Abstract
Delivery of anti-inflammatory steroids concurrently to both anterior and posterior segments of the eye is a challenge. The anterior ocular structures limit topical delivery. Injection can be disproportionately and repeatedly invasive and selective for only one ocular hemisphere. We developed a novel implant that can compensate for the limited conveyance of topical medicine and reduce the repetitive invasiveness of injection from the capsular bag allowing dexamethasone (DXM) delivery to both the anterior and posterior chambers. To establish proof of concept, microparticles were prepared with PLGA [poly(d,l-lactide-co-glycolide), 50:50, MW. 7000–17000], hydroxypropyl methyl cellulose (HPMC), and DXM by oil-in-water emulsion/solvent evaporation technique. Zeatsizer Nano and SEM (scanning electron microscopy) results showed microspheres in the range of 8 ± 1 μM. The target load of DXM in the microparticles was ~20.0% with a % recovery of 99.9% (w/w). Dose related pharmacokinetics with near zero order kinetics was observed for up to 6 weeks in rabbits with intracapsular bag implants. DXM flow was bidirectional from the endocapsular space and significant concentrations were found in the anterior and posterior chambers after up to 6 weeks. Whereas, with topical drops the exposure was minimal in all the ocular tissues with greater systemic exposure. Intraocular pressure was normal in all of the study groups, slit lamp biomicroscopy examinations revealed that no cells or fibrin formation in the anterior and posterior chamber with implants but flare, cells and fibrin was present in the topical drops group. Histological examination revealed normal tissues and no signs of inflammation in all the groups. The implant did not migrate to the center of the eye or obstruct the visual axis. We believe these findings demonstrate the feasibility of drug delivery from the capsular bag to the anterior and posterior segments effectively compared to topical alternatives.
Keywords: bioerodible, implant, intraocular, uveitis, dexamethasone, PLGA, HPMC, anti-inflammatory, cataract, capsular bag, bidirectional, drug delivery, topical drops
1. Introduction
Sustained delivery of anti-inflammatory agents to both anterior and posterior hemispheres of the eye, with minimal systemic exposure, is a significant challenge in ocular drug delivery. Implants fabricated using biodegradable polymer PLGA [poly (DL-lactide-co-glycolide)] have been widely utilized as carriers for bioactive molecules and pose a possible solution to limitations surrounding ocular drug penetration [1]. PLGA has been demonstrated to be biocompatible and biodegradable. It is approved by the FDA for specific human clinical applications [2].
Ophthalmic diseases are most commonly treated by topical instillation of eye drops. These formulations unfortunately face technical problems (e.g., solubility, stability, and preservation) and have clinical limitations (e.g., limited efficacy, corneal/scleral permeability, systemic toxicity and compliance). Further, within two minutes after instillation over 80% of the topical product is eliminated via the nasolacrimal drainage system limiting ocular penetration of the drug to less than 1% of the administered dose [3].
Ocular inflammation (as can occur in uveitis and after cataract surgery) is often difficult to treat due to poor tissue permeability [4–6]. While topical administration can reach the anterior segment, it is less effective at reaching the posterior segment, where cystoid macular edema or vitritis could be sequelae of inflammation. Furthermore, compliance with topical eye drops is poor [7–11]. Oral or intravenous administration can reach the posterior segment but are associated with non-specific accumulation of drugs in other organs and results in related adverse effects. In addition the blood-retina barrier hinders drug diffusion into the posterior segment [12]. Intravitreal injections effectively deliver drug to the posterior segment but have complications of retinal detachment, bleeding, or infection [13–20]. Sustained release of medication from a biocompatible and biodegradable polymer matrix would be a significant advance in therapy of conditions such as inflammation where delivery of medication to the anterior and posterior segment is important.
The FDA has approved intravitreal implants for intraocular drug delivery from biodegradable or non-biodegradable polymers. Implants are introduced into the vitreous humor through an incision in the ocular pars plana located posterior to the lens and anterior to the retina. There is only one currently approved product containing corticosteroid in a biodegradable matrix that is delivered in this method: Ozurdex™ (Allergan Inc.). It is a dexamethasone implant that has a therapeutic duration of 6–9 months and is approved for the treatment of macular edema following branch or central retinal vein occlusion [21, 22] and posterior segment non-infectious uveitis [23]. Two other implants are approved for intravitreal delivery in a non-biodegradable matrix. These include Vitrasert™ (Bausch & Lomb), a ganciclovir preparation indicated for CMV (cytomegalovirus) retinitis, lasting 6 months [24], and Retisert™ (Bausch & Lomb), has fluocinolone acetonide, a corticosteroid with approximately 2 years life time for the treatment of severe non-infectious posterior segment uveitis [25]. There are no approved delivery systems for extended drug delivery to the anterior segment.
Each of these poses several limitations. They are intended for posterior segment drug delivery and deliver only minimal concentrations to the anterior segment of the eye. With exception of Ozurdex™, these implants require removal after reservoir depletion. Vitreous hemorrhage, endophthalmitis, and retinal detachment are other potential complications of these drug delivery systems which are placed in the vitreous [26–28].
In the present study we sought to determine whether DXM could be delivered to both the anterior and posterior segments of the eye from within the lens capsule, which lies in between the two. This would allow concurrent treatment of both segments of the eye for post-cataract inflammation or uveitis without topical, oral or intravitreal therapy.
