Summary
The VKORC1 Asp36Tyr single nucleotide polymorphism (SNP) is one of the most promising predictors of high warfarin dose, but data on its population prevalence is incomplete. We determined the frequency of this SNP in participants from seven countries on four continents and investigated its effect on warfarin dose requirement. 1000 samples were analyzed to define the population prevalence of this SNP. Those samples included individuals from Egypt, Ghana, Sudan, Kenya, Saudi Arabia, Peru and African Americans from the United States. 206 Egyptian samples were then used to investigate the effect of this SNP on warfarin dose requirements. This SNP was most frequent among Kenyans and Sudanese, with a minor allele frequency (MAF) of 6% followed by Saudi Arabians and Egyptians with a MAF of 3% and 2.5%, respectively. It was not detected in West Africans, based on our data from Ghana, and a large cohort of African Americans. Egyptian carriers of the VKORC1 Tyr36 showed higher warfarin dose requirement (57.1±29.4 mg/week) than those with the Asp36Asp genotype (35.8±16.6 mg/week; P<0.03). In linear regression analysis, this SNP had the greatest effect size among the genetic factors (16.6 mg/week increase in dose per allele), and improved the warfarin dose variability explained in Egyptians (model R2 from 31% to 36.5%). The warfarin resistant VKORC1 Asp36Tyr appears to be confined to north-eastern Africa and nearby Middle-Eastern populations, but in those populations where it is present, it has a significant influence on warfarin dose requirement and the percent of warfarin dose variability that can be explained.
Keywords: Warfarin, Pharmacogenetics, VKORC1 Asp36Tyr, polymorphism, Egyptians
Introduction
Warfarin was first approved for clinical use in 1954, and since then, it has been the mainstay oral anticoagulant therapy for treatment and prevention of thromboembolic events. However, its narrow therapeutic index and wide inter-patient variability in dose requirements for a therapeutic warfarin effect make it an extremely challenging pharmacological agent to utilize clinically (1-3).
Numerous pharmacogenetic studies have been conducted with warfarin, and have shown that warfarin dose requirements, risk of bleeding and time to reach a stable warfarin dose are influenced by demographic factors (age, gender and ethnicity), clinical factors (smoking, concurrent medications, illness and diet) and genetic variables (4-7). In 2007, and again in 2010, the Food and Drug administration (FDA) updated the warfarin label with information on warfarin pharmacogenetics. The 2010 update included a dosing guidance based on genetic factors, specifically CYP2C9 and VKORC1 polymorphisms, which are strongly associated with warfarin dose requirements, with the variant alleles leading to lower warfarin dose (1, 8-11). The inclusion of the CYP2C9 and VKORC1 warfarin sensitivity polymorphisms with clinical factors explain more than 50% of the warfarin dose variability in those of European ancestry, however, less variability was explained in other ethnic populations (1, 9, 12, 13). Thus, it is important to identify other genetic or clinical factors that may help improve the prediction of warfarin dose requirements in non-Europeans. It is also clear that even in whites, there is a substantial portion of the variability yet to be explained, and it is important to note that most of the genetic factors identified to date help to explain requirements for a low dose of warfarin; the genetic underpinnings for high warfarin dose requirements, or warfarin resistance, are poorly understood.
The one variant that has been most strongly associated with high warfarin dose requirements is the VKORC1 coding Asp36Tyr (D36Y; rs61742245) variant. This variant appears to exhibit large differences in population prevalence. For example, it is relatively common in Ethiopians with minor allele frequency (MAF) of 15%, and Ashkenazi Jews (MAF 4%), less common in Israeli Jews (MAF 1.5%) and Arab Muslims in Israel (MAF 1%), and has a MAF of 0.5% in Sephardic, Yemenite, and North African Jews (10, 14-18). On the other hand, it was absent in over 700 non-Jewish Caucasian controls, 180 Israelis of Druze descent, 220 Han Chinese, 240 Southeast Indians and 213 South African individuals (17, 19-22).
The primary objective of this study was to better define the population frequencies of this variant, through testing of populations in seven countries on four continents, including five African and Middle Eastern countries, the United States (African Americans), and Peru. We also investigated the effect of VKORC1 Asp36Tyr polymorphism on warfarin dose requirements in Egyptians.
