Abstract
Background and Purpose
Future demographic changes predict an increase in the number of patients with atrial fibrillation. As long-term anticoagulation for the prevention of ischemic strokes becomes more prevalent, the burden of warfarin-associated intracerebral hemorrhage (W-ICH) is likely to grow. However, little is known about the clinical aspects and pathophysiologic mechanisms of W-ICH. This study describes the development of a mouse model of W-ICH in which hematoma growth and outcomes can be correlated with anticoagulation parameters.
Methods
CD-1 mice were treated with warfarin (2 mg/kg per 24 hours) added to drinking water. ICH was induced by stereotactic injection of collagenase type VII (0.075 U) into the right striatum. Hemorrhagic blood volume was quantified by means of a photometric hemoglobin assay 2 and 24 hours after hemorrhage induction. Neurologic outcomes were assessed on a 5-point scale.
Results
The international normalized ratio in nonanticoagulated mice was 0.8±0.1. After 24 (W-24) and 30 (W-30) hours of warfarin pretreatment, international normalized ratio values increased to 3.5±0.9 and 7.2±3.4, respectively. Compared with nonanticoagulated mice, mean hemorrhagic blood volume determined 24 hours after hemorrhage induction was found to be 2.5-fold larger in W-24 mice (P=0.019) and 3.1-fold larger in W-30 mice (P<0.001, n=10 per group). Mortality at 24 hours after hemorrhage induction was 0% in nonanticoagulated mice, 10% in W-24 mice, and 30% in W-30 mice. Hematoma enlargement between 2 and 24 hours after hemorrhage induction was −1.4% for nonanticoagulated mice, 22.9% for W-24 mice, and 62.2% for W-30 mice.
Conclusions
This study characterizes the first experimental model of W-ICH. It may be helpful in gaining further insights into the pathophysiology of W-ICH and may be used for testing the efficacy of treatment strategies, such as hemostatic therapy, in this severe subtype of stroke.
Keywords: cerebral hemorrhage, warfarin, mice
The number of patients with atrial fibrillation will increase at least 2.5-fold within the next 5 decades, reflecting the growing proportion of elderly individuals.1,2 In parallel, the widespread use of oral anticoagulants for the prevention of atrial fibrillation-associated ischemic stroke will increase in importance. Consequently, a growing burden of warfarin-associated intracerebral hemorrhage (W-ICH), the most serious complication of long-term anticoagulation, can be expected.3–5 At present, ≈8000 to 10 000 cases of W-ICH are estimated to occur in the United States every year.6 W-ICH is an iatrogenic but particularly deadly form of stroke, with a shortterm mortality rate >50% that has not improved over time.6–8
To date, our knowledge on the clinical aspects of W-ICH is mainly based on retrospective studies and patient series. The majority of these have been limited by difficulties to adjust for a number of confounding factors. Some studies suggested a larger hematoma size in patients with W-ICH compared with spontaneous ICH in nonanticoagulated patients,9–11 but others did not confirm this finding.12,13 Whether a correlation exists between the intensity of anticoagulation and hematoma size remains controversial.9,12,14 More consistently, a higher rate of hematoma expansion after a certain time point12,15 and a worse clinical outcome5,8,11,12 have been reported in W-ICH patients.
At the present time, no widely accepted consensus exists about the optimal treatment for W-ICH. Current treatment guidelines for W-ICH may reflect mostly expert opinions rather than results from randomized, clinical trials.4,6 The underlying pathophysiologic mechanisms of W-ICH remain murky, in part due to the lack of suitable animal models. Activation of the coagulation cascade and the production of thrombin are known to be important steps in cell damage and blood– brain barrier disruption after ICH. It therefore seems plausible that W-ICH that potentially contains noncoagulated blood will induce a different tissue reaction than spontaneous ICH.16,17
This study aimed to characterize a mouse model of W-ICH that can be used as a reliable basis for future studies on the clinical and pathophysiologic aspects of the disease, as well as for preclinical testing of new therapeutic approaches.18 As a first point of interest, the influence of the level of anticoagulation on hemorrhagic blood volume, hematoma size, hematoma enlargement, and neurologic outcome was studied.