2. Materials and Methods
2.1. Materials
PLGA or poly(d,l-lactide-co-glycolide; 50:50, Mw-7,000–17,000, acid terminated), Hydroxypropyl methylcellulose (HPMC, 2600–5600 cP), dexamethasone, poly vinyl alcohol (PVA, 90.0 kDa), dichloromethane, acetonitrile, methanol, ammonium acetate, acetic acid were purchased from Sigma-Aldrich, USA. Standard RC dialysis tubing (MWCO 1,000 Da) and weighted closures were purchased from Spectrum Labs, USA. Custom 2.0 mm die sets were purchased from International Crystal Labs, USA. Thermo high-purity C18 HPLC column was obtained from Thermo Scientific, USA. The bench top pellet press was from Carver Instruments, USA. Dexamethasone sodium phosphate 0.1% ophthalmic solution and ciprofloxacin hydrochloride 0.3% ophthalmic solution were from Falcon pharmaceuticals, USA. Ketamine was from VEDCO, Xylazine from LLOYD laboratories and euthanasia solution was from VETONE, USA.
2.2. Methods
2.2.1.Pilot pharmacokinetic Study
To establish proof of concept, two New Zealand White (NZW) rabbits were injected with a 50.0 μL suspension of DXM (prepared in BSS, 5.0 mg/mL) into the endocapsular space immediately after phacoemulsification followed by intraocular lens (IOL) insertion. One rabbit each was sacrificed at 3 and 6 hr post injection, followed by collection of ocular tissues and blood samples with analysis by mass spectroscopy method to observe the injectate distribution.
2.2.2.Preparation of PLGA Microspheres and Residual Solvent Content
DXM loaded PLGA microspheres were prepared using standard oil-in-water (o/w) emulsion-solvent extraction method. 160.0 mg PLGA was dissolved in 4.0 mL methylene chloride and 1.0 mL acetonitrile. 40.0 mg DXM and 10 mg of HPMC was dispersed in this PLGA solution by vortexing for 5.0 min. This organic phase with floating HPMC particles was emulsified in 20.0 mL of a 2.0% (w/v) PVA solution and homogenized at 16,000 rpm for 2.0 min. The resultant emulsion was poured into 200.0 mL of a 2.0% (w/v) PVA solution and stirred at 12,000 rpm in an ice bath for 6.0 min. The contents were stirred for 8.0 hr at room temperature on a magnetic stirrer to evaporate the dichloromethane and acetonitrile, allowing the formation of a turbid particulate suspension. Microparticles were then separated by centrifugation at 15,000 rpm for 10.0 min. The pellets are washed two times with deionized water, re-suspended in deionized water, and freeze-dried to obtain lyophilized particles. Residual methylene chloride (CH2Cl2) and acetonitrile present in DXM-PLGA microparticles was analyzed by gas chromatography-mass spectrometry (GC-MS) method [29].
2.2.3. Particle Size Analysis
Mean particle size of microparticles was analyzed by Zetasizer Nano Z (Malvern Instruments Inc, MA). Approximately 2.0 mg of microspheres were dispersed in 5.0 ml of 0.2% (w/v) PVA solution, diluted 5 times with deionized water and used for particle size analysis. All measurements were conducted in triplicate and the results are reported as mean ± SD.
2.2.4. Drug Loading
10.0 mg of microparticle powder was weighed and dissolved in 10.0 ml of acetonitrile. This solution was filtered (Millex® HV, PVDF 0.45 μm syringe filter) and the DXM concentration was determined by the LC/MS/MS method as described below. Drug loading was determined as percent drug loading = (weight of drug loaded/weight of microspheres) ×100. All measurements were done in triplicate and the results are reported as mean ± SD.
2.2.5. Preparation of Implants
BDI (Bioerodible Dexamethasone Implant) implants were prepared using bench top pellet press (Carver instruments, USA) in a 2.0 mm die set made for this study (ICL, USA). In brief, 1.6 mg of microparticle powder was weighed and transferred into the opening of the micro collar. The solid anvil with the polished face was placed on the collar and pressure (2.0 ton) was applied on the die set for 20.0 sec. After releasing pressure, the implant (2.0 mm diameter and ~1.5 mm thickness) was collected. Implants with promising in vitro release kinetics were sterilized by ethylene oxide gas before implantation in rabbits.
2.2.6. Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS)
Quantification of DXM from all collected samples (in vitro and in vivo) was performed using a validated LC/MS/MS method. In brief, all study samples were allowed to equilibrate to room temperature. For the preparation of standards 45.0 μL of corresponding blank matrix (95.0 μL for in vitro samples) + 5.0 μL of known standard of DXM sample + 10.0 μL of internal standard (triamcinolone acetonide, 20.0 μg/mL) was added in 1.5 mL eppendorf tubes. After brief vortexing, 1.0 mL of tert-butyl methyl ether was added to all tubes and centrifuged at 15,000 rpm for 8.0 min. Supernatant was transferred to glass tubes and the solution was evaporated to dryness at 45.0°C under a stream of nitrogen using Zymark Turbovap LV evaporator work station. The residue was reconstituted in 120.0 μL of mobile phase (10.0 mM ammonium acetate and acetonitrile with 2.0% acetic acid 10:90 ratio), vortexed, transferred to glass vials and 30.0 μL was injected onto MS/MS for analysis. Plasma, aqueous humor, vitreous humor, and IOL samples were analyzed by following the above procedure. Analyzing iris/ciliary body and retina/choroid required one additional step. In brief, internal standard and standard drug solution was added to blank iris/ciliary body or retina/choroid tissues, vortexed, 1.0 mL of tert-butyl methyl ether was added and homogenized for 2.0 min using Polytron homogenizer (Kinematica Inc, USA), and analyzed by following the above procedure. The lower limit of quantitation was 2.0 ng/mL for in vivo samples and 1.0 ng/mL for in vitro samples (unpublished data, manuscript in progress).
