Abstract
Studies on various compounds of inorganic phosphate, as well as on organic phosphate added by post-translational phosphorylation of proteins, all demonstrate a central role for phosphate in biomineralization processes. Inorganic polyphosphates are chains of orthophosphates linked by phosphoanhydride bonds that can be up to hundreds of orthophosphates in length. The role of polyphosphates in mammalian systems, where they are ubiquitous in cells, tissues and bodily fluids, and are at particularly high levels in osteoblasts, is not well understood. In cell-free systems, polyphosphates inhibit hydroxyapatite nucleation, crystal formation and growth, and solubility. In animal studies, polyphosphate injections inhibit induced ectopic calcification. While recent work has proposed an integrated view of polyphosphate function in bone, little experimental data for bone are available. Here we show in osteoblast cultures producing an abundant collagenous matrix that normally shows robust mineralization, that two polyphosphates (PolyP5 and PolyP65, polyphosphates of 5 and 65 phosphate residues in length) are potent mineralization inhibitors. Twelve-day MC3T3-E1 osteoblast cultures with added ascorbic acid (for collagen matrix assembly) and β-glycerophosphate (a source of phosphate for mineralization) were treated with either PolyP5 or PolyP65. Von Kossa staining and calcium quantification revealed that mineralization was inhibited in a dose-dependent manner by both polyphosphates, with complete mineralization inhibition at 10 μM PolyP. Cell proliferation and collagen assembly were unaffected by polyphosphate treatment, indicating that polyphosphate inhibition of mineralization results not from cell and matrix effects but from direct inhibition of mineralization. This was confirmed by showing that PolyP5 and PolyP65 bound to synthetic hydroxyapatite in a concentration-dependent manner. Tissue-nonspecific alkaline phosphatase (TNAP, ALPL) efficiently hydrolyzed the two PolyPs as measured by Pi release. Importantly, at the concentrations of polyphosphates used in this study which inhibited bone cell culture mineralization, the polyphosphates competitively saturated TNAP, thus potentially interfering with its ability to hydrolyze mineralization-inhibiting pyrophosphate (PPi) and mineralizing-promoting β-glycerophosphate (in cell culture). In the biological setting, TNAP may regulate mineralization by shielding the essential inhibitory substrate pyrophosphate from TNAP degradation, and in the same process, delay the release of phosphate from this source. In conclusion, the inhibition of mineralization by polyphosphates is shown to occur via direct binding to apatitic mineral and by mixed inhibition of TNAP.
Keywords: polyphosphates, phosphate, bone, biomineralization, extracellular matrix, osteoblasts
Introduction
Amongst many other biological functions, phosphate (Pi) has long been known to be important in skeletal and dental mineralization, where orthophosphate (PO43−) and calcium mineral ions combine to form the apatitic mineral phase – a carbonate-substituted form of hydroxyapatite – found in bones and teeth. Also important in bone is phosphate's cell signaling function during osteoblast differentiation [1, 2], and its ability to modulate gene expression relevant to mineralization processes [3]. Furthermore, other phosphorylated small-molecule and protein species such as pyrophosphate [4], inositol hexakisphosphate [5], and some phosphoproteins (and fragments/peptides derived from them) [4, 6, 7] act to regulate mineralization by directly interacting with hydroxyapatite crystal surfaces, as well as by indirectly modulating levels or activities of other mineralization inhibitors. Phosphate can also be found as inorganic linear polyphosphates – long polymers of three to several hundred orthophosphates bound by energy-rich phosphoanhydride bonds [8–10]. Polyphosphates have been extensively studied in prokaryotic and lower eukaryotic organisms [10], however, only more recently have they been found in a variety mammalian cells and tissue fluids [11–16], but their biological roles at these sites have not been elucidated. Based on the biochemical properties of polyphosphates, and their predominant localization within nuclei, mitochondria and the plasma membrane, several roles for polyphosphates including phosphate storage [17], cation sequestration [18], counter-ion actions for basic amino acids [19], regulation of intracellular adenylate nucleotides [20], and modulation of cellular responses to stress have been proposed [21].
Although polyphosphates are now known to be ubiquitous in mammalian cells and tissue fluids, they have been reported to be in particularly high amounts in human osteoblast-like cells, suggesting a potential role in bone biology [12, 15, 22] – additionally supported by the fact that these cells also show exopolyphosphatase activity [12, 15]. Recently, electron-dense, calcium-and phosphate-rich polyphosphate granules have been identified at sites of bone resorption and in calcified cartilage [22]. These authors propose that where concentrations of phosphate and calcium ions exceed supersaturation limits for hydroxyapatite, polyphosphates can act as an ion storage depot to locally decrease the concentration of mineral ions (with polyphosphates in turn sequestering calcium) to levels below supersaturation. It is also proposed by these authors that when bone mineralization proceeds, enzymes such as tissue-nonspecific alkaline phosphatase (TNAP, ALPL) release the ions from the polyphosphate-calcium complex to be used in apatitic crystal formation and growth. Despite this possible scenario that would necessarily occur outside the bone cells, it is not yet known how polyphosphate granules observed intracellularly are exported or formed in the extracellular matrix where mineralization occurs.
Early studies performed in vivo over 40 years ago to determine the effects of polyphosphate treatment on mineralization in animals showed that subcutaneous injection of polyphosphates (then called Graham's salt, a heterogeneous mixture of long-chain linear and cyclic polyphosphates) could inhibit induced models of aortic calcification [23–25]. The mechanism underlying this inhibition was proposed at that time to be the same as that of pyrophosphate inhibition of mineralization – another inhibitory phosphate derivative – occurring by direct binding of these molecules to hydroxyapatite crystal surfaces to prevent crystal growth. Other studies performed in vitro were consistent with this mineralization-inhibiting function, where polyphosphates varying in length from having 2–27 phosphates were shown to act as strong stabilizers of amorphous calcium phosphate and strong inhibitors hydroxyapatite crystal growth with equal or better potency than pyrophosphate (PPi) [26, 27]. The key role that PPi plays in regulating physiologic and pathologic mineralization has now been well studied both in animal models and in humans, where the Pi/PPi ratio is known to be a critical determinant in allowing for the progression of mineralization [28, 29]. Despite abundant data showing mineralization inhibition in various systems, it is surprising that previous suggestions stating that longer phosphate polymers might act in much the same manner as PPi [26] has not lead to further bone studies and a determination of a potential role for polyphosphates in inhibiting bone mineralization. Given that polyphosphates are found at relatively high concentrations in osteoblast-like cells [12], and that they potently inhibit hydroxyapatite formation in cell-free crystal growth assays [26, 30], we aimed to determine whether polyphosphates could inhibit extracellular matrix mineralization in a well-established osteoblast cell culture model.
Materials and methods
Reagents
Sodium phosphate glass type 5 (PolyP5) and type 65 (PolyP65) were obtained from Sigma-Aldrich (St. Louis, MO, USA), and have been certified to be 5 and 65 phosphates in chain length. All other reagents were obtained from Invitrogen (Carlsbad, CA, USA) unless otherwise specified.