Methods
Study population
A total of 1000 samples were included in the analysis to define population prevalence. Those samples included individuals from Egypt, Ghana, Sudan, Kenya, Saudi Arabia, Peru and African Americans from the United States, as shown in Table 1. All participants provided informed consent and the study protocol was approved by relevant local Institutional Review Boards.
Table 1.
VKORC1 Asp36Tyr genotype prevalence in the 7 studied populations.
| Asp36Tyr Genotypes |
Tyr36Allele Frequency, % |
||||
|---|---|---|---|---|---|
| Population | Na | Tyr/Tyr | Asp/Tyr | Asp/Asp | |
| Kenya | 41 | 1 | 3 | 37 | 6 |
| Sudan | 43 | 0 | 5 | 38 | 6 |
| Saudi Arabia | 85 | 0 | 5 | 80 | 3 |
| Egypt | 206 | 0 | 10 | 196 | 2.5 |
| Ghana | 85 | 0 | 0 | 85 | <0.5b |
| Peru | 71 | 0 | 0 | 71 | <0.7b |
| African | |||||
| American | 469 | 0 | 0 | 469 | <0.1b |
Number of individuals genotyped from each population.
Tyr36allele frequency (< X %), where X is the frequency if there had been one heterozygous subject in a given population that we tested.
207Egyptian patients were enrolled while taking chronic warfarin therapy (Marevan®; GlaxoSmithKline, Cairo, Egypt) for various indications as previously described (23). Eligible patients were those who were taking stable weekly doses of warfarin for three consecutive clinic visits, occurring over a minimum time period of 2 months. A stable weekly maintenance dose of warfarin was defined as a dose that did not vary by more than 10% between clinic visits. The international normalized ratio (INR) at each of the three visits had to be in the patient’s specific goal INR range. Liver cirrhosis, advanced malignancy, hospitalization within the earlier 4 weeks, and febrile/diarrheal illness within the past 2 weeks were the exclusion criteria of this study. The Egyptian warfarin pharmacogenetic study was approved by the Research Ethics Committee at the Faculty of Medicine, Ain Shams University, Cairo, Egypt.
Genotyping procedure
VKORC1 Asp36Tyr polymorphism was genotyped at the University of Florida, University of North Carolina and University of Illinois at Chicago. Genotyping was done using the same protocol at all 3 sites including PCR followed by pyrosequencing as previously described (24). The following forward: biotinylated-5′TCTACGCGCTGCACGTGA-3′; and reverse PCR primers: 5′-AGAAGACGCGCGAACAGCT-3′ along with reverse sequencing primer: 5′-AGCGCGCGGTAATCCC-3′ were used in this analysis.
The standard PCR reaction mixture used for the amplification of the target sequence consisted of 12.75 ul including 6.5 ul of ABI PCR master mix with Taq DNA polymerase, 1 ul dimethylsulfoxide (Sigma-Aldrich, St Louis, Missouri, USA), 1 ul of each primer (Invitrogen, Carlsbad, California), 1.25 ulof purified water, and 2 ul of genomic DNA. An annealing temperature of 58°C was used for the PCR reaction.
Statistical analysis
The mean weekly warfarin dose in the Egyptian cohort was calculated by taking the average of the warfarin dose for three consecutive clinic visits that was documented for each patient. Categorical variables were represented as percentages. Numerical variables were represented as mean ± standard deviation or median and interquartile range as appropriate. Hardy-Weinberg equilibrium was assessed by allele counting and x2 analysis with one degree of freedom, and Fisher’s exact test for populations with small sample size (e.g. Kenyans) (25). . Median weekly warfarin dose differences by VKORC1 Asp36Tyr genotypes were evaluated by nonparametric methods (Mann-Whitney U-test)
A stepwise linear regression model was used to assess the explanatory power of VKORC1 Asp36Tyr polymorphism in relation with mean weekly warfarin dose and whether this polymorphism improved our previous explanatory model in Egyptians (23). This regression analysis included our previous genetic variables [VKORC1 (rs9923231), CYP2C9*2*3*4*5*8, CYP4F2 (rs2108622), APOE (rs429358 and rs7412), and CALU (rs339097)] which were available for 195 patients only. Additionally, it included non-genetic factors that may contribute to warfarin dose requirements. The VKORC1 Asp36Tyr was coded: Asp/Tyr as 1 and Asp/Asp (wild type) as 0. Square root transformation of the dose was applied to improve model fit and limit heteroscedascity. Statistical significance was defined as p < 0.05. All statistical analyses were carried out with SPSS (version 17.0 for windows; SPSS Inc., Chicago, Illinois, USA) and SAS 9.2 (SAS Institute Inc., Cary, NC) software.
