Abstract
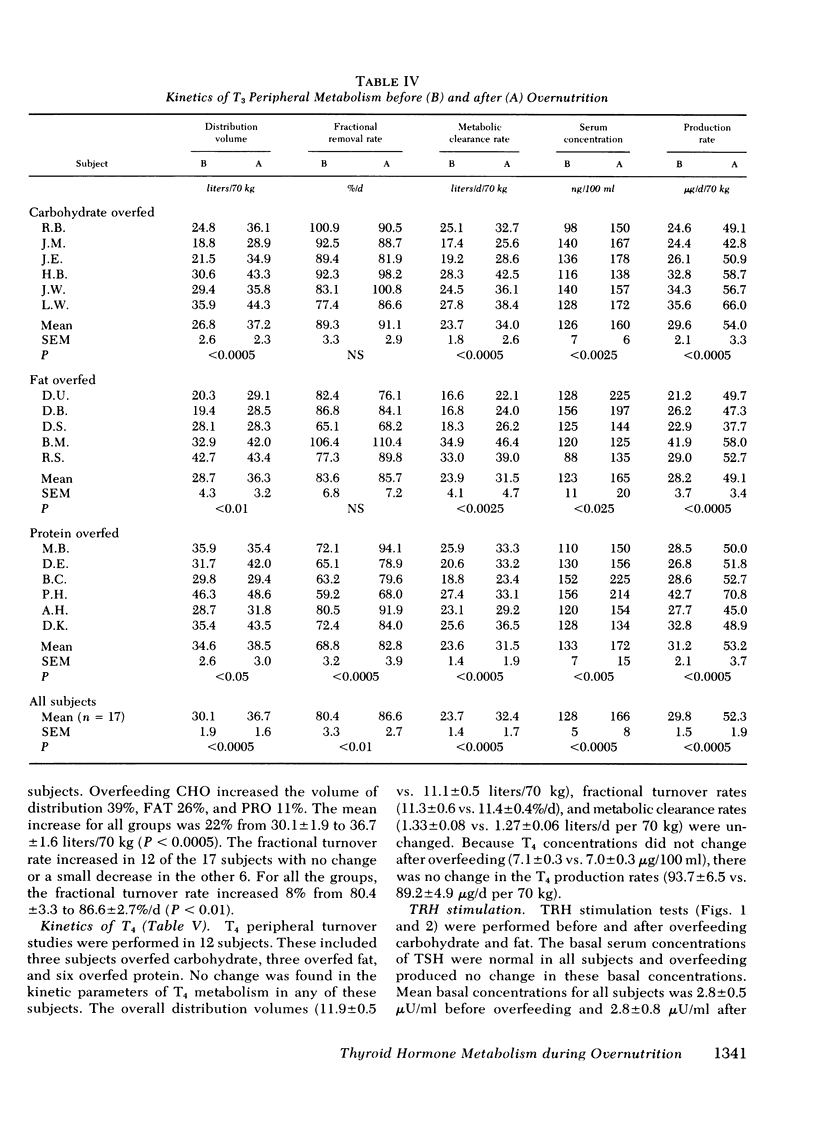
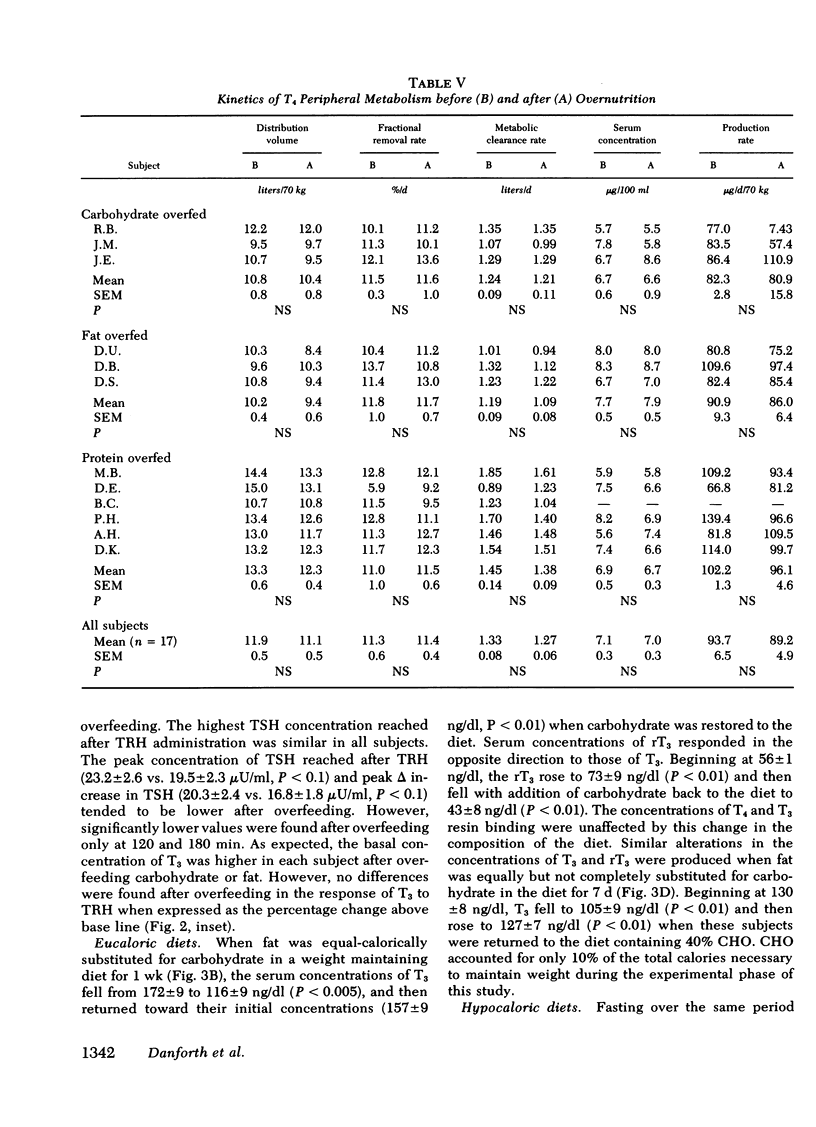
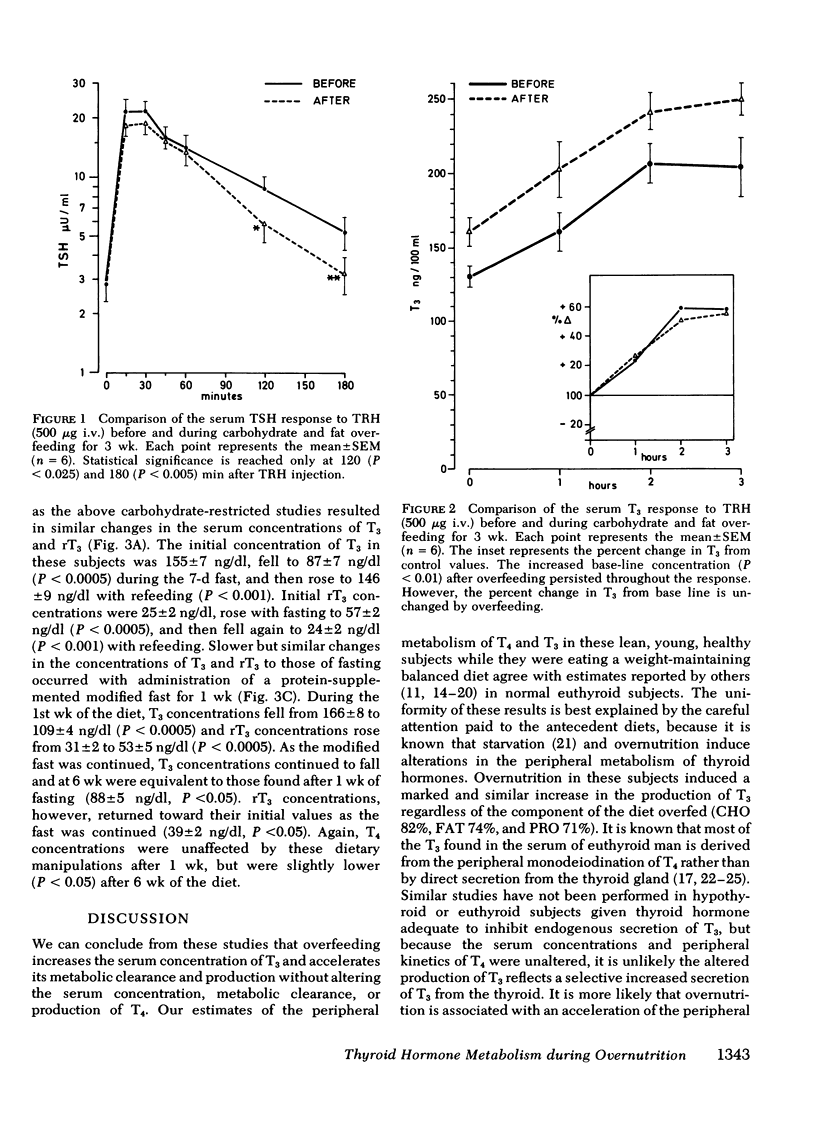
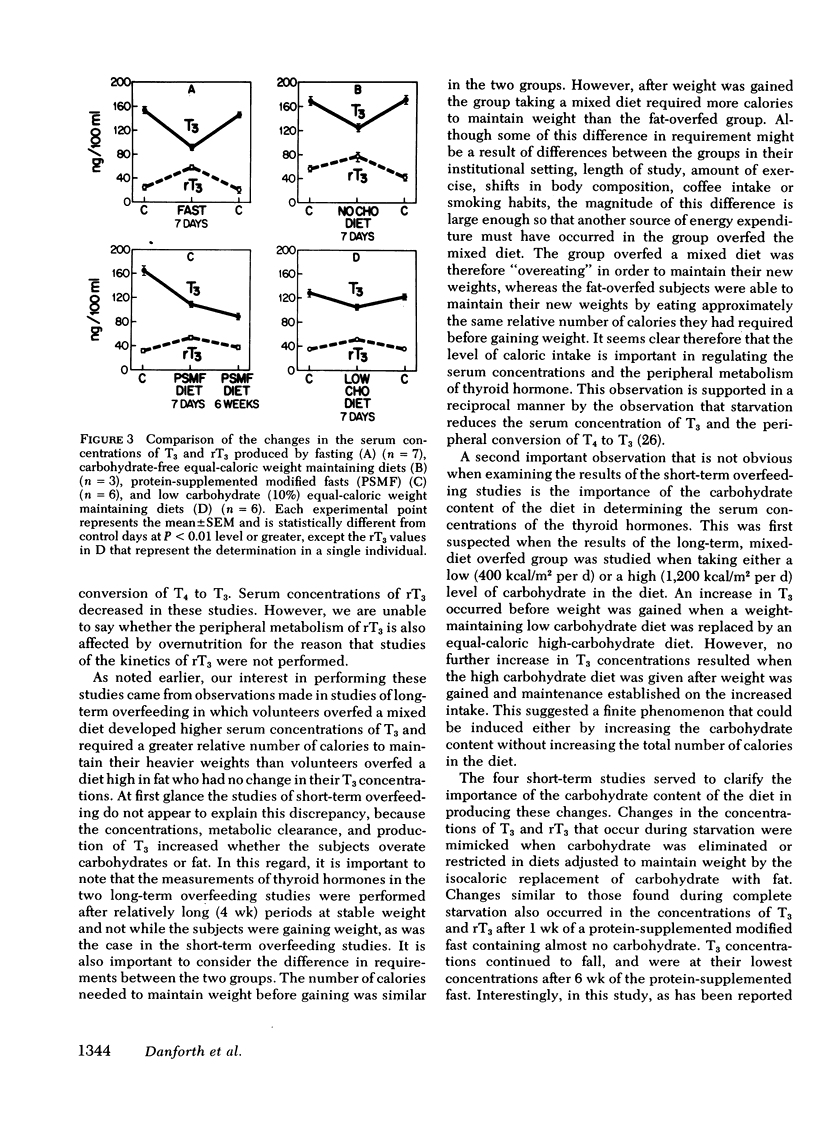
Diet-induced alterations in thyroid hormone concentrations have been found in studies of long-term (7 mo) overfeeding in man (the Vermont Study). In these studies of weight gain in normal weight volunteers, increased calories were required to maintain weight after gain over and above that predicted from their increased size. This was associated with increased concentrations of triiodothyronine (T3). No change in the caloric requirement to maintain weight or concentrations of T3 was found after long-term (3 mo) fat overfeeding. In studies of short-term overfeeding (3 wk) the serum concentrations of T3 and its metabolic clearance were increased, resulting in a marked increase in the production rate of T3 irrespective of the composition of the diet overfed (carbohydrate 29.6 +/- 2.1 to 54.0 +/- 3.3, fat 28.2 +/- 3.7 to 49.1 +/- 3.4, and protein 31.2 +/- 2.1 to 53.2 +/- 3.7 microgram/d per 70 kg). Thyroxine production was unaltered by overfeeding (93.7 +/- 6.5 vs. 89.2 +/- 4.9 microgram/d per 70 kg). It is still speculative whether these dietary-induced alterations in thyroid hormone metabolism are responsible for the simultaneously increased expenditure of energy in these subjects and therefore might represent an important physiological adaptation in times of caloric affluence. During the weight-maintenance phases of the long-term overfeeding studies, concentrations of T3 were increased when carbohydrate was isocalorically substituted for fat in the diet. In short-term studies the peripheral concentrations of T3 and reverse T3 found during fasting were mimicked in direction, if not in degree, with equal or hypocaloric diets restricted in carbohydrate were fed. It is apparent from these studies that the caloric content as well as the composition of the diet, specifically, the carbohydrate content, can be important factors in regulating the peripheral metabolism of thyroid hormones.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azizi F. Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism. 1978 Aug;27(8):935–942. doi: 10.1016/0026-0495(78)90137-3. [DOI] [PubMed] [Google Scholar]
