Abstract
The presence in cancer tissue of Ag-specific, activated tumor infiltrating CD8+ T cells proves that tumors express Ags capable of eliciting immune response. Therefore, in general, tumor escape from immune-mediated clearance is not attributable to immunological ignorance. However, tumor-infiltrating lymphocytes are defective in effector phase function, demonstrating tumor-induced immune suppression that likely underlies tumor escape. Since exocytosis of lytic granules is dependent upon TCR-mediated signal transduction, it is a reasonable contention that tumors may induce defective signal transduction in tumor infiltrating T cells. In this review, we consider the biochemical basis for antitumor T cell dysfunction, focusing on the role of inhibitory signaling receptors in restricting TCR-mediated signaling in tumor-infiltrating lymphocytes.
Immune response to cancer is apparent; equally apparent is that tumors grow, implying escape from antitumor immunity (1) or defective antitumor immune responses (2). Multiple candidate mechanisms to account for failure of anti-tumor immunity have been described that involve a variety of cell types, factors, and mechanistic considerations (3). In murine models wherein tumor-bearing mice can be immunized with a variety of Ags (4), and patients in whom tumor-reactive Abs and T cells are commonly found (5), cancer does not cause defective systemic immune responses. Thus, tumor itself, or the host response, causes Ag-specific immune tolerance, almost certainly in the priming, and unequivocally in the effector phase of adaptive immunity, primarily in antitumor T cells resident in tumor tissue (6–8).
Priming of antitumor immune response is ineffectual to eliminate tumors
Detectable priming of antitumor T cells occurs during tumor growth but, because vaccination of patients can dramatically increase the frequency of antitumor T cells [in some cases resulting in a reduced rate of tumor growth (9)], either endogenous priming of antitumor immune response is insufficient to engender successful tumor elimination in patients receiving no therapy, or the effector phase is suppressed, or both. Analysis of APCs in murine tumors has shown that dendritic cells (DCs) are frequently defective in some aspect of priming: Ag capture, cytokine expression, costimulatory function, or migration to proximal lymph node (10). This results in diminished initiation of adaptive response to tumor Ags. In some cases, tumor DCs have been shown to be not only defective at priming but also tolerogenic (11, 12). Why tumor DCs do not function effectively as occurs in response to pathogens in which infection is resolved [e.g., Listeria monocytogenes (13)] is unclear but may be related to the kinetics of tumor growth (i.e., the dose of Ag available for priming, continual low amounts, as well as the lack of robust danger signals) (14). Similar observations have been made for DCs isolated from cancer patients (15, 16). An additional consideration is that, because many tumor Ags are closely related to self, cognate TCRs expressed in antitumor T cells that survive thymic selection are likely of low affinity and likely have enhanced activation requirements.
Furthermore, two immunosuppressive cell types, regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), have been shown to accumulate in tumors, both of which are thought to restrict the priming (17) and effector (18, 19) phases of adaptive immune response. Depletion or inactivation of Tregs (20) or MDSCs (21) enhances experimental immunotherapy in preclinical models, although data from clinical trials are less robust. The basis by which either Tregs or MDSCs inhibit priming is not definitively known, but these cells can produce a variety of molecules that are known to inhibit both DCs and T cell function including: TGFβ-1, IL-10, reactive oxygen and nitrogen species, and enzymes that are thought to either deplete the microenvironment of certain amino acids [arginine (22), tryptophan (23), and/or cysteine (24)] or produce toxic metabolites (25), therein leading either to a state of metabolic quiescence or induction of apoptosis in tumor-infiltrating lymphocytes (TILs) [although the notion of immune modulation by tryptophan metabolites has been questioned (26)]. Alternatively, or in addition, altered nitrogen metabolism in the context of enhanced production of reactive oxygen species is thought to produce highly reactive oxygen and nitrogen species that are capable of modifying both the cell surface (27) and enzyme activity within antitumor T cells (28). Postcoculture with T cells in vitro, MDSC-mediated production of reactive nitrogen has been shown to modify TCRs, resulting in diminished recognition by tetra-mer and reduced Ag-dependent lysis and cytokine release, a phenotype that may reflect inhibitory activity of those cells in tumor-draining lymph nodes (27, 29). Soluble bioactive molecules produced by Tregs and MDSCs that inhibit adaptive immunity, presumably by direct action on APCs in the priming phase, are also capable of potentially impacting other antitumor immune cell types in proximity, including NK cells and CD4+ and CD8+ T cells in the effector phase. In a mechanistic variation, purified MDSCs obtained from tumor (30) or peripheral lymphoid tissue (31) have been shown to cause apoptosis in activated T cells in vitro, implying a different role for MDSCs in dysregulation of the effector phase. However, TILs in situ are not appreciably apoptotic (32), thus arguing that the proapoptotic activity of either MDSCs or tumor cells is a function of in vitro analysis. Collectively considered, the extent to which MDSCs inhibit T cell signaling and function may reflect the tissue site of interaction with T cells (lymphoid or tumor) and as such may contribute to antitumor T cell dysfunction in addition to other candidate mechanisms, as described below.
The suppressed phenotype of TILs reflects the mechanism of the acquired functional defect and involves interference with the TCR-mediated signaling pathway
TILs have long been recognized as being deficient in cytokine release, proliferation, and lytic function (6). One potential mechanism for blockade of effector phase involves galectins, a family of carbohydrate binding proteins made in a variety of cells that have multiple functions but are thought to restrict the mobility of cell-surface T cell proteins important in Ag recognition and signaling (33). Such a mechanism has received support from several lines of investigation but is as yet incompletely understood and undoubtedly complex because exposure of immune cells to some galectins induce tolerance or death (34); in contrast, other galectins change the phenotype of T cells (35), whereas yet others enhance DCs and CD8+ T cell numbers (36).