Results
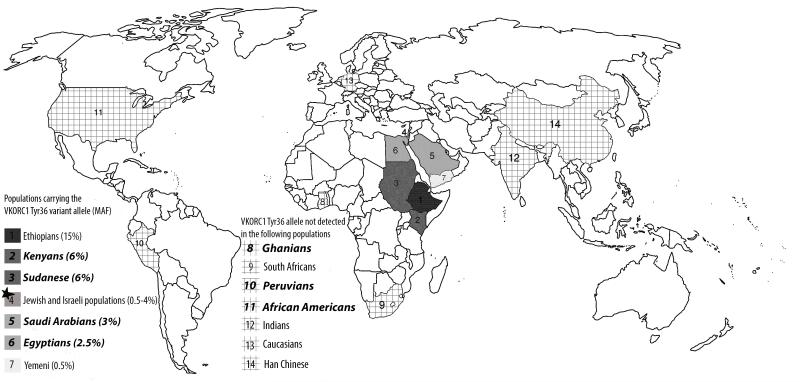
Complete genotyping data were available for 1000 samples. All genotypes were in Hardy-Weinberg equilibrium. One Egyptian sample was removed from the analysis since it was not successfully genotyped. Prevalence of the VKORC1 Asp36Tyr SNP in the populations studied is shown in Table 1. These data reveal the presence of the VKORC1 Asp36Tyr SNP in all East African countries studied, along with Saudi Arabia. However, this SNP was not detected in the one West African country (Ghana), in African Americans (who derive largely from West Africa) (26, 27) and in Peruvians. The prevalence of this SNP was highest among Kenyans and Sudanese with a MAF of 6%, followed by Saudi Arabians and Egyptians with a MAF of 3% and 2.5%, respectively. ▶Figure 1 displays a world map that indicates the countries from which Asp36Tyr frequency data have been previously reported with the new data from this study. Countries in which this SNP is prevalent are shown in decreasing shades of gray, highlighting the greatest prevalence of this SNP in northeast Africa and western parts of the Middle East, and its absence or being very rare in the other studied populations.
Figure 1. World map showing VKORC1 Asp36Tyr geographical distribution.
Populations having the variant were listed in a descending order according to the VKORC1 Asp36Tyr frequency in each studied population. Populations having the VKORC1 Asp36Tyr highest frequency were represented by dark gray color, followed by lighter gray colors in populations with lower frequency. Cross hatches represent populations in which this variant was not detected . The countries in bold italic represent the seven populations included in our study; the remainder represent previously studied populations (14-16, 21, 22, 32, 33).★ Jewish and Israeli populations include: Ashkenazi Jews (4%), Israeli Jews (1.5%), Arab Muslims in Israel (1%), Sephardic Jews (0.5%) and North African Jews (0.5%).
Ten patients in the Egyptian warfarin pharmacogenetic study were carriers of the VKORC1 Asp36Tyr variant. Those carriers had a stable warfarin dose (57.1 ± 29.4 mg) significantly higher than non-carriers (35.8 ± 16.6 mg/week, P = 0.03); as shown in ▶Fig. 2). The range of the warfarin dose requirement among the VKORC1 Asp36Tyr carriers was 21 mg/week to 98 mg/week. Among the 15 patients requiring ≥ 70 mg/week (10 mg/day) in this cohort, five (33%) were VKORC1 Asp36Tyr carriers. Among the other five VKORC1 Asp36Tyr carriers, four of them carried variants in VKORC1 (−1639 G>A, rs9923231) or other genes that associate with lower dose requirements, as shown in Table 2.
Figure 2. Box plot showing the effect of VKORC1 Asp36Tyr polymorphism on warfarin dose requirements in Egyptians.