Cell culture
MC3T3-E1 (clone 14) pre-osteoblast cells – a generous gift from Dr. Renny T. Franceschi (University of Michigan) – were maintained in complete media (minimum essential medium supplemented with 10% fetal bovine serum (Hyclone, Waltham, MA, USA), 0.225 mM L-aspartic acid, 2 mM L-glutamine, and 1X penicillin/streptomycin). To induce cell differentiation, cells were plated at 50,000 cells/cm2 in tissue culture plates and allowed to adhere for 24 h at 37°C/5% CO2 in a humidified incubator before treatment with osteogenic media (complete media supplemented with 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate) containing either PolyP5 or PolyP65. Media was changed every 48 h and assayed as described below.
Mineralization quantification
After 12 days of culture in differentiation media, mineralization in osteoblast cultures was visualized by von Kossa staining using a 5% silver nitrate (w/v) incubation followed by exposure of the culture dishes to bright light. Quantification of mineral was determined by measuring the insoluble calcium content in each culture as follows. Mineralized osteoblast cultures were rinsed with phosphate-buffered saline (PBS), decalcified using 0.5 N hydrochloric acid (HCl), and the supernatant was assayed for calcium using a calcium assay kit (Sekisui Diagnostics, Charlottetown, PEI, Canada). Calcium content was normalized to protein content, as measured by the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA).
Histology
Mineralizing MC3T3-E1 cultures were treated with 10 μM PolyP5 or PolyP65 at the indicated days. After 12 days of culture, cells were washed twice in PBS and fixed in 1% glutaraldehyde for 15 min at room temperature. The cell layer was then scraped into a glass vial and washed three times in PBS, before dehydration in a graded ethanol series followed by embedding in LR White acrylic resin (London Resin Company, Berkshire, UK) which was polymerized at 55°C for 2 days. The embedded cell layer was sectioned at 0.5 μm using a Leica EM UC6 ultramicrotome (Leica, Wetzlar, Germany) and stained for mineral by von Kossa staining (5% silver nitrate) followed by counterstaining with toluidine blue. The sections were mounted on glass slides, coverslipped, and viewed and photographed using a Leitz DMRBE (Leica, Wetzlar, Germany) optical microscope equipped with a 3-CCD Sony DXC-950 camera (Sony, Tokyo, Japan).
Hydroxyapatite binding assay
A 20 mg/ml stock hydroxyapatite bead (5 μm average diameter, Berkeley Advanced Biomaterials, Berkeley, CA, USA) slurry was generated by washing the beads as received from the supplier three times in Tris-buffered saline, pH 7.0 (TBS), and then reconstituting in TBS. The indicated amount of hydroxyapatite (HAP) was aliquoted into tubes, and spun down at 2100 × g. The supernatant was removed and 0.5 mM PolyP5 or PolyP65 in TBS was added to each tube. The slurry was incubated with end-over-end rotation for 2 h, spun down, and supernatants were removed. This was performed twice before hydrolyzing the supernatants with HCl (final concentration 1 N HCl) at 90°C for 10 min. Hydrolyzed supernatants were assayed for phosphate by mixing 25 μl supernatant with 200 μl 50% acetone/25% 10 M H2SO4/25% 10 mM ammonium molybdate and 20 μl 1 M citric acid. The reaction was mixed for 2–3 min and the absorbance was read spectrophotometrically at 405 nm.
HAP-bound polyphosphate enzymatic assay
In 50 mM Tris (pH 7.2), 20 μg of either PolyP5 or PolyP65 were adsorbed onto 2 mg synthetic HAP beads for 2 h at room temperature. Adsorbed polyphosphate was then incubated with 0.5 units of bovine kidney alkaline phosphatase (Sigma Aldrich, St. Louis, MO, USA) for 1 h at 37°C; phosphate release was measured colorimetrically with a spectrophotometer using the ammonium molybdate method, and compared to polyphosphate alone (without HAP beads). Polyphosphate bound to HAP was verified using the method outlined above.
Ionized calcium measurements
Osteogenic media was combined with PolyP5 or PolyP65 at the indicated concentrations and allowed to stand for 1 h at room temperature. The media was then mixed in a 1:1 ratio with a constant complexation buffer (0.23 M ammonia/0.01 M ammonium chloride/ 0.4 M potassium chloride/ 0.2 M iminodiacetic acid), as adapted from Morais et al [31]. Free calcium ions were measured using a calcium ion-sensing electrode (Fisher Scientific, Waltham, MA, USA). Calcium measurements were compared to calcium chloride standards.
Cell viability
MC3T3-E1 osteoblast cells were cultured in complete media containing either 10 μM PolyP5 or PolyP65. On days 0, 1, 3, 6, 9 and 12 of culture, the media was removed, replaced with 0.25 mg/ml thiazolyl blue tetrazolium bromide, and incubated at 37°C/5% CO2 for 3 h. The cells were then lysed by the addition of dimethyl sulfoxide, and the absorbance in each well was read spectrophotometrically at 565 nm.
Collagen quantification
Mineralizing MC3T3-E1 osteoblast cultures were washed three times with PBS, and stained with Bouin's fluid (70% v/v saturated aqueous picric acid/9.25% v/v formaldehyde/5% v/v glacial acetic acid) for 1 h at room temperature. Excess Bouin's fluid was removed and the cultures were rinsed with water before staining with Picrosirius Red solution (1 mg/mL Direct Red (Sigma Aldrich, St. Louis, MO, USA) in saturated aqueous picric acid) for 1 h at room temperature. Excess stain was removed by washing twice with 0.01 N HCl, before drying and imaging using a flatbed scanner. The stain was then removed using 0.1 N sodium hydroxide and quantified colorimetrically by reading absorbance at 562 nm in a spectrophotometer. Collagen type I from calf skin (Sigma Aldrich, St. Louis, MO, USA) was used as a standard.
Alkaline phosphatase activity assay
Alkaline phosphatase activity was measured by washing MC3T3-E1 osteoblast cultures twice with PBS, and harvesting the cells in 10 mM Tris, pH 7.4/0.2% IGEPAL. Cell lysates were obtained by sonication, and alkaline phosphatase activity was measured using SIGMAFAST™ p-nitrophenyl phosphate tablets (Sigma Aldrich, St. Louis, MO, USA), as per the manufacturer's instructions. Alkaline phosphatase from bovine intestinal mucosa (Sigma Aldrich, St. Louis, MO, USA) was used as a standard. Alkaline phosphatase activity was normalized to protein content.
Semi-quantitative polymerase chain reaction (PCR)
RNA from 12-day mineralizing osteoblast cultures was extracted using Trizol®, according to the manufacturer's instructions. RNA was treated with DNaseI (New England Biolabs, Ipswich, MA, USA), and then analyzed for gene expression of bone sialoprotein (Bsp), collagen type I (Colla1), osteocalcin (Ocn), tissue-nonspecific alkaline phosphatase (Alpl), ectonucleotide pyrophosphatase/phosphodiesterase 1 (Enpp1), progressive ankylosis protein (Ank), and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) using the SuperScript™III One-Step RT-PCR System with Platinum® Taq DNA Polymerase and the primers listed in Table 1.
Table 1.