Our laboratory has pursued a murine model of TIL dysfunction wherein the defective phenotype was transient, being regained upon purification and culture in vitro (37). CD8+ TILs have hallmarks of proximal TCR-mediated signaling blockade, interpreted to be the basis of defective lytic function [in freshly isolated TILs assayed in vitro (38), as judged inferentially because tumor cells proximal to TILs in situ are not TUNEL+ (32) or evaluated by confocal microscopy wherein lytic granules and the microtubule organizing center fail to polarize to the immune synapse (38, 39)]. Considered with other TIL phenotypes [cell cycle arrest, lack of Ag-dependent cytokine release in vitro, and the inability to arrest migration in tumor tissue (40, 41)], TILs appear to be deficient in Ag-dependent TCR-mediated signal transduction. A detailed biochemical analysis of TCR signaling was investigated in a murine model (42) wherein nonlytic TILs were shown to be triggered in that p56lck is activated upon recognition of cognate tumor cells (becoming phosphorylated on Y394) but the activation signal does not penetrate deeper into the signaling cascade because ZAP70 is not activated (37), calcium flux is extinguished (37) and activation of LFA-1–mediated TIL adhesion is deficient (43) [shown to require p56lck activity (44)]. After initial p56lck activation, Src homology region 2 domain-containing phosphatase (SHP)-1 was rapidly activated and localized to the immunological synapse coincident with dephosphorylation of p56lck Y394. Reversal of both defective proximal signaling and effector function is rapidly achieved by purification of TILs and brief culture in vitro in the absence of tumor. In a similar manner, reintroduction of the signaling block is rapidly achieved by coculture of TILs with tumor [and not with MDSCs or syngeneic but antigenically distinct tumor cells (42)], observations that are consistent with a mechanism involving a fast-acting biochemical inhibitory switch, one that requires contact with cognate tumor for activation (42).
TILs express inhibitory receptors that mediate negative signaling and effector phase dysfunction
Considered collectively, the requirement for tumor cell contact to induce the signaling defect and the rapid kinetics of induction of the TIL signaling block (42) implies that tumor-induced inhibition of TIL signaling involves a ligand–receptor interaction, one similar to that characteristic of inhibitory receptors expressed on NK cells (45). Cell surface inhibitory signaling receptors (IRs) that contain cytoplasmic ITIMs are expressed in a wide variety of immune cells and function in homeostatic regulation of immune responses wherein they negatively regulate signaling mediated by Ag (activating) receptors. Most IRs function in concert with triggering of the Ag receptor in a manner analogous to that of costimulatory receptors (e.g., B7), with the distinction that the activating signal is dampened or abrogated, and function in the homeostasis of normal responses important during cell differentiation and activation (46).
As a consequence of ligand binding, typically IRs are activated by tyrosine phosphorylation on a consensus structure ([I/V/L/S]-X-Y-X-X-[L/V]) by a kinase that is associated with the Ag receptor. Upon ITIM phosphorylation, phosphatase(s) are recruited and likely activated by the kinase associated with the Ag receptor. The activated protein tyrosine phosphatase (PTP) rapidly dephosphorylates proximal substrates, typically the Src family kinase associated with the Ag receptor (e.g., p56lck, but also additional proximal kinases [ZAP70] or adaptor proteins [TCRζ, Vav-1]). Thus, coordinately with Ag-dependent activation of p56lck, IRs are tyrosine phosphorylated, leading to recruitment of PTP and inactivation of the activation signal. In the course of induction of an activating immune response wherein a sufficiently strong positive signal is provided to the T cell such that a sustained triggering event occurs (i.e., a high concentration of cognate Ag presented by an appropriately activated APC), PTP-mediated inactivation of proximal signaling occurs following cell activation, reflecting the downregulation of T cell activation during differentiation of naive cells into effector cells. It makes sense conceptually that IRs on effector T cells restrict inadvertent expression of effector phase functions until the T cell recognizes a target cell expressing cognate Ag (as discussed below). Thus, IRs function as part of a system that integrates positive (activating) and negative (inhibitory) signals, therein both maintaining tonic balance and influencing cell activation thresholds. We hypothesize that the activity of IRs can be considered as cell- or organ-specific rheostats that control the magnitude or extent of T cell activation, and, because there are >100 human genes containing ITIM sequences, it is likely that IRs play an important role in regulation of immune responses. A compilation of IRs that can be expressed in T cells is shown in Table I.
Table I.
IR expression in T cells
| IR | Family | Ligand | Features | Reference |
|---|---|---|---|---|
| CD5 | Scavenger (cysteine-rich) | gp150, CD72 | Has a pseudo-ITAM; regulates FasL/caspase 8 activation; inhibits Ca flux and ERK activation; no PTP involvement | 54–58 |
| CD22 (Siglec-2) | Siglec | Sialic acid | Multiple ligands, ITIM | 59, 60 |
| CD31 (PECAM-e) | Ig superfamily | CD31, CD38, αvβ3 integrin | CD31 is expressed widely, ITIM | 61 |
| CD33 (Siglec-3) | Siglec | Sialic acid | ITIM | 62 |
| CD38* | Cyclic ADP ribose hydrolase | CD31 | No ITIM or PTP involvement, enzyme is soluble in serum, has both + and − function | 63 |
| CD66a (CEACAM-1) | Ig superfamily | Multiple | 18 genes in family; is expressed widely; multiple splice variants; has both + and − function; those with ITIM are inhibitory; function depends on oligomerization | 64–67 |
| CD72 | Cysteine-rich | CD5, CD100 | ITIM | 68 |
| CD73* | Ecto-5′-nucleotidase | GPI-linked, adenosine production from tumor | 69 | |
| CD85 (PIR-A/B in mice) | LILR | MHC class I | Some family members lack ITIM | 70 |
