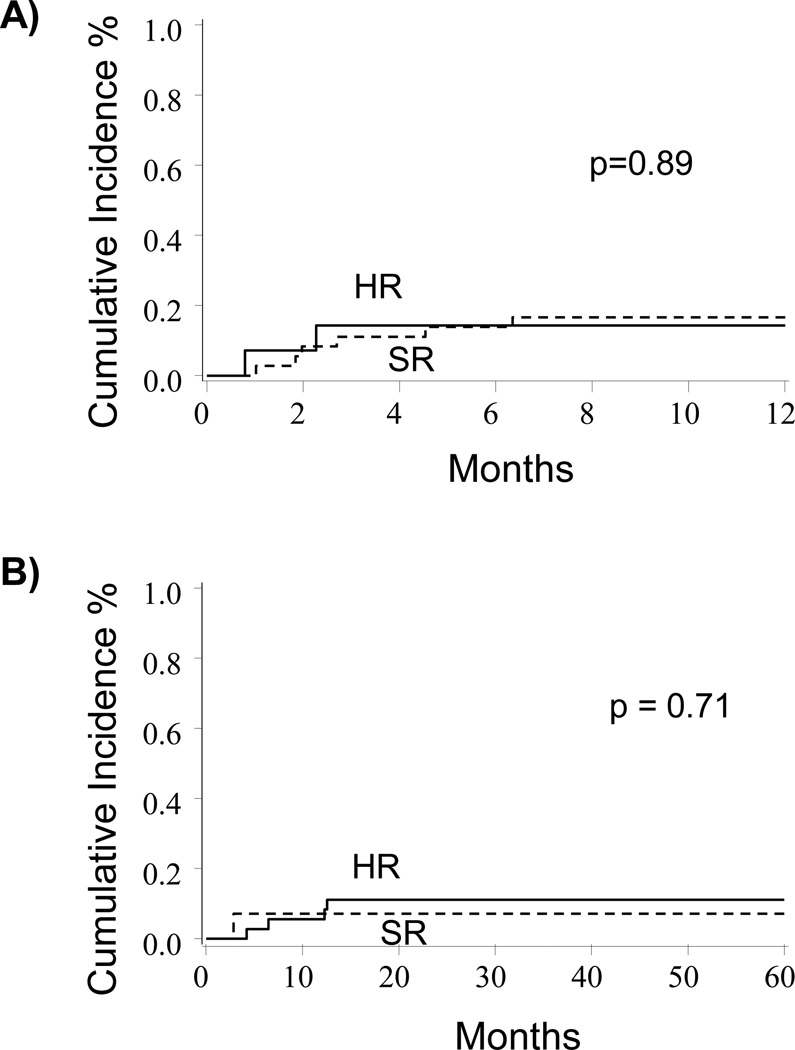
In univariate analysis, the cumulative incidence of TRM at 1-year was similar between HR and SR patients (17%, 95% CI: 5–29% vs. 14%, 95% CI: 0–32%; p=0.89, Figure 2A). This similarity persisted in multivariate analysis where no significant differences were found (HR 0.50, 95% CI: 0.04–5.46, p=0.57, Table 2). Similarly, univariate analysis showed no significant differences in TRM between the two graft sources (marrow vs. UCB), presence of GVHD, or recipient CMV seropositivity prior to HCT. Causes of TRM for the HR patients included organ failure (n=1), infection (n=2), GVHD (n=2), graft failure (n=2), compared to infection (n=2) for the SR patients.
Figure 2. TRM and relapse for HR and SR patients.
A) The cumulative incidence of TRM for HR vs. SR patients (17% vs. 14%, p=0.89). B) The cumulative incidence of relapse for HR vs. SR patients (11% vs. 7%, p=0.71).
Disease risk status (HR versus SR) was not a significant factor in the development of grade II–IV acute GVHD (36%, 95% CI 20–52% vs. 14%, 95% CI 0–32%; p=0.13), grade III–IV acute GVHD (11%, 95% CI 1–21% vs. 7%, 95% CI 0–20%; p=0.67) or chronic GVHD (22%, 95% CI 9–36% vs. 0%, 95% CI 0–0%; p=0.06). In the multivariate analyses, there were no significant differences in the development of acute GVHD grade II–IV (HR 0.35, 95% CI 0.08–1.58; p=0.17) or grade III–IV (HR 1.1, 95% CI 0.14–8.75; p=0.93) between HR and SR patients. Chronic GVHD was significantly different between the two risk groups in multivariate analysis as only HR patients developed chronic GVHD (HR 0; p<0.01), a likely result of the graft source discrepancies between these two groups.