Primers used for semi-quantitative RT-PCR
| Gene Name | Forward Primer (5' to 3') | Reverse Primer (5' to 3') |
|---|---|---|
| Bsp | AACAATCCGTGCCACTCA | GGAGGGGGCTTCACTGAT |
| Colla1 | GAGGCATAAAGGGTCATCGTGG | CATTAGGCGCAGGAAGGTCAGC |
| Ocn | CTGGCCCTGGCTGCGCTCTGT | GGTCCTAAATAGTGATACCGTAGATGC |
| Opn | CTGCTAGTACACAAGCAGACA | CATGAGAAATTCGGAATTTCAG |
| Alpl | GGGGACATGCAGTATGAGTT | GGCCTGGTAGTTGTTGTGAG |
| Enpp1 | GCTTTGAAAGGACGTTCAGC | GTATGTGCCGGACTTGACCT |
| Ankh | TTACTCGCCTTCCCTGAAGA | AAAACCCGCTTTCCAAAACT |
| Gapdh | TTGGCAAAGTGGAGATTGTTG | TTCAGCTCTGGGATGACCTT |
Expression and preparation of test enzymes
Expression plasmids containing a secreted epitope-tagged TNAP were transfected into COS-1 cells for transient expression [32]. The medium was replaced to Opti-MEM® media 24 h later, and the serum-free media containing secreted proteins was collected 60 h after electroporation. In order to keep the TNAP metalized and active during dialysis (to remove phosphate), and for subsequent assays, the conditioned medium was dialyzed against TBS containing 1 mM MgCl2 and 20 μM ZnCl2 and filtrated through a 2 μm cellulose acetate filter.
Polyphosphate assay and enzymatic measurements
All kinetic measurements were performed in triplicate at 25°C. For the measurement of relative catalytic activity, 3 μl (0.7 μg/ml) of a secreted epitope-tagged TNAP was used [32]. Recombinant TNAP was incubated with different concentration of polyphosphates in 50 mM Tris-HCl buffer, pH 7.2 for 1 h with and without the addition of 0.5 mM of MgCl2. For determination of the kinetic parameters, the Pi released was measured spectrophotometrically at 650 nm using the PiColorLock Gold (Innova Biosciences, Cambridge, UK). Competition studies were carried out in the presence of 0.5 mM of p-nitrophenyl phosphate (pNPP) in 50 mM Tris-HCl buffer, pH 7.2. p-nitrophenol, the product of the reaction, was read at 405 nm in the presence of different polyphosphate concentrations. In addition, double-reciprocal plots were constructed for the competition at pH 9.8 (1 M diethanolamine buffer, containing 20 μM and 1 mM MgCl2) upon assessment of pNPP (0.2–3.0 mM) hydrolysis for each PolyP5 and PolyP65 concentration (0–2.0 mM). The presence of PPi contamination in PolyP5 and PolyP65 (15 μM) was measured in comparison to a standard PPi solution (15 μM). The concentration of PPi was determined by adsorption on activated charcoal of UDP-D-[6-3H] glucose (Amersham Pharmacia, Piscataway, NJ, USA) from the reaction product 6-phospho [6-3H] gluconate, as described previously [33].
Results
Polyphosphates inhibit osteoblast culture mineralization
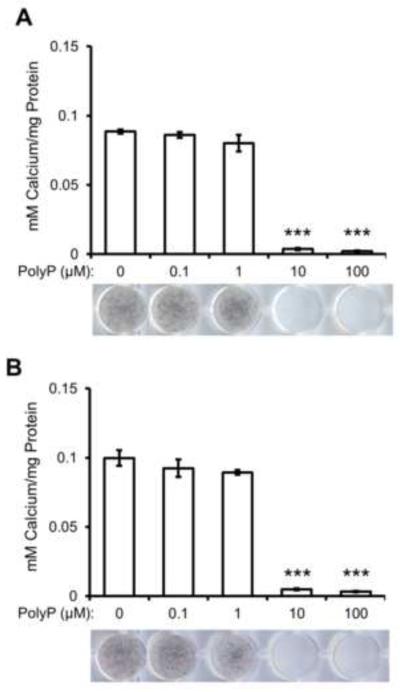
In this study, we sought to determine if polyphosphates had an effect on osteoblast culture mineralization. MC3T3-E1 osteoblast cells were cultured with osteogenic media in the presence of PolyP5 or Poly65 for 12 days. Figure 1 shows that both PolyP5 (Fig. 1A) and PolyP65 (Fig. 1B) dose-dependently decrease the amount of mineralization in 12-day mineralizing osteoblast cultures. Polyphosphate preparations were found to have some PPi contamination; in order to ensure that the observed inhibition of mineralization was caused by PolyP5 and PolyP65, and not the contaminating PPi, the calculated concentration of PPi in each case were added to mineralizing osteoblast cultures alone for 12 days, and mineralization was not inhibited (data not shown).
Figure 1. Effect of polyphosphates on MC3T3-E1 osteoblast culture mineralization.
MC3T3-E1 osteoblast cells cultured in 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate, and (A) PolyP5 or (B) PolyP65 for 12 days. Mineralization in the culture plates was visualized by von Kossa staining (black) and mineralization was quantified using ImageJ. Both PolyPs dose-dependently inhibit mineralization. Data are presented as mean values ± SE. ***p<0.001, Student's t-test relative to the 0 μM PolyP treatment.
Osteoblast culture mineralization is inhibited by direct binding of PolyPs to hydroxyapatite
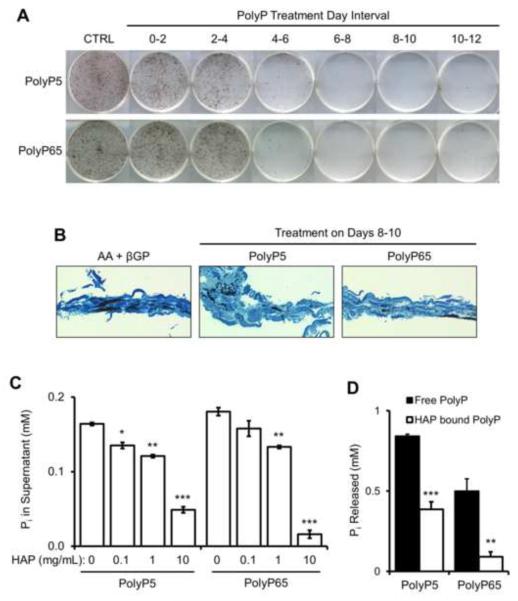
Previous studies in our lab have shown that days 0–6 of culture are involved with osteoblast differentiation and extracellular matrix production, while days 6–12 are predominantly involved with extracellular matrix mineralization. To identify at which stage of osteoblast differentiation and mineralization polyphosphates affect most strongly, mineralizing osteoblast cells were treated with 10 μM PolyP5 or PolyP65 at 2-day intervals over the 12-day course of mineralization. Cultures were allowed to progress to day 12, where mineralization was analyzed using von Kossa staining. Figure 2A shows that mineralization was mostly affected when polyphosphates were added in the 6–12 day interval. Smaller mineralization foci can be seen in histological sections of these cultures (Fig. 2B), suggesting that the mode of inhibition is direct binding to apatite mineral. To determine if this was possible, synthetic hydroxyapatite beads were incubated with a solution of 0.5 mM PolyP5 or PolyP65, the beads were then spun down in a centrifuge, and the supernatant was measured for PolyP. With increasing amounts of HAP, Figure 2C shows that the solutions of PolyP5 and PolyP65 were being depleted, indicating that they were bound to the HAP. Since the addition of polyphosphates inhibited osteoblast culture mineralization even when given only in the 4–6-day interval, we sought to determine if polyphosphates once bound to HAP could still be hydrolyzed by TNAP. In Figure 2D, both PolyP5 and PolyP65 bound to HAP and exposed to TNAP showed significantly less release of Pi than from the unbound polyphosphates.