| CD94/NKG2A (KLRD1) | C-type lectin | HLA-E | CD94 has two forms: 39 kDa lacks ITIM, 43 kDa has ITIM (and recruits SHP-1); is expressed on NK cells, memory T cells, and DCs | 45 |
| CD152 (CTLA-4) | Ig superfamily | B7-1/2 | Also is expressed on Tregs; no ITIM (has YVKM that mediates biding of PI3K, PP2A, and SHP-2); CD276-B7-H3 and CD276-B7-H4 are orphan ligands | 23, 71–74 |
| CD155 (TIGIT) | Ig superfamily | Poliovirus receptor | Enhances DC-mediated Treg production; ITIM | 75 |
| CD158a | KIR | MHC class I | 14 genes in family; different members have + or − function; ITIM | 76, 77 |
| CD159a (NKG2A) | Ig superfamily | Pairs with CD94 | Is a multigene family; some lack ITIM | 78 |
| CD160 | Ig superfamily | HVEM | GPI-anchored, lacks ITIM; is expressed on CD44+ memory cells | 79 |
| CD161 (NKR-P1A) | C-type lectin | LLT1 | Is expressed on NK and CD8+ T cells | 80 |
| CD169 (Siglec-1) | Siglec | Sialic acid | Lacks ITIM; expressed on macrophage and activated DCs (may be activating on macrophage but inhibitory on DCs) | 81 |
| CD170 (Siglec-5) | Siglec | Sialic acid | CD33 related; ITIM | 82 |
| CD172a (SIRP-A) | Ig superfamily | CD47 | Recruits SHP-1/2; may interact with: Csk, SLAP-130, Grb2; is expressed in DCs and mast cells; some forms are activating; ITIM | 83, 84 |
| CD223 (LAG-3) | Ig superfamily | MHC class II | Widely expressed on lymphocytes; is related to CD4; effect is reversed by IL-2 | 85 |
| CD244 (2B4) | CD2 family | CD48 and CD244 | Is expressed on NK cells and T cells; has ITSM; binding to CD48 is inhibitory; binding to CD244 is activating | 86, 87 |
| CD272 (BTLA) | Ig superfamily | HVEM | ITIM | 88, 89 |
| CD279 (PD-1) | Ig superfamily | PDL-1 (CD274 = B7H1) and PDL-2 (CD273 = B7DC) | Recruits SHP-1/2, ITIM, and ITSM | 90, 91 |
| CD300a (IRp60) | Ig superfamily | Widely expressed; ITIM | 92, 93 | |
| CD305 (LAIR-1) | Ig superfamily | Collagen | Recruits SHP-1/2; may interact with Csk; ITIM | 94 |
| CD328 (Siglec-7) | Siglec | Sialic acid | Expressed in myeloid, NK cells, subsets of T cells; ITIM | 95 |
| gp49B-1 | Ig superfamily | αvβ3 integrin | ITIM | 96 |
| KLRG1 (MAFA-1) | C-type lectin | Cadherin | Expressed on T cells and NK cells; ITIM | 97–99 |
| Ly49 | C-type lectin | MHC class I | Part of a multigene family; some are activating; ITIM | 100 |
| MGL | C-type lectin | CD45 | Expressed on immature DCs | 101 |
| Galectin-3 | Galectin | β-galactoside | 15-member family; intracellular but translocates to surface upon activation | 102 |
| β1 integrin | Integrin | VCAM-1, soluble integrins | Recruits SHP-1; when bound to soluble integrin or ligated with monomeric Ab is inhibitory | 103 |
| TIM-3 | Ig superfamily | Galectin-9 | Multigene family; some activate (e.g., TIM-1); expressed on various immune cell types | 104 |
| T cell Src-binding proteins | May sequester p56lck away from functional signaling complexes, therein reducing T cell responsiveness | |||
| LAT | Modulates inhibitory feedback loop via Dok2/SHIP binding; homolog (LAB) induced upon activation | 105, 106 | ||
| PAG/Cbp | Ubiquitous; recruits Csk, ERM, RasGAP; also affects Ras | 107 | ||
| LIME | Deletion has only a modest effect on peripheral T and B cells | 108, 109 | ||
| SIT | Interacts with SHP-2 | 110 | ||
| TSAd | Rapidly induced in primary T cells by activation | 111–113 |
The common names of receptors are noted in parentheses. CD38 and CD73 (marked with *) are not true IRs per se but are involved in local production of adenosine thought to be immunosuppressive. Although not all IRs contain canonical ITIM elements (e.g., CTLA-4), an ITIM or a functional homolog on the prototypic IR functions to recruit SHP-1 to phosphorylated ITIM and thus into proximity with PTP targets.
Cbp, C-terminal Src kinase binding protein; Csk, C-terminal Src kinase; FasL, Fas ligand; HVEM, herpesvirus entry mediator; KIR, killer Ig-related (or Ig-like) receptor; KLRG1, killer cell lectin-like receptor G-1; LAIR-1, leukocyte-associated Ig-like receptor-1; LAT, linker for activation of T cells; LILR, leukocyte Ig-like receptor; LIME, LCK-interacting molecule; LLT1, lectin-like transcript-1; MAFA-1, mast cell function-associated Ag-1; MGL, macrophage galactose-type lectin; PAG, protein associated with glycosphingolipid-enriched microdomains; TIM-3, T cell Ig and mucin domain-3.
The majority of IRs are transmembrane plasma membrane proteins for which the extracellular portion contains recognition elements that govern ligand interaction. The variety of ligand–receptor interaction is considerable in terms of both the number of IRs and the number and type of ligands, leading to the consideration that T cell activation is under the constant influence, if not control, by this regulatory system. Because some IR ligands are widespread (e.g., MHC class I, sialic acid, collagen, and certain integrins), the notion of constant involvement of IR in the control of T cell activation seems plausible. Some IRs mediate homophilic interaction (e.g., CD31), therein restricting activation to a limited number of cell types; others interact with MHC Ag-presenting molecules both class I (CD85, CD158, Ly49) or class II (CD223), implying enhanced function during interaction with APCs.
Many IRs belong to families that share certain structural motifs, such as the Ig superfamily (e.g., CD31, CD66a, CD152) or the Siglec family (e.g., CD22, CD33, CD170). Because many IRs share related ligands (e.g., sialic acid), it is reasonable to consider that there exists overlap or redundancy in the types of target cells that can inhibit a given T cell. Almost all IRs contain at least one ITIM motif in their cytoplasmic domain, which, when phosphorylated, recruits SHP-1 (e.g., CD22), although some IRs contain sequence motif variants (e.g., immunoreceptor tyrosine-based switch motif, or ITSM), which are associated with recruitment of a related phosphatase SHP-2 (e.g., CD152 and CD279). In addition, some IRs have several closely related variants (e.g., CD66), some of which are restricted in terms of cell expression or lack a canonical ITIM sequence (e.g., CD152 or CD160). Variant IRs lacking ITIM can be activating rather than inhibitory in function, possibly due to contributions to T cell–target adhesion.