2.2.7. In vitro Release Studies
BDI implants containing 300.0 μg of DXM were placed in a dialysis bag (7 Spectra/por®, MWCO 10 kDa) containing 1.0 ml of BSS (pH 7.4). The bag was presealed at one end with a weighted closure after which BSS and implant were introduced and the other side of the bag was closed. Sodium azide (0.025% w/v) was used as a preservative. The dialysis bag containing the implant was placed in drug release medium (100.0 ml BSS, pH 7.4) in an amber colored beaker. The beakers were incubated at 37.0°C while stirring the contents at 70.0 rpm in the dark room owing to DXM light sensitivity. At 1, 4, 7, 15 and 22 days complete release medium was collected and replaced with fresh medium maintained at 37.0°C. The dissolution medium removed at each time interval was analyzed by LC/MS/MS method, and the amount of DXM in the release medium was determined. All in vitro samples were collected and analyzed in triplicate.
2.2.8. Scanning Electron Microscopy (SEM)
Surface morphology and the size of the microparticles were visualized using scanning electron microscope (Hitachi S-2460N; Variable Pressure SEM) at different magnifications ranging from ×2,000 to ×8,000. Samples were analyzed after they were gold sputter coated. SEM images of the microparticles were taken before and after making an implant. Explant images were taken at week 1, 2 and 4 from the commencement of in vivo study.
2.2.9. Slit Lamp Biomicroscopy
After BDI implantation, all eyes were evaluated by slit-lamp biomicroscopy before euthanasia. Anterior and posterior chamber cells, flare, synechiae and formation of fibrin were noted if present.
2.2.10. Intraocular Pressure Measurement
Corneal surface IOP was recorded with a Tono-Pen AVIA® Applanation Tonometer (Reichert Technologies, USA). Three consecutive measurements were taken for each eye and an average value was used for comparison. To minimize circadian oscillation, IOP measurements were taken at 3:00 pm in all rabbits.
2.2.11. Pharmacokinetic Analysis
Pharmacokinetic parameters were calculated using a non-compartmental analysis tool in Phoenix WinNonlin professional software (Version 6.2.1). The area under the tissue concentration-time curve (AUC0-t), peak tissue concentration (Cmax), time for the peak tissue concentration (Tmax), and lowest tissue concentration (Clast) were obtained for individual tissue in low dose, high dose, and positive control groups.
2.3. In vivo Studies
2.3.1. Animals
New Zealand White (NZW) rabbits (2.8 ± 0.6 Kg, female) were purchased from an approved breeding facility, USA. All animal procedures were performed according to animal care protocols approved by the Institutional Animal Care and Use Committee (IACUC) in accordance with the requirement of Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. Each animal is housed in an individual numbered cage at 19.0–23.0°C with a 12.0 h light-dark cycle and allowed free access to food and water.
2.3.2. Study Design
All the study animals were acquainted to study room conditions once they are out of quarantine and randomized. The positive control group and both BDI implant groups underwent phacoemulsification with insertion of IOL in both eyes of each animal. Group III and IV received one and two implants per eye respectively. BDI implants were placed in the inferior fornix of the capsular bag immediately after phacoemulsification and IOL insertion.
Group I: Normal control group; n=8
Group II: Phacoemulsification and IOL insertion- Topical DXM drops containing 0.1% DXM (up to 4 weeks with tapering) and ciprofloxacin drops up to 5 days (t.i.d.)- positive control group- n=6
Group-III: Phacoemulsification and IOL insertion- BDI (PLGA-50:50, M.W. 7,000 to 17,000 with 10% HPMC) low dose (one implant per eye) and ciprofloxacin drops up to 5 days (t.i.d.) after surgery- n=8
Group-IV: Phacoemulsification and IOL insertion- BDI (PLGA-50:50, M.W. 7,000 to 17,000 with 10% HPMC) high dose (two implants per eye) and ciprofloxacin drops up to 5 days (t.i.d.) after surgery- n=8
2.3.3. Sample Collection and Storage
Following BDI implant insertion, two rabbits from each low dose, high dose, positive control and normal control groups were sacrificed at 1, 2, 4 weeks, and at week 6 (only low, high dose, normal control) and eyes were enucleated and collected in tubes containing balanced salt solution (BSS). All the animals were anesthetized by injecting cocktail of ketamine and xylazine intramuscularly prior to euthanasia. Animals were euthanized by injecting 2.0 mL of euthanasia solution intravenously through marginal ear vein. Individual tissue samples (aqueous humor, vitreous humor, IOL, iris/ciliary body and retina/choroid) were separated and stored in a freezer below −70.0°C until bio-analysis. Part of tissues was immediately transferred to biopsy cassettes submersed in neutral buffered formalin 10.0% for sectioning/staining followed by histological examination. Approximately 0.5 mL whole blood was collected (before injecting euthanasia solution), placed into labeled micro-centrifuge tubes (1.5 mL capacity), and centrifuged @ 6,000 rpm for 5.0 min, serum was collected and stored in a freezer.