Relapse
Rates of leukemia relapse were low in both groups of patients in this study. In univariate analysis, there was no significant difference in relapse at 5-years between HR and SR patients (11%, 95% CI 1–21% vs. 7%, 95% CI 0–20%; p=0.71, Figure 2B) which continued in the multivariate model (HR 0.79, 95% CI 0.08–8.07; p=0.84). Patients who received an UCB allo-HCT with 4/6 HLA mismatch alleles (n=11) reported no relapses, compared to patients matched at 5/6 or 6/6 HLA alleles (p<0.01). As well, there were no relapses reported in patients who experienced grade II–IV acute GVHD (n=15) compared to patients who did not (p<0.01).
DISCUSSION
In this single institution retrospective analysis of 50 pediatric/young adult patients with AML who received an allo-HCT in CR1 from 2001 to 2010, we found no significant difference in survival or relapse between those with or without high-risk disease. As previous studies in pediatric AML have typically included transplant data from older time periods (4, 16–19) when rates of TRM were found to be greater and supportive care measures (including antifungal therapy) less advanced, we investigated whether outcomes for patients with HR AML would now be comparable to SR AML patients.
It has been well documented that children with HR AML who are treated with chemotherapy alone have higher rates of treatment failure (relapse) and inferior survival compared to those with SR disease (6, 8, 9, 20). Creutzig and colleagues reported the long-term outcomes of the BFM AML pediatric trials spanning from 1978 to 1998. (21) In the earlier studies (AML-BFM 78 and AML-BFM 83) SR and HR patients were treated with chemotherapy alone without any recommendation for allo-HCT and reported 5-year EFS of 38±4% and 47±4% respectively. In AML-BFM 87 and 93, HR patients who had an available matched related donor were allocated to allo-HCT in CR1 (n=39), reporting similar EFS at 5-years of 64±3% compared to the HR patients treated with chemotherapy alone 62±3% (n=317).
Whether allo-HCT can improve survival in patients with HR AML is debated. Most of the pediatric AML transplant literature reporting outcomes in HR patients have included earlier periods and typically have not found a benefit to HCT (4, 18–21); as a result, there is limited data on HCT outcomes for pediatric HR AML in the current era. For instance, the BFM group reported results of AML-BFM 98 (study period: 1998– 2004) which allocated HR patients with an available MSD to allo-HCT in CR1 and those without a MSD to receive chemotherapy. The only HR group which appeared to benefit from allo-HCT were those with MLL rearrangements, where HCT provided significantly improved outcomes compared to chemotherapy alone (5-year OS 94± 6% (n=18) vs. 52± 7% (n=49), p=0.01). The outcomes of the other HR subgroups did not show differences between MSD allo-HCT and chemotherapy alone, questioning the role of HCT for patients with HR disease. More recently, Koh and colleagues reported their experience using allo-HCT for 29 children with either HR or advanced AML. (22) This data included a 10-year period between 1998 and 2008 and reported improved 3-year OS and EFS of 77% (95% CI 65–99%) and 70% (95% CI 57–93%) respectively and particularly low TRM (7%, 95% CI 0–44%).
Several reports have demonstrated inferior outcomes for acute leukemia patients who undergo allo-HCT in ≥CR2, compared to CR1 patients. (23–27) Gassas and colleagues reported a comparison of children with AML who underwent allo-HCT in CR1 (n=47) versus CR2 (n=23) with the CR2 patients having inferior survival (3-yr OS: 51% ±11 vs. 74% ±7; p=0.05) and much greater TRM (38% ±11 vs. 11% ±5; p=0.01) compared to patients transplanted in CR1. (26) Thus, postponing allo-HCT based on the unavailability of a MSD, particularly in patients with HR disease who are at greater risk of treatment failure, may not be the most prudent approach. This becomes particularly relevant in the current era, where HCT outcomes for acute leukemia have improved. These improvements have been largely from the use of high-resolution HLA-typing and enhanced supportive care (including improved antifungal agents) which have resulted in similar outcomes regardless of donor source (28–33) as well as the ability to identify minimal residual disease in patients prior to HCT which has proven to impact post-HCT survival. (34–36) The Children’s Oncology Group (COG) is currently exploring the concept of taking children with HR AML to allo-HCT with best available donor in first remission in a large prospective Phase III clinical trial (AAML1031). The results of this study will be critical in determining whether allo-HCT remains the best treatment today for children and young adults with HR AML.