There are examples of IRs that contain no evident cytoplasmic motifs for recruitment of a PTP (e.g., β1 integrins or CD160, which is also unusual in being GPI anchored that can be released in soluble form), but which nonetheless function to recruit SHP-1. In these cases, perhaps the IR interacts with a cytoplasmic adapter protein that in turn is responsible for binding and recruiting a PTP into proximity with regulatory proteins in the signaling cascade wherein cell activation can be inhibited. In addition to their major role as rheostats of Ag-dependent signaling, there may be other nonsignaling functions of IRs that affect the behavior or activity of immune cells. For example, several IRs are mediators of adhesion (e.g., CD22, CD66a, β1 integrins), a function that may predominate in situations in which a T cell interacts with cells that express ligands for a given IR but do not express cognate Ag.
Functionally defective T cells in viral infection or cancer have been shown to express IRs (e.g., PD-1, LAG-3, or CTLA-4), and experimental therapeutic intervention based upon blocking IRs is extant. Tumor cells often express ligands (counterreceptors) for IRs and, as such, when the tumor cell is recognized by Ag-specific CTL, deliver a negative signal that blocks (or partially blocks) the TIL activation signal, thus restricting effector phase functions, to the detriment of the host. The phenotype of suppression of T cell activation by IRs was shown in 1997 in a mouse model wherein cytokine release and cytotoxicity was blocked upon engagement of NKG2A/CD94 by tumor MHC class I (47). Biochemical analysis of signaling showed that TCRζ was phosphorylated, demonstrating the cells were triggered, but ZAP70 was not activated, thus explaining how the functional defect was induced in that delivery of the activation signal deeper into the TCR signaling pathway was abrogated. [The detailed biochemical phenotype and functional defect was demonstrated more recently in a study of TILs (42)].
The factors that influence the basis of IR-mediated control of T cell activation are incompletely known but likely involve the following considerations. First, expression of IR ligands on the various cells that T cells contact—DCs during priming, endothelia during transit to the tissues, and ultimately target cells—is undoubtedly important in that receptor ligation is required for function and likely influenced by the differentiation and activation status of the cell. We hypothesize that interaction of IR with cognate ligands (on DCs, endothelia, or tumor cells) functions to recruit/stabilize IR into proximity with the Ag receptor/associated p56lck so that, in turn, the recruited PTP will be in proximity to the target kinase (Fig. 1). During activation of a productive immune response, DCs receive appropriate danger signals leading to their state of full competency, and we hypothesize that this includes modest (or repressed) expression of IR ligands: either levels of a given ligand, or the type/number of IR ligands, or both. A testable corollary of this notion is that suboptimal DC activation, such as that leading to differentiation of DCs having an inhibitory or tolerogenic phenotype, may lead to enhanced expression of inhibitory receptor ligands. Putative involvement of IR ligands in suppression of DC function may be in addition to expression of other mediators of inhibition (e.g., IDO) and insufficient levels of costimulatory molecules and activation-associated factors (IL-12).
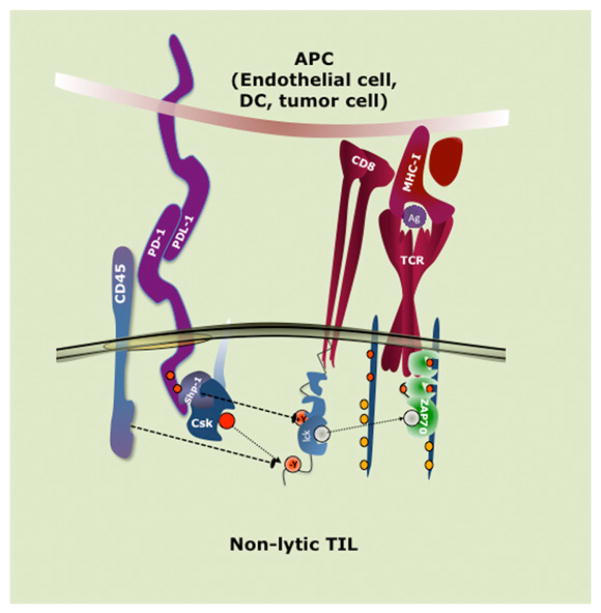
FIGURE 1.
Negative signaling in T cells mediated by IRs. T cells express a given inhibitory receptor (or multiple IRs, although only PD-1 is shown) for which the ligand can be expressed on DCs, endothelial cells, or tumor cells. When bound by its cognate ligand, IRs are phosphorylated on their ITIM and/or ITSM (which in turn reveals a PTP binding site, typically SHP-1 but possibly SHP-2) and brought into proximity to a Src kinase and become bound by PTP. PTP becomes activated by tyrosine phosphorylation, likely also by Src, and dephosphorylates proximal substrates, including the activating Src. Diminished p56lck-mediated activation of downstream signaling raises the activation threshold for the T cell.
Secondly, in a similar manner, the differentiation and activation state of the T cell may control the type and number of IRs that are expressed. For example, perhaps naive T cells express a different repertoire of IR than do effector or memory T cells, reflecting differing activation requirements of T cells in different differentiation states. According to this notion, for example, a memory T cell may express an IR for which the cognate ligand is expressed on endothelial cells that the T cell must traverse en route to interaction with its cognate Ag-expressing tissue/target cell. Thus, a T cell may express multiple IRs, some or any of which may not function until interaction with its cognate ligand occurs. Expression and activity of IRs in this context can be considered to provide another level of safety against inadvertent cytokine release or degranulation of lytic cells that may occur during extravasation that is mediated by activated adhesion molecules that, if they use inside-out signaling, may stimulate inadvertent effector phase activity. In other words, IR activity may be protective against tissue damage by raising the T cell activation threshold, therein restraining effector phase function until an authentic target cell is engaged. Supporting this notion, it was recently reported that multiple IRs are coexpressed on CD8+ T cells in chronically infected mice (48). Both of the IRs in that example (PD-1 and LAG-3) were functional, as shown by in vivo blockade experiments.