Figure 2. Direct inhibition action of polyphosphate by binding to hydroxyapatite mineral.
MC3T3-E1 osteoblasts were cultured in osteogenic media and treated with either 10 μM PolyP5 or PolyP65 for the indicated 2-day intervals. After 12 days of culture, mineral was stained in the plates (A) by the von Kossa method (black), or (B) the cell/matrix layer was embedded in LR White acrylic resin, sectioned perpendicular to the culture dish plane, and sections were stained with the von Kossa method followed by counterstaining with toluidine blue. By both methods, less mineral is seen after PolyP treatment starting at days 4–6 when the matrix starts to mineralize; earlier treatments when matrix is being first secreted and assembled had little effect. (C) Direct binding of PolyPs to hydroxyapatite (HAP) was determined by incubating the indicated concentrations of synthetic HAP beads in either 0.5 mM PolyP5 or PolyP65 solutions, and quantifying the remaining concentration of PolyP in the supernatant (by acid hydrolysis and phosphate quantification). Increasing amounts of HAP clearly leads to increasing depletion of PolyPs from solution. Data are presented as mean values ± SE. *p<0.05, **p<0.01, ***p<0.001 Student's t-test relative to 0 mg/ml HAP. (D) When 20 μg PolyP are bound to 2 mg HAP, hydrolysis of the polyphosphates by TNAP is less than that of polyphosphate alone. Adsorption of polyphosphate onto HAP was verified by supernatant acid hydrolysis followed by phosphate quantification. Data are presented as mean values ± SE. **p<0.01, ***p<0.001 Student's t-test relative to free PolyP.
Calcium chelation by polyphosphate does not affect mineralization inhibition
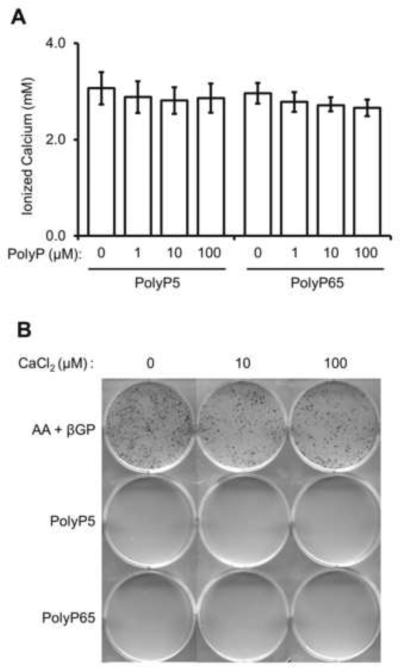
As polyphosphate is a known chelator of calcium ions, we investigated if this effect was enough to inhibit mineralization in osteoblast cultures. Firstly, the amount of free, ionized calcium was measured in osteogenic media after the addition of PolyPs. Figure 3A shows that increasing levels of both PolyP5 and PolyP65 were able to chelate small amounts of ionized calcium (calcium chelation ranged from 100–300 μM). To determine if this depletion of calcium was sufficient to inhibit mineralization, additional calcium – in the form of calcium chloride – was added to mineralizing osteoblast cultures treated with 10 μM PolyP5 or PolyP65. Figure 3B shows that the addition of calcium in PolyP-treated osteoblast cultures did not allow mineralization to occur, indicating that this does not contribute to the inhibition. Furthermore, we examined whether adding calcium to PolyPs prior to addition to the cultures – to generate calcium-polyphosphate salt – would have any effect on mineralization, and we found that unlike a previous study using SaOS-2 cells [34], calcium-polyphosphate salt was also able to inhibit mineralization (data not shown). The disparity in these observations is likely attributable to the different cell lines used, as SaOS-2 cells have been known to show high alkaline phosphatase activity [35], but a less developed (if any) assembled extracellular matrix.
Figure 3. Effect of polyphosphates on calcium chelation.
(A) Various concentrations of PolyP5 and PolyP65 were added to osteogenic media and ionized calcium content was measured using an ion-sensing calcium electrode. Minimal amounts of calcium are chelated by the polyphosphates. Data are presented as mean values ± SE. (B) MC3T3-E1 osteoblast cultures were treated with 10 μM PolyP5 or PolyP65 with the addition of the indicated concentrations of calcium chloride. After 12 days of treatment, culture plates were analyzed for mineralization by von Kossa staining (black). No effect from the additional calcium is seen for the inhibition of mineralization by the two polyphosphates.
Polyphosphates do not affect osteoblast differentiation or proliferation
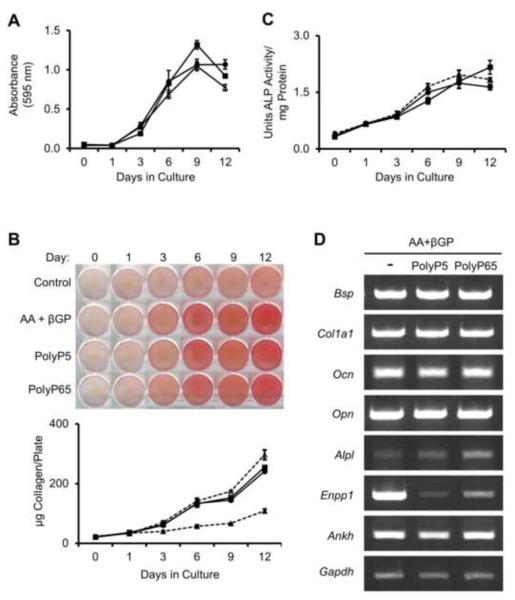
A lack of mineralization can be due to several factors including cell toxicity or altered expression of genes critical to osteoblast differentiation and mineralization. To investigate if PolyPs could affect osteoblast cell proliferation, MC3T3-E1 cells were assayed for cell viability using thiazolyl blue tetrazolium bromide over the course of 12 days. As shown in Figure 4A, cell viability in the presence of 10 μM PolyP5 or PolyP65 was not significantly different from that seen in cultures not treated with polyphosphate. Furthermore, collagen production (Fig. 4B) and alkaline phosphatase activity (Fig. 4C), two markers of osteoblast differentiation, were unaltered in polyphosphate-treated MC3T3-E1 cultures. Gene expression analysis, as shown in Fig. 4D, also show that osteogenic markers were unchanged; however, there was a marked decrease in Enpp1 expression, and an increase in Alpl expression when treated with PolyP65.
