Abstract
Objective:
Tooth loss is a common problem and since current tooth replacement methods cannot counter balance with biological tooth structures, regenerating natural tooth structures has become an ideal goal. A challenging problem in tooth regeneration is to find a proper clinically feasible cell to seed.This study was designed to investigate the odontogenic potential of human bone marrow mesenchymal stem cells (HBMSCs) for seeding in tooth regeneration.
Materials and Methods:
In this experimental study, three pregnant Sprague Dawley (SD) rats were used at the eleventh embryonic day and rat fetuses were removed surgically using semilunar flap under general anesthesia. The primary mandible was cut using a stereomicroscope. The epithelial and mesenchymal components were separated and the dissected oral epithelium was cultured for 3 days. We used flow cytometry analysis to confirm presence of mesenchymal stem cells and not hematopoietic cells and to demonstrate the presence of oral epithelium. Bone marrow mesenchymal stem cells (BMSCs) and cultured oral epithelium were then co-cultured for 14 days. BMSCs cultured alone were used as controls. Expression of two odontogenic genes Pax9 and DMP1 was assessed using quantitative reverse transcription- polymerase chain reaction (RT-PCR).
Results:
Expression of two odontogenic genes, Pax9 and DMP1, were detected in BMSCs co-cultured with oral epithelium but not in the control group.
Conclusion:
Expression of Pax9 and DMP1 by human BMSCs in the proximity of odontogenic epithelium indicates odontogenic potential of these cells.
Keywords: Pax9, DMP1, Bone Marrow Stem Cells, Odontogenesis
Introduction
It is well known that dental structures are derived from ectoderm and mesenchymal layers during embryogenesis which are almost destroyed after the formation of tooth structures (1). Therefore dental tissue exhibits a limited response to damages. Tooth loss will happen to most of people and may affect their life quality (2-5). Despite the technical improvements, the current tooth replacement methods and dental materials cannot counterbalance biological tooth structures (6). Stem cell-based tooth regeneration is a biological technique that aims to regenerate histological, morphological and functionally tooth like structures. Stem cells (SCs) are undifferentiated cells with multi-lineage differentiation and self-renewal capacity (7-9).
There are two types of stem cells according to their differentiation potential: embryonic stem cells (ESCs) (8) and somatic stem cells (also known as adult stem cells or mesenchymal stem cells) (10). Because of the limited usage of ESCs due to the ethical limitations (11), mesenchymal stem cells (MSCs) are applied as a more common source in tissue engineering (12). Post natal stem cells have been obtained from several sources such as periosteum, adipose tissue, skin, hair follicle, skeletal muscle, brain and bone tissue (13,14). In different studies six types of dental MSCs have been reported including: permanent dental pulp stem cells (DPSC) (15), stem cells from human exfoliated deciduous teeth (SHED) (16), human infantile dental stem cells (IDSC) (17, 18), periodontal ligament stem cells (PDLSC) (19), apical papilla stem cells (SCAPs) (20, 21) and dental follicle progenitors (22). Bone marrow is known as the best source of MSCs (17, 23). The origin of MSCs is different from follicle driven stem cells: they originate from mesoderm; however, the dental stem cells are driven from the neural crest (24). It has been demonstrated that the mouse BMSCs can differentiate into odontoblast and produce tooth-like structures in the proximity of embryonic dental epithelium (25-27). It has also been shown that produced tooth bud in the in vitro environment can be transferred into the adult mandible (26). BMSCs can also be extracted from the mandibular bone and it seems that the mandibular BMSCs have high osteogenic capacity (28). Nevertheless their count is much lower than iliac crest (29).
Dentin matrix protein1 (DMP1) is expressed in pulp and odontoblast cells during odontogenesis and facilitates mineral nucleus formation in special locations. It also prevents spontaneous calcium phosphate sedimentation in non-arbitrary sites (30). DMP1 is expressed before the expression of Dentin sialophosphoprotein (DSPP) gene (25) and regulates DSPP gene transcription, which indicates complete odontoblastic differentiation (31). Simultaneous expression of Paired box gene 9 (Pax9), MSX1 and Lhx7 is characteristic of dental ectomesenchymal tissue (32) and it has been suggested that these three genes can be used as an early event for the determination of odontogenic capacity (28). Expression of Pax9 is mandatory for progression of tooth development; hence in its absence dental development will stop in bud stage. DMP1 is not specific for dental tissue and is expressed by other hard tissue cells such as osteoblasts, osteocytes, ameloblasts and cementoblasts and functions by influencing the mineralization process of these tissues. It has also been demonstrated that DMP1 is expressed in the brain tissue of mouse and cow (33). In the present study, we used human BMSCs since these cells are more available than dental stem cells and assessed the expression of odontogenic genes in these cells.