In a murine tumor model (MCA38), lytic defective CD8+ antitumor TILs were shown to express several IRs (Fig. 2), further supporting the notion that tumor-induced blockade of CTL signaling suppresses the effector phase and thus abets tumor escape. The observation that multiple distinct IRs are expressed in TILs illustrates the apparent redundancy of their potential functional regulation (CD5, CD85, CD94-NKG2A, and CD279). Curiously, postrecovery of TIL signaling and lytic function, the level of those IRs remained unchanged in purified lytic TILs, arguing against a role for any of those IRs in mediating TIL signaling defects. A similar observation was made in a pathogen model wherein multiple IRs were expressed in CD8+ T cells, but lytic function was not inhibited (49), suggesting that if any of the IRs detected by our flow analysis influence signaling in nonlytic TILs, perhaps the context of expression relative to target cell interaction may be important in their activity. This may be an important consideration because some IRs are expressed at elevated levels upon T cell activation. It is also possible that the IRs observed expressed in TILs do not function in the tumor environment; perhaps instead their ligands are expressed on endothelial cells where they can participate in restriction of the TIL lytic phase (or on other cells that contact the TIL during its transit into the tumor). Thus, it is possible that another as yet unidentified IR controls TIL function. Also of interest is the observation that multiple IRs are expressed in normal splenocytes (Fig. 2), raising the possibility that quiescent naive (or memory) cells depend on IRs to maintain tonic balance, possibly permitting a rapid response to a strong activation signal.
FIGURE 2.
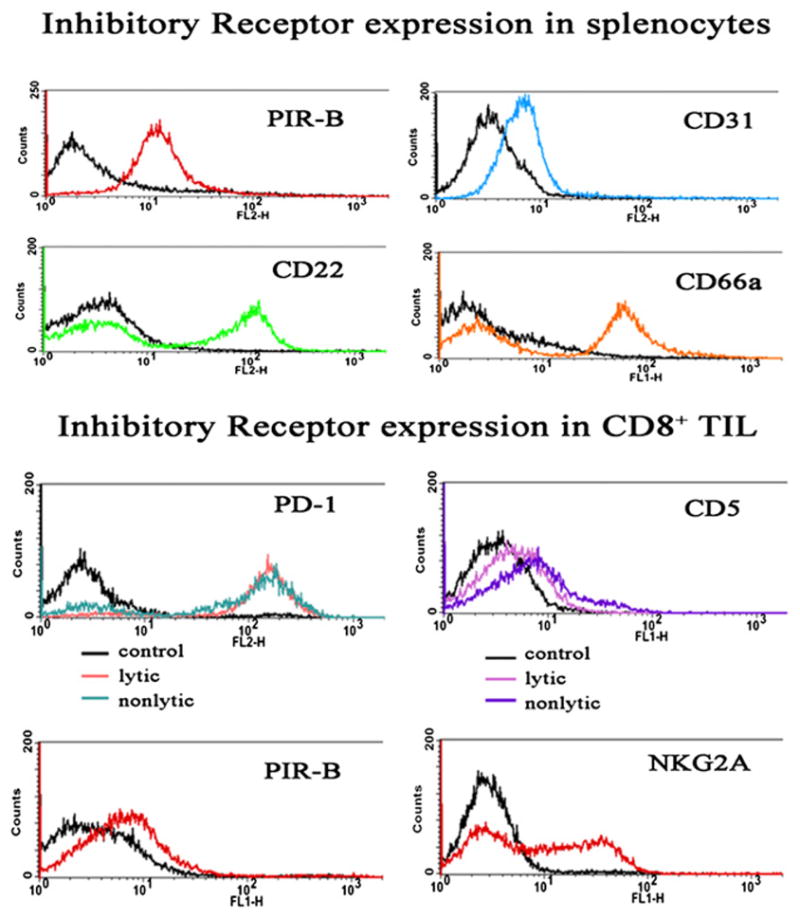
Expression of IRs in nonlytic and lytic MCA38-derived TILs. Splenocytes or TILs were prepared from control or MCA38 tumor-bearing mice as described (42) and analyzed by flow cytometry for expression of the indicated IRs. Isotype-matched nonimmune Ig was used as labeling control, shown in each panel as the black tracing. Staining of nonlytic TILs for expression of additional IRs was made (anti-CD22, anti-CD31, anti-Ly49 [C/I,G2, A, and D], and anti-CD66a) and were consistently negative (dns).
Several molecules are included in the list of IRs (Table I) even though they are not, strictly speaking, IRs, having very small extracellular domains unlikely to permit interaction with an extracellular ligand and no canonical ITIM. However, functionally, these proteins (LAT, PAG, LIME, SIT, and TSAd) may play a similar role as that of ITIM-bearing IRs in that by binding to p56lck its kinase function may be repressed, therein having the equivalent inhibitory effect on TIL function as does recruitment and activation of a PTP.
Several unanswered questions arise in consideration of the role of IRs in tumor escape from antitumor immune response. For example, if tumors express ligands for IRs that are capable of mediating blockade of effector functions at the site of the tumor, why can adoptively transferred T cells eliminate tumor? Although not definitively known, we hypothesize that priming or culture conditions for T cells in vitro cause downregulation of IRs, thus permitting effector phase functions upon adoptive transfer. Another conundrum arises in consideration of why transplantable regressor tumors fail to grow. Again, although not known, we hypothesize that either regressor tumors lack expression of IR ligands (and thus do not impede the effector phase), or priming of antitumor immune response during early-stage growth of this class of tumor does not elicit expression of IRs.
Conclusions
To summarize the potential role of IRs in the regulation of antitumor immunity: 1) Different IRs can be expressed by a given T cell at any time during its activation or differentiation; 2) A given IR may function to inhibit T cell responses depending upon the levels and activity of PTP able to interact with the IR; 3) Multiple different IRs can be expressed in a given T cell simultaneously; therefore, activity of a given IR is influenced by interaction of the T cell with cells expressing IR ligands; 4) Tumors can subvert antitumor immunity by expressing IR ligands.