In summary, we report similar outcomes for children and young adults with AML when transplanted in first remission, regardless of their risk status (HR or SR). Based on the overall advancements in HLA-typing, improvements in supportive care therapies and the ability to identify minimal residual disease in patients prior to HCT, outcomes for pediatric patients with HR AML are achieving new heights. With a greater than 70% overall survival predicted for children with HR AML who receive best available donor allo-HCT, reported not only by our own group but by others as well (22), HCT in CR1 should remain the standard of care for these patients. As new discoveries and advances occur in chemotherapy, what continued role allo-HCT will play in the consolidative setting for patients with AML will need to be addressed. As this was a single institution retrospective study with a relatively small cohort of pediatric and young adult patients, further studies in both children and adults should be pursued to verify our findings of similar outcomes in HR and SR AML patients following allo-HCT in CR1.
Acknowledgments
The NCI CA96028 (M.J.B.), Children’s Cancer Research Fund (M.J.B., J.E.W, M.R.V.), and the University of Minnesota Pediatric Leukemia Program supported this work. This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest to disclose.
Authors’ Contributions
MJB: conceived the study, reviewed data and writing of the manuscript; JEW: led or co-led each of the clinical transplant trials for AML, reviewed data and writing of the manuscript; CU: reviewed data and writing of the manuscript; QC: statistical analysis; MRV: reviewed data and writing of the manuscript.
References
- 1.Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120:1165–1174. doi: 10.1182/blood-2012-05-378943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods WG. Curing childhood acute myeloid leukemia (AML) at the half-way point: promises to keep and miles to go before we sleep. Pediatr Blood Cancer. 2006;46:565–569. doi: 10.1002/pbc.20646. [DOI] [PubMed] [Google Scholar]
- 4.Horan JT, Alonzo TA, Lyman GH, et al. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: the Children's Oncology Group. J Clin Oncol. 2008;26:5797–5801. doi: 10.1200/JCO.2007.13.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hospital MA, Thomas X, Castaigne S, et al. Evaluation of allogeneic hematopoietic SCT in younger adults with adverse karyotype AML. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.49. [DOI] [PubMed] [Google Scholar]
- 6.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 7.Riley LC, Hann IM, Wheatley K, Stevens RF. Treatment-related deaths during induction and first remission of acute myeloid leukaemia in children treated on the Tenth Medical Research Council acute myeloid leukaemia trial (MRC AML10). The MCR Childhood Leukaemia Working Party. Br J Haematol. 1999;106:436–444. doi: 10.1046/j.1365-2141.1999.01550.x. [DOI] [PubMed] [Google Scholar]
- 8.Meshinchi S, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Oncologist. 2007;12:341–355. doi: 10.1634/theoncologist.12-3-341. [DOI] [PubMed] [Google Scholar]
- 9.Harrison CJ, Hills RK, Moorman AV, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. 2010;28:2674–2681. doi: 10.1200/JCO.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- 10.Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 11.Reinhardt D, Kremens B, Zimmermann M, et al. No Improvement of Overall-Survival in Children with High-Risk Acute Myeloid Leukemia by Stem Cell Transplantation in 1st Complete Remission. ASH Annual Meeting Abstracts. 2006;108:320-. [Google Scholar]
- 12.Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116:2205–2214. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- 13.Meshinchi S, Alonzo T, Gerbing RB, et al. Minimal Residual Disease Detection by Four-Color Multidimensional Flow Cytometry Identifies Pediatric AML Patients at High Risk of Relapse. ASH Annual Meeting Abstracts. 2007;110:1429-. [Google Scholar]
- 14.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012;18:861–873. doi: 10.1016/j.bbmt.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods WG, Kobrinsky N, Buckley J, et al. Intensively timed induction therapy followed by autologous or allogeneic bone marrow transplantation for children with acute myeloid leukemia or myelodysplastic syndrome: a Childrens Cancer Group pilot study. J Clin Oncol. 1993;11:1448–1457. doi: 10.1200/JCO.1993.11.8.1448. [DOI] [PubMed] [Google Scholar]