Materials and Methods
Isolation and culture of human bone marrow cells
Human bone marrow stem cells were obtained from Iran Transplant Productions Bank.
Isolation and culture of rat oral epithelium
Male and female sexually mature SD rats (3 females and one male) were placed in the same cage overnight. The following morning, if a plug was observed in the female rat’s vagina, then the fetal age was considered day 0. The pregnant SD rats were carefully weighed at both 0 and 11th embryonic days. Pregnant SD rats were used at the eleventh embryonic day (each pregnant rat has 8-10 fetuses) and rat fetuses were removed surgically under general anesthesia using Ketamin-Xylazine (1 ml/100 g/IP). Uterus was exposed using a semilunar flap (Fig 1). The primary mandible was cut and after the separation from the attached tissues was washed in PBS solvent for 5 minutes at room temperature, and cultured in medium (1:1 mix of DMEM, nutrient mixture ham’s F-12 medium, 1% penicillinstreptomycin) (Invitrogen, USA).
Fig 1.
Rat fetuses were removed at the eleventh embryonic day.
The mesenchymal and epithelial components were separated by incubating cells for 60 minutes in a solution (44 mM NaHCO3; 54 mM KCl; 110 mM NaCl; 0.9 mM NaH2PO4, 1mM sodium pyrovate, 42 mM phenol red pH=7.5, containing 1% penicillin-streptomycin, 1.4 mg/ml pronase and 0.1 mg/ml DNase, collagenase BB).
The epithelial cells were isolated using gentle movement and were cultured for 2 hours at 37˚C in a media (collection media with 5% FCS, 120 IU/ml insulin) unattached cells after washing were seeded in a count of 5×105 cells /250 μl and incubated in 5% CO2, in humidity for 3 days. This passage was repeated for 4 times.
Flow cytometry analysis
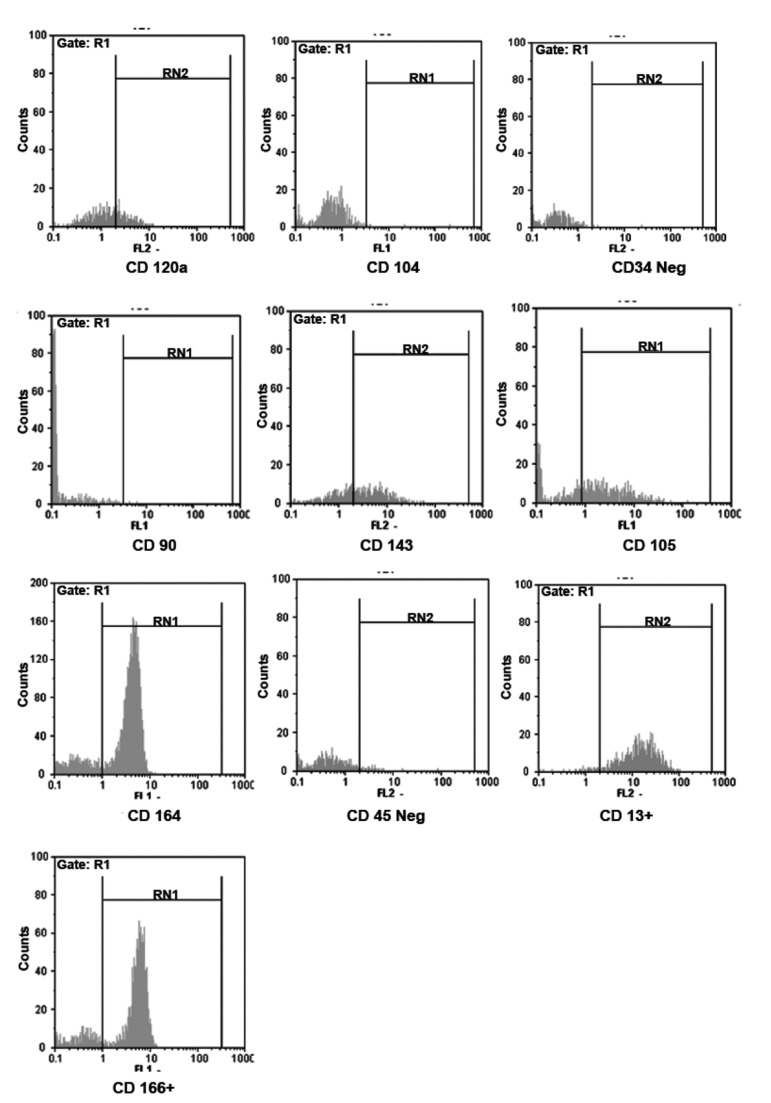
After 4 times passage, cultured bone marrow cells were trypsinized and incubated with primary monoclonal antibodies against CD13, CD90, CD105, CD166, -34FITC and -45FITC to confirm presence of mesenchymal stem cells and not hematopoietic cells. Cultured embryonic epithelial cells were assessed for CD104, CD120a, CD143, and CD164 (Fig 2).