3. Results
3.1. Pilot pharmacokinetics
Analysis of all the ocular tissues revealed, the flow of DXM was bidirectional with significant concentrations found both in the anterior and posterior segments of the eye. With this we have established proof of concept that, it is feasible to deliver drugs both to the anterior and posterior segments with minimal systemic exposure (data not shown).
3.2. Particle Size, Drug and Residual Solvent Content
Microspheres prepared with standard o/w method resulted in uniform microparticles with mean diameters ranging from 1–8 μm as analyzed with Zetasizer Nano Z with spherical and smooth surfaces evidenced in SEM (scanning electron microscopy) images (Fig. 1A). Interestingly, when the microparticles were pelleted using a benchtop pellet press by applying 2.0 tons of pressure up to 20.0 sec, the surface of the microparticles became flattened (Fig. 1. B), however, that didn't affect the DXM loading in microparticles. The target load of DXM in the microparticles was ~20.0% with a % recovery of 99.9% (w/w). There was no detectable concentration of methylene chloride or acetonitrile present in microparticles as analyzed by GC/MS (Limit of Detection: ~1.0 pg in 10.0 mg of sample).
Fig. 1.
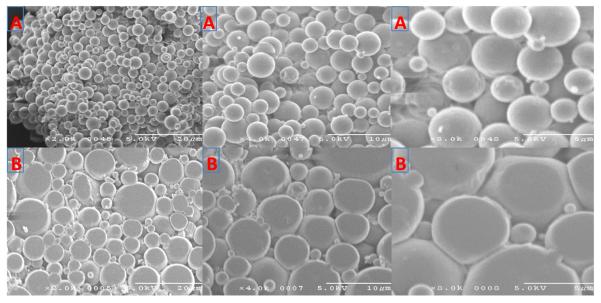
SEM images of dexamethasone loaded PLGA microspheres and implant in 2×, 4× and 8× magnifications (from left to right). (A) Microparticles. (B) Manufactured implants.
3.3. In vitro Release Kinetics
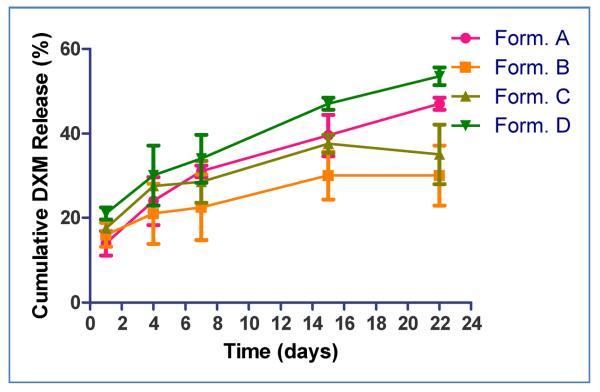
Results of in vitro release kinetics are presented in Fig. 2. All batches exhibited a biphasic release pattern with initial burst release on day 1 followed by a slow and sustained release profile. As predicted, the burst effect was slightly higher with implants containing HPMC. Formulation D (Fig.2.) showed favorable in vitro release kinetics, and was selected to study in vivo pharmacokinetics and preliminary pharmacodynamics in NZW rabbits.
Fig. 2.
In vitro release kinetics of dexamethasone from BDI implants (containing 300 μg of DXM). Data are presented as Mean ± SD (n=3) [Form#. A: PLGA 50:50, M.W. 7,000–17,000; Form#. B: PLGA 65:35, M.W. 17000–32,000; Form#. C: PLGA 50:50, M.W. 7,000–17000 (50%), PLGA 65:35, M.W. 17,000–32,000 (50%); Form#. D: PLGA 50:50, M.W. 7,000–17,000 with 10% hydroxypropyl methylcellulose (HPMC)]; #Form: Formulation
3.4. Clinical Observations
A total of 16 animals (32 eyes) received the BDI. Ophthalmic evaluations during the study were within normal limits. Immediately after implantation in the capsular bag, the implants were located inferior to the IOL. After four weeks the BDI implant transitioned from a round pellet to an elongated mass, typically in the original implantation site. With erosion and degradation, they became smaller (Fig. 3.). By day 42, all implants were fully eroded and not visible. With topical drops, synechiae and redness were evident up to 4 weeks.
Fig. 3.
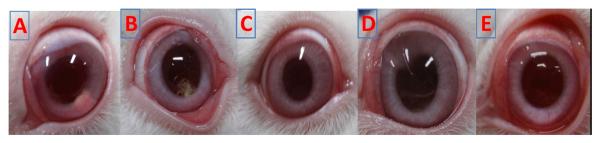
Clinical photographs of NZW rabbits before sacrifice with BDI A) week 1, B) week 4, and C) week 6 and Topicals D) week 1 and E) week 4.