Materials and Methods
Animals
All experiments were performed according to an institutionally approved protocol in accordance with the National Institute of Health’s guide for the care and use of laboratory animals. For the entire study, male CD-1 mice age 12 to 16 weeks were used. One set of animals (n=46) was used to determine values of the international normalized ratio (INR) at baseline and at certain time points after warfarin administration. A second set of animals (n=99) was used to study hemorrhagic blood volume, hematoma size, hematoma enlargement, and neurologic outcome after hemorrhage induction in both anticoagulated and nonanticoagulated (control, C) mice.
Warfarin Administration
Because warfarin is known to be highly soluble in water,19 we chose to administer warfarin by oral uptake through bottled drinking water. A 5-mg Coumadin tablet (warfarin sodium, crystalline; Bristol Myers Squibb) was dissolved by stirring in 375 mL tap water. If one assumes a body weight of 40 g and a water consumption of 15 mL/100 g per 24 hours (see www.fau.edu/research/ovs/VetData/mouse.php), this dosage corresponds to a warfarin uptake of 0.08 mg (2 mg/kg) per mouse during 24 hours. Similar doses of warfarin were previously shown to effectively prolong prothrombin time in rodents.20,21 Warfarin administration in the drinking water was always started at ≈10 AM.
INR Measurements
While the mice were under deep anesthesia, a peritoneal midline incision was performed and 0.6 mL blood was drawn from the inferior caval vein with a 1-mL syringe and a 25-gauge needle. Blood was transferred to glass tubes (BD Vacutainer) containing 66.6 µL citrate. Measurements of INR values and prothrombin time were performed on a routine analyzer platform in the Hematology Laboratory of Massachusetts General Hospital. Reference values for prothrombin time in CD-1 mice have been published elsewhere.22
ICH Induction
While the mice were under isoflurane anesthesia (1.5% to 2%) with spontaneous respiration in a nitrous oxide/oxygen mixture, a small borehole was drilled, and a 32-gauge 0.5-µL microinjection needle (Hamilton, 7000 series) was slowly lowered into the right striatum at the following coordinates from the bregma: 0.0 mm anterior, 2 mm lateral, and 3.5 mm depth. During a period of 5 minutes, 0.5 µL of saline containing 0.075 U collagenase VII-S (Sigma) or saline alone was injected.23 The needle was left in place for 10 minutes and then slowly removed over 5 minutes. Afterward, the borehole was sealed with bone wax, the scalp was sutured closed, and the mice were allowed to recover. The whole surgical procedure lasted ≈35 minutes for each mouse. A heat lamp was used to maintain animal body temperatures.
Measuring Hemorrhagic Blood Volume by Quantitative Hemoglobin Content Determination
Two or 24 hours after hemorrhage induction, mice under deep (5%) isoflurane anesthesia underwent transcardial perfusion with 30 mL phosphate-buffered saline. Brains were removed, separated into left and right hemispheres, and placed in glass tubes containing 3 mL phosphate-buffered saline. After 30 seconds of homogenization, ultrasound was applied for 1 minute to lyse erythrocytic membranes. After centrifugation for 30 minutes (13 000 rpm, 4°C), 250 µL supernatant was added to 1000 µL Drabkins reagent. With use of a photometer, absorption rates were determined at 540 nm, and hemorrhagic blood volumes were calculated for the entire brain (both hemispheres) on the basis of a standard curve. Mice that were found dead in their cages 24 hours after hemorrhage induction were not eligible for transcardial perfusion before measurements were made. In these cases, we subtracted 2.1 µL from the total hemorrhagic blood volume determined for these brains. This value was found to be the mean difference in hemorrhagic blood volume between 3 unperfused and 3 perfused brains (data not shown).