To fully investigate the possibility that experimental tumor therapy based upon the notion that interference with IR–ligand interaction may enable more vigorous antitumor T cell functions, future research efforts might involve the following considerations. Firstly, it will be important to know if different tumors use the same or different mechanisms to induce T cell defects. Therefore, categorization of TILs from different tumor types in terms of the biochemical basis of signaling defect is an objective. Because some candidate mechanisms of defective TIL function are controversial [loss of TIL TCRζ (27, 42, 50, 51) or significant TIL apoptosis in situ due to Fas ligand expression by tumor cells (52)], it will be important to confirm in different laboratories any candidate mechanism of TIL defective signaling. In addition, it will be important to know the full panoply of IRs expressed in TILs. Therefore, detailed understanding of IR expression in TILs will be imperative. Similarly, it will be important to know the identity of IR ligands expressed in tumor cells and also to understand induction of IR ligand expression in tumor. Once characterized in terms of candidate TIL IR and tumor ligand expression, rational design and testing of inhibitors of either IRs or ligands can be made and may include blocking Ab, small molecule inhibitors, or aptamers (53). Finally, design and testing of systems for delivery of IR/ligand inhibitors should be considered. Because many IRs are expressed on non-T cells and if inhibited may be deleterious, perhaps a linked combination of targeting molecules can be employed to enhance targeting specificity—for example, using tetramers or T cell activation Ags.
Acknowledgments
This work was supported by National Institutes of Health Grants F31CA136164 (to E.J.V.-C.) and R01CA108573 (to A.B.F.).
Abbreviations used in this paper
- Cbp
C-terminal Src kinase binding protein
- Csk
C-terminal Src kinase
- DC
dendritic cell
- FasL
Fas ligand
- HVEM
herpesvirus entry mediator
- IR
inhibitory signaling receptor
- ITSM
immunoreceptor tyrosine-based switch motif
- KIR
killer Ig-related (or Ig-like) receptor
- LAIR-1
leukocyte-associated Ig-like receptor-1
- LAT
linker for activation of T cells
- LILR
leukocyte Ig-like receptor
- LIME
LCK-interacting molecule
- LLT1
lectin-like transcript-1
- MAFA-1
mast cell function-associated Ag-1
- MDSC
myeloid-derived suppressor cell
- MGL
macrophage galactose-type lectin
- PAG
protein associated with glycosphingolipid-enriched micro-domains
- PTP
protein tyrosine phosphatase
- SHP
Src homology 2 domain-containing tyrosine phosphatase
- TIL
tumor-infiltrating lymphocyte
- TIM-3
T cell Ig and mucin domain-3
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Parmiani G, Pilla L, Castelli C, Rivoltini L. Vaccination of patients with solid tumours. Ann Oncol. 2003;14:817–824. doi: 10.1093/annonc/mdg246. [DOI] [PubMed] [Google Scholar]
- 2.Zwirner NW, Croci DO, Domaica CI, Rabinovich GA. Overcoming the hurdles of tumor immunity by targeting regulatory pathways in innate and adaptive immune cells. Curr Pharm Des. 2010;16:255–267. doi: 10.2174/138161210790170175. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radoja S, Rao TD, Hillman D, Frey AB. Mice bearing late-stage tumors have normal functional systemic T cell responses in vitro and in vivo. J Immunol. 2000;164:2619–2628. doi: 10.4049/jimmunol.164.5.2619. [DOI] [PubMed] [Google Scholar]
- 5.Parmiani G. Tumor-infiltrating T cells—friend or foe of neoplastic cells? N Engl J Med. 2005;353:2640–2641. doi: 10.1056/NEJMp058236. [DOI] [PubMed] [Google Scholar]
- 6.Radoja S, Frey AB. Cancer-induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune-mediated killing. Mol Med. 2000;6:465–479. [PMC free article] [PubMed] [Google Scholar]
- 7.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Liénard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 8.Lüscher U, Filgueira L, Juretic A, Zuber M, Lüscher NJ, Heberer M, Spagnoli GC. The pattern of cytokine gene expression in freshly excised human metastatic melanoma suggests a state of reversible anergy of tumor-infiltrating lymphocytes. Int J Cancer. 1994;57:612–619. doi: 10.1002/ijc.2910570428. [DOI] [PubMed] [Google Scholar]
- 9.Koido S, Hara E, Homma S, Namiki Y, Ohkusa T, Gong J, Tajiri H. Cancer vaccine by fusions of dendritic and cancer cells. Clin Dev Immunol. 2009;2009:657369. doi: 10.1155/2009/657369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127:77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 13.Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 2007;9:1208–1215. doi: 10.10110/2/076/j.micinf.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerner MY, Mescher MF. Antigen processing and MHC-II presentation by dermal and tumor-infiltrating dendritic cells. J Immunol. 2009;182:2726–2737. doi: 10.4049/jimmunol.0803479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 16.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pachéco Y, Lebecque S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 17.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 18.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 19.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 20.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–11028. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 22.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 23.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber WP, Feder-Mengus C, Chiarugi A, Rosenthal R, Reschner A, Schumacher R, Zajac P, Misteli H, Frey DM, Oertli D, et al. Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Eur J Immunol. 2006;36:296–304. doi: 10.1002/eji.200535616. [DOI] [PubMed] [Google Scholar]
- 26.Löb S, Königsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 27.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota AM. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- 29.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saio M, Radoja S, Marino M, Frey AB. Tumor-infiltrating macrophages induce apoptosis in activated CD8(+) T cells by a mechanism requiring cell contact and mediated by both the cell-associated form of TNF and nitric oxide. J Immunol. 2001;167:5583–5593. doi: 10.4049/jimmunol.167.10.5583. [DOI] [PubMed] [Google Scholar]
- 31.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radoja S, Saio M, Frey AB. CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ. J Immunol. 2001;166:6074–6083. doi: 10.4049/jimmunol.166.10.6074. [DOI] [PubMed] [Google Scholar]