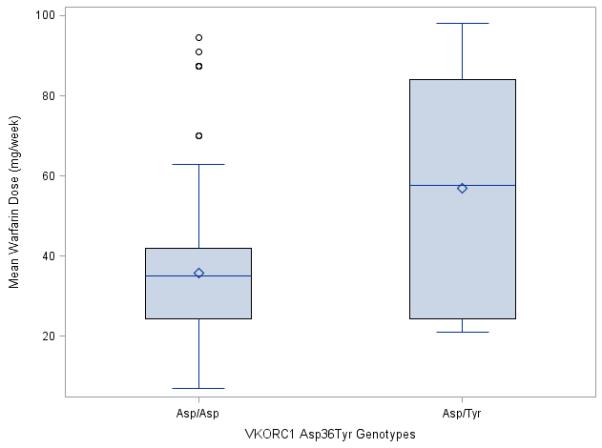
The box represents the values from the 25 to 75% percentile. The horizontal line represents the median. The diamond represents the mean. The vertical line extends from the minimum to the maximum value, excluding outlier and extreme values which are marked as closed circles. Nonparametric test (Mann-Whitney U test) was used to compare the warfarin dose requirements between the two groups, showing a significant difference of P = 0.03.
Table 2.
VKORC1 −1639G>A, and CYP2C9*2*3*4*5 and *8 genotypes distribution among the ten VKORC1 Asp36Tyr variant carriers in the Egyptian cohort.
| Sample Number |
VKORC1 Asp36Tyr |
VKORC1 −1639G>A# |
CYP2C9 # | Weekly Warfarin dose (mg/week) |
|---|---|---|---|---|
| 1 | Asp/Tyr | WT♣ | WT | 98 |
| 2 | Asp/Tyr | WT | WT | 87.5 |
| 3 | Asp/Tyr | G/A | WT | 84 |
| 4 | Asp/Tyr | WT | WT | 77 |
| 5 | Asp/Tyr | G/A | WT | 70 |
| 6 | Asp/Tyr | WT | WT | 45.5 |
| 7 | Asp/Tyr | G/A | WT | 38.5 |
| 8 | Asp/Tyr | G/A | WT | 24.5 |
| 9 | Asp/Tyr | G/A | WT | 24.5 |
| 10 | Asp/Tyr | G/A | *3/*3 | 21 |
VKORC1 (−1639G>A, rs9923231), CYP2C9 tested for *2, *3, *4, *5, and *8 which were previously published (23).
WT refers to a wild type genotype (G/G for VKORC1 and *1/*1 for CYP2C9).
We previously published a multivariate regression analysis of factors significantly influencing warfarin dose requirements in Egyptians, and showed genetic and clinical variables that explained (model R2) 31% of the warfarin dose variability (23). The VKORC1 Asp36Tyr variant was significantly associated with warfarin dose variability when added to the previous regression analysis, and increased the model R2 to 36.5%, Table 3).
Table 3.
Linear Regression stepwise modeling association between mean weekly warfarin dose (square root transformed) as a dependent variable and genetic and non-genetic factors as independent variables.
| Predictor | Coefficient | Standard Error | PartialR2(%) | Pvalue |
|---|---|---|---|---|
| Intercept | 8.19 | 0.319 | ||
| VKORC1# variant | −0.62 | 0.131 | 11.5 | <0.0001 |
| Age (decades) | −0.32 | 0.05 | 9.2 | <0.0001 |
| CYP2C9# variant | −0.56 | 0.17 | 6.6 | 0.002 |
| Pulmonary Embolism | 1.21 | 0.54 | 2.6 | 0.026 |
| APOE ε2 | −0.59 | 0.21 | 2.7 | 0.006 |
| VKORC1 Asp36Tyr§ | 0.96 | 0.38 | 2.0 | 0.013 |
| Smoking Status | 0.58 | 0.25 | 1.9 | 0.021 |
| Model R2 | 36.5 | <0.0001 |
VKORC1 (−1639G>A; rs9923231), CYP2C9 *2, *3, *4, *5, and *8
None of the VKORC1 Tyr36 allele carriers had pulmonary embolism
Discussion
The VKORC1 Asp36Tyr (rs61742245) SNP was first described as a warfarin resistance SNP in 2007 (14, 28), but its frequency has previously been reported in limited numbers of populations. We provide substantial additional data on population prevalence in this study, and our data, combined with the existing literature provides strong evidence for the restriction of this genetic variant to northeastern Africa and neighboring Middle-Eastern regions. However, we did not detect any variant carriers among those tested from Ghana or our large cohort of African Americans, who derive largely from West Africa, suggesting this allele is absent or very rare among west Africans.