3.5. Pharmacokinetic Profile
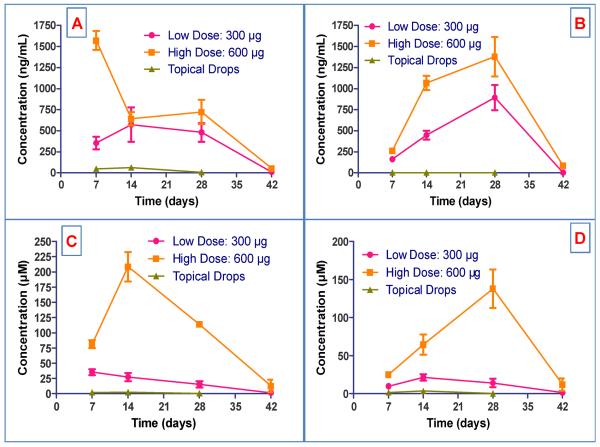
DXM concentrations in all the collected samples were analyzed by a validated LC/MS/MS method; results are presented in Fig. 4.
Fig.4.
Pharmacokinetic profile of BDI implant and topical DXM drops in ocular tissues of NZW rabbits. (A) Aqueous humor. (B) Vitreous humor. (C) Iris/Ciliary body. (D) Retina/choroid.
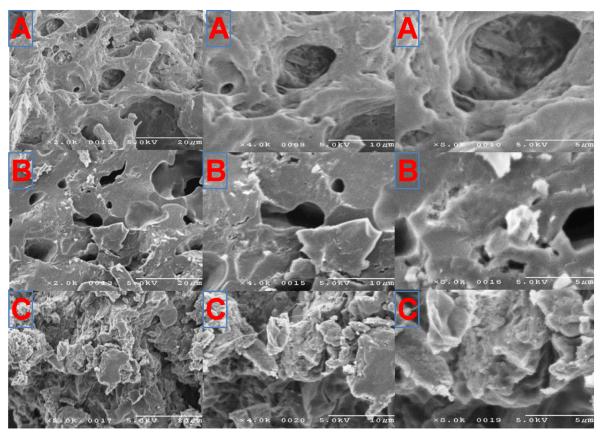
Dose related exposure (AUC0-t) was observed in all tissues by doubling the dose. Tmax in vitreous humor was 28 ± 0 days irrespective of the dose and it was most rapid in the aqueous humor (7 ± 0 days) with the high dose (600 μg). The exposure of DXM in retina/choroid with both the doses was significantly higher than after topical drops. DXM delivery was bidirectional when the BDI implant was placed inside the capsular bag. The implant eroded slowly over 4 weeks, and by week 6 it had disappeared. SEM photomicrographs of explants are presented in Fig. 5.
Fig. 5.
SEM photomicrographs of explants presented in 2×, 4× and 8 × magnifications (from left to right). (A) Week 1, (B) Week 2, and (C) Week 4.
After standard of care treatment with eye drops for 4 weeks, (frequency of drop administration 4×/day in week 1, with a graduated weekly taper over 4 weeks), cumulative exposure (AUC0-t) was minimal in aqueous humor and negligible in the vitreous humor, retina/choroid and iris/ciliary body. More importantly, with the BDI implant, therapeutic concentrations of DXM were found up to week 6 with minimal systemic exposure (40.0 ng/mL, high dose). In contrast, use of topical DXM drops resulted in minimal local tissue exposure but systemic exposure was substantially higher (>150.0 ng/mL during week 1). A summary of mean pharmacokinetic (PK) parameters for BDI implant (low and high dose) vs. topical DXM drops in aqueous humor, vitreous humor, retina/choroid and iris/ciliary body are shown in Table 1 and 2.
Table 1.
Pharmacokinetics of BDI vs. topical drops in aqueous humor and vitreous humor of NZW rabbits.
| Parameter | Low dose: 300 μg | High dose: 600 μg | Topical Drops | |||
|---|---|---|---|---|---|---|
| Aqueous humor | Vitreous humor | Aqueous humor | Vitreous humor | Aqueous humor | Vitreous humor | |
| Cmax (ng/mL) | 650 ± 109 | 892 ± 151 | 1570 ± 113 | 1379 ± 233 | 62 ± 24 | 3 ± 0 |
| Tmax (day) | 19 ± 8 | 28 ± 0 | 7 ± 0 | 28 ± 0 | 14 ± 0 | 16 ± 11 |
| AUC0-t (day*ng/mL) | 15231 ± 361 | 18317 ± 2435 | 28202 ± 3369 | 32933 ± 4027 | 1023 ± 320 | 61 ± 5 |
| Clast (ng/mL) | 8 ± 3 | 2 ± 1 | 52 ± 18 | 85 ± 23 | 6 ± 2 | 2 ± 1 |
Table 2.