- 18.Dini G, Boni L, Abla O, et al. Allogeneic bone marrow transplantation in children with acute myelogenous leukemia in first remission. Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) and the Gruppo Italiano per il Trapianto di Midollo Osseo (GITMO) Bone Marrow Transplant. 1994;13:771–776. [PubMed] [Google Scholar]
- 19.Klingebiel T, Pession A, Paolucci P, Rondelli R. Autologous versus allogeneic BMT in AML: the European experience. Report of the EBMT--Pediatric Diseases Working Party. Bone Marrow Transplant. 1996;18(Suppl 2):49–52. [PubMed] [Google Scholar]
- 20.Zwaan CM, Meshinchi S, Radich JP, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 21.Creutzig U, Zimmermann M, Ritter J, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 22.Koh KN, Park M, Kim BE, Bae KW, Im HJ, Seo JJ. Favorable outcomes after allogeneic hematopoietic stem cell transplantation in children with high-risk or advanced acute myeloid leukemia. J Pediatr Hematol Oncol. 2011;33:281–288. doi: 10.1097/MPH.0b013e318203e279. [DOI] [PubMed] [Google Scholar]
- 23.Beck JC, Cao Q, Trotz B, et al. Allogeneic hematopoietic cell transplantation outcomes for children with B-precursor acute lymphoblastic leukemia and early or late BM relapse. Bone Marrow Transplant. 2011;46:950–955. doi: 10.1038/bmt.2010.217. [DOI] [PubMed] [Google Scholar]
- 24.Nemecek ER, Ellis K, He W, et al. Outcome of myeloablative conditioning and unrelated donor hematopoietic cell transplantation for childhood acute lymphoblastic leukemia in third remission. Biol Blood Marrow Transplant. 2011;17:1833–1840. doi: 10.1016/j.bbmt.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borgmann A, Baumgarten E, Schmid H, et al. Allogeneic bone marrow transplantation for a subset of children with acute lymphoblastic leukemia in third remission: a conceivable alternative? Bone Marrow Transplant. 1997;20:939–944. doi: 10.1038/sj.bmt.1701013. [DOI] [PubMed] [Google Scholar]
- 26.Gassas A, Ishaqi MK, Afzal S, Finkelstein-Shechter T, Dupuis A, Doyle J. A comparison of the outcomes of children with acute myelogenous leukemia in either first or second complete remission (CR1 vs CR2) following allogeneic hematopoietic stem cell transplantation at a single transplant center. Bone Marrow Transplant. 2008;41:941–945. doi: 10.1038/bmt.2008.16. [DOI] [PubMed] [Google Scholar]
- 27.Bunin NJ, Davies SM, Aplenc R, et al. Unrelated donor bone marrow transplantation for children with acute myeloid leukemia beyond first remission or refractory to chemotherapy. J Clin Oncol. 2008;26:4326–4332. doi: 10.1200/JCO.2008.16.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DH, Kwon YJ, Lim J, et al. Comparable outcomes of HLA-matched unrelated and HLA-identical sibling donor bone marrow transplantation for childhood acute myeloid leukemia in first remission. Pediatr Transplant. 2009;13:210–216. doi: 10.1111/j.1399-3046.2008.00997.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith AR, Baker KS, Defor TE, Verneris MR, Wagner JE, Macmillan ML. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009;15:1086–1093. doi: 10.1016/j.bbmt.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhang MJ, Davies SM, Camitta BM, Logan B, Tiedemann K, Eapen M. Comparison of outcomes after HLA-matched sibling and unrelated donor transplantation for children with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2012;18:1204–1210. doi: 10.1016/j.bbmt.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey J, Green A, Cornish J, et al. Improved survival in matched unrelated donor transplant for childhood ALL since the introduction of high-resolution matching at HLA class I and II. Bone Marrow Transplant. 2012;47:1294–1300. doi: 10.1038/bmt.2012.8. [DOI] [PubMed] [Google Scholar]
- 33.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venditti A, Maurillo L, Buccisano F, et al. Pretransplant minimal residual disease level predicts clinical outcome in patients with acute myeloid leukemia receiving high-dose chemotherapy and autologous stem cell transplantation. Leukemia. 2003;17:2178–2182. doi: 10.1038/sj.leu.2403138. [DOI] [PubMed] [Google Scholar]
- 36.Laane E, Derolf AR, Bjorklund E, et al. The effect of allogeneic stem cell transplantation on outcome in younger acute myeloid leukemia patients with minimal residual disease detected by flow cytometry at the end of post-remission chemotherapy. Haematologica. 2006;91:833–836. [PubMed] [Google Scholar]