Measuring Hematoma Size by Computer-Assisted Outlining of Brain Slices
Two and 24 hours after hemorrhage induction, mice were humanely killed under deep (5%) isoflurane anesthesia. Brains were removed and cut in slices according to a 1-mm mouse brain matrix. Under standardized conditions, images of the brain slices were taken with a digital camera. Hematoma size was calculated by ImageJ software (http://rsb.info.nih.gov/ij/). First, a binary image was created by using the software’s “make binary” function, in which the hematoma areas appear black and the remaining brain tissue, white. Second, an automated measurement of hematoma size (ie, black areas on the binary image) was performed. In 10 randomly selected brains, we compared the hematoma size as derived from the automated measurement with the results of a manually performed volumetry by a coworker who was blinded to all other results of the study. Linear regression analysis showed a very high correlation between the 2 methods (R2=0.976, P<0.001; unstandardized residuals: minimum=−4.66 mm3, maximum=6.23, SD=3.24).
Outcome Assessment
At 2 and 24 hours after hemorrhage induction, neurologic deficits were assessed on a 5-point outcome scale and a standard hanging wire test. For the 5-point scoring scale, mice were placed at the center of a stainless steel plate. Their spontaneous motion activity was videotaped for 60 seconds. If mice stopped moving during the observation period or did not move at all, they were picked up by the tail and again placed at the center of the plate to stimulate movements. In addition, a 30-second sequence was recorded to document forepaw activity when the mice were suspended by the tail. In a blinded fashion, videotapes were rated for neurologic status on a simple 5-point scale, which was adapted from a previously published version24: 0=no apparent deficit; 1=slight deficit, eg, extension deficit of right forepaw or slight instability during walking, but no circling; 2=circling to the right with at least some straight movements and some covering of distance; 3=heavy circling to the right without straight movements or no movements at all; and 4=death. For the hanging wire test, mice were gently placed on the wire until they had achieved a firm grip with their paws. The period of time to falloff was recorded. A maximum of 60 seconds of hanging was allowed, and the test was repeated 3 times for every mouse. Presurgical training was not performed. Twenty-four hours after hemorrhage induction, weight loss as an indirect parameter of stroke severity was also determined (percentage weight loss=[prehemorrhage weight—posthemorrhage weight]/prehemorrhage weight ×100).
Statistical Analysis
We used SPSS version 12.0 (SPSS Inc, Chicago, Ill) for statistical analysis. Hemorrhagic blood volume and hematoma size were compared between groups with the t test. Statistical analysis of ordinal data was performed with the χ2 test. The Pearson and Spearman rank tests were used for correlation analysis between hemorrhagic blood volume, hematoma size, and outcome.
Results
INR Kinetics
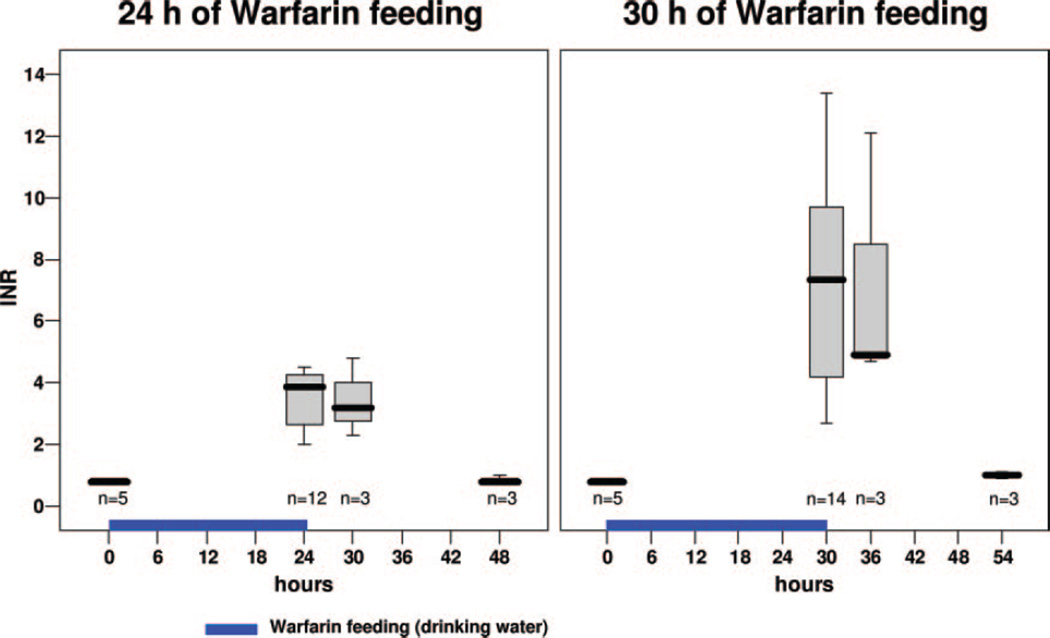