In our previous Egyptian warfarin pharmacogenetic study, we reported significant association with warfarin sensitivity markers in the VKORC1, CYP2C9 and APOE but did not investigate genetic markers explaining warfarin resistance. The current study is consistent with the previous literature on VKORC1 Asp36Tyr (14-16, 29, 30), and showed that Egyptian carriers of the VKORC1 Asp36Tyr variant require higher warfarin doses than wild type carriers (P = 0.03). The inclusion of this variant in our previous regression analysis improved the percent of variability explained by an absolute 5.5% when compared to our previous model. Moreover, this variant had the largest effect size among the significantly associated genetic variants in our linear regression model, resulting in a 16.6 mg/week higher warfarin dose per allele.
A total of 10 patients were identified as carriers of VKORC1 Asp36Tyr polymorphism, five of those patients required a mean warfarin dose of 83.3 ± 10.6 mg/week, while the other 5 carriers required a mean warfarin dose of 31 ± 10.6 mg/week. The lower dose requirements in this latter group is likely explained in part by the fact that four of the five had variants in VKORC1 (−1639G>A, rs9923231) or other genes that associate with lower warfarin dose requirements. This lower dose might also be explained by other warfarin sensitive genetic variants or environmental factors like diet that were not investigated in our study.
It is of interest to note that only 33% of warfarin resistance cases (dose requirements ≥ 10 mg/day) carried this variant allele, meaning there is a large portion of patients within this high dose group for which the insensitivity to warfarin remains unexplained. This is consistent with a recent study by Watzka et al., who screened for VKORC1 mutations in 626 individuals with marked warfarin resistance. They found only six (or < 1%) of these patients carrying the Asp36Tyr variant, three from Russia, one from Turkey and an African American (30). Moreover, a recent study by Kurnik et al., who studied the effect of the VKORC1 Asp36Tyr polymorphism on warfarin maintenance dose in 210 Israelis, consistently showed that only 31% of patients taking a warfarin dose ≥ 10 mg/day carried this variant allele (31). They also showed that the presence of a single allele of this variant more than doubled the maintenance warfarin dose, and by adding this variant to the International Warfarin Pharmacogenetic Consortium (IWPC) dose prediction model, a significantly better performance in the warfarin dose prediction was detected (R2 from 27% to 47.2%)(31). The data from the current study, from Watzka, et al. (30) and Kurnik et al.(31) studies suggest that in those populations where VKORC1 Asp36Tyr is prevalent, it explains a portion of warfarin resistance, yet in all population groups, including those where VKORC1 Asp36Tyr is prevalent, substantial additional work is needed to better understand the genetic underpinnings of warfarin insensitivity.
In conclusion, this study provides greater insight into the population prevalence of the VKORC1 Asp36Tyr, and suggests that it occurs primarily in northeastern African and Middle-Eastern populations. Our data on warfarin dose requirements in Egyptian carriers of the variant allele further support the importance of the variant in leading to insensitivity to warfarin therapy. The improvement in the prediction of warfarin dose variability suggests that the inclusion of this SNP in dosing algorithms in those populations where the SNP is prevalent, particularly for those in northeastern Africa and the Middle-East, may lead to improved warfarin dose prediction. However, this polymorphism appears to be unimportant to West Africans, African Americans and Peruvians, along with other populations where it has not been detected (e.g. Europeans and Asians) (19, 20). Further research is required to explain the genetic or other causes of warfarin resistance.
Supplementary Material
Acknowledgements
This work was funded in part by U01 GM074492 grant, as part of the National Institute of Health Pharmacogenomics Research Network (J.A.J). The Pharmacogenetics for Every Nation Initiative (PGENI.org) supported the studies in Ghana, Kenya, Sudan, Saudi Arabia, and Peru (H.L.M). This study was also supported by the University of Illinois at Chicago College of Pharmacy Hans Vahlteich Research Award (L.H.C.); the National Institutes of Health (grant K23 HL089808-01A2, M.P.).
