Abstract
Endothelial progenitor cells (EPCs) may provide novel opportunities for therapeutic angiogenesis after ischemic diseases. However, it is unclear how the angiogenic potential of EPCs might be affected by an inflammatory environment. We examine how the potent cytokine interleukin-1β (IL-1β) affects angiovasculogenic responses in EPCs in culture. Mononuclear cells isolated from mouse spleen were plated on fibronectin-coated wells and grown in EGM-2MV media. Endothelial progenitor cells were phenotyped using multiple markers (UEA-Lectin, ac-LDL, CD133, CD34, vWillebrand Factor, Flk-1) and to identify the IL-1 Receptor-I. We quantified cell and colony counts and performed MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide) and Matrigel assays, in vitro, under control and IL-1β (10 ng/mL) conditions. Endothelial progenitor cells exposed to IL-1β increased in the number of cells and colonies compared with untreated cells, without any effect on cell metabolic integrity. Furthermore, IL-1β treatment augmented EPC angiogenic function, significantly increasing the number of vessel-like structures in the Matrigel assay. An early phosphorylation of ERK1/2 occurred after IL-1β stimulation, and this pathway was inhibited if IL-1 Receptor-I was blocked. Our results suggest that IL-1β is a potent stimulator of in vitro angiogenesis through ERK signaling in mouse EPCs. Further studies are warranted to assess how interactions between proinflammatory environments and EPC responses may be leveraged to enhance therapeutic angiogenesis.
Keywords: angiogenesis, cytokine, MAP kinase, neuroinflammation, spleen, stroke
Introduction
Classically, the formation of new blood vessels was thought to be mediated exclusively by embryogenic vasculogenesis followed by the sprouting of endothelial cells from preexisting vessels during angiogenesis (Pepper, 1997; Risau and Flamme, 1995). In the last decade, this standard dogma was overturned when Asahara and colleagues reported the existence of circulating bone marrow-derived CD34+ hematopoietic progenitor cells from adult humans with endothelial characteristics (Asahara et al, 1997). Those cells, named endothelial progenitor cells (EPCs), were capable of differentiating, ex vivo, into endothelial-phenotyped cells, and now comprise a new model for endothelial generation and vessel repair.
Angiogenic responses will likely play an important role in multiple brain diseases such as cerebral ischemia, dementia, or brain trauma (Issa et al, 1999; Morgan et al, 2007; Pogue and Lukiw, 2004). Of special interest is the idea of promoting angiogenesis, perhaps through growth factor (e.g., vascular endothelial growth factor (VEGF)) stimulation, to improve and restore perfusion and function in ischemic brain after stroke. However, some caveats may exist in terms VEGF-induced alterations in BBB permeability (Valable et al, 2005; Zhang et al, 2000). An emerging alternate approach is to use cell-based therapies to potentiate angiovasulogenic responses in early stages of cerebral ischemia. It has been shown that neovascularization and neuronal regeneration can be successfully induced after delayed systemic administration of human cord blood-derived CD34 + in a mouse model of cerebral ischemia (Taguchi et al, 2004). More recently, Ohta and colleagues have showed that hyperacute administration of ex vivo expanded bone marrow-derived EPCs reduced both infarct and neurologic deficits in a focal ischemia-reperfusion rat model, by attenuating endothelial dysfunction (Ohta et al, 2006).
Within this context of using cell-based therapies in stroke, it is important to ask whether the inflammatory environment in the blood stream and brain might influence critical cell functions such as survival, adhesion, proliferation, or growth factor secretion. In animal models of stroke or brain trauma, multiple cytokines and chemokines can be upregulated (Offner et al, 2006; Kamm et al, 2006). Other authors have studied the role of additional cytokines on EPCs-angiogenic responses reporting opposite effects: while tumor necrosis factor-α compromises EPCs function (He et al, 2004; Seeger et al, 2005), interleukin (IL)-6 appears to increase EPC proliferation, migration and in vitro angiogenic-like tubulogenesis (Fan et al, 2008). Here, we focus on IL-1β, a potent immunoregulatory and proinflammatory cytokine secreted by a variety of activated immune cells. In human astrocytes, IL-1β induced a pattern of gene expression to favor vascular permeability involving the HIF-VEGF axis (Argaw et al, 2006). In human umbilical vein endothelial cells, IL-1β increases angiogenesis by augmenting VEGF protein and mRNA expression concomitant to a down-regulation of ANG-1, an endothelial cell stability factor (Fan et al, 2004). In bone marrow-derived cells, IL-1β triggered the increase in adhesion, survival, and endothelial differentiation (Qin et al, 2006). However, a recently published study by Henrich and colleagues suggests a harmful role for this cytokine, as IL-1β compromised cell survival in peripheral blood isolated human EPCs (Henrich et al, 2007). In a broader sense, IL-1β has been described to have both beneficial and detrimental actions in neuroinflammation and stroke. In this study, we isolated, characterized, and cultured EPCs from mouse spleen, which acts as a reservoir of peripheral blood and bone marrow-derived cells (Patschan et al, 2006), and, in vitro, we exposed them to an inflammatory environment through IL-1β stimulation. Our goal is to assay EPC responses and to assess whether IL-1β stimulation can affect the potential vasculogenic response of these EPCs in vitro.
Materials and methods
Endothelial Progenitor Cell Isolation and Culture
Spleens were used for the obtaining of EPCs for mice as described earlier (Brühl et al, 2004). For each independent experiment, a pool of 10 to 20 spleens from 10 weeks old CD-1 males were obtained and kept in HBSS solution (1 mmol/LEDTA and 4% BSA). Under the hood, spleens were mechanically minced, placed at 37°C for 15 mins and run thorough a 40-µm nylon membrane to obtain cell suspension. Mononuclear cells (MNCs) were obtained by density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences Corp) as described earlier (He et al, 2004). Isolated MNCs were shortly washed with red blood cells lysis solution (150 mmol/L NH4Cl, 10 mmol/L NaHCO3 and 0.1 mmol/L EDTA in distilled water) and gently washed twice with complete growth media EGM-2 MV (CC-3202; Lonza, Walkersville, MD, USA) consisting of endothelial basal medium-2, 5% fetal bovine serum, hEGF, VEGF, hFGF-B, IGF-1, ascorbic acid, and heparin. Isolated MNCs were finally resuspended in EGM-2MV and 3 × 107 MNCs per well were seeded on fibronectin-coated (Sigma, St Louis, MO, USA) six-well plates and incubated in a 5% CO2 incubator at 37°C. Under daily observation, first media change was performed 3 days after plating and, thereafter, media was changed every 2 days. Attached and spindle-shaped cells were observed from first media change at day 3 and cell colonies (> 50 tight cells) appeared between days 10 and 15. At 3 to 4 weeks, subconfluent colonies were trypsinized for 3 mins and subsequently cultured in T-25 flasks in EGM-2MV to obtain late outgrowth EPC.