In C animals, mean INR values were found to be 0.8±0.1 (range, 0.8 to 0.9; n=5), corresponding to a mean prothrombin time of 10.3±0.6 seconds (range, 9.7 to 11.1 seconds). After 24 hours of warfarin administration, INR values were elevated (mean=3.5±0.9; range, 2.0 to 4.5; n=12) and reached the therapeutic span used in humans. After 30 hours of warfarin administration, INR values had increased to values beyond the human therapeutic range (mean=7.2±3.4; range, 2.7 to 13.4; n=14; see Figure 1). After 48 hours of warfarin administration, INR values exceeded the upper limit of the laboratory test range (n=2; INR >21; data not shown). On termination of warfarin administration, INR values remained stable for 6 hours and then dropped to normal values within the next 18 hours. In view of these results, we decided to use C animals as well as animals after 24 (W-24) and 30 (W-30) hours of warfarin treatment for investigating hemorrhagic blood volume, hematoma size, hematoma extension, and neurologic outcome (see study protocol in Figure 2).
Figure 1.
INR values before and after 24 hours (left) and 30 hours (right) of warfarin administration in drinking water. INR values were also determined 6 and 24 hours after withdrawal of warfarin administration.
Figure 2.
Experimental study protocol. C indicates control mice (no warfarin pretreatment); W-24, mice with 24 hours of warfarin feeding in drinking water; and W-30, mice with 30 hours of warfarin feeding in drinking water. ICH induction is the time point of ICH induction via stereotactic administration of collagenase into the right striatum. In all 3 groups, neurologic outcome, hemorrhagic blood volume (HBV), and hematoma size (HS) were determined 2 and 24 hours after hemorrhage induction.
ICH Induction
After 24 and 30 hours of warfarin treatment, mice demonstrated no observable signs of inner or external bleeding. All animals survived the surgical procedure of ICH induction. As demonstrated by sham-operated C, W-24, and W-30 animals (n=3 per group), the surgical procedure itself, including craniotomy, needle insertion into the striatum, and application of 0.5 µL saline, did not cause relevant intracerebral or subarachnoid bleeding. Figure 3 shows representative images of the needle track through the brain in sham-operated animals. Trace amounts of blood along the needle track and around the insertion point at the brain surface appeared to increase in parallel with INR values, but parenchymal (ie, striatal) hematoma or subarachnoid hemorrhage could not be identified. Furthermore, neurologic deficits were not apparent in sham-operated mice 24 hours after surgery (blindly rated; score=0 for all 9 sham-operated animals), and weight loss was not detected (C = 2.0±1.7%, W-24= −1.7± 1.5%, W-30=1.0±1.7%; see Figure 5C). Within the entire study, only 1 of 66 warfarin-treated animals developed a superficial subarachnoid hemorrhage on the ipsilateral cortex. This animal was excluded and replaced within the study protocol. Basal subarachnoid hemorrhages did not occur, although the intracerebral hematoma sometimes reached the outer parts of the cortex in severe cases.
Figure 3.
Left, Representative brain slices of the needle track 24 hours after sham operation (ie, administration of 0.5 µL saline into the right striatum) in C mice as well as in mice after 24 (W-24) and 30 (W-30) hours of warfarin pretreatment. Right, Representative brain slices 24 hours after hemorrhage induction by collagenase administration into the right striatum. In anticoagulated mice, the hematoma appeared to be slightly larger but much more dense compared with that in C mice. W-30 mice had amounts of still-noncoagulated blood.
Figure 5.