- Balsam A., Ingbar S. H. Observations on the factors that control the generation of triiodothyronine from thyroxine in rat liver and the nature of the defect induced by fasting. J Clin Invest. 1979 Jun;63(6):1145–1156. doi: 10.1172/JCI109408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam A., Ingbar S. H. The influence of fasting, diabetes, and several pharmacological agents on the pathways of thyroxine metabolism in rat liver. J Clin Invest. 1978 Aug;62(2):415–424. doi: 10.1172/JCI109143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R., Zucchelli G. C., Giannessi D., Pilo A., Mariani G., Carpi A., Toni M. G. Evaluation of triiodothyronine (T3) kinetics in normal subjects, in hypothyroid, and hyperthyroid patients using specific antiserum for the determination of labeled T3 in plasma. J Clin Endocrinol Metab. 1978 Feb;46(2):203–214. doi: 10.1210/jcem-46-2-203. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman L. E., Vagenakis A., Downs P., Foster A. E., Sterling K., Ingbar S. H. Effects of replacement doses of sodium L-thyroxine on the peripheral metabolism of thyroxine and triiodothyronine in man. J Clin Invest. 1973 May;52(5):1010–1017. doi: 10.1172/JCI107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A., Sakoloff C., Staeheli V., Vallotton M. B., Ingbar S. H. Radioimmunoassays of 3,5,3'-triiodo-L-thyronine with and without a prior extraction step. Acta Endocrinol (Copenh) 1975 Sep;80(1):58–69. doi: 10.1530/acta.0.0800058. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Dimond R. C., Harvey G. S., O'Brian J. T., Georges L. P., Bruton J., Wright F. D., Wartofsky L. Glucose modulation of alterations in serum iodothyronine concentrations induced by fasting. Metabolism. 1979 Apr;28(4):291–299. doi: 10.1016/0026-0495(79)90098-2. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Lukes Y., Wright F. D., Wartofsky L. Reduction in hepatic triiodothyronine binding capacity induced by fasting. Endocrinology. 1977 Oct;101(4):1331–1334. doi: 10.1210/endo-101-4-1331. [DOI] [PubMed] [Google Scholar]