Immunophenotyping of Spleen-Derived Endothelial Progenitor Cells
Fluorescent chemical detection of the so-called ‘early EPCs’ with spindle shape was performed on attached cells after 5 to 12 days in culture. Direct fluorescent staining was used to detect lectin binding with FITC labeled Ulex Europaeus Agglutinin (UEA)-1 (Sigma, St Louis, MO, USA) along with uptake of 1,19-dioctadecyl-3,3,39,39-tetramethylindocarbocyanine (DiI) labeled acetylated low density lipoprotein (ac-LDL; Molecular Probes, Eugene, OR, USA). Cells were first incubated with 5 µg/ml ac-LDL at 37°C for 2 h in EGM-2 MV, washed and fixed with 4% paraformaldehyde for 10 mins. After washes with PBS, the cells were incubated with FITC-UEA-1 (10 µg/ml) for 1 h. In parallel, surface-antigen expression of markers such as CD133 (Prominin-1), CD34, Flk-1 (VEGFR2) and von Willebrand Factor (vWF) were performed. Briefly, cells were washed with PBS twice and fixed with 4% paraformaldehyde for 15 mins. Later, blocking was performed with 1%BSA in PBS for 1 h followed by permeabilitzation with 0.1% Triton X-100 in PBS for 10 mins. Primary antibodies (Santa Cruz, CA, USA) were diluted 1:200 in blocking buffer and cells incubated overnight at 4°C. After 16 to 18 h incubation, cells were washed with PBS and secondary antibodies added at 1:1000 in PBS for 1 h at RT. Finally, all cells were counter-stained with Vectashield mounting media with DAPI (Vector Laboratories; Burlingame, CA, USA) and cells visualized with a fluorescent microscope. Cells showing doublepositive fluorescence for Lectin/ ac-LDL uptake and positivity CD133, CD34, vWF, and Flk-1 markers were identified as differentiating early EPCs. Additional immunostaining for IL-1β Receptor-I (R&D Systems, Minneapolis, MN, USA) was performed on growing early EPCs following the same protocol. Negative controls were performed to detect autofluorescence or secondary antibody background.
IL-1β Treatments
Lyophilized IL-1β was purchased from R&D Systems (rhIL-1β/IL-1F2) and diluted in a PBS solution at 10µg/mL stock solution and froze at −20°C until use. For treatments, IL-1β stock solution was directly diluted in culture media. A set of dose-dependant preliminary experiments were performed to ensure IL-1β stimulation on EPCs at 1 ng/mL, 5 ng/mL, and 10ng/mL. To further show IL-1β specific activity, additional experiments were performed earlier blocking the IL-1 Receptor-I (5 µmg/mL for 5 h). The treatment regimen consisted in continuous or acute exposure to IL-1β. Groups exposed to continuous treatment, IL-1β was given at the moment of seeding (day 0) and again when first media change was performed (day 3). For colony formation assessment, chronic treatment was repeated at days 5 and 7 before colony counting at day 10. However, the group exposed to acute stimulation was treated with IL-1β only at day 3 and for 24 h. Finally, a nontreatment group was always included as a control. All the results were obtained from experiments performed at least per duplicate in a minimum of three independent assays.
Western Blot
Endothelial progenitor cells were grown 12-well plates and at day 5 were used for western blotting. Five hours before IL-1β treatment, the cells were washed twice with endothelial basal medium and then incubated with or without IL-1 Receptor-I antibody. After 15-min IL-1β treatment (10 ng/mL), EPCs were rinsed twice with ice-cold phosphate buffered saline and collected into 75 µL SDS sample buffer. Samples were heated at 95°C for 5 mins, and then each sample (20 µL per lane) was loaded onto 4% to 20% Tris-glycine gels. After electorophoresis and transferring to polyvinylidene difluoride membranes, the membranes were blocked in Tris-buffered saline containing 0.1% Tween 20% and 0.2% I-block (Tropix) for 120 mins at room temperature. Membranes were then incubated overnight at 4°C with monoclonal anti-p-ERK antibody or antiactin antibody followed by incubation with peroxidase-conjugated secondary antibodies and visualization by enhanced chemiluminescence (Amersham).
Cell Number Quantitation
A 3 × 107 MNCs per well were seeded on fibronectincoated six-well plates. After 3 days in culture, cells were gently washed with complete media and fresh EGM-2MV was replaced with appropriate treatment. Using an inverted microscope, pictures were taken in five random fields (200 × magnification). Cells were kept in the incubator and, at day 5, pictures were taken again following the same protocol. Later on, the number of total and spindle-shaped cells was counted on photomicrographs taken by a masked investigator.
MTT Assay
Measurement of the reduction of 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT) to produce a dark blue formazan product was performed to assesses the integrity of mitochondrial function as a measure of cell viability. Briefly, 1×107 MNCs were seeded on 12-well plates in EGM-2MV and only the group receiving continuous IL-1β stimulation was treated. Later, at day 3, cells were gently washed twice with endothelial basal medium-2 and treatment with IL-1β was added to both groups receiving continuous and acute treatment in basal fresh media. After 24 h incubation, MTT solution (Sigma, St Louis, MO, USA) was prepared at 0.5mg/mL with fresh basal media and added to cells. After 90mins incubation at 37°C, the medium was replaced by dimethyl sulfoxide to solubilize the formazan. The absorbance of this colored solution was quantified by spectrophotometry at 570 nm wavelength. Dimethyl sulfoxide absorbance was substracted to all sample measurements to allow standaritzation, each sample was measured per duplicate to obtain a mean value and results for treatments are expressed as a percentage of the control group absorbance.