Figure 4. Effect of polyphosphates on osteoblast proliferation and differentiation.
MC3T3-E1 cultures with added 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate (Δ) were treated with either 10 μM PolyP5 (●) or PolyP65 (■) for 12 days. (A) Cell proliferation was measured by the thiazolyl blue tetrazolium bromide assay, and (B) collagen deposition was visualized and quantified using picrosirius red staining (red), and compared to control MC3T3-E1 cultures without osteogenic media (▲). (C) Alkaline phosphatase activity was measured spectrophotometrically using p-nitrophenyl phosphate as a colorimetric substrate. Data are presented as mean values ± SE, and show essentially no change in extracellular matrix production and alkaline phosphatase activity after PolyP treatment. (D) MC3T3-E1 cultures were treated as indicated with PolyP for 12 days, and RNA was extracted for RT-PCR analysis of the osteoblast differentiation markers bone sialoprotein (Bsp), collagen I (Col1a1), osteocalcin (Ocn), and osteopontin (Opn), as well as for the phosphate/pyrophosphate-regulating genes alkaline phosphatase (Alpl), ectonucleotide pyrophosphatase/phosphodiesterase 1 (Enpp1), and ankylosis progressive homolog (Ankh). Expression of all marker genes was relative to Gapdh expression. Again, except for a reduction in the expression of Enpp1, these marker genes related to matrix production and mineralization remain essentially unchanged after PolyP treatment.
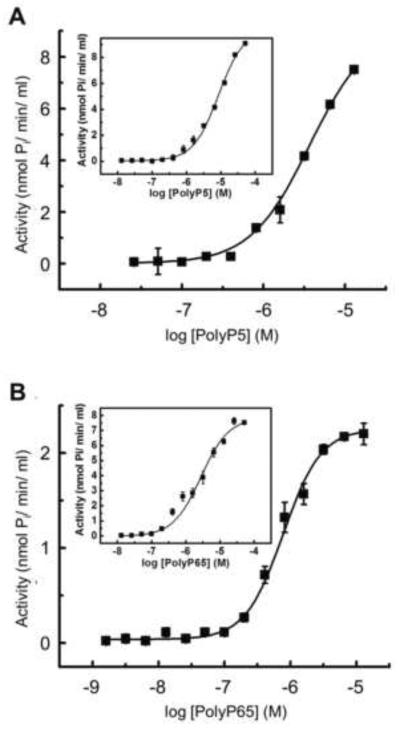
Kinetic analysis of polyphosphate hydrolysis catalyzed by recombinant TNAP
To understand the role of TNAP in PolyP-mediated mineralization inhibition, we investigated the kinetic properties of PolyP5 and PolyP65 as a function of chain length and at physiological pH (pH 7.2). TNAP was able to individually and linearly hydrolyze PolyP5, PolyP65 and the contaminant PPi over a time interval of 3 h (data not shown) although at 1 h of incubation very little hydrolysis of PPi was detected (data not shown). To assure a correct kinetic measurement of PolyP5 and PolyP65 degradation, all the kinetic experiments were performed with a 1 h incubation. Analysis of the reactions showed that recombinant TNAP hydrolyzed both PolyPs under physiologic conditions (Fig. 5). In the absence of added divalent metals, a plateau was easily reached between 10–100 μM of PolyP65, but saturation was not fully achieved for PolyP5 in this concentration range. A better catalytic efficiency was suggested for the long-chain PolyP, as indicated by the lower Km of 0.8 μM and 3.7 μM for PolyP65 and PolyP5, respectively (Fig. 5). Since TNAP activity is positively modulated by Mg2+, we also performed the same analysis in the presence of 0.5 mM MgCl2. This treatment enhanced the maximal activity for hydrolysis of PolyP65, whereas it also decreased affinity as observed by a 3–4 fold increase in Km values (8.6 and 2.8 μM for PolyP5 and PolyP65, respectively) (insets in Fig. 5). Given that hydrolysis of PolyPs by TNAP generates more Pi as TNAP levels are increased, Pi levels in the cultures should be increased with the addition of PolyP, yet inhibition of mineralization still occurs, underscoring the potency of the PolyP inhibition.
Figure 5. Kinetic analysis of polyphosphate hydrolysis catalyzed by recombinant TNAP.
Kinetic activity of TNAP on (A) PolyP5 and (B) PolyP65 was measured based on released Pi as described in Material and Methods. Shown are the reaction velocities (activity) as a function of the log of substrate concentration. Insets show kinetic analysis in the presence of 0.5 mM of MgCl2 for (A) PolyP5 and (B) PolyP65. Data are presented as means ± SE.
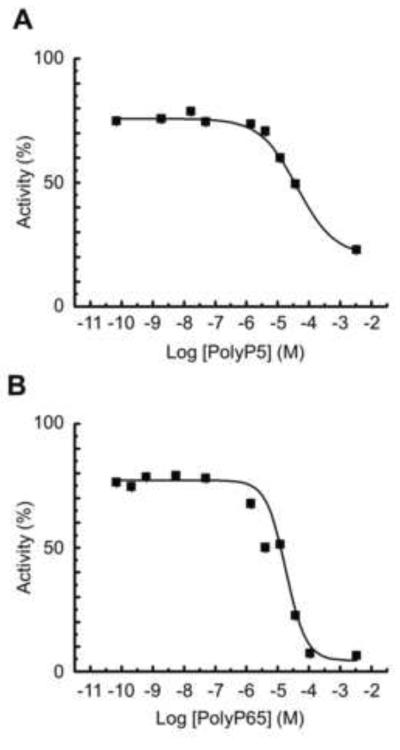
Inhibition of degradation of pNPP phosphate by polyphosphates
To further investigate the interaction of PolyP5 and PolyP65 with the TNAP catalytic site, competition studies were done with pNPP as a substrate, both at pH 7.2 and 9.8. Figure 6 shows the dose-dependent inhibition at pH 7.2 of pNPP hydrolysis by PolyP5 and PolyP65, with a calculated Ki of 2.9 μM and 0.9 μM respectively, in the absence of added Mg2+. These values were very similar to the Kms determined in the absence of added Mg2+ as reported above, i.e. they confirm that PolyP5 and PolyP65 are substrates for TNAP and that they can competitively interfere with known TNAP substrates. Competitive substrate inhibition was further illustrated at pH 9.8 (data not shown), where both PolyP5 and PolyP65 could dose-dependently compete with 0.25 mM pNPP. However, double reciprocal plots of enzyme activity versus the PolyP concentration at this pH revealed a more complex type of inhibition, i.e. a mixed type, illustrating that PolyP5 and PolyP65, in addition to being able to bind to the active site of TNAP, also bind in a noncompetitive manner to the enzyme-substrate complex. These findings, not surprisingly, illustrate that long polyphosphate substrates can interact with binding sites outside the catalytic pocket, a finding which was not investigated in greater detail in the present study.
Figure 6. Inhibition of pNPP degradation by TNAP after addition of PolyP5 (A) and PolyP65 (B).