- 33.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 34.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 35.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 36.Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol. 2008;181:7660–7669. doi: 10.4049/jimmunol.181.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koneru M, Schaer D, Monu N, Ayala A, Frey AB. Defective proximal TCR signaling in inhibits CD8+ tumor-infiltrating lymphocyte lytic function. J Immunol. 2005;174:1830–1840. doi: 10.4049/jimmunol.174.4.1830. [DOI] [PubMed] [Google Scholar]
- 38.Radoja S, Saio M, Schaer D, Vukmanovic S, Frey AB. CD8+ tumor infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001;167:5042–5051. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- 39.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breart B, Lemaître F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67:11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koneru M, Monu N, Schaer D, Barletta J, Frey AB. Defective adhesion in tumor infiltrating CD8+ T cells. J Immunol. 2006;176:6103–6111. doi: 10.4049/jimmunol.176.10.6103. [DOI] [PubMed] [Google Scholar]
- 44.Morgan MM, Labno CM, Van Seventer GA, Denny MF, Straus DB, Burkhardt JK. Superantigen-induced T cell:B cell conjugation is mediated by LFA-1 and requires signaling through Lck, but not ZAP-70. J Immunol. 2001;167:5708–5718. doi: 10.4049/jimmunol.167.10.5708. [DOI] [PubMed] [Google Scholar]
- 45.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 2008;29:578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carena I, Shamshiev A, Donda A, Colonna M, Libero GD. Major histocompatibility complex class I molecules modulate activation threshold and early signaling of T cell antigen receptor-gamma/delta stimulated by nonpeptidic ligands. J Exp Med. 1997;186:1769–1774. doi: 10.1084/jem.186.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, Raulet DH. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 50.Franco JL, Ghosh P, Wiltrout RH, Carter CR, Zea AH, Momozaki N, Ochoa AC, Longo DL, Sayers TJ, Komschlies KL. Partial degradation of T-cell signal transduction molecules by contaminating granulocytes during protein extraction of splenic T cells from tumor-bearing mice. Cancer Res. 1995;55:3840–3846. [PubMed] [Google Scholar]
- 51.Levey DL, Srivastava PK. T cells from late tumor-bearing mice express normal levels of p56lck, p59fyn, ZAP-70, and CD3 zeta despite suppressed cytolytic activity. J Exp Med. 1995;182:1029–1036. doi: 10.1084/jem.182.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Restifo NP. Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med. 2000;6:493–495. doi: 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNamara JO, II, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 54.Friedlein G, El Hage F, Vergnon I, Richon C, Saulnier P, Lécluse Y, Caignard A, Boumsell L, Bismuth G, Chouaib S, Mami-Chouaib F. Human CD5 protects circulating tumor antigen-specific CTL from tumor-mediated activation-induced cell death. J Immunol. 2007;178:6821–6827. doi: 10.4049/jimmunol.178.11.6821. [DOI] [PubMed] [Google Scholar]
- 55.Gary-Gouy H, Lang V, Sarun S, Boumsell L, Bismuth G. In vivo association of CD5 with tyrosine-phosphorylated ZAP-70 and p21 phospho-zeta molecules in human CD3+ thymocytes. J Immunol. 1997;159:3739–3747. [PubMed] [Google Scholar]
- 56.Lenz LL. CD5 sweetens lymphocyte responses. Proc Natl Acad Sci USA. 2009;106:1303–1304. doi: 10.1073/pnas.0812579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van de Velde H, von Hoegen I, Luo W, Parnes JR, Thielemans K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature. 1991;351:662–665. doi: 10.1038/351662a0. [DOI] [PubMed] [Google Scholar]
- 59.Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr Opin Immunol. 2005;17:290–297. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Nitschke L, Carsetti R, Ocker B, Köhler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 61.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 62.Crocker PR, Varki A. Siglecs in the immune system. Immunology. 2001;103:137–145. doi: 10.1046/j.0019-2805.2001.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- 64.Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, Blumberg RS. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and co-stimulate human T cell function. J Immunol. 2004;172:3535–3543. doi: 10.4049/jimmunol.172.6.3535. [DOI] [PubMed] [Google Scholar]
- 65.Chen T, Zimmermann W, Parker J, Chen I, Maeda A, Bolland S. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J Leukoc Biol. 2001;70:335–340. [PubMed] [Google Scholar]
- 66.Chen Z, Chen L, Qiao SW, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol. 2008;180:6085–6093. doi: 10.4049/jimmunol.180.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y, Nadler MJ, Brennan LA, Gish GD, Timms JF, Fusaki N, Jongstra-Bilen J, Tada N, Pawson T, Wither J, et al. The B-cell trans-membrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr Biol. 1998;8:1009–1017. doi: 10.1016/s0960-9822(07)00421-6. [DOI] [PubMed] [Google Scholar]
- 69.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verbrugge A, Rijkers ES, de Ruiter T, Meyaard L. Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur J Immunol. 2006;36:190–198. doi: 10.1002/eji.200535226. [DOI] [PubMed] [Google Scholar]
- 71.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 72.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 73.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 74.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 75.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 76.Guerra N, Guillard M, Angevin E, Echchakir H, Escudier B, Moretta A, Chouaib S, Caignard A. Killer inhibitory receptor (CD158b) modulates the lytic activity of tumor-specific T lymphocytes infiltrating renal cell carcinomas. Blood. 2000;95:2883–2889. [PubMed] [Google Scholar]
- 77.Guerra N, Michel F, Gati A, Gaudin C, Mishal Z, Escudier B, Acuto O, Chouaib S, Caignard A. Engagement of the inhibitory receptor CD158a interrupts TCR signaling, preventing dynamic membrane reorganization in CTL/tumor cell interaction. Blood. 2002;100:2874–2881. doi: 10.1182/blood-2002-02-0643. [DOI] [PubMed] [Google Scholar]