Tube Formation Assay
Matrigel reduced growth factor (BD; San José, CA, USA) was used as a substrate to assess tube formation capacity as an in vitro measurement of functional angiogenesis. Standard 24-well plates were coated with 200 µL of cold Matrigel and allowed to solidify at 37°C for 30 mins. Afterwards, outgrown EPCs (passages 3 to 6) were added onto matrigel-coated wells at seeding density of 4 × 104 cells per well in EGM-2MV media containing IL-1β treatment or not. Even distribution was ensured by microscope visualization. Cells were incubated for 24 h at 37°C, and tube formation was assessed by counting the number of circular structures (named rings) and number of branching points (named tube joints) per low powered field (100 × magnification) in three random fields. Counting was masked for treatment.
Statistical Analyses
SPSS 12.0 package was used for statistical analyses. Statistical significance was evaluated using the unpaired t-test to compare differences between the two groups and ANOVA followed by Bonferroni tests for multiple comparisons. Values are given in mean ± s.e.m. and some are expressed as a percentage versus the control group. Differences at P < 0.05 were considered to be statistically significant.
Results
Characterization of Murine Endothelial Progenitor Cells
Mononuclear cells obtained from mouse spleen contained a subpopulation that ended up displaying characteristics and markers representative of EPCs, when grown in fibronectin-coated plates in specific growth factor media (EGM-2MV). There remains a lack of full consensus in the literature regarding the precise nomenclature of EPCs. In this study, we follow a previously defined terminology (Hur et al, 2004). Early cells with spindle shape were termed ‘early EPCs,’ whereas ‘outgrowth EPCs’ referred to late cells derived from discretely identified growing colonies.
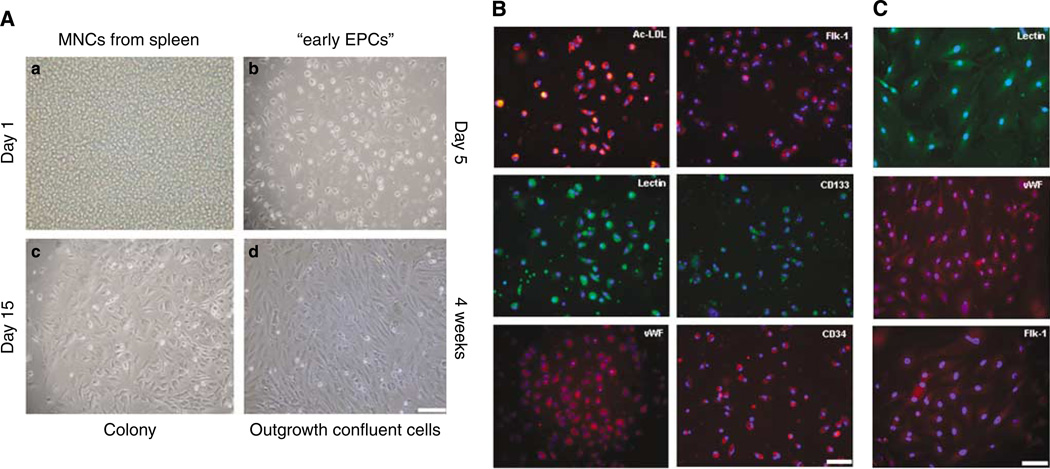
Overall, splenic MNCs seeded at high concentration (3 × 106 cell/cm2) at day 1 presented with a round shape (Figure 1A–a) and only some cells were attached to the bottom of the plate after the first media change at day 3. In our system, early EPCs with spindle shape started to appear from day 3 (Figure 1A–b) together with round differentiating cells. The panel of markers including endothelial, haematopoietic, and progenitor markers showed our growing enriched EPC population (Figure 1B). After 10 days in culture, some differentiated groups of cells appeared as colonies, comprising groups of 50 or more cells with typical cobblestone shape in the center of the colony, peripheral expansion, and continued growth over the next several weeks (Figure 1A, c–d). Cells from subconfluent expanded colonies were harvested and continued on a fibronectin coating, to obtain outgrowth EPCs. Immuno-phenotyping showed their endothelial lineage (Figure 1C).
Figure 1.
Spleen-derived EPCs phenotyping: (A) Seeded cells at day 1 (a), after 5 days in culture attached cells showing characteristic spindle shape of enriched-early EPC population (b), edge of a expanding colony (c), and confluent outgrowth EPCs (d). Bar =100 µm. (B) EPCs between day 5 and 12 were able to uptake acetylated low density lipoprotein (ac-LDL) and bind to a Lectin (Ulex Europaeus Aglutinin). Endothelial progenitor cells showed positive staining for endothelial, progenitor, and haematopoietic cell markers such as vWF, Flk-1, CD133, and CD34. Blue signal corresponds to nuclear DAPI staining. Bar= 100 µm. (C) Endothelial immunophenotyping to outgrowth cells at early passage. Blue signal corresponds to nuclear DAPI staining. Bar=50 µm.