Reaction mixtures containing Tris-HCl pH 7.2 and 0.5 mM pNPP were assayed for alkaline phosphatase activity in the presence of different concentrations of inhibitory PolyP5 and PolyP65. Data are presented as mean values ± SE.
Discussion
Pyrophosphate has already been well-established as an inorganic mineralization inhibitor in bone [4, 26]; however, it is surprising that longer-chain inorganic phosphates – such as polyphosphates – have not been studied in greater detail given their reported abundant presence in bone cells [12, 15, 22]. Here, we show that short-chain (PolyP5) and long-chain (PolyP65) polyphosphates are potent inhibitors of osteoblast culture mineralization, and that this inhibition is caused in part by direct binding of the polyphosphates to the apatitic mineral with no changes occurring in osteoblast differentiation and proliferation. This observation is consistent with previous cell-free studies [26, 27, 36], and animal studies [23–25], which show polyphosphates to be inhibitors of hydroxyapatite growth. However, these results differ from more recent work showing polyphosphates and calcium-polyphosphate complexes as inducers of osteoblast differentiation and mineralization [34, 37, 38]. These recent studies revealed that the addition of polyphosphates to bone cell cultures acted as a phosphate source [37, 38] and increased gene expression of osteoblast differentiation markers. Furthermore, as part of that work, it was proposed that polyphosphates could physically stabilize fibroblast growth factor 2, and activate a fibroblast growth factor pathway, thus stimulating osteoblastic differentiation [39, 40]. However, unlike the current study, a very high concentration of polyphosphate (100-fold higher) was used which suggests different effects for different concentrations of this extracellular phosphate species. Indeed, it has previously been reported that polyphosphate concentrations in osteoblast-lineage cells are higher in undifferentiated cells than in mature osteoblasts, an observation which may indicate a regulatory role for polyphosphates throughout the differentiation process [15].
In prokaryotes, polyphosphate catalysis occurs via endopolyphosphatases which cleave long-chain polyphosphates into shorter-chain polyphosphates, or by exopolyphosphatases which remove terminal phosphates from long-chain polyphosphates [10, 15]. In mammalian cells, only exopolyphosphatase activity has been detected in osteoblast cell lysates [12], although there are some reports of endopolyphosphatase activity in brain [41] and synovial fluid [15]. In the current literature, the only exopolyphosphatase activity reported for bone has been that attributed to tissue-nonspecific alkaline phosphatase [22]; however, this is the first time that the kinetics of polyphosphate hydrolysis by TNAP have been defined. In the present study, we observed that the addition of Mg2+ enhanced the activity of TNAP on PolyP65, but not on PolyP5, which could be explained by the chelating properties of polyphosphates. In the absence of added MgCl2, PolyP65 more potently chelates traces of divalent ions in solution, reducing the maximal enzymatic reaction rate. In the presence of excess (0.5 mM) Mg2+, TNAP is allosterically activated and the substrate is chelated, leading to a higher enzyme activity (most pronounced with PolyP65) and a slightly compromised active site positioning, as reflected by a mildly elevated Km. In addition, we have also investigated the effects of divalent ions on enzyme function in greater detail for the TNAP-mediated hydrolysis of the two PolyPs in a dose-dependent manner (data not shown). Collectively, these results are aligned with a previous study involving mammalian intestinal alkaline phosphatase [42], which demonstrated that MgCl2, CaCl2 and Zn-acetate all interfere with polyphosphate catalysis, further illustrating the importance of metal ion chelation during hydrolysis of polyphosphates by TNAP. In osteoblast culture mineralization studies, which commonly add as a supplement an organic phosphate source such as β-glycerophosphate, TNAP expression and activity are critical for its hydrolysis and for the removal of inhibitory pyrophosphate [4]. However, in the present study we show that osteoblast culture mineralization is fully inhibited by addition of 10 μM PolyP5 or PolyP65, and that importantly, at this concentration, both PolyPs competitively saturated TNAP, therefore potentially interfering with its ability to hydrolyze mineralization-inhibiting pyrophosphate and mineralizing-promoting β-glycerophosphate. Thus, polyphosphates in the biological setting may regulate mineralization by shielding the essential inhibitory substrate pyrophosphate from TNAP degradation, and in the same process, delay the release of phosphate from this source. In addition, the competitive inhibition of TNAP by polyphosphates could cause an increase in extracellular PPi, which has been shown to modulate the expression of Enpp1, Ank, Alpl and Opn seemingly to compensate for increased levels of this mineralization inhibitor [4, 43]. For Enpp1, this is consistent with the present study which demonstrates that polyphosphate treatment of MC3T3-E1 cultures increases Alpl expression and decreases Enpp1 expression.
The current paradigm of phosphate regulation of bone mineralization is based on the ratio of phosphate-to-pyrophosphate (Pi:PPi) as being critical to either inhibiting or promoting hydroxyapatite crystal growth in the extracellular matrix. Indeed, long ago it was proposed that not only could pyrophosphate be a physiologic determinant of mineralization regulator, but that other longer-chain phosphate species might also act as inhibitors [15, 25, 26]. Considering the model in which the Pi:PPi ratio is a key determinant regulating mineralization, it now seems that these longer-chain species may also play a regulatory role, and that the importance of a Pi:PPi:PolyP composite ratio needs to be considered. In this scenario, Pi would act as a signaling molecule and a source of Pi for hydroxyapatite formation, PPi as an inhibitor that can be quickly and efficiently removed (by TNAP) and to also produce Pi, and PolyPs as longer-lasting inhibitors that act by direct binding to mineral and also by shielding PPi from TNAP but which then can also serve as a rich Pi source as they are completely cleaved to lose their inhibitory activity. These activities may be modulated by decreasing available polyphosphate for TNAP cleavage through (protective) polyphosphate binding to mineral – an observation also seen with PPi [7]. Since long-chain polyphosphates are abundantly produced early in osteoblast differentiation, inhibition of mineralization can be sustained over time (despite high TNAP levels in this cell type) until an appropriate threshold is reached coincident with the required organic matrix maturation necessary for physiologic mineralization. Understanding such an interplay between these determinants might be advantageously harnessed for tissue engineering strategies where mineralization fronts are required to create robust implantable constructs for orthopedic applications, as has been recently reported [44].