Pharmacokinetics of BDI vs topical drops in retina/choroid and iris/ciliary body of NZW rabbits.
| Parameter | Low dose: 300 μg | High dose: 600 μg | Topical Drops | |||
|---|---|---|---|---|---|---|
| Retina/Choroid | Iris/CB | Retina/Choroid | Iris/CB | Retina/Choroid | Iris/CB | |
| Cmax (μM) | 21 ± 4 | 35 ± 5 | 117 ± 40 | 209 ± 24 | 3 ± 1 | 3 ± 2 |
| Tmax (day) | 14 ± 0 | 7 ± 0 | 23 ± 8 | 14 ± 0 | 14 ± 0 | 9 ± 4 |
| AUC0-t (day*μM) | 455 ± 61 | 759 ± 132 | 2226 ± 1105 | 3913 ± 685 | 48 ± 16 | 42 ± 27 |
| Clast (μM) | 1.3 ± 0.6 | 1.5 ± 0.5 | 12 ± 8 | 13 ± 10 | 0.2 ± 0.1 | 0.5 ± 0.3 |
3.6. Intraocular Pressure (IOP)
IOP was normal in all groups, and there were no significant differences among groups (all p's>0.05). There was no significant rise in IOP in any group over the course of the study, although there was a non-significant trend for topical drops to increase the intraocular pressure over 4 weeks (Table 3).
Table 3.
Effect of BDI implant with low, high dose and topical drops on IOP in NZW rabbits.
| Time (days) | Intraocular Pressure (mm Hg) | |||
|---|---|---|---|---|
| Control group | Low Dose: 300 μg | High Dose: 600 μg | Topical Drops | |
| 7 | 8 ± 2 | 8 ± 2 | 9 ± 3 | 9 ± 2 |
| 14 | 11 ± 1 | 7 ± 2 | 6 ± 1 | 13 ± 2 |
| 28 | 9 ± 1 | 10 ± 2 | 8 ± 2 | 15 ± 5 |
| 42 | 12 ± 2 | 9 ± 2 | 12 ± 4 | NA |
3.7. Slit Lamp Examination
There were no signs of anterior or posterior chamber inflammation observed on slit lamp biomicroscopy after BDI usage. Slit lamp photographs are presented in Fig. 6 (A, B). However, in eyes treated with topical DXM drops, flare, fibrin, cells and synechiae are seen up to 4 weeks Fig. 6 (C, D).
Fig. 6.
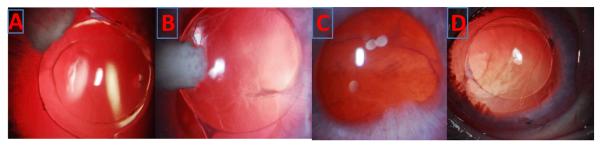
Slit Lamp photographs show endocapsular implant and clear lens with BDI implant# A) week 1 B) week 4 and topical group show flare, fibrin and synechiae C) week 1 and D) week 4. # both low and high dose groups
3.8. Histological Examination
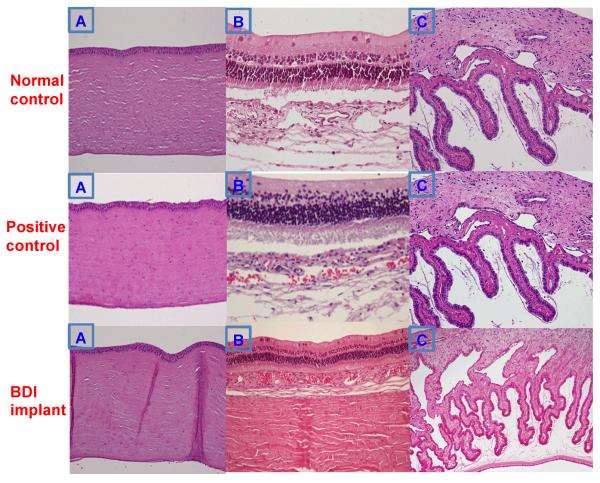
Histological examination of ocular tissues - cornea, iris/ciliary body and retina/choroid/sclera showed normal tissues in all the groups (Fig. 7). The histological data also reveals the biocompatibility of the tested formulation in this novel space.
Fig. 7.
Histology of rabbit ocular tissues at 4 weeks. (A) Cornea. (B) Retina, choroid, and sclera. (C) Iris and ciliary body. Original magnification ×40
Discussion
The delivery of anti-inflammatory agents to both anterior and posterior segments of the eye with minimal systemic exposure is a significant challenge. We have shown that the Bioerodible Dexamethasone Implant (BDI), designed to fit within the inferior fornix of the capsular bag, adjacent to the IOL, readily supersedes the limitations of both invasive and noninvasive delivery modalities.
The BDI implant was manufactured with components which have all been used safely in the eye. Bioerodible poly(d,l-lactide-co-glycolide), has been used in the Ozurdex implant, while hypromellose or hydroxypropyl methylcellulose (HPMC) is a semisynthetic, inert, viscoelastic polymer used as a irrigating liquid during cataract surgery [30]. Prior studies have successfully demonstrated the use of biodegradable polymeric systems in ocular [29] and non-ocular environments [31]. Others have evaluated the parameters that influence drug release kinetics in vitro [32–35].
Following BDI placement, sustained release of drug was observed up to 42 days and exhibited near zero order kinetics. In the aqueous and vitreous humor the exposure was dose related, while in retina/choroid and iris/ciliary body the exposure was dose dependent (AUC0-t).