Endothelial Progenitor Cell Viability/Proliferation is Increased by IL-1β
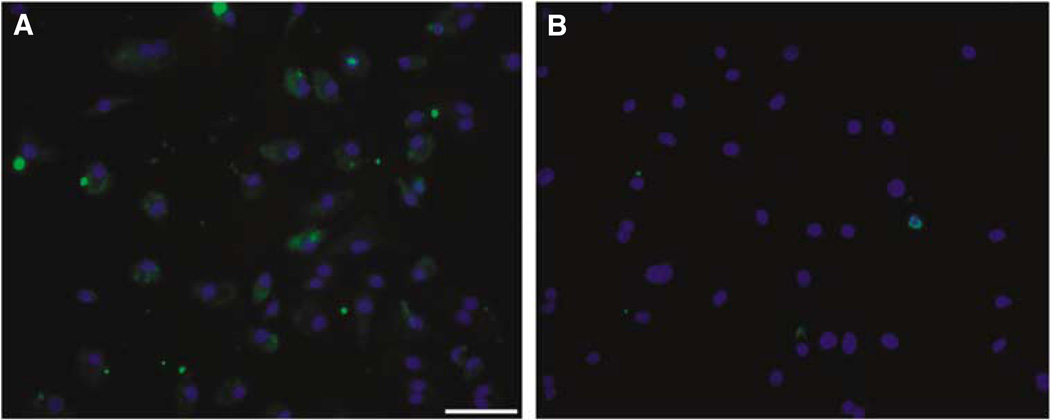
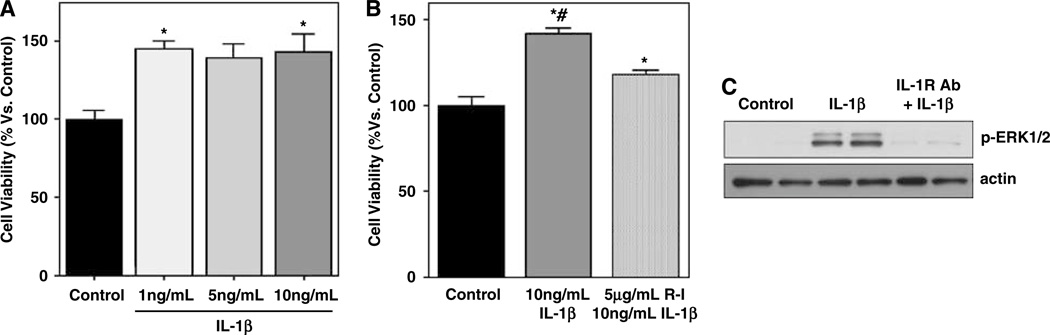
Immunostaining confirmed the presence of IL-1 Receptor-I in our early EPC cultures (Figure 2). Early EPCs were then exposed to IL-1β (0, 1, 5, and 10 ng/ mL) for 24 h and quantification of MTT reduction was obtained as an indirect and integrated measure of both EPC viability and proliferation. None of the IL-1β concentrations showed toxic effects. Endothelial progenitor cell viability/proliferation was increased by IL-1β, with the most reproducible responses occurring at 10ng/mL concentrations (Figure 3A). To further support specificity, we tested IL-1β together with an IL-1 Receptor-1 antagonist. Whereas stimulation with 10ng/mL of IL-1β resulted in a 142.1%±2.8% increase in cell viability/proliferation, cotreatment with the receptor blocker decreased responses down to 117.4%±2.9% (Figure 3B).
Figure 2.
IL-1 Receptor-I immunostaining. (A) Photomicrograph showing EPC positivity for IL-1 Receptor-I at day 6 of culture (green). (B) Photomicrograph showing a negative control. Blue signal corresponds to DAPI nuclear staining. Bar= 100 µm.
Figure 3.
(A) MTT assay showing a dose-response study for IL-1β stimulation. (B) Graph showing that the increase of MTT by IL-1β is reduced by blocking the IL-1 Receptor-I. (C) Representative western blot showing the induction of phosphorylated ERK1/2 at 15 mins after IL-1β treatment. This activation was almost totally prevented when IL-1β receptors were blocked with an IL-1 Receptor-I antibody. Equal loading was confirmed by actin content. *P < 0.05 versus control and #P < 0.05 versus IL-1 Receptor-I blocking.
Mitogen-Activated Protein Kinase Signaling Pathways
To support the signaling effects of IL-1β, we assessed the ERK mitogen-activated protein (MAP) kinase pathway. Immunoblots showed that phospho-ERK1/2 levels were clearly increased after IL-1β stimulation of the early EPCs (Figure 3C). Cotreatment with the IL-1 Receptor-I blocker almost completely abolished the phosphorylation of ERK1/2 (Figure 3C). Taken together with the viability/proliferation data, these findings suggest that ERK signaling is critical for IL-1β-induced responses in our EPC system.
IL-1β Increases Endothelial Progenitor Cells Proliferation In Vitro
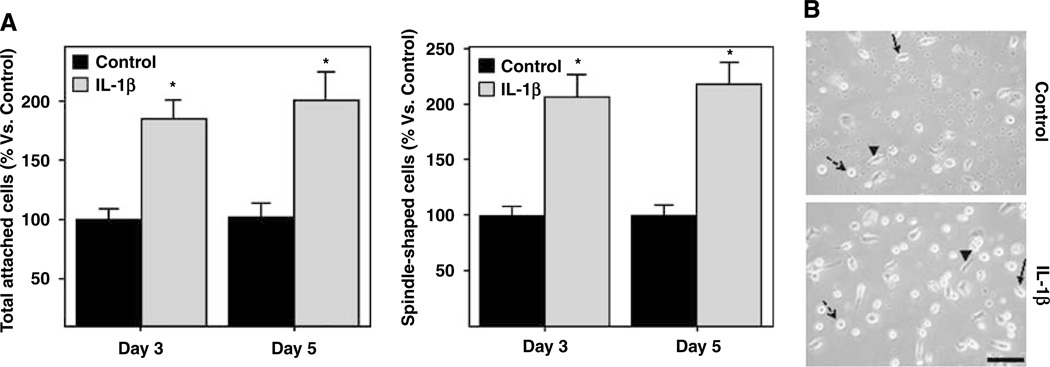
On day 3, a total of 42.1±3.7 cells per field were counted under IL-1β-treated conditions versus 22.9±2.2 for controls, reflecting in a 184% increment (P = 0.001; Figure 4A). By day 5, a total of 32.3±3.9 cells per field were counted under IL-1β-treated conditions versus 16.6±1.8 in control wells, resulting in a 199% increment (P =0.005; Figure 4A). The number of differentiated EPCs with spindle shape was also higher in the IL-1β-treated group than in the controls at day 3 (18.3±1.7 and 8.8±0.7, respectively) and at day 5 (17.5±1.5 and 8±0.8, respectively), resulting in a 206% and 218% mean increments when compared with control conditions (P < 0.001; Figure 4A). Representative cellular density and EPC differentiation at day 5 in control versus IL-1β conditions are shown in Figure 4B. Although the absolute number of cells decreased between days 3 and 5, this was not affected by the treatment (P > 0.1).
Figure 4.
Cell counting of total and spindle-shaped cells. (A) Bar graphs represent the percentage of IL-1β-treated cells versus corresponding control. IL-1β significantly increased the number of both total and EPC differentiated cells at days 3 and 5. (B) Cell density observed at day 5 after seeding in both control and treatment conditions. Notice the presence of round cells (dashed arrow), spindle-shaped cells (arrow head), and some cells in process of differentiation (arrow). Three independent experiments with 2 or 3 wells per condition were quantified. *P < 0.05. Bar=100 µm.