Grants and financial support: U01 GM074492 NIH grant (J.A.J.), University of Illinois at Chicago College of Pharmacy Hans Vahlteich Research Award (L.H.C.); K23 HL089808-01A2 NIH grant (M.P.).
Footnotes
Conflict of interest:
The authors declared no conflict of interest.
This article is not an exact copy of the original published article in Thrombosis and Haemostasis. The definitive publisher-authenticated version of (Thromb Haemost 2013 109:) is available online at: (http://www.schattauer.de/en/magazine/subject-areas/journals-a-z/thrombosis-and-haemostasis.html).
References
- 1.Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009 Feb 19;360(8):753–64. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginsburg GS, Voora D. The long and winding road to warfarin pharmacogenetic testing. J Am Coll Cardiol. 2010 Jun 22;55(25):2813–5. doi: 10.1016/j.jacc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JA. Warfarin pharmacogenetics: a rising tide for its clinical value. Circulation. 2012 Apr 24;125(16):1964–6. doi: 10.1161/CIRCULATIONAHA.112.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Schwarz UI, Ritchie MD, et al. Relative contribution of CYP2C9 and VKORC1 genotypes and early INR response to the prediction of warfarin sensitivity during initiation of therapy. Blood. 2009 Apr 23;113(17):3925–30. doi: 10.1182/blood-2008-09-176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aquilante CL, Langaee TY, Lopez LM, et al. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin Pharmacol Ther. 2006 Apr;79(4):291–302. doi: 10.1016/j.clpt.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Jonas DE, McLeod HL. Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol Sci. 2009 Jul;30(7):375–86. doi: 10.1016/j.tips.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005 Oct 1;106(7):2329–33. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JA, Cavallari LH, Beitelshees AL, et al. Pharmacogenomics: application to the management of cardiovascular disease. Clin Pharmacol Ther. 2011 Oct;90(4):519–31. doi: 10.1038/clpt.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limdi NA, Wadelius M, Cavallari L, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood. 2010 May 6;115(18):3827–34. doi: 10.1182/blood-2009-12-255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005 Jun 2;352(22):2285–93. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008 Sep;84(3):326–31. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schelleman H, Chen J, Chen Z, et al. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin Pharmacol Ther. 2008 Sep;84(3):332–9. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavallari LH, Perera MA. The future of warfarin pharmacogenetics in under-represented minority groups. Future Cardiol. 2012 Jul;8(4):563–76. doi: 10.2217/fca.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loebstein R, Dvoskin I, Halkin H, et al. A coding VKORC1 Asp36Tyr polymorphism predisposes to warfarin resistance. Blood. 2007 Mar 15;109(6):2477–80. doi: 10.1182/blood-2006-08-038984. [DOI] [PubMed] [Google Scholar]
- 15.Aklillu E, Leong C, Loebstein R, et al. VKORC1 Asp36Tyr warfarin resistance marker is common in Ethiopian individuals. Blood. 2008 Apr 1;111(7):3903–4. doi: 10.1182/blood-2008-01-135863. [DOI] [PubMed] [Google Scholar]
- 16.Scott SA, Edelmann L, Kornreich R, et al. Warfarin pharmacogenetics: CYP2C9 and VKORC1 genotypes predict different sensitivity and resistance frequencies in the Ashkenazi and Sephardi Jewish populations. Am J Hum Genet. 2008 Feb;82(2):495–500. doi: 10.1016/j.ajhg.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efrati E, Elkin H, Sprecher E, et al. Distribution of CYP2C9 and VKORC1 risk alleles for warfarin sensitivity and resistance in the Israeli population. Curr Drug Saf. 2010 Jul 2;5(3):190–3. doi: 10.2174/157488610791698299. [DOI] [PubMed] [Google Scholar]