- Carlson H. E., Drenick E. J., Chopra I. J., Hershman J. M. Alterations in basal and TRH-stimulated serum levels of thyrotropin, prolactin, and thyroid hormones in starved obese men. J Clin Endocrinol Metab. 1977 Oct;45(4):707–713. doi: 10.1210/jcem-45-4-707. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R., Steinberg M., Searle G. L. Metabolic clearance rate of L-triiodothyronine in man: a comparison of results by single-injection and constant infusion methods. J Clin Endocrinol Metab. 1971 Oct;33(4):624–629. doi: 10.1210/jcem-33-4-624. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. An assessment of daily production and significance of thyroidal secretion of 3, 3', 5'-triiodothyronine (reverse T3) in man. J Clin Invest. 1976 Jul;58(1):32–40. doi: 10.1172/JCI108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J., Solomon D. H., Chopra U., Wu S. Y., Fisher D. A., Nakamura Y. Pathways of metabolism of thyroid hormones. Recent Prog Horm Res. 1978;34:521–567. doi: 10.1016/b978-0-12-571134-0.50018-1. [DOI] [PubMed] [Google Scholar]
- Croxson M. S., Hall T. D., Kletzky O. A., Jaramillo J. E., Nicoloff J. T. Decreased serum thyrotropin induced by fasting. J Clin Endocrinol Metab. 1977 Sep;45(3):560–568. doi: 10.1210/jcem-45-3-560. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Chopra I. J. Effect of carbohydrate and noncarbohydrate sources of calories on plasma 3,5,3'-triiodothyronine concentrations in man. J Clin Endocrinol Metab. 1979 Apr;48(4):577–581. doi: 10.1210/jcem-48-4-577. [DOI] [PubMed] [Google Scholar]
- DeGroot L. J., Coleoni A. H., Rue P. A., Seo H., Martino E., Refetoff S. Reduced nuclear triiodothyronine receptors in starvation-induced hypothyroidism. Biochem Biophys Res Commun. 1977 Nov 7;79(1):173–178. doi: 10.1016/0006-291x(77)90076-6. [DOI] [PubMed] [Google Scholar]