Fig 2.
Flow cytometric analysis to confirm presence of oral epithelium (CD104, CD120, CD143 and CD164) and bone marrow mesenchymal stem cells (CD13, CD90, CD105, CD166, -34FITC and -45FITC).
Co-culture
The two single cell suspensions with the approximate count of 5×105/ml (BMSCs and oral epithelium of rat fetus) were co-cultured in proximity to each other with 2:1 proportion for 14 days in DMEM (containing 15% FCS, 1% ATB and 10ng/ml insulin growth factor (IGF)). After wards they were incubated at 37˚C and 5% CO2. As the control group, BMSCs were used alone. An E300, Eclipse, Nikon inverted microscope (made in Japan) was used for cytological study.
Quantitive RT-PCR
Quantitive RT-PCR was performed to assess DMP1 and Pax9 gene expression using RBC mRNA purificant kit (Metabion, Germany). RNA was reverse transcribed into cDNA by means of RTGO (Metabion, Germany). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and DMP1 genes were used as positive and negative control respectively. Following primers were used for RT-PCR:
DMP1 forward primer: 5´-CCCGCAGAACCTGAAGATG- 3´
DMP1 reverse primer: 5´-GACCCGGCAAAACAGGTAG- 3´
Fragments: 1060bp
Pax9 forward primer: 5´-GCCCACGTTGCTGCTTAGATTGAAA- 3´
Pax9 reverse primer: 5´-CTCCCTCCCTTCCCGGCTCT- 3´
Fragment: 240bp
GAPDH forward primer: 5´-TGATGACATCAAGAAGGTGGTGAAG- 3´
GAPDH reverse primer: 5´-TCCTTGGAGGCCATGTGGGCCAT- 3´
Fragment: 240bp
After computing the annealing temperature according to METABION co. instruction, these genes were polymerized using the RT co. kit and results were analyzed after Electrophoresis and taking photographs.
Ethical considerations
The number of included animals, their nutrition and maintenance status were supervised by a specialist veterinarian. The bone marrow donors were aware of the usage of their tissue samples in this study and signed the informed consent. The present study was approved by the Ethical Committee of Dental Faculty, Shahid Beheshti Medical Sciences University.
Results
Cultured bone marrow cells expressed CD13, CD90, CD105, CD166, -34FITC and -45FITC which indicates they are mesenchymal stem cells and not hematopoietic cells (Fig 2).
Cultured embryonic epithelial cells showed positive reaction with CD104, CD120a, CD143, and CD164 which indicates the presence of odontogenic epithelium (Fig 2).
Aggregation of polygonal cells with round to oval nuclei, granular cytoplasm and desmosome junctions were observed. Mesenchymal cells with centrally located pale staining nuclei located near polygonal cells (Fig 3).
Fig 3.
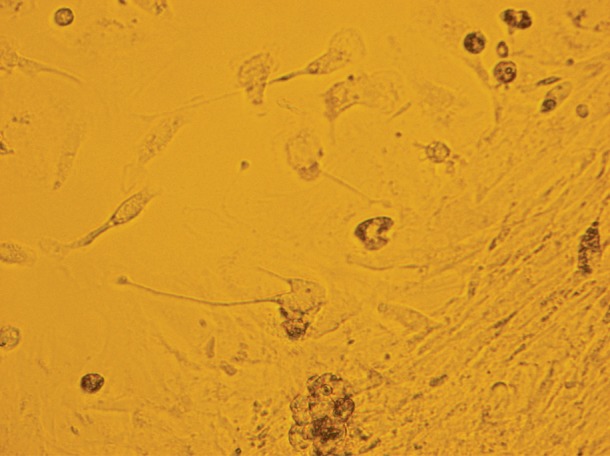
2816×2112- Aggregation of polygonal epithelial cells with round to oval nuclei. Mesenchymal cells with centrally located pale staining nuclei located near epithelial cells.