A, Neurologic outcome in C mice and in mice with 24 (W-24) and 30 (W-30) hours of warfarin pretreatment, as assessed on a 5-point scale. B, Results of the hanging wire test. The time period to falloff was recorded, and a maximum of 60 seconds of hanging was allowed. C, Percentage of body weight loss 24 hours after hemorrhage induction or sham operation.
Hemorrhagic Blood Volume Assessed by Quantitative Hemoglobin Content Determination
Two hours after hemorrhage induction, hemorrhagic blood volume (Figure 4) was 7.1± 1.7 µL in C animals, 14.4±3.2 µL in W-24 animals, and 13.5±8.5 µL in W-30 animals (C vs W-24, P=0.002; C vs W-30, P=0.134; n=5 per group). Twenty-four hours after hemorrhage induction, hemorrhagic blood volume was 7.0±1.5 µL in C animals. Warfarin-treated animals showed a 2.5-fold and a 3.1-fold increase in mean hemorrhagic blood volume compared with untreated animals (W-24=17.7±9.1 µL, P=0.019; W-30=21.9± 11.9 µL, P<0.001; n= 10 per group). Hemorrhagic blood volumes did not significantly differ between the W-24 and the W-30 groups (P=0.128). To assess the question of hematoma growth, we calculated the relative increase in hemorrhagic blood volume from 2 to 24 hours after hemorrhage induction, which was found to be −1.4% for C animals, 22.9% for W-24 animals, and 62.2% for W-30 animals.
Figure 4.
Left, Hemorrhagic blood volume measured 2 and 24 hours after hemorrhage induction by quantitative hemoglobin content determination in C mice and in mice with 24 (W-24) and 30 (W-30) hours of warfarin pretreatment. Right, Hematoma size measured 2 and 24 hours after hemorrhage induction by computer-assisted outlining of brain slices in C, W-24, and W-30 mice.
Hematoma Size Assessed by Computer-Assisted Outlining of Brain Slices
Between C and W-24 animals, mean hematoma size 24 hours after hemorrhage induction was not significantly different (32.3±7.7 vs 37.4±7.0 mm3, P=0.143), but the W-30 group showed a significantly larger hematoma size (64.4±24.8 mm3) compared with C animals (P<0.001) and with W-24 animals (P=0.001, n=10 per group). Correlation between hemorrhagic blood volume (measured by quantitative hemoglobin content determination) and hematoma size (determined by outlining of brain slices) revealed nonsignificant results for the 2-hour groups (R = −0.122, P=0.666) and a trend toward significance for the 24-hour groups (R=0.332, P=0.073). However, it has to be mentioned that assessment of hematoma size is not yet practicable in W-30 animals, as some blood was found to be fluid even after 24 hours, thus aggravating precise measurement due to smudging and smearing effects (see Figure 3). The given values for hematoma size of the W-30 group therefore should be interpreted with caution.
Mortality and Neurologic Outcomes
In parallel with an increasing intensity of anticoagulation, the 24-hour mortality rate increased from 0 of 20 in C animals to 2 of 20 in W-24 animals and to 6 of 20 in W-30 animals (C vs W-24, P=0.147; C vs W-30, P=0.008). Figure 5A shows the neurologic outcome at 2 and 24 hours after hemorrhage induction. Even at 2 hours after hemorrhage induction, the number of animals with a good or moderate outcome (score=0 to 2) was significantly diminished in the W-24 and W-30 groups compared with the C group (P=0.007, P=0.025, respectively). Twenty-four hours after hemorrhage induction, there was a trend for W-24 mice to have a worse outcome compared with C mice (P=0.113), but only the W-30 group reached the level of significance (P=0.003). A significant correlation was obtained between outcome and hemorrhagic blood volume (R = −0.379, P=0.039) as well as between outcome and hematoma size (R = −0.473, P=0.008).
Figure 5B shows the results from the hanging wire test. The proportion of mice that were able to hang on the wire for at least 60 seconds was significantly lower in W-24 compared with C animals (P=0.005). For the W-30 group, this comparison did not reach significance (P=0.242). However, owing to the high mortality rate in the W-30 group, a certain positive selection bias may have occurred.