Although mammalian cells have only been reported to accumulate polyphosphates intracellularly in nuclei, mitochondria, plasma membrane and dense-granule organelles [11, 14] – subcellular locations that suggest a role in energy storage or transport [45] – a number of recent studies suggest a role for extracellular polyphosphates, particularly in bone biology [22, 34, 37–39]. The present study, performed with a commonly used osteoblast culture model that produces an abundant extracellular matrix and that has a temporo-spatial mineralization pattern similar to bone, provides new data for polyphosphates in the inhibition of extracellular matrix mineralization. Prokaryotic polyphosphate anabolism has been well-characterized, and several enzymes have been identified that synthesize polyphosphate including the polyphosphate kinases (PPKs) – the principal enzymes responsible for their synthesis in bacteria [10], however a homologue has yet to be identified in higher eukaryotes [46]. Evidence for extracellular polyphosphate production in bacteria has been shown by PPK localization and activity on the outer plasma membrane of N. meningitidis [47]. Although an enzyme for polyphosphate biosynthesis has not been identified in higher eukaryotes, it is clear that polyphosphates are an abundant molecular species in mammalian cells [12] and in the extracellular matrix of bone [22]. One possibility for the latter localization may be via a dietary route as is seen for another phosphate species – inositol hexakisphosphate (IP6, phytate) – also commonly found in mammalian cells and fluids [48–51]. While IP6 likewise can be synthesized intracellularly de novo by mammalian cells as part of several metabolic pathways [52], in plants where it is in very high concentrations, it serves as a major phosphate storage molecule [53]. In animal studies, increasing the consumption of IP6-rich foods modulates IP6 levels in tissues and body fluids [51], which in turn inhibited vascular calcification and urolithiasis in animal models of pathologic calcification [54]. Consistent with this, in humans, increased dietary IP6 associates with reduced incidence of kidney stones [55]. Related to bone physiology, IP6 has recently been shown to be an inhibitor of osteoblast culture mineralization [5]. Given that subcutaneous injections of polyphosphates can reduce ectopic calcification [23–25], it seems possible that excessive consumption of polyphosphate-rich foods might have an effect on extracellular polyphosphate levels and subsequently on physiologic mineralization.
Conclusions
Polyphosphates are shown here to be potent inhibitors of MC3T3-E1 osteoblast culture extracellular matrix mineralization. This inhibition of mineralization by polyphosphates is shown to occur via direct binding to apatitic mineral and by mixed inhibition of TNAP – the enzyme that cleaves inhibitory pyrophosphate and generates free phosphate for hydroxyapatite crystal growth.
Acknowledgements
This work was funded by research grants from the Canadian Institutes of Health Research (CIHR) to MDM, and grants DE12889 and AR53102 from the National Institutes of Health, USA. MDM is a member of the FRQ-S Reseau de Recherche en Santé Buccodentaire et Osseuse and the FRQ-S Groupe de Recherche Axé sur la Structure des Protéines, and the Jamson T.N. Wong Laboratories in Calcified Tissue Research of the McGill Centre for Bone and Periodontal Research. BH and TKM performed the experiments in this study, and all authors participated in drafting the manuscript and revising it critically for important intellectual content, and all authors approved the final version of the submitted manuscript.
Abbreviations
- Pi
phosphate
- PO43−
orthophosphate
- PolyP
polyphosphate
- PPi
pyrophosphate
- TNAP, ALPL
Tissue-nonspecific alkaline phosphatase
- PBS
phosphate-buffered saline
- HCl
hydrochloric acid
- TBS
Tris-buffered saline
- HAP
hydroxyapatite
- pNPP
p-nitrophenyl phosphate
- PPK
polyphosphate kinase
- IP6
inositol hexakisphosphate
- SE
standard error of the mean
References
- [1].Tenenbaum HC. Role of Organic Phosphate in Mineralization of Bone in vitro. J Dent Res. 1981;60:1586–1589. doi: 10.1177/0022034581060003S0801. [DOI] [PubMed] [Google Scholar]
- [2].Beck GR., Jr. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. 2003;90:234–43. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- [3].Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282:15872–83. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- [5].Addison WN, McKee MD. Inositol hexakisphosphate inhibits mineralization of MC3T3-E1 osteoblast cultures. Bone. 2010;46:1100–7. doi: 10.1016/j.bone.2010.01.367. [DOI] [PubMed] [Google Scholar]
- [6].Addison WN, Masica DL, Gray JJ, McKee MD. Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res. 2010;25:695–705. doi: 10.1359/jbmr.090832. [DOI] [PubMed] [Google Scholar]
- [7].Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–49. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- [8].Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–6. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- [10].Kulaev I, Vagabov V, Kulakovskaya T. The Biochemistry of Inorganic Polyphosphates. 2nd ed. John Wiley & Sons, Ltd; West Sussex, England: 2004. [Google Scholar]
- [11].Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–22. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- [12].Leyhausen G, Lorenz B, Zhu H, Geurtsen W, Bohnensack R, Muller WE, Schroder HC. Inorganic polyphosphate in human osteoblast-like cells. J Bone Miner Res. 1998;13:803–12. doi: 10.1359/jbmr.1998.13.5.803. [DOI] [PubMed] [Google Scholar]
- [13].Jimenez-Nuñez MD, Moreno-Sanchez D, Hernandez-Ruiz L, Benítez-Rondán A, Ramos-Amaya A, Rodríguez-Bayona B, Medina F, Brieva JA, Ruiz FA. Myeloma cells contain high levels of inorganic polyphosphate which is associated with nucleolar transcription. Haematologica. 2012;97:1264–1271. doi: 10.3324/haematol.2011.051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–7. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- [15].Schroder HC, Kurz L, Muller WE, Lorenz B. Polyphosphate in bone. Biochemistry (Mosc) 2000;65:296–303. [PubMed] [Google Scholar]
- [16].Gabel NW, Thomas V. Evidence for the occurrence and distribution of inorganic polyphosphates in vertebrate tissues. J Neurochem. 1971;18:1229–42. doi: 10.1111/j.1471-4159.1971.tb00222.x. [DOI] [PubMed] [Google Scholar]
- [17].Harold FM. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966;30:772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dunn T, Gable K, Beeler T. Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem. 1994;269:7273–7278. [PubMed] [Google Scholar]
- [19].Cramer CL, Davis RH. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J Biol Chem. 1984;259:5152–7. [PubMed] [Google Scholar]
- [20].Lorenz B, Batel R, Bachinski N, Muller WE, Schroder HC. Purification and characterization of two exopolyphosphatases from the marine sponge Tethya lyncurium. Biochim Biophys Acta. 1995;1245:17–28. doi: 10.1016/0304-4165(95)00067-l. [DOI] [PubMed] [Google Scholar]
- [21].Lorenz B, Münkner J, Oliveira MP, Kuusksalu A, Leitão JM, Müller WEG, Schröder HC. Changes in metabolism of inorganic polyphosphate in rat tissues and human cells during development and apoptosis. Biochim Biophys Acta. 1997;1335:51–60. doi: 10.1016/s0304-4165(96)00121-3. [DOI] [PubMed] [Google Scholar]