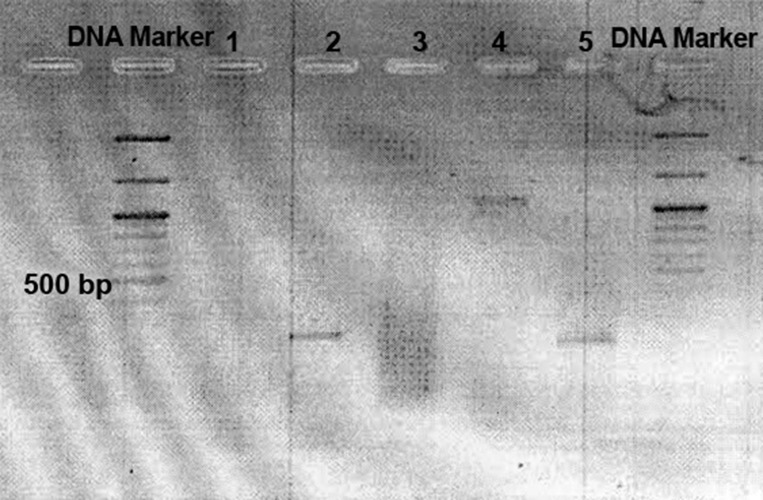
Using RT-PCR, both DMP1 and Pax9 genes were expressed by HBMSCs after 14 days proximity with rat odontogenic epithelium. According to the control group (BMSCs alone) RT-PCR, the result was negative (Fig 4).
Fig 4.
Inverted electrophoresis picture. 1. BMSCs did not express DMP1 gene when they were not in proximity to oral epithelium (as negative control), 2. Pax9 expression by BMSCs after proximity, 3. Extracted mRNA, 4. DMP1 expression of BMSCs after proximity, and 5. GAPDH expression by BMSCs without proximity (as positive control).
Discussion
It is well known that the interaction between inner enamel epithelium and dental papilla mesenchymal cells leads to odontoblastic and ameloblastic differentiation during tooth development. These specialized cells secrete specific mineralized materials (enamel and dentin). In the present study we replaced the epithelial cells derived from primary mouth of the rat with human BMSCs to investigate the odontogenic potential of human BMSCs. Expression of two odontogenic genes (Pax9 and DMP1) by human BMSCs, indicates odontogenic capacity of these cells. Similar studies used different animals including SD rat (26, 28), 1-CR rat and SD rat (34) as mesenchymal or epithelial cell sources. We used SD rat with respect to maintenance cost and animal size. Because the first histological signs of the rat tooth development as first Branchial arch thickening become evident at day 11, this study was designed based on 11 days old rat fetuses (4).
We seeded mesenchymal and epithelial cells near to a supposition line in a liquid medium without full interconnection. This method was used due to its simplicity and speed. Previous studies have used different methods. In some studies epithelial cells were centrifuged to get cell aggregation and cells were then seeded in a semi-solid medium near mesenchymal cells (28, 35). Other methods such as transferring mesenchymal cells into a translucent membrane and adding epithelial pieces after centrifuging mesenchymal cells (19), using collagen scaffold and nano hydroxyl apatite (36), using poliglicolic acid (PGA) and poly coglycolic copolymer (PLGA) scaffolds (2, 37), silk fibron (1, 38, 39) and using a mixture of mesenchymal and epithelial cells without attention to their integration (34), were also performed.
Odontogenic potential of odontogenic and non-odontogenic stem cells has been reported in the literature (14, 15, 17, 25-27). Ohazama et al. reported that among the three kinds of non-odontogenin mesenchymal stem cells (neural stem cells, BMSCs and Embryonic stem cells), BMSCs had the best odontogenic results (26). Nakatsuka and colleagues reported differentiation of odontogenic stem cells into mature dental structures such as dentin, pulp, cementum and PDL, but could not differentiate into ameloblast (11). However, two other studies have demonstrated BMSC differentiation into ameloblast-like cells (28, 29). These cells have different origin from dental follicle stem cells (which are more differentiated): BMSCs originate from mesoderm whereas the origin of dental follicle stem cells is the neural crest (24). Comparing with DPSCs and SCAPs (two odontogenic stem cells), BMSCs have different multipotentiality profile. Some studies have reported a greater tendency to odontogenic/ osteogenic differentiation in BMSCs (40, 41), while others suggest that DPSCs and SCAPs have more tendency to differentiate into odontogenic/osteogenic lineages (41-45).