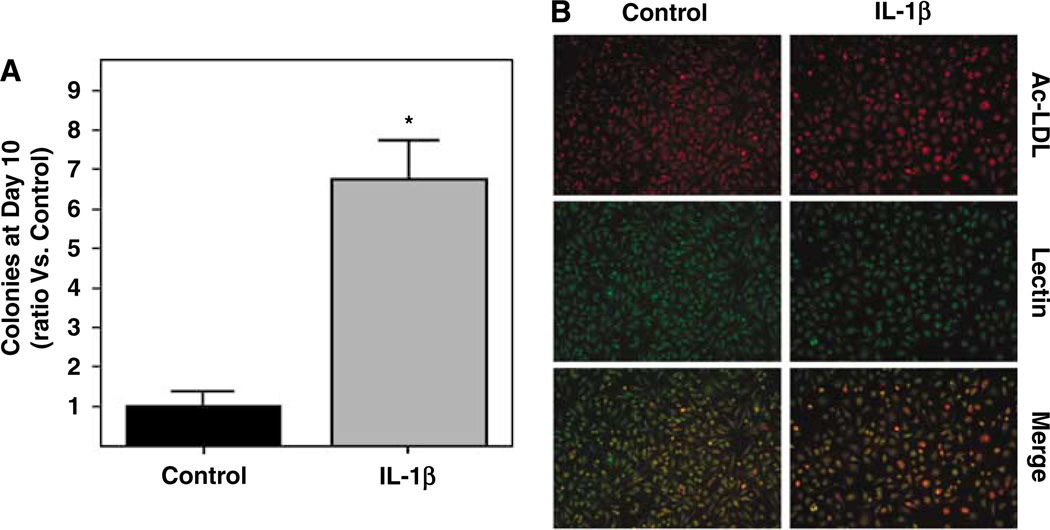
The number of colonies, defined as clusters of expanding EPCs with 50 cells or more, was significantly increased by continuous IL-1β treatment compared with untreated cells. Whereas some control wells did not yield any colonies at all on day 10, all IL-1β-stimulated cells formed colonies. A six-fold increase in the number of colonies was detected (P < 0.001, Figure 5A). Additional immunostaining performed on appearing colonies confirmed the endothelial phenotype of these late-outgrowth EPCs (both in treated and nontreated conditions, Figure 5B).
Figure 5.
IL-1β treatment increased the number of expanding colonies at day 10 of culture. (A) Bar graphs representing the number of colonies expressed as a ratio versus control conditions. *P < 0.05. Three independent experiments with 2 or 3 wells per condition were quantified. (B) Colonies of EPCs showed Lectin binding and ac-LDL uptake both in control and IL-1β treatment conditions.
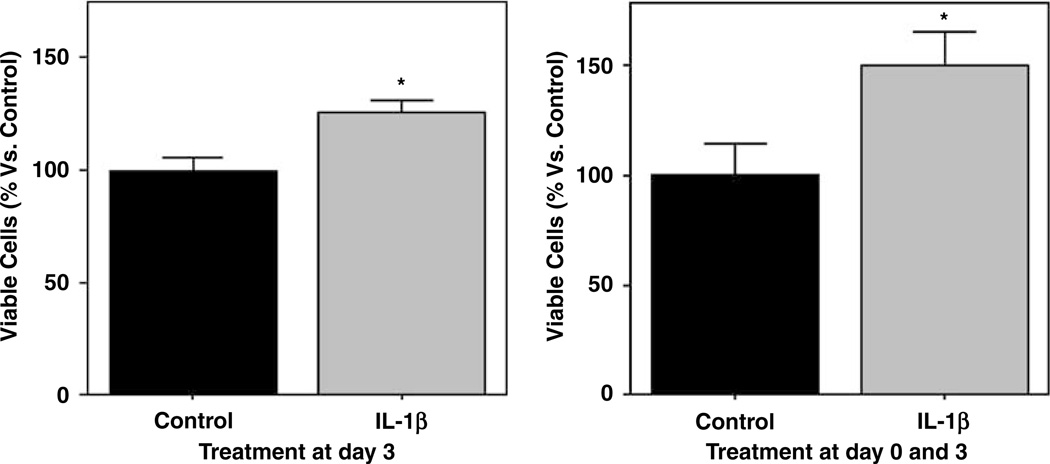
Because gross cell counting can incorrectly include dying cells, another measurement of cell proliferation was performed. The standard MTT assay showed that the percentage of viable cells was significantly increased by IL-1β treatment compared with controls (Figure 6). Sustained IL-1β treatment starting on day 0 and continued for 4 days raised the mean MTT values to 149% compared with controls (P= 0.029). Acute IL-1β stimulation on just day 3 alone for 24h also significantly elevated MTT values by 126% compared with controls (P= 0.005; Figure 6).
Figure 6.
Bar graphs representing MTT assay results to assess cell viability/proliferation. The percentage of viable cells after 24 h and 4 days with continuous IL-1β treatment increased significantly. The increase in the number of viable cells after 24h treatment at day 3 confirm the effect of IL-1β on EPC proliferation, whereas the continuous treatment from day 0 and during 4 days explain an effect on both attachment and proliferation. Three independent experiments with 3 wells per condition were quantified *P < 0.05.
IL-1β Amplifies Matrigel Tube Formation
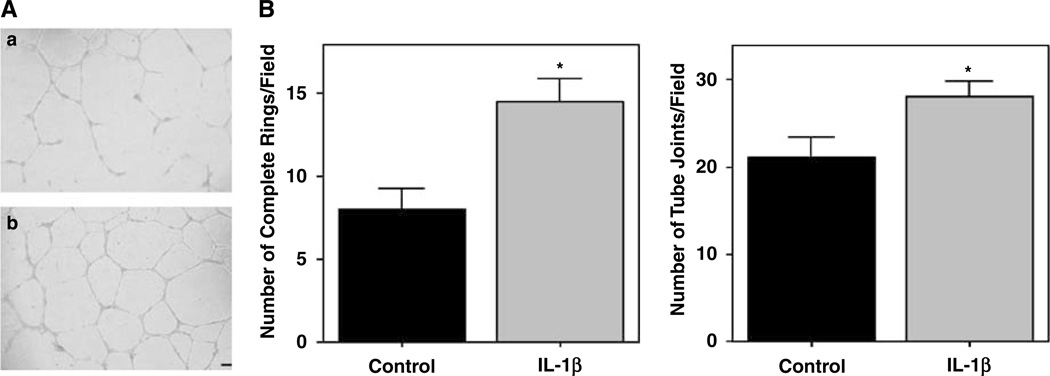
In vitro angiogenesis was assessed by tube formation on a standard Matrigel assay. Outgrown EPCs were used for these assays. IL-1β stimulation appeared to amplify the vasculogenic-like function with the clear formation of more tube-like structures compared with controls (Figure 7A). Two different parameters were increased by IL-1β: the number of complete ring structures (14.5±1.4 versus 8±1.2; P=0.005) and the number of tube joints or branches that the cells were able to establish between each other (28.1±1.7 versus 21.1±2.2; P=0.029; Figure 7B).