- 18.Bodin L, Perdu J, Diry M, et al. Multiple genetic alterations in vitamin K epoxide reductase complex subunit 1 gene (VKORC1) can explain the high dose requirement during oral anticoagulation in humans. J Thromb Haemost. 2008 Aug;6(8):1436–9. doi: 10.1111/j.1538-7836.2008.03049.x. [DOI] [PubMed] [Google Scholar]
- 19.Cen HJ, Zeng WT, Leng XY, et al. CYP4F2 rs2108622: a minor significant genetic factor of warfarin dose in Han Chinese patients with mechanical heart valve replacement. Br J Clin Pharmacol. 2010 Aug;70(2):234–40. doi: 10.1111/j.1365-2125.2010.03698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burmester JK, Berg RL, Glurich I, et al. Absence of novel CYP4F2 and VKORC1 coding region DNA variants in patients requiring high warfarin doses. Clin Med Res. 2011 Nov;9(3-4):119–24. doi: 10.3121/cmr.2011.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavani A, Naushad SM, Rupasree Y, et al. Optimization of warfarin dose by population-specific pharmacogenomic algorithm. Pharmacogenomics J. 2012 Aug;12(4):306–11. doi: 10.1038/tpj.2011.4. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, Gregersen N, Krause A. Novel CYP2C9 and VKORC1 gene variants associated with warfarin dosage variability in the South African black population. Pharmacogenomics. 2011 Jul;12(7):953–63. doi: 10.2217/pgs.11.36. [DOI] [PubMed] [Google Scholar]
- 23.Shahin MH, Khalifa SI, Gong Y, et al. Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics. 2011 Mar;21(3):130–5. doi: 10.1097/FPC.0b013e3283436b86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh S, King CR, Garsa AA, et al. Pyrosequencing of clinically relevant polymorphisms. Methods Mol Biol. 2005;311:97–114. doi: 10.1385/1-59259-957-5:097. [DOI] [PubMed] [Google Scholar]
- 25.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005 May;76(5):887–93. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ely B, Wilson JL, Jackson F, et al. African-American mitochondrial DNAs often match mtDNAs found in multiple African ethnic groups. BMC Biol. 2006;4:34. doi: 10.1186/1741-7007-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bress A, Patel SR, Perera MA, et al. Effect of NQO1 and CYP4F2 genotypes on warfarin dose requirements in Hispanic-Americans and African-Americans. Pharmacogenomics. 2012 Dec;13(16):1925–35. doi: 10.2217/pgs.12.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Ambrosio RL, D’Andrea G, Cafolla A, et al. A new vitamin K epoxide reductase complex subunit-1 (VKORC1) mutation in a patient with decreased stability of CYP2C9 enzyme. J Thromb Haemost. 2007 Jan;5(1):191–3. doi: 10.1111/j.1538-7836.2006.02261.x. [DOI] [PubMed] [Google Scholar]
- 29.Harrington DJ, Gorska R, Wheeler R, et al. Pharmacodynamic resistance to warfarin is associated with nucleotide substitutions in VKORC1. J Thromb Haemost. 2008 Oct;6(10):1663–70. doi: 10.1111/j.1538-7836.2008.03116.x. [DOI] [PubMed] [Google Scholar]
- 30.Watzka M, Geisen C, Bevans CG, et al. Thirteen novel VKORC1 mutations associated with oral anticoagulant resistance: insights into improved patient diagnosis and treatment. J Thromb Haemost. 2011 Jan;9(1):109–18. doi: 10.1111/j.1538-7836.2010.04095.x. [DOI] [PubMed] [Google Scholar]
- 31.Kurnik D, Qasim H, Sominsky S, et al. Effect of the VKORC1 D36Y variant on warfarin dose requirement and pharmacogenetic dose prediction. Thromb Haemost. 2012 Sep 27;108(4):781–8. doi: 10.1160/TH12-03-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burmester JK, Berg RL, Glurich I, et al. Absence of novel CYP4F2 and VKORC1 coding region DNA variants in patients requiring high warfarin doses. Clin Med Res. 2011 Nov;9(3-4):119–24. doi: 10.3121/cmr.2011.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cen HJ, Zeng WT, Leng XY, et al. CYP4F2 rs2108622: a minor significant genetic factor of warfarin dose in Han Chinese patients with mechanical heart valve replacement. Br J Clin Pharmacol. 2010 Aug;70(2):234–40. doi: 10.1111/j.1365-2125.2010.03698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.