- Dimond R. C., Rosen S. W. Chromatographic differences between circulating and pituitary thyrotropins. J Clin Endocrinol Metab. 1974 Aug;39(2):316–325. doi: 10.1210/jcem-39-2-316. [DOI] [PubMed] [Google Scholar]
- Edozien J. C., Niehaus N., Mar M. H., Makoui T., Switzer B. R. Diet-hormone interrelationships in the rat. J Nutr. 1978 Nov;108(11):1767–1776. doi: 10.1093/jn/108.11.1767. [DOI] [PubMed] [Google Scholar]
- Glass A. R., Mellitt R., Burman K. D., Wartofsky L., Swerdloff R. S. Serum triiodothyronine in undernourished rats: dependence on dietary composition rather than total calorie or protein intake. Endocrinology. 1978 Jun;102(6):1925–1928. doi: 10.1210/endo-102-6-1925. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Hinerfeld L., Braverman L. E., Vagenakis A. G. The role of sulfhydryl groups on the impaired hepatic 3',3,5-triiodothyronine generation from thyroxine in the hypothyroid, starved, fetal, and neonatal rodent. J Clin Invest. 1979 Mar;63(3):516–524. doi: 10.1172/JCI109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Inada M., Kasagi K., Kurata S., Kazama Y., Takayama H., Torizuka K., Fukase M., Soma T. Estimation of thyroxine and triiodothyronine distribution and of the conversion rate of thyroxine to triiodothyronine in man. J Clin Invest. 1975 Jun;55(6):1337–1348. doi: 10.1172/JCI108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubiz W., Bigler A. H., Kumagai L. F., West C. D. Estimation of thyroxine production rates in non-steady states. J Clin Endocrinol Metab. 1972 Jun;34(6):1009–1015. doi: 10.1210/jcem-34-6-1009. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicod P., Burger A., Staeheli V., Vallotton M. B. Radioimmunoassay for 3,3',5'-triiodo-L-thyronine in unextracted serum: method and clinical results. J Clin Endocrinol Metab. 1976 May;42(5):823–829. doi: 10.1210/jcem-42-5-823. [DOI] [PubMed] [Google Scholar]