Weight loss after 24 hours was significantly higher in W-24 animals compared with C animals (see Figure 5C; P=0.012). However, weight loss was not significantly different between C and W-30 animals (P=0.187). Once again, a selection bias may have occurred because of the high mortality rates in the latter group.
Discussion
Our study characterizes for the first time an experimental model of W-ICH in mice that may provide a reliable basis for studying both the clinical and pathophysiologic aspects of the disease as well as for performing preclinical therapeutic studies. Administering the highly water-soluble warfarin (2 mg/kg) in the drinking water for 24 hours resulted in well-reproducible INR values that closely mirrored the therapeutic range used for long-term anticoagulation in humans. After 30 hours of warfarin treatment, INR values increased beyond the therapeutic range. In human studies, as many as 20% of W-ICH patients were identified as having INR values >4.5 at hemorrhage onset.25 Thus, this model can simulate the human condition of effective anticoagulation with both therapeutic INR values and INR values beyond the therapeutic range.
A previous study investigated the time course of anticoagulant effects in mice after oral administration of warfarin at a single dose of 3 mg/kg and found a sharp peak in prothrombin time prolongation 18 hours after treatment.21 Both baseline prothrombin time values and the maximum ≈ 6-fold prothrombin time prolongation are comparable to our findings. Besides being easier to handle than oral feeding at a single time point, administration of warfarin in drinking water may result in a flatter INR kinetics curve with more stable values during collagenase-induced hematoma formation. However, the INR values in our mouse model showed a certain diversity, particularly after a 30-hour feeding period. This might be due to either individual differences in warfarin metabolism or individual variations in daily water consumption, leading to different amounts of warfarin intake.
Our model of W-ICH was derived from the well-established collagenase model of ICH in mice,23 but modifications were undertaken to avoid intraperitoneal injections of anesthetic drugs. Despite having impaired coagulation, sham-operated animals of both the W-24 and W-30 groups did not show parenchymal or subarachnoid hemorrhage. Considering the entire study, the risk for subarachnoid hemorrhage was low (1/66 anticoagulated animals). These data suggest that the model of collagenase-induced ICH can be reliably and safely applied with a 32-gauge needle in anticoagulated mice.
Our study is the first to provide experimental data on how hemorrhagic blood volume and neurologic outcome depend on the level of anticoagulation. Our data suggested a 2.5-fold increase in hemorrhagic blood volume after 24 hours in warfarin-treated animals with INR values near the therapeutic range used in humans compared with those in C animals. W-ICHs that occurred with INR values beyond the therapeutic range resulted in a 3.1-fold increase of hemorrhagic blood volume. A caveat is that we did not determine individual INR values in animals used for hemorrhage induction. So we could not directly assess whether a relation existed between INR values and hemorrhagic blood volumes, an issue that was previously discussed controversially in human studies.9,12,14 However, in a comparison between W-24 and W-30 animals as groups, a 106% increase in mean INR value was accompanied by only a 24% increase in mean hemorrhagic blood volume. These data suggest the existence of a certain limit of hemorrhagic blood volume rather than a linear relation. In concordance with clinical studies,8 we found that an increasing mortality rate appeared to track with increasing INR values.
Interestingly, despite a higher hemorrhagic blood volume (measured by quantitative hemoglobin content determination) in W-24 animals compared with C animals, hematoma size (measured by computer-assisted outlining of brain slices) was not significantly different between these groups. Thus, we speculate that the density of the hematoma (ie, the ratio of hemorrhagic blood volume and hematoma size) was higher in W-ICH (0.47 µL/mm3) than in non W-ICH (0.22 µL/mm3) animals. The increased density of the hematoma may lead to a greater degree of tissue destruction within the clot, which may worsen neurologic outcome (see Figure 3). Our findings in part explain the discrepant results regarding hematoma size in human W-ICH studies. Whereas some authors reported significantly larger hematoma size in W-ICH,9–11 others did not.12,13 However, a worse clinical outcome in W-ICH was consistently reported.5,8,11,12 In our study, the larger hemorrhagic blood volumes in W-ICH mice were unambiguously matched with a higher mortality rate and a worse neurologic outcome compared with C mice. In this context, it might be interesting to investigate in our model whether and when the large amounts of blood entering the brain lead to a critical increase in intracranial pressure and subsequent cerebral herniation. Future studies may focus on comparing these parameters between W-ICH and ICH that occurs in nonanticoagulated animals.