- 78.Gunturi A, Berg RE, Forman J. The role of CD94/NKG2 in innate and adaptive immunity. Immunol Res. 2004;30:29–34. doi: 10.1385/IR:30:1:029. [DOI] [PubMed] [Google Scholar]
- 79.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 80.Rosen DB, Cao W, Avery DT, Tangye SG, Liu YJ, Houchins JP, Lanier LL. Functional consequences of interactions between human NKR-P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J Immunol. 2008;180:6508–6517. doi: 10.4049/jimmunol.180.10.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirchberger S, Majdic O, Steinberger P, Blüml S, Pfistershammer K, Zlabinger G, Deszcz L, Kuechler E, Knapp W, Stöckl J. Human rhinoviruses inhibit the accessory function of dendritic cells by inducing sialoadhesin and B7-H1 expression. J Immunol. 2005;175:1145–1152. doi: 10.4049/jimmunol.175.2.1145. [DOI] [PubMed] [Google Scholar]
- 82.Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J Biol Chem. 2005;280:19843–19851. doi: 10.1074/jbc.M502041200. [DOI] [PubMed] [Google Scholar]
- 83.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 84.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175:7781–7787. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 85.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 86.McNerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol Immunol. 2005;42:489–494. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 87.Vacca P, Pietra G, Falco M, Romeo E, Bottino C, Bellora F, Prefumo F, Fulcheri E, Venturini PL, Costa M, et al. Analysis of natural killer cells isolated from human decidua: Evidence that 2B4 (CD244) functions as an inhibitory receptor and blocks NK-cell function. Blood. 2006;108:4078–4085. doi: 10.1182/blood-2006-04-017343. [DOI] [PubMed] [Google Scholar]
- 88.Crawford A, Wherry EJ. Editorial: Therapeutic potential of targeting BTLA. J Leukoc Biol. 2009;86:5–8. doi: 10.1189/JLB.0209076. [DOI] [PubMed] [Google Scholar]
- 89.Derré L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, Olive D, Speiser DE. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175:7989–7995. doi: 10.4049/jimmunol.175.12.7989. [DOI] [PubMed] [Google Scholar]
- 93.Cantoni C, Bottino C, Augugliaro R, Morelli L, Marcenaro E, Castriconi R, Vitale M, Pende D, Sivori S, Millo R, et al. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J Immunol. 1999;29:3148–3159. doi: 10.1002/(SICI)1521-4141(199910)29:10<3148::AID-IMMU3148>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 94.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7:283–290. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 95.Nicoll G, Ni J, Liu D, Klenerman P, Munday J, Dubock S, Mattei MG, Crocker PR. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 96.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gründemann C, Bauer M, Schweier O, von Oppen N, Lässing U, Saudan P, Becker KF, Karp K, Hanke T, Bachmann MF, Pircher H. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- 98.Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203:289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olcese L, Lang P, Vély F, Cambiaggi A, Marguet D, Bléry M, Hippen KL, Biassoni R, Moretta A, Moretta L, et al. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 101.van Vliet SJ, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 2006;7:1200–1208. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 102.Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, Maverakis E, Yang RY, Hsu DK, Dustin ML, Liu FT. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci USA. 2009;106:14496–14501. doi: 10.1073/pnas.0903497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mary F, Moon C, Venaille T, Thomas ML, Mary D, Bernard A. Modulation of TCR signaling by beta1 integrins: role of the tyrosine phosphatase SHP-1. Eur J Immunol. 1999;29:3887–3897. doi: 10.1002/(SICI)1521-4141(199912)29:12<3887::AID-IMMU3887>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 104.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 105.Zhu M, Koonpaew S, Liu Y, Shen S, Denning T, Dzhagalov I, Rhee I, Zhang W. Negative regulation of T cell activation and autoimmunity by the transmembrane adaptor protein LAB. Immunity. 2006;25:757–768. doi: 10.1016/j.immuni.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 106.Lineberry N, Fathman CG. T cell anergy: where it’s LAT. Immunity. 2006;24:501–503. doi: 10.1016/j.immuni.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 107.Brdicka T, Pavlistová D, Leo A, Bruyns E, Korínek V, Angelisová P, Scherer J, Shevchenko A, Hilgert I, Cerný J, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brdicková N, Brdicka T, Angelisová P, Horváth O, Spicka J, Hilgert I, Paces J, Simeoni L, Kliche S, Merten C, et al. LIME: a new membrane Raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J Exp Med. 2003;198:1453–1462. doi: 10.1084/jem.20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grégoire C, Simova S, Wang Y, Sansoni A, Richelme S, Schmidt-Giese A, Simeoni L, Angelisova P, Reinhold D, Schraven B, et al. Deletion of the LIME adaptor protein minimally affects T and B cell development and function. Eur J Immunol. 2007;37:3259–3269. doi: 10.1002/eji.200737563. [DOI] [PubMed] [Google Scholar]
- 110.Marie-Cardine A, Kirchgessner H, Bruyns E, Shevchenko A, Mann M, Autschbach F, Ratnofsky S, Meuer S, Schraven B. SHP2-interacting transmembrane adaptor protein (SIT), a novel disulfide-linked dimer regulating human T cell activation. J Exp Med. 1999;189:1181–1194. doi: 10.1084/jem.189.8.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sundvold-Gjerstad V, Granum S, Mustelin T, Andersen TC, Berge T, Shapiro MJ, Shapiro VS, Spurkland A, Lea T. The C terminus of T cell-specific adapter protein (TSAd) is necessary for TSAd-mediated inhibition of Lck activity. Eur J Immunol. 2005;35:1612–1620. doi: 10.1002/eji.200425638. [DOI] [PubMed] [Google Scholar]
- 112.Spurkland A, Brinchmann JE, Markussen G, Pedeutour F, Munthe E, Lea T, Vartdal F, Aasheim HC. Molecular cloning of a T cell-specific adapter protein (TSAd) containing an Src homology (SH) 2 domain and putative SH3 and phosphotyrosine binding sites. J Biol Chem. 1998;273:4539–4546. doi: 10.1074/jbc.273.8.4539. [DOI] [PubMed] [Google Scholar]
- 113.Sundvold V, Torgersen KM, Post NH, Marti F, King PD, Røttingen JA, Spurkland A, Lea T. T cell-specific adapter protein inhibits T cell activation by modulating Lck activity. J Immunol. 2000;165:2927–2931. doi: 10.4049/jimmunol.165.6.2927. [DOI] [PubMed] [Google Scholar]