Figure 7.
Effect of IL-1β on angiogenesis in vitro. Tube formation assays on a Matrigel matrix showed a strong potentiation of functional angiogenesis by IL-1β as more tube-like structures were formed. (A) Capillary-like morphogenesis after 24 h in control (a) and IL-1β (b) treatment conditions. Bar =100 µm. (B) The number of complete rings (circular structures) and tube joints (branching points between cells) showed a significant increase when EPCs were exposed to IL-1β treatment. Three independent experiments with 2 or 3 wells per condition were quantified. *P < 0.05.
Discussion
Endothelial progenitor cells represent a new frontier in cell-based therapies for stroke and other CNS diseases. Because circulating EPCs can be isolated from blood, they comprise a relatively easy source of cells with the potential for vascular and tissue repair. However, there are still many gaps in current knowledge. Definition and classification of EPC lineages and markers are still evolving. The true multipotency of these cells remains to be fully elucidated. And how EPCs might respond in normal versus a diseased or inflammatory milieu has to be sorted out. There is some controversy in this latter point. Some have proposed that inflammatory cytokines promote endothelial differentiation (Qin et al, 2006), whereas others propose that inflammation induces EPC cell death (Henrich et al, 2007). Our present study shows that IL-1β, a potent cytokine known to be upregulated in a broad range of neuroinflammatory responses after stroke and other brain diseases, enhances the angiogenic responses of mouse-spleen-derived EPCs in vitro without any effect on cell metabolic integrity. These data suggest that EPC responses might be augmented as an endogenous repair response in stroke.
Because acute neuroprotective therapies for stroke have not been successful thus far, attention has begun to turn to regenerative strategies to promote repair. Emerging studies now suggest that neurorecovery is potentially possible thorough various cell-based therapies. For example, Taguchi and colleagues showed that both neovascularization and neuron regeneration may be induced after delayed systemic administration of human cord blood-derived CD34 + in a mouse model of cerebral ischemia (Taguchi et al, 2004). Other efforts have shown that ex vivo expanded EPCs can reduce hind limb vascular injury (Kajiguchi et al, 2007), myocardial infarction (Kawamoto et al, 2001), and cerebral infarction (Ohta et al, 2006) in ischemic diseases and accelerate the recovery of endothelial function in damaged vessels (He et al, 2004). Of course, the underlying mechanisms are complex. Besides potentially serving as a source of cells, EPCs might also be beneficial due to their paracrine effects through the secretion of salutary factors such as VEGF (Urbich et al, 2005) and angiogenic cytokines such as IL-8 (He et al, 2005).
Nonetheless, future EPC-based therapies cannot be designed without knowing how a pathologic environment will challenge the angiogenic response of these cells. The neuroinflammatory response that follows brain ischemia will include a dysregulation in multiple cytokines, chemokines, and matrix proteases broadly related to cell death, apoptosis or tissue degradation. In general, most would predict that neuroinflammation will be harmful for EPCs. In fact, other studies have showed that EPC function is compromised when exposed to tumor necrosis factor-α (He et al, 2004; Seeger et al, 2005), a relevant cytokine known to be significantly upregulated after stroke (Offner et al, 2006) or brain trauma (Williams et al, 2007). However, recent studies also suggest that some neuroinflammatory factors might counter-intuitively augment the angiogenic response of EPCs. Hence, growth factors such as GM-CSF (Takahashi et al, 1999), chemokines such as SDF-1 (Yamaguchi et al, 2003), and cytokines such as IL-6 (Fan et al, 2008) appear to increase EPC survival, adhesion, differentiation, or proliferation.
Our results here show that mouse-spleen-derived EPCs exposed to IL-β improved the angiogenic response by (1) increasing the number of EPCs in culture, (2) enhancing cell viability/proliferation, (3) improving the in vitro angiogenic functionality, and (4) some of these effects may operate through ERK MAP kinase signaling. To begin with, exposing spleen MNC to IL-1β rapidly increased the number of attached cells and promoted their differentiation into EPCs. Although the absolute number of cells naturally decreases between days 3 and 5 in control conditions, this is not affected by IL-1β treatment, indicating that we cannot exclusively establish protective effects on cell survival. But overall, the percentage of both total and differentiated EPCs was significantly higher under IL-1β conditions compared with controls.