We used the BMSCs due to its good results as well as its availability and being more accessible compared with odontogenic stem cells. Because of delayed expression of DMP1compared with Pax9 we used DMP1 as a negative control knowing that loss of DMP1 expression leads to loss of Pax9 expression. We used GAPDH gene as positive control since it is expressed in all cell types and can be used as an index to measure the expression level of other genes by comparing the intensity of electrophoresis bands. This makes the results semiquantitative and more accurate.
Expression of different genes have been investigated by different authors as odontogenic differentiation indicators including Amelogenin, Ameloblastin, dentin phosphoprotein (DPP), dentin sialoprotein (DSP) (28), Pax9 and DMP (25) and Lhx7 and Msx1 (26, 37). Zhang et al. reported that in the absence of Pax9 tooth development will stop at bud stage (5). According to Li et al. DSPP expression indicates odontogenic differentiation and DMP1 is expressed in the time interval between Pax9 and DMPP expression (25). In this study we demonstrated that proximity of human BMSCs with rat epithelium leads to induction of Pax9 and DMP1 by BMSCs. This finding is consistent with the finding of Li et al. who reported Pax9, DMP1 and DSPP expression by rat BMSCs (25).
Conclusion
Expression of Pax9 and DMP1 by human BMSCs in the proximity of rat odontogenic epithelium in liquid medium is suggestive of odontogenic potential of these cells. Although this is the early stage of using stem cells for the production of tooth structures, it has the potential of natural tooth becoming the replacement of the lost teeth.
More studies on the HBMSCs are recommended to establish odontoblast cells with the ability to secrete dentin matrix and develop the ectodermal component of the tooth bud to produce ameloblasts and enamel.
Acknowledgments
We would like to thank Iran Transplant Productions Bank for providing HBMSCs. This study was financially supported by Research Center of Dental Faculty, Shahid Beheshti Medical Sciences University. There is no conflict of interest in this study.
References
- 1.D’souza RN. Development of the tooth and it’s supporting tissues. In: Nanci A, editor. Tencate’s oral histology: development, structure and function. 7th ed. Missouri: Mosby Elsevier; 2008. pp. 79–108. [Google Scholar]
- 2.Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83(7):523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 3.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81(10):695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 4.Zarb GA. Edentulous predicament. In: Zarb GA, Bolender CL, editors. Prosthodontic treatment for edentulous patients, complete denture and implant supported prosthese. 12th ed. Missouri: Mosby Elsevier; 2004. pp. 18–33. [Google Scholar]
- 5.Zhang YD, Chen Z, Song YQ, Liu C, Chen YP. Making a tooth: Growth factors, transcription factors and stem cells. Cell Res. 2005;15(5):301–316. doi: 10.1038/sj.cr.7290299. [DOI] [PubMed] [Google Scholar]
- 6.Yen AH, Sharpe PT. Regeneration of teeth using stem cell-based tissue engineering. Expert Opin Biol Ther. 2006;6(1):9–16. doi: 10.1517/14712598.6.1.9. [DOI] [PubMed] [Google Scholar]
- 7.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dental Traumatol. 2001;17(4):185–187. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 8.Wei X, Ling J, Wu L, Liu L, Xiao Y. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–708. doi: 10.1016/j.joen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/ dentin regeneration and bioroot engineering. J Endod. 2008;34(6):645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen BJ, Gilissen C, Roelofs H, Schaap-Oziemlak A, Veltman JA, Raymakers RA, et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010;19(4):481–490. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsuka R, Nozaki T, Uemura Y, Matsuoka Y, Sasaki Y, Shinohara M, et al. 5-Aza-2'-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch Oral Biol. 2010;55(5):350–355. doi: 10.1016/j.archoralbio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30(4):196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hui JH, Ouyang HW, Hutmacher DW, Goh JC, Lee EH. Mesenchymal stem cells in musculoskeletal tissue engineering: a review of recent advances in National University of Singapore. Ann Acad Med Singapore. 2005;34(2):206–212. [PubMed] [Google Scholar]