The time to reach maximum concentration (Tmax) was comparatively slower at 14 ± 0 days with low dose but was rapid (7 ± 0 days) following the high dose therapy in aqueous humor. Vitreous humor Tmax was the same (28 ± 0 days) in low and high dose groups. We attributed this distinction to differential aqueous humor flow as this compartment is known to manifest higher turnover of fluid. DXM distributed to all ocular tissues and concentrations were measurable up to 42 days post implantation. DXM delivery was bidirectional and posterior segment concentrations were comparable to anterior segment concentrations by day 14 with high dose, which no other marketed ocular implants, can achieve. Retina/choroid and iris/ciliary body concentrations were higher compared to aqueous and vitreous humor, likely due to the lipophilic nature of DXM which is conducive to tissue accumulation. Drug concentration observed in the ocular tissue fit, from highest to lowest, the following hierarchy: iris/ciliary body, retina/choroid, vitreous humor, aqueous humor, and finally, serum. All the tissue concentrations are expected to be within the therapeutic window and there was no sign of inflammation or toxicity clinically, from slit lamp or histological examination.
With respect to intraocular pressure, while steroid induced glaucoma is a complication of long term steroid treatment. A retrospective case study in patients received fluocinolone acetonide (FA) intravitreal implant concluded that, patients receiving FA implants have a significant risk of increased IOP that frequently necessitates glaucoma surgery [36]. However, BDI did not induce any significant increase in IOP in this animal model throughout the study period (6 weeks).
Analysis of intraocular lenses from rabbits receiving BDI implants revealed that DXM was bound to the IOL (~0.1 to 0.3 μg/mL) in a dose and concentration dependent manner. This subsided, correspondingly, over time with decreasing local tissue concentration. Similar results were observed in animals treated with topical DXM drops in positive control group. Although lens clarity was not affected, whether this observation represents deleterious consequences to the IOL is unclear and will require further study.
Systemic exposure was minimal in both BDI groups. Serum concentrations of DXM were <40.0 ng/mL in all the rabbits up to day 42. As expected, and in line with published results [13–15], topical DXM drops led to much greater systemic exposure.
Above we describe the design and development of the bioerodible implant system with a dimension of 2.0 mm diameter and ~1.5 mm thickness, which can be inserted easily through standard small-incision cataract surgery wound. We tested this system, in vivo, and showed that it continuously releases DXM over a period of 42 days in NZW rabbits. The PLGA is a proven polymer with established biocompatibility that degrades to lactic and glycolic acid, both of which are further degraded into carbon dioxide and water before elimination. Drug-loaded implants are beneficial in that the polymer matrix is readily absorbed while drug is slowly delivered to both the front and back of the eye without obstructing vision. We will optimize the loading dose of the BDI in future GLP toxicity studies in rabbits. We will explore whether this form of continuous, bidirectional release of DXM will be able to control postoperative inflammation and uveitis without regard to patient compliance and therefore enhance clinical outcomes.
References
- [1].Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharmaceutical research. 1991;8:713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- [2].Suggs LJ, Mikos AG. Synthetic Biodegradable Polymers for Medical Applications. American Institute of Physics; Woodbury: 1996. [Google Scholar]
- [3].Shell JW. Ophthalmic drug delivery systems. Survey of ophthalmology. 1984;29:117–128. doi: 10.1016/0039-6257(84)90168-1. [DOI] [PubMed] [Google Scholar]
- [4].Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, BenEzra D, Behar-Cohen FF. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Investigative ophthalmology & visual science. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- [5].Baheti U, Siddique SS, Foster CS. Cataract surgery in patients with history of uveitis. Saudi Journal of Ophthalmology. 2012;26:55–60. doi: 10.1016/j.sjopt.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nair A, Thevenot P, Hu W, Tang L. Nanotechnology in the Treatment and Detection of Intraocular Cancers. Journal of biomedical nanotechnology. 2008;4:410–418. doi: 10.1166/jbn.2008.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. The British journal of ophthalmology. 1990;74:477–480. doi: 10.1136/bjo.74.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gupta R, Patil B, Shah BM, Bali SJ, Mishra SK, Dada T. Evaluating eye drop instillation technique in glaucoma patients. Journal of glaucoma. 2012;21:189–192. doi: 10.1097/IJG.0b013e31820bd2e1. [DOI] [PubMed] [Google Scholar]
- [9].Hennessy AL, Katz J, Covert D, Protzko C, Robin AL. Videotaped evaluation of eyedrop instillation in glaucoma patients with visual impairment or moderate to severe visual field loss. Ophthalmology. 2010;117:2345–2352. doi: 10.1016/j.ophtha.2010.03.040. [DOI] [PubMed] [Google Scholar]
- [10].Quek DT, Ong GT, Perera SA, Lamoureux EL, Aung T. Persistence of patients receiving topical glaucoma monotherapy in an Asian population. Archives of ophthalmology. 2011;129:643–648. doi: 10.1001/archophthalmol.2010.345. [DOI] [PubMed] [Google Scholar]
- [11].Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An objective evaluation of eyedrop instillation in patients with glaucoma. Archives of ophthalmology. 2009;127:732–736. doi: 10.1001/archophthalmol.2009.96. [DOI] [PubMed] [Google Scholar]