- [22].Omelon S, Georgiou J, Henneman ZJ, Wise LM, Sukhu B, Hunt T, Wynnyckyj C, Holmyard D, Bielecki R, Grynpas MD. Control of vertebrate skeletal mineralization by polyphosphates. PLoS One. 2009;4:e5634. doi: 10.1371/journal.pone.0005634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Casey PA, Casey G, Fleisch H, Fussel RG. The effect of polyphloretin phosphate, polyoestradiol phosphate, a diphosphonate and a polyphosphate on calcification induced by dihydrotachysterol in skin, aorta and kidney of rats. Experientia. 1972;28:137–8. doi: 10.1007/BF01935714. [DOI] [PubMed] [Google Scholar]
- [24].Schibler D, Russell RG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci. 1968;35:363–72. [PubMed] [Google Scholar]
- [25].Fleisch H, Schibler D, Maerki J, Frossard I. Inhibition of aortic calcification by means of pyrophosphate and polyphosphates. Nature. 1965;207:1300–1. doi: 10.1038/2071300b0. [DOI] [PubMed] [Google Scholar]
- [26].Fleish H, Neuman WF. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961;200:1296–300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- [27].Francis MD. The inhibition of calcium hydroxypatite crystal growth by polyphosphonates and polyphosphates. Calcif Tissue Res. 1969;3:151–62. doi: 10.1007/BF02058658. [DOI] [PubMed] [Google Scholar]
- [28].Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- [29].Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millán JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Omelon S, Grynpas M. Polyphosphates affect biological apatite nucleation. Cells Tissues Organs. 2011;194:171–5. doi: 10.1159/000324845. [DOI] [PubMed] [Google Scholar]
- [31].Morais S, Carvalho GS, Sousa JP. Potentiometric determination of total and ionized calcium in osteoblast-like cell culture medium. Electroanalysis. 1996;8:1174–1178. [Google Scholar]
- [32].Kozlenkov A, Le Du MH, Cuniasse P, Ny T, Hoylaerts MF, Millan JL. Residues determining the binding specificity of uncompetitive inhibitors to tissue-nonspecific alkaline phosphatase. J Bone Miner Res. 2004;19:1862–72. doi: 10.1359/JBMR.040608. [DOI] [PubMed] [Google Scholar]
- [33].Johnson K, Vaingankar S, Chen Y, Moffa A, Goldring MB, Sano K, Jin-Hua P, Sali A, Goding J, Terkeltaub R. Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes. Arthritis Rheum. 1999;42:1986–97. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [34].Muller WEG, Wang X, Diehl-Seifert B, Kropf K, Schlossmacher U, Lieberwirth I, Glasser G, Wiens M, Schroder HC. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011;7:2661–71. doi: 10.1016/j.actbio.2011.03.007. [DOI] [PubMed] [Google Scholar]
- [35].Murray E, Provvedini D, Curran D, Catherwood B, Sussman H, Manolagas S. Characterization of a human osteoblastic osteosarcoma cell line (SAOS-2) with high bone alkaline phosphatase activity. J Bone Miner Res. 1987;2:231–8. doi: 10.1002/jbmr.5650020310. [DOI] [PubMed] [Google Scholar]
- [36].Amjad Z. The influence of polyphosphates, phosphonates, and poly(carboxylic acids) on the crystal growth of hydroxyapatite. Langmuir. 1987;3:1063–1069. [Google Scholar]
- [37].Kawazoe Y, Shiba T, Nakamura R, Mizuno A, Tsutsumi K, Uematsu T, Yamaoka M, Shindoh M, Kohgo T. Induction of calcification in MC3T3-E1 cells by inorganic polyphosphate. J Dent Res. 2004;83:613–8. doi: 10.1177/154405910408300806. [DOI] [PubMed] [Google Scholar]
- [38].Hacchou Y, Uematsu T, Ueda O, Usui Y, Uematsu S, Takahashi M, Uchihashi T, Kawazoe Y, Shiba T, Kurihara S, Yamaoka M, Furusawa K. Inorganic polyphosphate: a possible stimulant of bone formation. J Dent Res. 2007;86:893–7. doi: 10.1177/154405910708600917. [DOI] [PubMed] [Google Scholar]
- [39].Kawazoe Y, Katoh S, Onodera Y, Kohgo T, Shindoh M, Shiba T. Activation of the FGF signaling pathway and subsequent induction of mesenchymal stem cell differentiation by inorganic polyphosphate. Int J Biol Sci. 2008;4:37–47. doi: 10.7150/ijbs.4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shiba T, Nishimura D, Kawazoe Y, Onodera Y, Tsutsumi K, Nakamura R, Ohshiro M. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J Biol Chem. 2003;278:26788–92. doi: 10.1074/jbc.M303468200. [DOI] [PubMed] [Google Scholar]
- [41].Kumble KD, Kornberg A. Endopolyphosphatases for long chain inorganic polyphosphate in yeast and mammals. J Biol Chem. 1996;271:27146–51. doi: 10.1074/jbc.271.43.27146. [DOI] [PubMed] [Google Scholar]
- [42].Lorenz B, Schroder HC. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim Biophys Acta. 2001;1547:254–61. doi: 10.1016/s0167-4838(01)00193-5. [DOI] [PubMed] [Google Scholar]
- [43].Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].St-Pierre J-P, Pilliar RM, Grynpas MD, Kandel RA. Calcification of cartilage formed in vitro on calcium polyphosphate bone substitutes is regulated by inorganic polyphosphate. Acta Biomater. 2010;6:3302–9. doi: 10.1016/j.actbio.2010.02.033. [DOI] [PubMed] [Google Scholar]
- [45].Pavlov E, Aschar-Sobbi R, Campanella M, Turner RJ, Gomez-Garcia MR, Abramov AY. Inorganic polyphosphate and energy metabolism in mammalian cells. J Biol Chem. 2010;285:9420–8. doi: 10.1074/jbc.M109.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rao NN, Gomez-Garcia MR, Kornberg A. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem. 2009;78:605–47. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- [47].Tinsley CR, Manjula BN, Gotschlich EC. Purification and characterization of polyphosphate kinase from Neisseria meningitidis. Infect Immun. 1993;61:3703–3710. doi: 10.1128/iai.61.9.3703-3710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vallejo M, Jackson T, Lightman S, Hanley MR. Occurrence and extracellular actions of inositol pentakis- and hexakisphosphate in mammalian brain. Nature. 1987;330:656–8. doi: 10.1038/330656a0. [DOI] [PubMed] [Google Scholar]
- [49].Heslop JP, Irvine RF, Tashjian AH, Jr., Berridge MJ. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- [50].Szwergold BS, Graham RA, Brown TR. Observation of inositol pentakis- and hexakis-phosphates in mammalian tissues by 31P NMR. Biochem Biophys Res Commun. 1987;149:874–81. doi: 10.1016/0006-291x(87)90489-x. [DOI] [PubMed] [Google Scholar]
- [51].Grases F, Simonet BM, Vucenik I, Perello J, Prieto RM, Shamsuddin AM. Effects of exogenous inositol hexakisphosphate (InsP(6)) on the levels of InsP(6) and of inositol trisphosphate (InsP(3)) in malignant cells, tissues and biological fluids. Life Sci. 2002;71:1535–46. doi: 10.1016/s0024-3205(02)01927-6. [DOI] [PubMed] [Google Scholar]
- [52].Letcher AJ, Schell MJ, Irvine RF. Do mammals make all their own inositol hexakisphosphate? Biochem J. 2008;416:263–70. doi: 10.1042/BJ20081417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Raboy V. myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry. 2003;64:1033–43. doi: 10.1016/s0031-9422(03)00446-1. [DOI] [PubMed] [Google Scholar]
- [54].Grases F, Isern B, Sanchis P, Perello J, Torres JJ, Costa-Bauza A. Phytate acts as an inhibitor in formation of renal calculi. Front Biosci. 2007;12:2580–7. doi: 10.2741/2256. [DOI] [PubMed] [Google Scholar]
- [55].Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Arch Intern Med. 2004;164:885–91. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]