- 14.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 15.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34(8):962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 20.Abe S, Yamaguchi S, Amagasa T. Multilineage cells from apical pulp of human tooth with immature apex. Oral Sci Int. 2007;4:455–458. [Google Scholar]
- 21.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 22.Song S, Song S, Zhang H, Cuevas J, Sanchez-Ramos J. Comparison of neuron-like cells derived from bone marrow stem cells to those differentiated from adult brain neural stem cells. Stem Cells Dev. 2007;16(5):747–756. doi: 10.1089/scd.2007.0027. [DOI] [PubMed] [Google Scholar]
- 23.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds AJ, Jahoda CA. Cultured human and rat tooth papilla cells induce hair follicle regeneration and fiber growth. Differentiation . 2004;72(9-10):566–575. doi: 10.1111/j.1432-0436.2004.07209010.x. [DOI] [PubMed] [Google Scholar]
- 25.Li ZY, Chen L, Liu L, Lin YF, Li SW, Tian WD. Odontogenic potential of bone marrow mesenchymal stem cells. J Oral Maxillofac Surg. 2007;65(3):494–500. doi: 10.1016/j.joms.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cellbased tissue engineering of murine teeth. J Dent Res. 2004;83(7):518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 27.Modino SA, Sharpe PT. Tissue engineering of teeth using adult stem cells. Arch Oral Biol. 2005;50(2):255–258. doi: 10.1016/j.archoralbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haïkel Y, et al. Bone marrow cells can give rise to ameloblast- like cells. J Dent Res. 2006;85(5):416–421. doi: 10.1177/154405910608500504. [DOI] [PubMed] [Google Scholar]
- 29.Keller L, Kuchler-Bopp S, Mendoza SA, Poliard A, Lesot H. Tooth engineering: searching for dental mesenchymal cells sources. Front Physiol. 2011;2: 7–7. doi: 10.3389/fphys.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado S, Al N, Sire JY. Evolutionary analysis of DMP1. Eur Cell Mater. 2007;14 (Suppl 2):10–10. [Google Scholar]
- 31.Mari-Buyé N, Garreta E, Semino C, Colominas C, Borrós S. Developing 3D printing of hydroxyapatite-like scaffold: from precursor to structure. Eur Cell Mater. 2007;14(Suppl 1):78–78. [Google Scholar]
- 32.Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36(5):781–789. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Minor Metab. 2004;22(5):430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, et al. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99(8):465–474. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]
- 35.Hu B, Nadiri A, Bopp-Küchler S, Perrin-Schmitt F, Lesot H. Dental epithelial histomorphogenesis in vitro. J Dent Res. 2005;84(6):521–525. doi: 10.1177/154405910508400607. [DOI] [PubMed] [Google Scholar]
- 36.Li ZY, wang HM, Bao TW. Tissue engineering teeth using bone marrow mesenchymal stem cells with odontogenic potential compounding collagen/nano-hdroxyapatite composite scaffold. Int J Oral Max Surg. 2009;38(5):525–526. [Google Scholar]
- 37.Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, et al. Tissue-engineered hybrid tooth and bone tissue engineering. Tissue Eng. 2005;11(9-10):1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 38.Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC. Bioengineered dental tissues grown in the rat jaw. J Dent Res. 2008;87(8):745–750. doi: 10.1177/154405910808700811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu WP, Zhang W, Asrican R, Kim HJ, Kaplan DL, Yelick PC. Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng Part A. 2008;14(4):549–557. doi: 10.1089/tea.2007.0227. [DOI] [PubMed] [Google Scholar]
- 40.Barnett ML. Molecular approaches to oral therapeutics: dentistry in the next millennium? J Dent Res. 1997;76(6):1236–1238. doi: 10.1177/00220345970760060101. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15(1):13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 42.Yamada Y, Fujimoto A, Ito A, Yoshimi R, Ueda M. Cluster analysis and gene expression profiles: a cDNA microarray system-based comparison between humandental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials. 2006;27(20):3766–3781. doi: 10.1016/j.biomaterials.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82(12):976–981. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- 44.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs.those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hara K, Yamada Y, Nakamura S, Umemura E, Ito K, Ueda M. Potential characteristics of stem cells from human exfoliated deciduous teeth compared with bone marrowderived mesenchymal stem cells for mineralized tissueforming cell biology. J Endod. 2012;37(12):1647–1652. doi: 10.1016/j.joen.2011.08.023. [DOI] [PubMed] [Google Scholar]