As described earlier, there are two kinds of EPCs with different functions and properties in this model system (Hur et al, 2004). In our hands, early cells with spindle shape appeared from round differentiating cells beginning from day 3. Later on, additional cells emerged with different morphology (larger cytoplasmatic surfaces and defined nuclei), which can be outgrown into expanding colonies, and finally expanded into monolayers. A recently published review (Hirschi et al, 2008) proposed a new scheme to classify EPC biology in culture. ‘Early outgrowth’ cells that do not form colonies (when obtained after 4 days of culture on fibronectin-coated plates) yield circulating angiogenic cells as potent regulators of any recruited or stimulated angiogenic response. These circulating angiogenic cells would comprise our classic ‘early EPCs’ defined by Hur and colleagues. However, Hirschi and colleagues proposed a new term of endothelial colony forming cells for highly proliferative expanding colonies that appear 7 to 14 days (after plating and discarding nonadherent cells) with truly in vivo vasculogenesis capacity. These ECFCs would comprise the ‘late outgrowth’ cells used in our present study. Exposing early EPCs to continuous IL-1β stimulus accelerated the appearance and expansion of these endothelial-like cells that bind lectin and take up ac-LDL. Outgrown cells from these colonies were functionally capable to shape vessel-like structures in vitro, consistent with other findings (Hur et al, 2004). Thereafter, exposure to IL-1β improved their functionality as they formed tubular structures and vascular connections on a Matrigel substrate. This exogenous modification on the functional angiogenic response of the outgrowth EPCs has been reported earlier by other authors to decrease with tumor necrosis factor-α (He et al, 2004) and increase with IL-6 stimulation (Fan et al, 2008). Our study shows for the first time that IL-1β stimulation triggers a strong in vitro angiogenic response. Additionally, our MTT measurements are consistent with these IL-1β effects on proliferation and metabolic integrity. Although the decrease of MTT reduction is a potential indication of cell toxicity, other authors have showed that the increase on cell viability together with the increase on cell number indicates an effect on cell proliferation (Jang et al, 2007; Wang et al, 2007). Our MTT results (increase on cell viability) and the fact that the number of EPCs was also increased in the presence of IL-1β indicate a role on proliferation function. The early and continuous stimulation from initial seeding could influence attachment, survival, and proliferation functions, but our experiments exposing EPCs to IL-1β at day 3 of culture (after initial attachment occurred) suggest that there might also be independent proproliferation actions on these EPCs. Of course, our MTT assays may reflect ‘prevention-of-death’ plus ‘enhanced-proliferation.’ Hence, the overall stimulatory effects we document here by cell counting and MTT assays will likely represent an integrated response of both survival plus/minus proliferation. Indeed, activation of the ERK MAP pathway here may be consistent with highly conserved mechanisms, which allow cells to respond to external stimuli such as IL-1β. ERK MAP kinase is traditionally thought to lie at the heart of signaling networks that govern proliferation, differentiation, and cell survival (Kolch, 2000). For progenitor cells, similar ERK activation profiles have been described for IL-6 and EPCs (Fan et al, 2008) and for VEGF-induced endothelial differentiation (Xu et al, 2008).
In general, our results suggest a proangiogenic role of IL-1β on EPCs. These data differ from a recent investigation performed by Henrich and colleagues whereby low doses of IL-1β (0.025 and 0.25 ng/mL) reduced the number of ac-LDL/Lectin positive cells and induced the number of apoptotic annexin V-positive EPCs (only at 0.25 ng/mL). One might hypothesize that technical differences including the source of EPCs (pheripheral blood MNCs in the Henrich study), the cell culture protocol, and the lower IL-1β concentration used could partially explain discrepancies in the results. An important point in terms of variance may especially relate to the source of EPCs. Different investigators have shown earlier that EPCs can be isolated from peripheral blood, umbilical blood cord, bone marrow, or spleen and then further expanded ex vivo. However, it is important to notice that no single definition exists for EPCs, and precise lineages of these vascular progenitor stem cells are still operationally characterized (Fadini et al, 2008; Prater et al, 2007). In our system, we isolated spleen-derived EPCs following standard procedures as reported by others (Brühl et al, 2004). These EPCs display endothelial characteristics such as the uptake of ac-LDL and Lectin binding and expressed endothelial marker proteins such as Flk-1 or vWF together with the primitive progenitor cell marker CD133 and hematopoietic progenitor CD34 antigen. These markers have been widely used by other investigators to phenotype the EPCs (Asahara et al, 1997; Friedrich et al, 2006; Peichev et al, 2000; Santhanam et al, 2008; Seeger et al, 2005).
Nevertheless, these findings alert us to the complex dualities for neuroinflammation. Depending on context, cytokines may cause cell death or promote angiogenesis and tissue repair. A few studies in humans have reported an increase in acute IL-1β blood level after stroke (Mazzotta et al, 2004) and in brain parenchyma after a cranial contusion (Holmin and Höjeberg, 2004). More detailed investigations into the temporal profile of IL-1β levels in blood and brain after these acute brain diseases are needed to determine the environmental conditions in which EPCs exist and to determine the best timing for potential EPC-based treatments.
In this investigation, we have been able to explore IL-1β angiogenic responses at different time points (from 24 h in the matrigel and MTT assays, and at 3 to 7 days also in the MTTor cell counting experiments). However, we acknowledge that faster responses may operate in vivo. Furthermore, there may be critical differences in terms of concentrations. In vivo human and rodent data suggests that systemic levels of IL-1β after ischemia, measured in peripheral blood may sometimes be in the picogram range (Mazzotta et al, 2004; Sotgiu et al, 2006; Fox et al, 2005). These are lower than our in vitro concentrations used here and in other cell signaling studies (Fan et al, 2008; Argaw et al, 2006; Sung et al, 2005; Ducut-Sigala et al, 2004, Fan et al, 2004). This is known to be a limitation and major difference between what might be happening in vivo versus the types of experiments usually performed in cell cultures. It is difficult to truly reconcile differences in signaling concentrations, but what happens at the cell surface in terms of local microconcentrations may be completely different from the overall bulk concentrations that may be assessed in plasma in vivo. The higher concentrations we (and many others) use for cell cultures may reflect local signaling microenvironments not reflected in bulk plasma averages. In any case, our concentrations used here are not different from what is normally reported in the cell signaling literature. Ultimately, pharmacologic or genetic studies are needed to elucidate the true actions of cytokines such as IL-1β on EPCs in vivo.
In conclusion, our study provides evidence of a proangiogenic response of spleen-derived EPCs when exposed to IL-1β, suggesting that acute inflammatory stages of stroke or brain trauma can surprisingly provide favorable conditions for cell-based angiogenic therapies by promoting endogenous repair by augmenting EPC functionality. Further dissection of the inflammatory signaling pathways dose-response and temporal profiles in EPCs is warranted before this promising approach can be truly translated into clinical applications for stroke and neurorecovery.
Acknowledgements
Supported in part by NIH grants P01-NS55104, P50-NS10828, R37-NS37074, R01-NS48422, R01-NS53560, and a Bugher award from the American Heart Association. AR is the recipient of a postdoctoral grant from Ministerio de Educacion y Ciencia (EXT2006/766) and MN is the recipient of a predoctoral grant from Fondo de Investigación Sanitaria (FIS06/471).
Footnotes
Disclosures
The authors report no conflict of interest.