Some clinical studies have reported that a blood sedimentation level reflecting uncoagulated blood can be found in W-ICH.25 Our study supports this finding, as we were able to detect uncoagulated blood in the brains of the W-30 group after 24 hours, leading to smearing effects when cutting the brains in a 1-mm matrix (see Figure 3). For this reason, quantification of hematoma size on 1-mm slices is difficult. From a technical standpoint, this mouse model of W-ICH is better quantified with the hemoglobin assay.
Our study design also allowed us to investigate hematoma enlargement. Previous studies in humans have described a higher rate of hematoma enlargement in W-ICH after a certain time point in comparison with ICH in nonanticoagulated patients.12,15 Our experimental model supports these clinical findings. In fact, we could not identify any hematoma growth between 2 and 24 hours after hemorrhage induction in C animals. In contrast, W-24 and W-30 animals showed considerable hematoma enlargement between 2 and 24 hours after hemorrhage induction (22.9% and 62.2%, respectively). Thus, our study supports the hypothesis that the time window of ongoing bleeding is prolonged in W-ICH. This is important, because it suggests that there may be a window of opportunity for hemostatic treatment to prevent further hematoma growth.6 However, this window may be narrow. By 2 hours, hemorrhagic blood volume was already 2.0-fold higher in W-24 animals and outcome was significantly worse compared with C animals. Thus, based on our animal model, treatment at very early time points might be most beneficial.
Several caveats have to be considered when translating our experimental results to human W-ICH. First, the time course and extent of collagenase-induced hematoma formation in the mouse model may be different from that in humans. We do not know whether a similar degree of anticoagulation (assessed by INR measurement) in mice and humans results in a similar relative increase in hemorrhagic blood volume. In this context, it is also important to mention that different types of coumarin with different half-lives are being used for long-term anticoagulation in humans (ie, coumadin, phenprocoumon). Furthermore, our model investigated collagenase-induced hematoma formation originating from the striatum. Translated into human W-ICH, this would mimic deep hemispheric hemorrhage rather than lobar hemorrhage, particularly if the latter arises from amyloid angiopathy or vascular malformations. Pathophysiologically, it remains unclear whether differences exist between W-ICH and ICH under normal coagulation conditions in terms of neuronal death, edema formation, blood–brain barrier damage, and inflammatory response. Further studies are needed to investigate the time course of these parameters after hemorrhage induction. This may include the issue of rebleeding, which was shown to occur more frequently in W-ICH, even at later time points.12 However, in the mouse model, it is very difficult to sustain an appropriate state of anticoagulation after hemorrhage induction because withdrawal of warfarin will result in a rather fast normalization of INR values (ie, within 24 hours).
In conclusion, our study characterized a reliable experimental model of W-ICH in mice. It may be helpful in obtaining further insight into the pathophysiology of human W-ICH and may be used for testing the efficacy of treatment strategies, such as hemostatic therapy, for ameliorating the devastating outcomes of this severe subtype of stroke.
Acknowledgments
Sources of Funding
This study was supported in part by a grant from the Deutsche Forschungsgemeinschaft and by National Institutes of Health grants R01-NS37074, R01-NS48422, R01-NS53560, and R01-NS56458.