- Nicoloff J. T., Low J. C., Dussault J. H., Fisher D. A. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest. 1972 Mar;51(3):473–483. doi: 10.1172/JCI106835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M., Robbins D. C., Horton E. S., Sims E. A., Danforth E., Jr Changes in serum concentrations of 3,3',5'-triiodothyronine and 3,5,3'-triiodothyronine during prolonged moderate exercise. J Clin Endocrinol Metab. 1979 Aug;49(2):242–246. doi: 10.1210/jcem-49-2-242. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Determination of common parameters fo iodothyronine metabolism and distribution in man by noncompartmental analysis. J Clin Endocrinol Metab. 1975 Aug;41(2):319–324. doi: 10.1210/jcem-41-2-319. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Letter: (to the editor). Erratum: revised calculations of common parameters of iodothyronine metabolism and distribution by noncompartmental analysis. J Clin Endocrinol Metab. 1975 Dec;41(06):1172–1173. doi: 10.1210/jcem-41-6-1172. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman C. S., Chambers J. B., Jr, Read V. H. The extrathyroidal conversion rate of thyroxine to triiodothyronine in normal man. J Clin Invest. 1971 Jun;50(6):1187–1196. doi: 10.1172/JCI106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnay G. I., O'Brian J. T., Bush J., Vagenakis A. G., Azizi F., Arky R. A., Ingbar S. H., Braverman L. E. The effect of starvation on the concentration and binding of thyroxine and triiodothyronine in serum and on the response to TRH. J Clin Endocrinol Metab. 1974 Jul;39(1):191–194. doi: 10.1210/jcem-39-1-191. [DOI] [PubMed] [Google Scholar]
- Schussler G. C., Orlando J. Fasting decreases triiodothyronine receptor capacity. Science. 1978 Feb 10;199(4329):686–688. doi: 10.1126/science.204004. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Sims E. A., Danforth E., Jr, Horton E. S., Bray G. A., Glennon J. A., Salans L. B. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- Sims E. A. Experimental obesity, dietary-induced thermogenesis, and their clinical implications. Clin Endocrinol Metab. 1976 Jul;5(2):377–395. doi: 10.1016/s0300-595x(76)80027-8. [DOI] [PubMed] [Google Scholar]
- Spaulding S. W., Chopra I. J., Sherwin R. S., Lyall S. S. Effect of caloric restriction and dietary composition of serum T3 and reverse T3 in man. J Clin Endocrinol Metab. 1976 Jan;42(1):197–200. doi: 10.1210/jcem-42-1-197. [DOI] [PubMed] [Google Scholar]