- [12].Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert opinion on drug delivery. 2007;4:371–388. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- [13].Weijtens O, Feron EJ, Schoemaker RC, Cohen AF, Lentjes EG, Romijn FP, van Meurs JC. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. American journal of ophthalmology. 1999;128:192–197. doi: 10.1016/s0002-9394(99)00129-4. [DOI] [PubMed] [Google Scholar]
- [14].Weijtens O, Schoemaker RC, Lentjes EG, Romijn FP, Cohen AF, van Meurs JC. Dexamethasone concentration in the subretinal fluid after a subconjunctival injection, a peribulbar injection, or an oral dose. Ophthalmology. 2000;107:1932–1938. doi: 10.1016/s0161-6420(00)00344-4. [DOI] [PubMed] [Google Scholar]
- [15].Weijtens O, Schoemaker RC, Romijn FP, Cohen AF, Lentjes EG, van Meurs JC. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 2002;109:1887–1891. doi: 10.1016/s0161-6420(02)01176-4. [DOI] [PubMed] [Google Scholar]
- [16].Short BG. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicologic pathology. 2008;36:49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- [17].Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert opinion on drug delivery. 2004;1:99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PubMed] [Google Scholar]
- [18].Ranta VP, Mannermaa E, Lummepuro K, Subrizi A, Laukkanen A, Antopolsky M, Murtomaki L, Hornof M, Urtti A. Barrier analysis of periocular drug delivery to the posterior segment. Journal of controlled release : official journal of the Controlled Release Society. 2010;148:42–48. doi: 10.1016/j.jconrel.2010.08.028. [DOI] [PubMed] [Google Scholar]
- [19].Lobo AM, Sobrin L, Papaliodis GN. Drug delivery options for the treatment of ocular inflammation. Seminars in ophthalmology. 2010;25:283–288. doi: 10.3109/08820538.2010.518522. [DOI] [PubMed] [Google Scholar]
- [20].Chang DT, Herceg MC, Bilonick RA, Camejo L, Schuman JS, Noecker RJ. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin Ophthalmol. 2009;3:345–355. doi: 10.2147/opth.s5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shiah J-G, Bhagat R, Nivaggioli T, Peng L, Chou D, Weber DA. Ocular implant made by a double extrusion process. Allergan, INC; United States: 2011. p. 36. [Google Scholar]
- [22].Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Investigative ophthalmology & visual science. 2011;52:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- [23].Allergan, Inc. Allergan receives FDA approval for Ozurdex® as treatment option for non-infectious uveitis affecting the posterior segment of the eye. 2010 Available online: http://agn.client.shareholder.com/releasedetail.cfm?ReleaseID=511060.
- [24].Bausch & Lomb Vitrasert® (ganciclovir intravitreal implant) 4.5 mg. Available online: http://www.bausch.co.nz/en_US/ecp/pharma/product/vitrasert.aspx.
- [25].Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Archives of ophthalmology. 2008;126:1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- [26].Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- [27].Dunn JP, Van Natta M, Foster G, Kuppermann BD, Martin DF, Zong A, Jabs DA. Complications of ganciclovir implant surgery in patients with cytomegalovirus retinitis: the Ganciclovir Cidofovir Cytomegalovirus Retinitis Trial. Retina. 2004;24:41–50. doi: 10.1097/00006982-200402000-00007. [DOI] [PubMed] [Google Scholar]
- [28].Haller JA, Bandello F, Belfort R, Jr., Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J, Li XY, Whitcup SM, Li J. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [29].Shelke NB, Kadam R, Tyagi P, Rao VR, Kompella UB. Intravitreal Poly(L-lactide) Microparticles Sustain Retinal and Choroidal Delivery of TG-0054, a Hydrophilic Drug Intended for Neovascular Diseases. Drug delivery and translational research. 2011;1:76–90. doi: 10.1007/s13346-010-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tahir MY, Baig RA, Ashraf KM, Babar M, Qazi ZA. Comparative Study of Endothelial Cell Loss after Phacoemulsification by Using 2% Hydroxypropyl Methylcellulose (HPMC) Versus 2.3% Sodium Hyaluronate (Healon 5) Pak. J Ophthalmol. 2008;24:175–178. [Google Scholar]
- [31].Cleek RL, Rege AA, Denner LA, Eskin SG, Mikos AG. Inhibition of smooth muscle cell growth in vitro by an antisense oligodeoxynucleotide released from poly(DL-lactic-co-glycolic acid) microparticles. Journal of biomedical materials research. 1997;35:525–530. doi: 10.1002/(sici)1097-4636(19970615)35:4<525::aid-jbm12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [32].Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel T. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. International journal of pharmaceutics. 2007;334:137–148. doi: 10.1016/j.ijpharm.2006.10.036. [DOI] [PubMed] [Google Scholar]
- [33].McGinity JW, O'Donnell PB. Preparation of microspheres by the solvent evaporation technique. Advanced drug delivery reviews. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- [34].Zolnik BS, Burgess DJ. Evaluation of in vivo-in vitro release of dexamethasone from PLGA microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2008;127:137–145. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [35].Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. Journal of controlled release : official journal of the Controlled Release Society. 2006;112:293–300. doi: 10.1016/j.jconrel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- [36].Bollinger K, Kim J, Lowder CY, Kaiser PK, Smith SD. Intraocular pressure outcome of patients with fluocinolone acetonide intravitreal implant for noninfectious uveitis. Ophthalmology. 2011;118:1927–1931. doi: 10.1016/j.ophtha.2011.02.042. [DOI] [PubMed] [Google Scholar]