Footnotes
Disclosures
None.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol. 2005;14:56–61. doi: 10.1111/j.1076-7460.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- 3.Kucher N, Castellanos LR, Quiroz R, Koo S, Fanikos J, Goldhaber SZ. Time trends in warfarin-associated hemorrhage. Am J Cardiol. 2004;94:403–406. doi: 10.1016/j.amjcard.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 4.Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–262. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty ML, Kissela B, Woo D, Kleindorfer D, Alwell K, Sekar P, Moomaw CJ, Haverbusch M, Broderick JP. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–121. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar MI, Hart RG, Kase CS, Freeman WD, Hoeben BJ, Garcia RC, Ansell JE, Mayer SA, Norrving B, Rosand J, Steiner T, Wijdicks EF, Yamaguchi T, Yasaka M. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:82–92. doi: 10.4065/82.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Sjoblom L, Hardemark HG, Lindgren A, Norrving B, Fahlen M, Sam-uelsson M, Stigendal L, Stockelberg D, Taghavi A, Wallrup L, Wallvik J. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: a Swedish multicenter study. Stroke. 2001;32:2567–2574. doi: 10.1161/hs1101.098523. [DOI] [PubMed] [Google Scholar]
- 8.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 9.Franke CL, de Jonge J, van Swieten JC, Op de Coul AA, van Gijn J. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21:726–730. doi: 10.1161/01.str.21.5.726. [DOI] [PubMed] [Google Scholar]
- 10.Radberg JA, Olsson JE, Radberg CT. Prognostic parameters in spontaneous intracerebral hematomas with special reference to anticoagulant treatment. Stroke. 1991;22:571–576. doi: 10.1161/01.str.22.5.571. [DOI] [PubMed] [Google Scholar]
- 11.Neau JP, Couderq C, Ingrand P, Blanchon P, Gil R. Intracranial hemorrhage and oral anticoagulant treatment. Cerebrovasc Dis. 2001;11:195–200. doi: 10.1159/000047638. [DOI] [PubMed] [Google Scholar]
- 12.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 13.Fogelholm R, Eskola K, Kiminkinen T, Kunnamo I. Anticoagulant treatment as a risk factor for primary intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1992;55:1121–1124. doi: 10.1136/jnnp.55.12.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berwaerts J, Dijkhuizen RS, Robb OJ, Webster J. Prediction of functional outcome and in-hospital mortality after admission with oral anticoagulant-related intracerebral hemorrhage. Stroke. 2000;31:2558–2562. doi: 10.1161/01.str.31.11.2558. [DOI] [PubMed] [Google Scholar]
- 15.Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J, Mueller S, Meyding-Lamade U, Strobl R, Mansmann U, Schwab S, Steiner T. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–1470. doi: 10.1161/01.STR.0000221786.81354.d6. [DOI] [PubMed] [Google Scholar]
- 16.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 17.Levine JM, Snider R, Finkelstein D, Gurol ME, Chanderraj R, Smith EE, Greenberg SM, Rosand J. Early edema in warfarin-related intracerebral hemorrhage. Neurocrit Care. 2007;7:58–63. doi: 10.1007/s12028-007-0039-3. [DOI] [PubMed] [Google Scholar]
- 18.Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 2005;36:e23–e41. doi: 10.1161/01.STR.0000155685.77775.4c. [DOI] [PubMed] [Google Scholar]
- 19.Palareti G, Legnani C. Warfarin withdrawal: pharmacokinetic-pharmacodynamic considerations. Clin Pharmacokinet. 1996;30:300–313. doi: 10.2165/00003088-199630040-00003. [DOI] [PubMed] [Google Scholar]
- 20.Dickneite G. Prothrombin complex concentrate versus recombinant factor VIIa for reversal of coumarin anticoagulation. Thromb Res. 2007;119:643–651. doi: 10.1016/j.thromres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Taniuchi Y, Kawasaki T, Hirayama F, Koshio H, Matsumoto Y, Iizumi Y. Comparison of the anticoagulant and antithrombotic effects of YM-75466, a novel orally-active factor Xa inhibitor, and warfarin in mice. Jpn J Pharmacol. 1998;78:191–197. doi: 10.1254/jjp.78.191. [DOI] [PubMed] [Google Scholar]
- 22.Lemini C, Jaimez R, Franco Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb Res. 2007;120:415–419. doi: 10.1016/j.thromres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998;29:2136–2140. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- 24.Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa K, Yanagihara C. Sedimentation level in acute intracerebral hematoma in a patient receiving anticoagulation therapy: an autopsy study. Neuroradiology. 1998;40:380–382. doi: 10.1007/s002340050604. [DOI] [PubMed] [Google Scholar]