- Stirling J. L., Stock M. J. Metabolic origins of thermogenesis induced by diet. Nature. 1968 Nov 23;220(5169):801–802. doi: 10.1038/220801a0. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulp O. L., Krupp P. P., Danforth E., Jr, Horton E. S. Characteristics of thyroid function in experimental protein malnutrition. J Nutr. 1979 Jul;109(7):1321–1332. doi: 10.1093/jn/109.7.1321. [DOI] [PubMed] [Google Scholar]
- Vagenakis A. G., Burger A., Portnary G. I., Rudolph M., O'Brian J. R., Azizi F., Arky R. A., Nicod P., Ingbar S. H., Braverman L. E. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975 Jul;41(1):191–194. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- Vagenakis A. G., Burger A., Portnary G. I., Rudolph M., O'Brian J. R., Azizi F., Arky R. A., Nicod P., Ingbar S. H., Braverman L. E. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975 Jul;41(1):191–194. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- Vagenakis A. G., Burger A., Portnary G. I., Rudolph M., O'Brian J. R., Azizi F., Arky R. A., Nicod P., Ingbar S. H., Braverman L. E. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975 Jul;41(1):191–194. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- Vinik A. I., Kalk W. J., McLaren H., Hendricks S., Pimstone B. L. Fasting blunts the TSH response to synthetic thyrotropin-releasing hormone (TRH). J Clin Endocrinol Metab. 1975 Mar;40(3):509–511. doi: 10.1210/jcem-40-3-509. [DOI] [PubMed] [Google Scholar]
- Wimpfheimer C., Saville E., Voirol M. J., Danforth E., Jr, Burger A. G. Starvation-induced decreased sensitivity of resting metabolic rate to triiodothyronine. Science. 1979 Sep 21;205(4412):1272–1273. doi: 10.1126/science.224460. [DOI] [PubMed] [Google Scholar]
- Woeber K. A., Sobel R. J., Ingbar S. H., Sterling K. The peripheral metabolism of triiodothyronine in normal subjects and in patients with hyperthyroidism. J Clin Invest. 1970 Apr;49(4):643–649. doi: 10.1172/JCI106275. [DOI] [PMC free article] [PubMed] [Google Scholar]