References
- Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Brühl T, Heeschen C, Aicher A, Jadidi AS, Haendeler J, Hoffmann J, Schneider MD, Zeiher AM, Dimmeler S, Rössig L. p21Cip1 levels differentially regulate turnover of mature endothelial cells, endothelial progenitor cells, and in vivo neovascularization. Circ Res. 2004;94:686–692. doi: 10.1161/01.RES.0000119922.71855.56. [DOI] [PubMed] [Google Scholar]
- Ducut-Sigala JL, Bottero V, Young DB, Shevchenko A, Mercurio F, Verma IM. Activation of transcription factor NF-kappaB requires ELKS, an IkappaB kinase regulatory subunit. Science. 2004;304:1963–1967. doi: 10.1126/science.1098387. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: Ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- Fan F, Stoeltzing O, Liu W, McCarty MF, Jung YD, Reinmuth N, Ellis LM. Interleukin-1beta regulates angiopoietin-1 expression in human endothelial cells. Cancer Res. 2004;64:3186–3190. doi: 10.1158/0008-5472.can-03-0407. [DOI] [PubMed] [Google Scholar]
- Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, Chen Y, Lawton MT, Young WL, Yang GY. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro . J Cereb Blood Flow Metab. 2008;28:90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM, Vexler ZS. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:e20–e25. doi: 10.1161/01.RES.0000205765.28940.93. E-pub 2006 Jan 26. [DOI] [PubMed] [Google Scholar]
- He T, Peterson TE, Katusic ZS. Paracrine mitogenic effect of human endothelial progenitor cells: role of interleukin-8. Am J Physiol Heart Circ Physiol. 2005;289:H968–H972. doi: 10.1152/ajpheart.01166.2004. [DOI] [PubMed] [Google Scholar]
- He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- Henrich D, Seebach C, Wilhelm K, Marzi I. High dosage of simvastatin reduces TNF-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1beta in vitro. J Surg Res. 2007;142:13–19. doi: 10.1016/j.jss.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmin S, Höjeberg B. In situ detection of intracerebral cytokine expression after human brain contusion. Neurosci Lett. 2004;369:108–114. doi: 10.1016/j.neulet.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Issa R, Krupinski J, Bujny T, Kumar S, Kaluza J, Kumar P. Vascular endothelial growth factor and its receptor, KDR, in human brain tissue after ischemic stroke. Lab Invest. 1999;79:417–425. [PubMed] [Google Scholar]
- Jang JH, Kim SK, Choi JE, Kim YJ, Lee HW, Kang SY, Park JS, Choi JH, Lim HY, Kim HC. Endothelial progenitor cell differentiation using cryopreserved, umbilical cord blood-derived mononuclear cells. Acta Pharmacol Sin. 2007;28:367–374. doi: 10.1111/j.1745-7254.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori K, Murohara T. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71:196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- Kamm K, Vanderkolk W, Lawrence C, Jonker M, Davis AT. The effect of traumatic brain injury upon the concentration and expression of interleukin-1beta and interleukin-10 in the rat. J Trauma. 2006;60:152–157. doi: 10.1097/01.ta.0000196345.81169.a1. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- Mazzotta G, Sarchielli P, Caso V, Paciaroni M, Floridi A, Floridi A, Gallai V. Different cytokine levels in thrombolysis patients as predictors for clinical outcome. Eur J Neurol. 2004;11:377–381. doi: 10.1111/j.1468-1331.2004.00798.x. [DOI] [PubMed] [Google Scholar]
- Morgan R, Kreipke CW, Roberts G, Bagchi M, Rafols JA. Neovascularization following traumatic brain injury: possible evidence for both angiogenesis and vasculogenesis. Neurol Res. 2007;29:375–381. doi: 10.1179/016164107X204693. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Ohta T, Kikuta K, Imamura H, Takagi Y, Nishimura M, Arakawa Y, Hashimoto N, Nozaki K. Administration of ex vivo-expanded bone marrow-derived endothelial progenitor cells attenuates focal cerebral ischemia-reperfusion injury in rats. Neurosurgery. 2006;59:679–686. doi: 10.1227/01.NEU.0000229058.08706.88. discussion 679–686. [DOI] [PubMed] [Google Scholar]
- Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Pepper MS. Manipulating angiogenesis. From basic science to the bedside. Arterioscler Thromb Vasc Biol. 1997;17:605–619. doi: 10.1161/01.atv.17.4.605. [DOI] [PubMed] [Google Scholar]
- Pogue AI, Lukiw WJ. Angiogenic signaling in Alzheimer’s disease. Neuroreport. 2004;15:1507–1510. doi: 10.1097/01.wnr.0000130539.39937.1d. [DOI] [PubMed] [Google Scholar]
- Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–1149. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- Qin SL, Li TS, Takahashi M, Hamano K. In vitro assessment of the effect of interleukin-1beta on angiogenic potential of bone marrow cells. Circ J. 2006;70:1195–1199. doi: 10.1253/circj.70.1195. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, d’Uscio LV, Peterson TE, Katusic ZS. Activation of endothelial nitric oxide synthase is critical for erythropoietin-induced mobilization of progenitor cells. Peptides. 2008;29:1451–1455. doi: 10.1016/j.peptides.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rössig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- Sotgiu S, Zanda B, Marchetti B, Fois ML, Arru G, Pes GM, Salaris FS, Arru A, Pirisi A, Rosati G. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol. 2006;13:505–513. doi: 10.1111/j.1468-1331.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- Sung MJ, Kim W, Ahn SY, Cho CH, Koh GY, Moon SO, Kim DH, Lee S, Kang KP, Jang KY, Park SK. Protective effect of alpha-lipoic acid in lipopolysaccharide-induced endothelial fractalkine expression. Circ Res. 2005;97:880–890. doi: 10.1161/01.RES.0000186522.89544.4D. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Wang XB, Huang J, Zou JG, Su EB, Shan QJ, Yang ZJ, Cao KJ. Effects of resveratrol on number and activity of endothelial progenitor cells from human peripheral blood. Clin Exp Pharmacol Physiol. 2007;34:1109–1115. doi: 10.1111/j.1440-1681.2007.04667.x. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Wei HH, Dave JR, Tortella FC. Acute and delayed neuroinflammatory response following experimental penetrating ballistic brain injury in the rat. J Neuroinflammation. 2007;4:17. doi: 10.1186/1742-2094-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu X, Jiang Y, Chu L, Hao H, Liu Z, Verfaillie C, Zweier J, Gupta K, Liu Z. MAPK/ERK signaling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med. 2008;12:2395–2406. doi: 10.1111/j.1582-4934.2008.00266.x. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]